Abstract
Pharmacotherapy has long been used to control viral diseases. However, its success is questionable because its use can negatively impact environmental and human health. An alternative solution is the use of functional foods and diets containing natural products, which tend to be more biodegradable than synthetic molecules and are less likely to generate resistance. Seaweed contains biologically active macronutrients and minerals that offer a natural alternative to synthetic molecules. Red seaweeds, in particular, are a rich source of anti-viral compounds. This study aimed to evaluate the effect of two edible red seaweeds, Pyropia columbina and Gracilaria chilensis cryo concentrates (RSCC), on the gene transcription levels in leukocyte proteins involved in antiviral response (INFγ, Mx, interleukin-6, cathelicidin, and lysozyme). The RSCCs were fed to fish (Salmo salar L.) at concentrations of 0.1, 1, or 10 g kg−1 for 56 days, and blood samples were collected at 8 weeks. The transcription levels of key genes associated with the antiviral response were analyzed by qRT-PCR using leukocyte mRNA as template. The Mx transcript level was significantly decreased (p < 0.05) with the RSCC diets, and lysozyme transcript levels were significantly increased (1 g kg−1P. columbina cryo concentrate). Cathelicidin, interleukin-6, and INFɣ had stable transcription levels. Importantly, RSCC modulated the immune-relevant gene that encodes the Mx antiviral protein in white blood cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence and appearance of new diseases continue to be one of the biggest obstacles to sustainable aquaculture. In salmonids, current strategies include the use of vaccines and antibiotics, but these can create resistant pathogens, host immunosuppression, and negative effects on the environment (Asif et al. 2018). Functional diets are an alternative that considers the inclusion of natural ingredients like marine seaweed.
Marine seaweeds can absorb and concentrate minerals, trace elements, vitamins, and amino acids used to meet their metabolic needs, this makes seaweed a valuable food source containing significant amounts of protein, vitamins, taurine (Sánchez-Machado et al. 2004; Marsham et al. 2007; Garcia-Vaquero and Hayes 2016), omega-3 fatty acids, and carotenoids (Ngo et al. 2011). They have a low ω6/ω3 ratio (Mohamed et al. 2012) and can make an important contribution to daily mineral intake.
Most seaweeds also have health benefits due to their low Na/K ratio with values of 0.14–0.16 (Mohamed et al. 2012). Compared to land plants, seaweeds contain 10 to 20 times the amount of minerals making them rich in macrominerals and trace elements (Mohamed et al. 2012; Moredo-Piñeiro et al. 2012). Seaweeds also have high ash content with values ranging from 20 to 50% on a dry basis (Pereira 2016). Red seaweeds are considered a particularly important source of many biologically active metabolites (El Gamal 2010), and are a major source of anti-viral compounds as has been shown using a variety of red seaweed extracts (Mohamed et al. 2012; Rhimou et al. 2015; Boulho et al. 2017; Castilla et al. 2017). However, few studies have been performed using whole seaweed cryo concentrates as an ingredient. We previously used two edible red seaweeds (Pyropia columbina and Gracilaria chilensis) cryo concentrates as a supplemental ingredient in salmon diet. Ex vivo assays showed that a diet supplemented with red seaweed significantly increased resistance against salmon anemia virus at the blood level and significantly increased the specific growth rate (39%) (Lozano et al. 2016).
Compared to mammals, fish immune systems have lower specificity and less robust response. They have limited immunoglobulins, low lymphocyte proliferation, and weak immunological memory, and this reduces the potential for long-term protection (Magnadóttir 2006). Thus, the innate or non-specific immunity might play a bigger role in fish than the specific or adaptive immunity (MacKenzie et al. 2004).
Innate immunity involves a large repertoire of biological mechanisms that include cellular and humoral components such as macrophages, natural killer (NK) cells, lysozymes, the complement system, cytokines such as interferon (IFN), and antimicrobial peptides including cathelicidins. All of these help regulate the receptors capable of recognizing the molecular patterns associated with foreign nucleic acids or viral surface glycoproteins. Interferon types I and II are induced as an innate antiviral immune response in vertebrates. Interferon type II, also called interferon gamma (IFN-ɣ), is produced by macrophages and cytotoxic cells (NK) (McBeath et al. 2007). Its most important function is the activation of the macrophages that are essential for the control of many microbial infections. It strongly stimulates the development of CD8+ T cell responses during an acute viral infection (Whitmire et al. 2005). In contrast, interleukin 6 (IL-6) is a vital innate immune cytokine necessary for promoting neutrophil-mediated viral clearance. IL-6 is produced by macrophages, dentric cells, mast cells, and other innate immune cells; it is a marker of inflammation (Dienz et al. 2012).
Lysozymes are produced by leukocytes (especially monocytes and neutrophils). These enzymes contribute to the antimicrobial activity of innate immunity (Saurabh and Sahoo 2008) and are found in immune-related cells such as macrophages, neutrophils, eosinophils, as well as epithelial and intestinal cells (Fletcher and White 1976). They can lyse Gram-positive bacteria and can coordinate with complement to lyse some Gram-negative bacteria. Lysozymes also have antimicrobial properties independent of their enzymatic activity (Paulsen et al. 2003); this significantly contributes to viral inhibition (Wei et al. 2012). Lysozyme mRNA expression and enzymatic activity increase in the presence of β-glucan and bacterial lipopolysaccharides (Paulsen et al. 2003; Ahmadi et al. 2014).
Cathelicidins are cationic peptides with antimicrobial activity that are constitutively present in granulocytes. These peptides are a part of the innate immune response in vertebrates and have been reported in different fish species (Broekman et al. 2013) including salmonids (Scocchi et al. 2009). Cathelicidins play an important role in fish innate immunity due to their antimicrobial activity and increased expression in response to pathogens (Chang et al. 2006; Broekman et al. 2013).
The Mx protein is an antiviral protein induced by interferon, the most important cytokine involved in innate immune system regulation (Haller et al. 2007). Mx is expressed in the cytoplasm and nucleus, and Mx expression has been shown to provide resistance against a broad range of viruses (Nygaard et al. 2000; Lester et al. 2012). It belongs to the dynamin superfamily of GTPases, and it might also play a role in intracellular transport, endocytosis, and in protein/vesicle transport via secretion and mitosis (Shirozu et al. 2016). The production of Mx in peripheral white blood cells has been reported as constitutive in human and other vertebrate leucocytes (Das et al. 2009). The alternative complement pathway is a powerful defense mechanism in lower vertebrates such as the teleosts (Ellis 2001; Morales et al. 2011; Kiron 2012). The complex innate defense mechanism can be constitutive or responsive in providing protection in the wild (Kiron 2012).
The aim of this study was to evaluate the effect of diets supplemented with two edible red seaweeds, Pyropia columbina and Gracilaria chilensis (Pereira 2016) cryo concentrates. The outcomes were levels of Mx, lysozyme, cathelicidin, IFN-ɣ, and IL-6 immune-relevant genes in uninfected Salmo salar blood leukocytes.
Materials and methods
Red seaweed cryo concentrates
Two edible red seaweeds P. columbina and G. chilensis were selected based on morphological description (Toledo et al. 2009) for the preparation of a cryo concentrate (CC). They were harvested from Ancud, Chile (41°52’S 73°19’W) during winter. The red seaweed cryo concentrates (RSCC) were obtained and cleaned, and the cryo concentrate was prepared using a freeze dryer. The RSCC was then vacuum packaged for subsequent grinding and inclusion in a balanced diet formulated by BioMar Chile SA (Castro, Chiloé Island, Chile).
Nutritional characterization of the RSCC
The nutritional characterization of the RSCC was performed versus reference samples of a standard national soybean meal made with ingredients produced nationally and provided by the Nutrition Laboratory, Faculty of Agricultural Sciences, University of Chile. The nutritional characterization was performed at the University of Missouri Agricultural Experiment Station Chemical Laboratories in Columbia, MO, USA and at the Nutrition Laboratory of the Department of Animal Production in the Faculty of Agronomic Sciences at the University of Chile.
The amino acid and fatty-acid profiles as well as proximate analyses were determined using the official methods of analysis of the Association of Official Analytical Chemists (AOAC): amino acid profile, AOAC 982.30; fatty acid profile, AOAC 996.06; proximate analyses and crude protein combustion analysis, AOAC 984.13A–D (2006); Kjeldahl method multiplying the nitrogen value by (6.25); ash determination, AOAC 942.05; sodium and potassium determination, AOAC 969.23 (2005); zinc determination, AOAC 999.11 (2005); calcium determination, AOAC 991.25 (2005); crude fat by extraction, AOAC 920.39A; crude fiber, AOAC 978.10 (2006); moisture content, AOAC 934.01 (2006); and total carbohydrates, crude “by difference” method, 100% − %(CP + ash + crude fat + M).
Experimental diets
The RSCC were ground to obtain 200 μm particles and mixed with a base of the BioMar Chile feed formulation in the following proportions to produce nine experimental diets: 0.1%, 1%, and 10% P. columbina or G. chilensis; 0.1 and 1% mixtures of both seaweeds in a 1:1 ratio; and a control diet with no supplemental seaweed (Table 1).
Experimental animals
Atlantic salmon (n = 486) with an initial mean body weight of 149.05 ± 32 g were randomly distributed into 27 experimental tanks with a continuous water flow of 6 L min−1, a summer photoperiod (LD 10:14), a mean temperature of 12 °C ± 0.52, and a tank volume of 200 L. Each treatment was performed in triplicate at the BioMar research center in Castro on Chiloé Island, Chile. The fish were acclimated for 2 weeks prior to feeding with the experimental diets for 8 weeks. Fish were fed twice daily to apparent satiation. Food intake was recorded daily, and the fish were weighed initially and at 8 weeks after initiating the diets.
Blood samples and isolation of leukocytes
Blood was collected from the fish after 8 weeks on the experimental and control diets. Three fish per tank were randomly selected and fasted for 24 h prior to sampling. To extract the blood, a fish was placed on an ice gel pack for approximately 30 s to reduce movement, and a 2-mL syringe was used to extract 0.5 mL of blood from the caudal vein. The blood was collected in a 2.0 mL cryovial with 0.8 mL of cold sterile Hank’s BSS (Gibco) medium (Fujiwara et al. 2001) without additives to prevent interference in the gene expression analysis (Lortat-Jacob et al. 1996; Johnson et al. 2003). After sampling, the fish were returned to the tank and recovered in about a minute. The blood was chilled in the cryovials on ice for 5 min before centrifuging at 258×g for 5 min at room temperature (RT). After centrifugation, the buffy coat and lymphocyte-rich plasma were collected in a 2.0-mL cryovial and placed in liquid nitrogen for transport. The cryovials were stored at − 80 °C in the Genetics and Aquaculture Laboratory in the Faculty of Agriculture at the University of Chile until the gene expression assay.
RNA isolation and cDNA synthesis
For RNA extraction, 750 μL of TRIzol was added to the white blood cells (leukocytes estimated to be 1.6% of the blood in salmonids, or ∼ 5.8 × 106 cells) (Fujiwara et al. 2001). The sample was then centrifuged at 12000×g for 10 min at 4 °C. The pellet was discarded, and the supernatant was incubated for 5–10 min at RT before adding 200 μL of chloroform. The sample was mixed by inversion for 15 s, incubated for 2–3 min at RT, and centrifuged at 12,000×g for 15 min at 4 °C before adding 1 μL of glycogen (50 mg mL−1) and 500 μL of isopropyl alcohol. The sample was then incubated for 10 min at RT and centrifuged for 10 min at 12000×g at 4 °C, and the supernatant was then removed. The RNA pellet was washed with ethanol (75%), centrifuged at 7500×g for 5 min at 4 °C, and allowed to dry for 10 min at RT after removal of the supernatant.
The RNA pellet was resuspended in RNAse-free water and incubated in a 55 °C water bath for 15 min. The quantity and purity of the extracted RNA was checked in a spectrophotometer (Biochrom WPA Biowave DNA Spectrophotometer); a 260/280 absorbance ratio of 1.8–2.1 was considered pure RNA.
Gene expression
Five genes (cathelicidin, lysozyme, IL-6, IFNɣ, and Mx) associated with the antiviral innate immune response were selected to evaluate the effects of the dietary ingredients on gene transcription levels in blood leukocytes. The elongation factor 1-alpha (EF1α) was used as a housekeeping gene. The primers were designed using the National Center for Biotechnology Information “Pick Primers” function (ncbi.nlm.nih.gov) and purchased from Integrated DNA Technologies, Inc., Santiago, Chile (Table 2).
Real-time polymerase chain reactions (qRT-PCR) were performed in a two-step system using Maxima SYBR Green/ROX qPCR Master Mix (2×; Thermo Scientific, Chile) and the Eco Real-Time PCR System (Illumina, USA). The first-strand cDNA was synthesized using Thermo Scientific RevertAid reverse transcriptase according to the supplier instructions.
PCR reactions were performed in duplicate in 48-well reaction plates in 20 μL reaction mixtures containing 10 μL of the 2× qPCR Master Mix, 0.3 μL forward primer, 0.3 μL reverse primer, 8.4 μL nuclease-free water, and 1 μL cDNA template. The wells were sealed with optical adhesive film, and the plate was centrifuged prior to amplification under the following conditions: 10 min at 95 °C (initial denaturation), followed by 40 cycles of 15 s at 95 °C (denaturation), and 60 s at 60 °C (annealing and extension). The amplification was confirmed by a melting curve analysis to verify that a single gene product had been amplified. The CT values provided by the real-time PCR instrumentation were imported to a Microsoft Excel spread sheet, and the gene expression fold changes were computed using the comparative 2–∆∆CT method, where ∆∆CT = (CT target gene − CT EF1α)treated sample − (CT target gene − CT EF1α)untreated control (Livak and Schmittgen 2001).
Statistical analysis
To test for differences between the dietary treatments on Mx, IL-6, IFNɣ, cathelicidin, and lysozyme gene expression, the data were subjected to a one-way analysis of variance (ANOVA) using SPSS Statistics version 22 (IBM Corporation, USA). All data were checked for homogeneity of variance prior to the ANOVA. When differences among groups were identified, multiple comparisons to the control were made using Dunnett’s post-hoc test. A difference was considered significant if p was < 0.05.
Results
Red seaweed cryo concentrates
The nutritional characterization of the RSCC showed higher taurine values in the amino acid profile. RSCC were characterized by high amounts of ash and analyzed minerals. The RSCCs had lower values for protein content than soybean meal and a wider range of amino acids. However, the taurine levels in RSCC were 16-fold greater than that in the soybean meal. The proximate and mineral analyses showed an ash content three times higher in the RSCC than in the soybean meal; potassium, calcium, and sodium levels were twice as high in the RSCC as in the soybean meal (Table 3).
There was also a difference in the fatty acid profiles between RSCC and the soybean meal. The RSCCs had high palmitic (16:0) and arachidonic acid (20:4n6) contents, while the soybean meal had a high linoleic acid (18:2n6) content and no arachidonic acid. Myristoleic fatty acids (9c-14:1) were also only present in the RSCC. Of the omega-3 fatty acids, eicosapentaenoic acid (EPA) (20:5n3) and DHA (22:6n3), EPA was only present in P. columbina CC, and docosahexaenoic acid (DHA) was only present in G. chilensis CC. Neither of these omega-3 fatty acids were present in the soybean meal (Table 4).
Experimental animals
No mortality was observed during the experimental period, and all experimental diets were well accepted by the animals; no toxic or pathological signs were observed. No significant differences were observed in the feed intake across groups regardless of RSCC supplement. The diet supplemented with 10% G. chilensis resulted in a significant increase in weight gain versus control diet (Table 5).
Gene expression
After 8 weeks, the qRT-PCR results showed that the Mx transcript levels in the blood leucocytes of the animals on the diets supplemented with RSCC were significantly lower (p < 0.05) than those fed with an unsupplemented control diet. All of the groups fed, the RSCC-experimental diets had negative values for Mx expression in their white blood cells at the end of the experiment (Fig. 1).
Effect of RSCC inclusion level on Mx expression in blood white cells (n = 9), expression levels are reported as fold change relative to untreated control. A Pyropia columbina CC, B Gracilaria chilensis CC, and Mix mixture of both seaweed cryo concentrates in a 1:1 ratio. *Significantly different from the control, p < 0.05, Dunnett’s test. Boxes represent the interquartile range (IQR) between the first and third quartiles (25th and 75th percentile), and the horizontal line defines the median
The lysozyme transcript levels showed that the constitutive expression significantly increased in the animals fed with the diet supplemented with 1.0% P. columbina CC. The lysozyme levels were increased in all animals fed with diets that included P. columbina CC, whether alone or mixed 1:1 with G. chilensis CC. In contrast, there was no effect on the lysozyme transcript levels for the animals fed with diets supplemented with only G. chilensis CC; their expression levels were similar to those observed in the control group (Fig. 2).
Effect of RSCC inclusion level on lysozyme expression in blood white cells (n = 9), expression levels are reported as fold change relative to untreated control. A Pyropia columbina CC, B Gracilaria chilensis CC, and Mix mixture of both seaweed cryo concentrates in a 1:1 ratio. * Significantly different from the control, p < 0.05, Dunnett’s test. Boxes represent the interquartile range (IQR) between the first and third quartiles (25th and 75th percentile), and the horizontal line defines the median
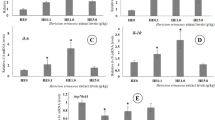
There were no significant changes in cathelicidin, IFNɣ, or IL-6 gene expression in any of the groups fed with the RSCC diets relative to the control diet. However, it is interesting that while no statistically significant changes were observed, there was a decrease in the cathelicidin and IFNɣ transcript levels especially for IFNɣ. The distribution of values was wide (Fig. 3a, b). In contrast, the IL-6 results suggest that its gene expression was induced. Although the values were not statistically significant, IL-6 expression increased in the animals fed with 0.1 and 1.0% mixed diets containing both P. columbina and G. chilensis CC (Fig. 3c).
Effect of RSCC inclusion level on (A) Cathelicidin, (B) INF-ɣ, and (C) IL6 expression in blood white cells (n = 9), expression levels are reported as fold change relative to untreated control. A Pyropia columbina CC, B Gracilaria chilensis CC, and Mix mixture of both seaweed cryo concentrates in a 1:1 ratio. Boxes represent the interquartile range (IQR) between the first and third quartiles (25th and 75th percentile), and the horizontal line defines the median
Dunnett’s test showed statistically significant differences between all groups fed with the RSCC-supplemented diets and the control-diet group (Table 6). There was a significant decrease in Mx expression in all groups fed with the supplemented diets, especially those groups fed with 1% P. columbina CC and 1% G. chilensis CC. These groups had − 1.25 ± 0.19 and − 1.05 ± 0.37-fold lower mRNA expression, respectively. A similar decrease was also observed for the 0.1% mixed-diet group (−1.19 ± 0.34). While this feed contained one tenth of the amount of seaweed CC, both seaweed cryo concentrates were incorporated. Finally, the lysozyme levels increased significantly (1.73 ± 0.06) in the group fed with a diet enriched with 1% P. columbina CC.
Discussion
The nutritional characterization showed that both RSCC materials had higher ash, taurine, potassium, calcium, sodium, as well as myristic and palmitic fatty acid contents than soybean meal. Myristoleic (9c-14:1) and arachidonic (20:4n6) fatty acids were present in both seaweed cryo concentrates but not in the soybean meal. The RSCC had ash content that was at least 3-fold higher than that in the soybean meal; the potassium and calcium contents were at least 1.5-fold higher in the marine seaweeds.
This in vivo study showed that the G. chilensis red seaweed concentrate increases weight gain; neither seaweed cryo concentrates had a significant effect on cathelicidin, IFN-ɣ, or IL-6 expression. Similar results have been reported for IFN-ɣ and IL-6 transcripts in the blood of pigs fed with brown seaweed Laminaria hyperborea and Laminaria digitata extracts where neither cytokine nor showed significant changes (Reilly et al. 2008). However, a significant increase in lysozyme expression was observed after 8 weeks in animals on the diet enriched with P. columbina (1.0%) concentrate. Similar results have been reported for enrichment diets made with free-dried powder (3%) from the red seaweed Asparagopsis taxiformis, garlic, and curcumin, each of these significantly increased the lysozyme transcript levels in the spleen of orbicular batfish (Reverter et al. 2016).
Marine oil-derived EPA inhibits arachidonic acid, which activates of NF-κB, and thus many inflammatory genes (Grimm et al. 2002). Diets with moderate levels of dietary linoleic acid (0.32 to 0.63% dry weight), a precursor to EPA and DHA, have been described as significantly enhancing anti-inflammatory responses (Chen et al. 2016; Statovci et al. 2017) and non-specific immunity via lysozyme and complement activity (Chen et al. 2016). Omega-3 polyunsaturated fatty acids can also activate the anti-inflammatory transcription factor PPAR-γ and inhibit NF-κB and production of its associated pro-inflammatory cytokine (Statovci et al. 2017). P. columbina concentrate was characterized by a high EPA content (44.19%), which was absent in G. chilensis. This suggests that the presence of the EPA in P. columbina is associated with the significantly increased lysozyme expression.
Interestingly, our results showed that experimental diets containing both RSCCs significantly decreased the expression of Mx transcript in peripheral white blood cells. This was observed in all doses of RSCC. Other studies have shown that Mx provides antiviral resistance for uninfected cells (Nygaard et al. 2000) and early protection for a several viral diseases (Lester et al. 2012; Purcell et al. 2012). The mechanism by which the RSCC supplementation decreases Mx expression in white blood cells is unknown. One hypothesis is that the high content of potassium, the primary intracellular cation (Halperin and Kamel 1998) provided by the RSCC, increased intracellular uptake and stimulated the sodium-potassium pump adenosine triphosphatase (Na+/K+-ATPase), a membrane-bound enzyme that imports potassium into cells by diffusion through a concentration gradient (Cantley et al. 1978; Skou and Esmann 1992; Goldstein et al. 2009).
The energy released in this reaction (Skou and Esmann 1992; Lingrel and Kuntzweiler 1994) plus the presence of the exogenous calcium ions from the RSCC could induce regulated exocytosis or, in this case, calcium-dependent exocytosis (Bi et al. 1995; Alés et al. 1999). This would decrease the Mx transcript in the white blood cells due to its participation in intracellular transport and protein export via secretion (Shirozu et al. 2016). Similar results have been reported in the liver of sea bream (Sparus aurata), where no Mx mRNA expression was detected in healthy animals or animals fed with a marine oil-based diet. Feeding the animals vegetable oils altered Mx transcription; this treatment markedly increased the basal Mx expression levels (Montero et al. 2008).
Conclusions
Our in vivo study is the first to show the potential of two edible red seaweed cryo concentrates (P. columbina and G. chilensis) to modulate the expression of the immune-relevant genes encoding the Mx antiviral protein in S. salar white blood cells within 8 weeks of administration. In addition, diets enriched with 1% P. columbina cryo concentrate significantly increased white blood cell lysozyme expression over a similar period.
References
Ahmadi PY, Farahmand H, Miandare HK, Mirvaghefi A, Hoseinifar SH (2014) The effects of dietary Immunogen® on innate immune response, immune related genes expression and disease resistance of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 37:209–214
Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G (1999) High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol 1:40–44
Asif MB, Hai FI, Price WE, Nghiem LD (2018) Impact of pharmaceutically active compounds in marine environment on aquaculture. In: HAI FI, Visavanathan C, Boopathy R (eds) Sustainable Aquaculture. Springer, Cham, pp 265–299
Bi GQ, Alderton JM, Steinhardt RA (1995) Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol 131:1747–1758
Boulho R, Marty C, Freile-Pelegrín Y, Robledo D, Bourgougnon N, Bedoux G (2017) Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J Appl Phycol 29:2219–2228
Broekman DC, Guḥmundsson GH, Maier VH (2013) Differential regulation of cathelicidin in salmon and cod. Fish Shellfish Immunol 35:532–538
Cantley LC, Cantley LG, Josephson L (1978) A characterization of vanadate interactions with the (Na,K)-ATPase. Mechanistic and regulatory implications J Biol Chem 253:7361–7368
Castilla V, Sepúlveda CS, García CC, Damonte EB (2017) Progress for antiviral development in Latin America. In: Ludert JE, Pujol FH, Arbiza J (eds) Human virology in Latin America. Springer, Cham, pp 439–460
Chang CI, Zhang YA, Zou J, Nie P, Secombes CJ (2006) Two cathelicidin genes are present in both rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Antimicrob Agents Chemother 50:185–195
Chen C, Sun B, Guan W, Bi Y, Li P, Ma J, Chen F, Pan Q, Xie Q (2016) N-3 essential fatty acids in Nile tilapia, Oreochromis niloticus: effects of linolenic acid on non-specific immunity and anti-inflammatory responses in juvenile fish. Aquaculture 450:250–257
Das BK, Ellis AE, Collet B (2009) Induction and persistence of mx protein in tissues, blood and plasma of Atlantic salmon parr, Salmo salar, injected with poly I:C. Fish Shellfish Immunol 26:40–48
Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, Rincon M (2012) Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol 5:258–266
El Gamal AA (2010) Biological importance of marine algae. Saudi Pharm J 18:1–25
Ellis AE (2001) Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25:827–839
Fletcher TC, White A (1976) The lysozyme of the plaice Pleuronectes platessa L. Comp Biochem Physiol B 55:207–210
Fujiwara A, Nishida-Umehara C, Sakamoto T, Okamoto N, Nakayama I, Abe S (2001) Improved fish lymphocyte culture for chromosome preparation. Genetica 111:77–89
Garcia-Vaquero M, Hayes M (2016) Red and green macroalgae for fish and animal feed and human functional food development. Food Rev Int 32:15–45
Goldstein I, Lerer E, Laiba E, Mallet J, Mujaheed M, Laurent C, Rosen H, Ebstein RP, Lichtstein D (2009) Association between sodium- and potassium-activated adenosine triphosphatase α isoforms and bipolar disorders. Biol Psychiatry 65:985–991
Grimm H, Mayer K, Mayser P, Eigenbrodt E (2002) Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr 87:S59–S67
Haller O, Staeheli P, Kochs G (2007) Interferon-induced Mx proteins in antiviral host defense. Biochimie 89:812–818
Halperin ML, Kamel KS (1998) Potassium. Lancet 352:135–140
Johnson ML, Navanukraw C, Grazul-Bilska AT, Reynolds LP, Redmer DA (2003) Heparinase treatment of RNA before quantitative real-time RT-PCR. Biotechniques 35:1140–1143
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol 173:111–133
Lester K, Hall M, Urquhart K, Gahlawat S, Collet B (2012) Development of an in vitro system to measure the sensitivity to the antiviral Mx protein of fish viruses. J Virol Methods 182:1–8
Lingrel JB, Kuntzweiler T (1994) Na+,K(+)-ATPase. J Biol Chem 269:19659–19662
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Lortat-Jacob H, Baltzer F, Grimaud J-A (1996) Heparin decreases the blood clearance of interferon-γ and increases its activity by limiting the processing of its carboxyl-terminal sequence. J Biol Chem 271:16139–16143
Lozano I, Wacyk JM, Carrasco J, Cortez-San Martín MA (2016) Red macroalgae Pyropia columbina and Gracilaria chilensis: sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J Appl Phycol 28:1343–1351
MacKenzie S, Bardolet LT, Balasch JC (2004) Fish health challenge after stress. Indicators of immunocompetence. Contrib Sci 2:443–454
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Marsham S, Scott GW, Tobin ML (2007) Comparison of nutritive chemistry of a range of temperate seaweeds. Food Chem 100:1331–1336
McBeath AJA, Snow M, Secombes CJ et al (2007) Expression kinetics of interferon and interferon-induced genes in Atlantic salmon (Salmo salar) following infection with infectious pancreatic necrosis virus and infectious salmon anaemia virus. Fish Shellfish Immunol 22:230–241
Mohamed S, Hashim SN, Rahman HA (2012) Seaweeds: a sustainable functional food for complementary and alternative therapy. Trends Food Sci Technol 23:83–96
Montero D, Grasso V, Izquierdo MS, Ganga R, Real F, Tort L, Caballero MJ, Acosta F (2008) Total substitution of fish oil by vegetable oils in gilthead sea bream (Sparus aurata) diets: effects on hepatic Mx expression and some immune parameters. Fish Shellfish Immunol 24:147–155
Morales B, Macgayver M, Rondón-Barragán IS (2011) Comparative biology of the complement system in fish. CES Med Vet Zootec 6:74–90
Moreda‐Piñeiro A, Peña‐Vázquez E, Bermejo‐Barrera P (2012) Significance of the presence of trace and ultratrace elements in seaweeds. In: Kim, S. (ed.) Handbook of marine macroalgae: biotechnology and applied phycology. Wiley-Blackwell, Chichester
Ngo D-H, Wijesekara I, Vo T-S, Ta QV, Kim S-K (2011) Marine food-derived functional ingredients as potential antioxidants in the food industry: an overview. Food Res Int 44:523–529
Nygaard R, Husgard S, Sommer A-I, Leong JA, Robertsen B (2000) Induction of Mx protein by interferon and double-stranded RNA in salmonid cells. Fish Shellfish Immunol 10:435–450
Paulsen SM, Lunde H, Engstad RE, Robertsen B (2003) In vivo effects of β-glucan and LPS on regulation of lysozyme activity and mRNA expression in Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol 14:39–54
Pereira L (2016) Edible seaweeds of the world. CRC Press, Boca Raton
Purcell MK, Laing KJ, Winton JR (2012) Immunity to fish rhabdoviruses. Viruses 4:140–166
Reilly P, O’Doherty JV, Pierce KM, Callan JJ, O'Sullivan JT, Sweeney T (2008) The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2:1465–1473
Reverter M, Saulnier D, David R, Bardon-Albaret A, Belliard C, Tapissier-Bontemps N, Lecchini D, Sasal P (2016) Effects of local Polynesian plants and algae on growth and expression of two immune-related genes in orbicular batfish (Platax orbicularis). Fish Shellfish Immunol 58:82–88
Rhimou B, Hassane R, Nathalie B (2015) Antiviral activity of the extracts of Rhodophyceae from Morocco. Afr J Biotechnol 9:7968–7975
Sánchez-Machado DI, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85:439–444
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39:223–239
Scocchi M, Pallavicini A, Salgaro R, Bociek K, Gennaro R (2009) The salmonid cathelicidins: a gene family with highly varied C-terminal antimicrobial domains. Comp Biochem Physiol B 152:376–381
Shirozu T, Sasaki K, Kawahara M, Yanagawa Y, Nagano M, Yamauchi N, Takahashi M (2016) Expression dynamics of bovine Mx genes in the endometrium and placenta during early to mid pregnancy. J Reprod Dev 62:29–35
Skou JC, Esmann M (1992) The Na, K-ATPase. J Bioenerg Biomembr 24:249–261
Statovci D, Aguilera M, MacSharry J, Melgar S (2017) The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 8:838
Toledo I, Avila M, Manríquez A et al (2009) Algas, insumo alternativo para la alimentación, de especies acuícolas. Pontificia Universidad Católica de Valparaíso. Escuela de Ciencias del Mar, Valparaiso
Wei S, Huang Y, Cai J, Huang X, Qin Q (2012) Molecular cloning and characterization of c-type lysozyme gene in orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol 33:186–196
Whitmire JK, Tan JT, Whitton JL (2005) Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med 201:1053–1059
Acknowledgments
This study was supported by Laboratorio de Genética y Biotecnología, Facultad de Ciencias Agronómicas, Universidad de Chile and BioMar Chile S.A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Lozano Muñoz, I., Wacyk, J., Perez, C. et al. Diets enriched in red seaweed (Pyropia columbina and Gracilaria chilensis) cryo concentrates modulate the immune-relevant gene encoding the Mx antiviral protein in salmon (Salmo salar) white blood cells. J Appl Phycol 31, 1415–1424 (2019). https://doi.org/10.1007/s10811-018-1595-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1595-y