Abstract
Holothuria scabra is the most valued and cultured tropical sea cucumber, given the great demand of this species for human consumption. However, despite its ecological and economic relevance, little is known regarding its immune responses under thermal stress. Here, the main goal was to study the response of sea cucumbers to temperature stress, assessing sub-organismal alterations and acclimation capacities of juveniles to temperature changes. After changing temperature (1 °C/day) for 6 days, organisms were exposed to temperature conditions of 21 °C (cold), 27 °C (control), and 33 °C (warm) over a 30 day period. At each 15-day interval (T0, T15, and T30), six replicates per condition were killed for biochemical analysis. Immune responses were addressed by studying the activity of phenoloxidase (PO) and prophenoloxidase (ProPO) in the coelomic fluid. Antioxidant defence responses—catalase (CAT), superoxide dismutase (SOD), and glutathione reductase (GR) enzymatic activities—were measured in the muscle and respiratory tree tissues, whereas oxidative damage was evaluated by measuring levels of superoxide radicals (ROS), DNA-strand breaks and lipid peroxidation (LPO). Juvenile H. scabra increased SOD and PO activities when temperature was elevated, and revealed low levels of ROS and damage in both cold and warm treatments throughout the experiment, confirming the organism’s moderate thermal stress. After the short acclimation period, the immune and antioxidant responses prevented damage and maintained homeostasis. This multi-biomarker approach highlights its usefulness to monitor the health of H. scabra and to gain insight concerning the use of this high-valued species in global-scale aquaculture from different temperature regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water temperature is a crucial factor influencing the physiological status of organisms in terms of growth rates, oxygen consumption and metabolism, or moulting process (e.g. Sierra et al. 1999; Zdanovich 1999; Dong et al. 2006). While it has been shown that changes in water temperature can evoke acute or chronic stress in a variety of organisms (Cheng and Chen 2000; Cheng et al. 2004; Coates et al. 2012), there is still some ambiguity concerning the effects of temperature variations. One common strategy to monitor the effects of temperature stress at lower biological organizational levels is the use of biomarkers (Peakall 1992; Menezes et al. 2006). Some of the most studied and applied biomarkers are parameters related with oxidative stress defence against reactive free radical production, such as superoxide anions (O ·−2 ), hydroxyl radicals (OH·), and hydrogen peroxide (H2O2) (Dröge 2003). The ability to regulate the production of these reactive oxygen species (ROS) and maintain “redox homeostasis” determines the health status of an organism (Ames et al. 1993).
It is generally known that temperature stress induces the generation of ROS (Valavanidis et al. 2006). Disturbing the balance between endogenous and exogenous ROS can cause a consequent incapacity of the antioxidant defences to respond, which may lead to oxidative damage in different target biomolecules and tissues (Sohal et al. 2002; Valavanidis et al. 2006). Superoxide radicals, for example, are known to have negative impacts on antioxidant vitamins (e.g. tocopherol, ascorbate) and enzyme activities [e.g. catalase (CAT), glutathione reductase (GR) and peroxidases], which can in turn result in DNA damage, enzymatic inactivation, or peroxidation in important cellular biomolecules, especially lipids (Kono and Fridovich 1982; Blum and Fridovich 1985; Valavanidis et al. 2006). Thus, antioxidants play a crucial role in the maintenance of cell integrity, homeostasis, and in prevention of oxidative damage (Vigo-Pelfrey 1990; Dix and Aikens 1993). Superoxide dismutase (SOD) and CAT provide the first line of defence in responses to oxidative damage. Initially, SOD converts the superoxide radicals to O2 and H2O2 and CAT in the next step transforms the H2O2 into O2 and H2O (Howcroft et al. 2009). Another important enzyme to protect the cells is GR, which reduces glutathione disulfide (GSSG) into two molecules of glutathione (GSH), which act as a non-enzymatic antioxidant (Saint-Denis et al. 2001).
Aside from enzymes involved in oxidative stress responses, environmental stress in marine invertebrates can also evoke immune responses through for instance the activity of phenoloxidase (PO) enzyme (Gomez-Jimenez et al. 2000). Phenoloxidase is responsible for the process of melanization, which is involved in wound healing and cellular defence responses (Ratcliffe et al. 1984; Rodriguez and Le Moullac 2000; Cerenius et al. 2008). Due to the cytotoxic nature of PO, this enzyme is usually stored in its inactive precursor form—pro-phenoloxidase (ProPO)—being activated only after external stimuli (Söderhäll and Cerenius 1998; Rodriguez et al. 2014). The ProPO activating system is described for many invertebrates and consists of a cascade of interactions between enzymes and their zymogens, inducing the production of PO as final product. Both PO and ProPO are well studied in arthropods such as crustaceans (Söderhäll and Unestam 1979) and insects (Laughton and Jothy 2011), but many open questions regarding their function and dynamics remain for non-arthropod invertebrates, including sea cucumbers.
Holothuria scabra is economically the most valuable tropical sea cucumber, given the high interest for the food industry (bêche-de-mer), as well as for pharmaceutical purposes (i.e. bioactive compounds) (Battaglene and Bell 1999; Hamel et al. 2001; Venugopal 2009; Bordbar et al. 2011). In addition, concerning their anatomy, sea cucumbers have unique organs/tissues with diverse functions (e.g. cellular aeration, locomotion, metabolism and regenerative processes), suitable for the study of oxidative stress and immune responses (Garcia-Arrarás and Dolmatov 2010), which make them good target tissues in the study of stress responses and oxidative and immune-related analysis. The respiratory tree, for example, is a well-developed structure responsible for cellular aeration and waste excretion (Spirina and Dolmatov 2001). Muscular system and body wall of sea cucumbers are also interesting organs to analyse since they are involved in the organisms’ locomotion and in the contraction movements in response to environmental stimuli (Motokawa and Tsuchi 2003).
These organisms also play an important ecological role as bioturbators (Uthicke 2001; Purcell et al. 2012). As shallow, bottom dweller species, they undergo seasonal and daily temperature fluctuations. Some studies demonstrate that H. scabra (Wolkenhauer 2008) and Apostichopus japonicus (Dong et al. 2006) seem to be adapted to temperature changes in terms of their burying and feeding habits, but very little is known regarding their mechanisms of adaptation and consequences for fitness in the long term.
Therefore, the main objective of the current study was to determine the effects of temperature stress (i.e. cold and warm) on immune and oxidative stress responses of juvenile H. scabra, using biochemical biomarkers involved in such processes, in order to understand the capacity of these organisms to cope with thermal stress and to find suitable markers for effect assessment on those levels.
Materials and methods
Test organism
Holothuria scabra (Jaeger, 1833) originated from the hatchery facilities of the Indonesian Research Centre for Oceanography (LIPI) on Lombok, Indonesia, were transported to the Alfred Wegener Institute, Helmholtz-Centre for Polar and Marine Research (AWI) in Bremerhaven, Germany, where they were maintained in recirculation systems for 14 days at 27 °C with a photoperiod of 12:12 h (light:dark) for acclimation. Sea cucumbers were observed and fed every second day with Algamac (Aquafauna—Bio Marine Inc.). To ensure optimal water quality, the aquaria water was continuously filtered and aerated. The water quality parameters, ammonia, pH and salinity were monitored regularly.
Experimental setup
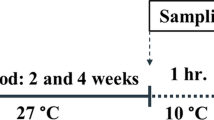
Experimental design followed previous work from Kühnhold et al. (2016). Briefly, after the acclimation period (14 days), 18 individuals were randomly assigned to each of three water temperature treatments: 21 °C (Cold), 27 °C (Control), and 33 °C (Warm). To achieve such temperatures, seawater temperature was decreased (for cold treatment) or increased (for warm treatment) by one degree per day over 6 days. Once the desired temperatures were reached, six individuals per tank were killed for further analysis, corresponding to day zero of the experiment (T0). Sampling was then performed at 15 days (T15) and 30 days (T30) of exposure to the different temperatures, with six replicates. Before killing the organisms, their coelomic fluid was collected using a 2-ml sterile syringe inserted through the body wall, for the assessment of immune responses. The procedure took no more than 20 s to ensure minimum effects of sampling on the immune responses. Then, muscle, respiratory tree, and body wall tissues were sampled for the oxidative stress-related endpoints (see below for sample processing details). All samples were subsequently stored at −80 °C until further analysis.
Tissue preparations
Immune responses
Following Jiang et al. (2014), two different fractions of coelomic fluid were prepared: Coelomocyte Lysate Supernatant (CLS) and Cell Free Supernatant (CFS). After centrifugation of extracted coelomic fluid at 500g for 10 min (4 °C), the supernatant (CFS) was stored at −80 °C, whereas the pellet was suspended with 1× PBS buffer to prepare the CLS fraction. After sonication for 5 min at 30-s intervals (UTR 200, Hielscher, Germany), the re-suspended pellets were centrifuged at 12,000g for 10 min (4 °C) and the obtained supernatant (CLS) was stored at −80 °C until further analysis.
Oxidative stress
According to different protocols and procedures, the oxidative stress-related parameters (except ROS) were measured in the muscle and respiratory tree tissues of sea cucumbers adapting the protocols more thoroughly described in Alves et al. (2016) and Silva et al. (2016). Both tissues were homogenized in K-phosphate buffer (0.1 M, pH 7.4) in a 1:4 proportion (w/v). Part of the homogenized tissue (150 μl) was transferred to a microtube containing 4 % BHT solution (2,6-dieter-butyl-4-methylphenol) to prevent tissue oxidation for further determination of lipid peroxidation (LPO), and another portion (50 μl) was separated for quantifying DNA-strand breaks. Samples were then centrifuged at 10,000g, for 20 min (4 °C). The resulting post-mitochondrial supernatant (PMS) was stored at −80 °C for further protein quantification and activity measurement of SOD, CAT and GR. For the determination of superoxide free radicals, as a measurement of ROS production, 50 mg of sea cucumber body wall was separated and kept at −80 °C until further analysis.
In all assays, K-phosphate buffer (0.1 M, pH 7.4) was used as blank. The spectrophotometric measurements were done at 25 °C in a synergy H1 Hybrid Multi-Mode microplate reader (Biotek® Instrument, Vermont, USA) and the enzymatic reactions were all previously optimized to ensure zero-order kinetic reactions (substrate in excess).
Biochemical analysis
Immune responses: phenoloxidase and pro-phenoloxidase
PO (monophenol, l-dopa:oxygen oxidoreductase, EC 1.14.18.1) and ProPO (zymogen form) activities were measured using the method partially described by Söderhäll (1981) with modification made by Laughton and Jothy (2011) and Jiang et al. (2014).
The activities of ProPO and PO were measured in both coelomic fluid fractions, i.e. CLS and CFS. PO activities were measured by adding 5 mM l-DOPA (l l-3,4-dihydroxyphenylalanine; Sigma, USA), dissolved in sodium cacodylate buffer (0.01 M, pH 7.4), to each fraction of the sample (CLS or CFS). For the blank reactions, seawater was used instead of the sample. The procedure for measuring ProPO was similar with the minor difference that chymotrypsin (0.25 mg/ml) was added to the sample, with a 10-min incubation prior to the addition of l-DOPA, to allow the activation of all phenoloxidase. The conversion of L-DOPA into dopachrome was determined spectrophotometrically at 490 nm (25 °C) with readings every 10 s for 5 min, giving an estimation of the enzyme activity. The final PO and ProPO activities were expressed as U/mg of protein, where 1U is defined as the amount of enzyme in the sample that, by converting the substrate, increases the absorbance by 0.001 per min.
Protein quantification
The soluble proteins were quantified according to the Bradford method (Bradford 1976), adapted from BioRad’s Bradford microassay set up in a 96-well flat-bottom plate, using bovine γ-globulin as a protein standard. In each well of the microplate, 10 μl of each sample was added along with 290 μl of Bradford reagent (in quadruplicates). After 15 min of agitation at 150 revs/min, absorbance was read at 600 nm and results were expressed in mg of protein/mL.
Antioxidant defences
The activity of SOD (EC 1.15.1.1) was measured performing an adaptation of the method described by McCord and Fridovich (1969), using the xanthine/xanthine oxidase-mediated reduction of cytochrome C. The reduction of cytochrome C was followed at 550 nm and SOD activity was expressed in U/mg of protein using an SOD standard of 1.5 U/ml, where 1 U represents the amount of enzyme in the sample that causes 50 % inhibition of cytochrome C reduction. CAT (EC 1.11.1.6) activity was estimated following the degradation of H2O2 at 240 nm, adapting the method described by Clairborne (1985). CAT activity was expressed in μmol/min/mg of protein, using a molar extinction coefficient of 40 M/cm. The activity of GR (EC 1.8.1.7) was estimated by measuring oxidation of NADPH in the process of reducing GSSG to glutathione (GSH) at 340 nm (Cribb et al. 1989). GR activity was calculated using a molar extinction coefficient of 6.2x103 M/cm and expressed in nmol/min/mg of protein.
Oxidative stress and damage
For the determination of superoxide free radicals production in the body wall of sea cucumber, the method of Drossos et al. (1995) was followed. Briefly, after adding Krebs buffer to the tissue, an incubation with cytochrome C (15 µM) was made at 37 °C. The presence of O2 − was determined by the capacity of the radicals to reduce cytochrome C, which was measured at 550 nm. Using a molar extinction coefficient of 19,000 M/cm (Wu et al. 2011), the amount of superoxide radicals produced was calculated and expressed in nmol O2 −/g wet weight.
Lipid peroxidation levels were assessed by measuring the content of thiobarbituric acid-reactive substances (TBARS), using the method described by Ohkawa et al. (1979) and Bird and Draper (1984), with modifications made by Wilhelm et al. (2001) and Torres et al. (2002). After the reaction with TBA 0.73 % (2-thiobarbituric acid) reagent, the absorbance of the samples was measured at 535 nm. The results were calculated using a molar extinction coefficient of 1.56 × 105 M/cm and expressed as nmol TBARS/mg of wet weight.
The DNA-strand breaks were measured using the DNA alkaline precipitation assay (Olive 1988), adapted from De Lafontaine et al. (2000). After the precipitation of SDS-associated nucleoproteins and genomic DNA, the remaining single and double-stranded DNA in the supernatant was mixed with Hoesch dye (1 μg/mL bisBenzimide, Sigma-Aldrich) and fluorescence was measured using an excitation/emission wavelength of 360/460 nm. Results were expressed as mg of DNA/mg of wet weight, using calf thymus DNA as standard to extrapolate DNA concentration.
Statistical analysis
Statistics was performed using Sigma Plot software for Windows, version 11.0 (SigmaPlot 1997). Data were first tested for normality and homoscedasticity using Kolmogorov–Smirnov and Levene tests, respectively. To determine statistically significant differences between the treatments and between each time point, a two-way analysis of variance (ANOVA) was applied. When significant differences were found, Holm–Sidak post hoc tests were used for multiple comparisons. Correlations between endpoints in different tissues, at each time point, were performed using Pearson correlations. The results are presented as means + standard error (SE). The significance level for all statistical analysis was set at p ≤ 0.05.
Results
No mortalities were registered at any treatment at any time point.
Immune responses
PO and ProPO activities in cell-free supernatant (CFS)
Although no significant differences were observed in the activity of PO and ProPO in CFS, the activities in both cases were higher in the warm treatment with a tendency for a decrease with the experiment duration (Fig. 1). In this CFS fraction, activities of ProPO and PO were found to be similar (within the same order of magnitude) in every treatment.
Immune responses: a phenoloxidase (PO) and b pro-phenoloxidase (ProPO) activities in the cell-free supernatant (CFS) fraction of Holothuria scabra coelomic fluid exposed to cold (21 °C), control (27 °C) and warm (33 °C) temperatures over different time periods (T0, T15, T30 days). Results express average values + standard error
PO and ProPO activities in coelomocyte lysate supernatant (CLS)
The PO activity in the CLS fraction followed the same pattern as ProPO, with progressively higher activities in the warm treatment over time of exposure (Fig. 2), which in the case of PO was found to be statistically significant at T30 (p = 0.005, Fig. 2a). Significant differences among different temperature treatments were found at the end of the experiment (T30), both for PO and ProPO (p = 0.004, Fig. 2a; and p = 0.011, Fig. 2b, respectively).
Immune responses: a phenoloxidase (PO) and b pro-phenoloxidase (ProPO) activities in the coelomocyte lysate supernatant (CLS) fraction of Holothuria scabra coelomic fluid exposed to cold (21 °C), control (27 °C) and warm (33 °C) temperatures over different time periods (T0, T15 and T30 days). Results express average values + standard error. a,bSignificant differences between cold, control and warm treatments within each time point (two-way ANOVA, Holm–Sidak, p < 0.05). A,BSignificant differences between time points within each temperature treatment (two-way ANOVA, Holm–Sidak, p < 0.05)
Contrary to the CFS fraction, in CLS ProPO activities were between 1000 and 2000× higher than PO activities. Despite the increase in the general immune response of the organisms in the warm treatment, the ratio between PO and ProPO remains constant among treatments with no significant differences being observed (Fig. 2c).
Oxidative stress-related endpoints
No significant changes in the activity of the tested antioxidant enzymes (SOD, CAT and GR) were observed, either in the muscle tissue or in the respiratory tree (Figs. 3, 4). However, although the effects were not statistically significant, in the muscle there was a trend for higher SOD activities in the warm treatment, compared to control and cold treatment (Fig. 3a). Antioxidant enzyme activity levels were usually higher in the respiratory tree (Fig. 4) than in the muscle (Fig. 3), independently of the treatments.
Oxidative stress-related responses: a superoxide dismutase (SOD), b catalase (CAT) and c glutathione Reductase (GR) enzymatic activities and levels of oxidative damage measured as d DNA damage and e lipid peroxidation (LPO) in the muscle tissue of Holothuria scabra exposed to cold (21 °C), control (27 °C) and warm (33 °C) temperatures over different time periods (T0, T15 and T30 days). f Reactive oxygen species (ROS) production in the body wall of Holothuria scabra exposed to the same conditions described for muscle. Results express average values + standard error. a,bSignificant differences between cold, control and warm treatments within each time point (two-way ANOVA, Holm–Sidak, p < 0.05). A,BSignificant differences between time points within each temperature treatment (two-way ANOVA, Holm–Sidak, p < 0.05)
Oxidative stress-related responses: a superoxide dismutase (SOD), b catalase (CAT) and c glutathione reductase (GR) enzymatic activities and levels of oxidative damage measured as d DNA damage and e lipid peroxidation (LPO) in the respiratory tree of Holothuria scabra exposed to cold (21 °C), control (27 °C) and warm (33 °C) temperatures over different time periods (T0, T15 and T30 days). Results express average values + standard error. a,bSignificant differences between cold, control and warm treatments within each time point (two-way ANOVA, Holm–Sidak, p < 0.05). A,BSignificant differences between time points within each temperature treatment (two-way ANOVA, Holm–Sidak, p < 0.05)
In relation to the parameters addressing oxidative damage, no effects of temperature were seen either in peroxidation of lipids or in higher levels of DNA-strand breaks (Figs. 3d, e, 4d, e).
The results of the ROS quantification in the body wall show that in the cold treatment, at T0, the organisms produced significantly less superoxide radicals than in the control treatment (p = 0.002—Fig. 3f). However, the levels of ROS in this treatment significantly increased with the duration of the experiment CA (p = 0.009), and no further differences were observed with the other temperature treatments.
Correlation analysis between all assessed biomarkers in H. scabra, relative to immune and oxidative stress responses, were performed separately for each time point (Tables S1–S3, supplementary material). At all time points, especially in muscle tissue, activities of CAT, SOD and GR correlated with each other positively, indicating that if one of these enzymes is activated or inhibited, the other enzymes follow the same pattern. Similarly, some positive correlations between DNA damage and LPO were observed. Moreover, GR (respiratory tree) and ProPO activating systems (CLS fraction) correlated negatively with ROS, while a positive correlation between DNA damage of the same tissue and ROS is apparent. Furthermore, PO activity of CLS correlated positively with SOD activities from both tissues.
Discussion
Temperature changes influence the growth rate, susceptibility, and the general health status of invertebrates (Hughes et al. 2003; Cheng et al. 2004; Wang et al. 2008; Purcell and Simutoga 2008; Hair 2012). The integrated antioxidant and ProPO activating systems are known as crucial components of invertebrates’ self-maintenance (Mathew et al. 2007), but little is known about these responses to different stress levels. The present study is the first to apply combined investigations of immune responses, cellular oxidative damage and antioxidant enzyme activities to assess stress responses in juvenile H. scabra at varying temperatures [i.e. cold (21 °C) and warm (33 °C)], and to assess their potential for easy-to-use, fast, and cost-effective multi-biomarker applications in aquaculture.
Immune responses
The nature of sea cucumbers as osmo-conformers (Coteur et al. 2004) indicates that any changes in water temperature may influence their coelomic fluids and particularly affect the activities of coelomocytes (Wang et al. 2008). The ProPO activating system is considered as the first line of defence in the immune response of invertebrates (Sritunyalucksana and Söderhäll 2000), where any reduction in PO activities affects the cellular defence of organisms (Mathew et al. 2007). This study confirmed that, similar to the Pacific oyster (Crassostrea gigas) (Hellio et al. 2007), PO activity is detectable in both fractions of the coelomic fluid in H. scabra. Most of PO was activated in the CFS fraction, seen by the similar activity between PO and ProPO, in contrast to the CLS fraction, where most PO remained in the inactive form, as expected at least under control conditions, given the cytotoxic nature of the by-products of the PO activating cascade (Tujula et al. 2001; Laughton and Jothy 2011).
In the CFS fraction, which represents the acellular fraction of the coelomic fluid (Gomez-Jimenez et al. 2000), there was a tendency for higher activities of both PO and ProPO activities in the warm treatment, mainly at the beginning of the experiment (Fig. 1). This is in agreement with the findings of a parallel study, where under the same warm temperature condition, the organisms were consuming more energy, possibly indicating the costs of the defence responses by inducing for instance these immune enzymes (Kühnhold et al. 2016). Similarly, Coates et al. (2012) reported that in horseshoe crabs (Limulus polyphemus), which have hemocyanin-derived phenoloxidase (Hc-PO), the activity of Hc-PO at the beginning of exposure was initially increased at the warmer treatment, but decreased again over a period of time, suggesting that temperature changes have limited effects on hemocyanin and PO activities. It is important to note that the coelomocytes of sea cucumbers and haemocytes of crustaceans display several common features (Tseng et al. 2009).
In the CLS fraction, this tendency for higher PO activities with increasing temperatures is also seen for both PO and ProPO activities, over the time of exposure. This increase in the total ProPO activity suggests that, along with the PO activation (in a much lower scale), probably more PO is being synthesized (Fig. 2). Although in this study the sea cucumbers responded with an increase in PO in the warmer treatment, the response of this enzyme to temperature changes seems to differ between species. For example, Vargas-Albores et al. (1998) reported that the yellowleg shrimp (Penaeus californiensis) had lower PO activity at higher temperature (32 °C) compared to colder treatment (18 °C). Moreover, Cheng and Chen (2000) and Cheng et al. (2004) reported that the PO and phagocytic activities in the giant freshwater prawn (Macrobrachium rosenbergii) and the Taiwan abalone (Haliotis diversicolor supertexta) at warmer treatments (34 °C) were lower than the ones reared at colder water (27–30 °C).
When comparing the ProPO activities between CFS and CLS factions, it is possible to observe that the activities are higher in the coelomocytes (CLS), indicating a higher potential for PO response in this fraction. These results show the importance of testing both cellular and acellular fractions of the coelomic fluid in order to accurately locate the PO activity. However, the different PO activities found in the two fractions might also indicate different types of PO enzymes (tyrosinase, laccase or catecholase). Although characterization of PO was already done for another holothurian species (Jiang et al. 2014) and this was the base for the methods employed here, it would be important for further studies to understand which specific enzymes are involved in PO activity in each fraction to more precisely target those reactions. Nevertheless, the present study represents already an important indication that PO induction might play an important role in H. scabra response to heat stress.
Oxidative stress-related endpoints
Regarding the oxidative stress-related endpoints, results showed that exposure to different temperatures had little impact on H. scabra. Various studies demonstrated that free radicals formed by a stressor could enhance the formation of malonaldehyde and therefore increase LPO (Di Pierro et al. 1992). Additionally, the heterogenic DNA molecules are susceptible to breakage and damage inflicted by elevated ROS levels (Cerutti 1985). In the present study, however, no signs of oxidative damage were observed in any temperature manipulation (Figs. 3, 4). In the beginning of the experiment (T0) specimens from the cold treatment exhibited even lower ROS levels, which resulted in lower levels of LPO in the same condition. These lower ROS levels can also be explained by the lower oxygen consumption rates (OCR) verified in a parallel study with the same exposure conditions (Kühnhold et al. 2016).
Increasing temperatures can stimulate oxidative stress and specific antioxidant responses in different classes of invertebrates. Although the antioxidant activities in H. scabra did not show a clear treatment response, induction of SOD was a constant trend observed in the warm treatment. Similar patterns of antioxidant response were observed by Ji et al. (2008) and Wang et al. (2008), in studies with A. japonicus, where at the beginning of exposure to higher temperatures, SOD and CAT activities measured in both body wall and respiratory tree, increased after a short exposure time (12 h). The tendency for higher activity of SOD is observed in the warm treatment mainly in the muscle, along with lower activities in the cold treatment (Fig. 3a). A similar pattern was reported for shrimps (Zhou et al. 2010) and the disk abalone Haliotis discus discus (Kim et al. 2007), where the activities of manganese superoxide dismutase (MnSOD) and copper superoxide dismutase (CuSOD) increased under heat stress. This SOD induction in the warm treatment in combination with the lack of significant changes in CAT and GR activities over the period of exposure, suggests that this enzyme is the most sensitive antioxidant enzyme among the ones tested, and likely plays an important role in ROS detoxification in juvenile H. scabra in response to thermal stress.
However, in the present study, the temperature stress resulted in only marginal increases in the antioxidant activities. This might be explained by the immediate induction of heat shock proteins, which reduce heat stress and oxidative stress in the organism, as reported previously for Haliotis tuberculata (Farcy et al. 2007). This cannot, however, be confirmed in the present work, since the expression of heat shock proteins was not studied.
Another observation of the present study was that the respiratory tree in the sea cucumbers had higher antioxidant potential than muscle (higher enzyme activities), similar to the gills in molluscs (Farcy et al. 2007; Box and Sureda 2009). Considering the functionality of the respiratory tree for oxygen circulation and gas exchange at its surface, and also the close contact with water, similarly to the gills in molluscs, this tissue is ought to be more susceptible to environmental changes. However, a clearer pattern of overall response to the induced thermal stress was observed in the muscle tissue. Further studies featuring more levels and higher intensity of treatment (i.e. lower and higher temperature thresholds) are needed in order to create a better understanding on the physiological thresholds and sensitivity of the antioxidant responses in this species.
In sum, immune and oxidative stress responses indicate that temperature manipulation applied in the present study was not severe enough to cause acute stress in juvenile H. scabra. This is in accordance with the general assumption that most of the sea cucumbers, reared in intertidal ponds, can tolerate temperature fluctuations from 20 to 30 °C (Dong et al. 2008). Furthermore, with this study it is possible to infer that immune responses through PO activity, and antioxidant activities (particularly SOD) in H. scabra seem to be efficient to reduce ROS production and oxidative damage under thermal variations. This is strengthened by the correlation analysis between biochemical responses throughout the duration of the experiment (Tables S1–S3, supplementary material), with positive correlations between SOD and PO activities and negative correlations between the activities of GR or PO enzymes and the levels of ROS. Therefore, this study highlights the importance of combining different endpoints into a multi-biomarker approach in order to gain a holistic picture of the processes and mechanisms underlying stress responses.
Conclusions
Juvenile H. scabra displayed sensitivity to thermal stress at the beginning of the experiment, especially in the warm treatment, and after a period of time they acclimated to the higher and lower temperatures. From an immune response-related point of view, PO and ProPO activities in the cell-free coelomic fluid were tendentiously increased in the warm treatment at T0, showing an early immune response with the temperature change. Antioxidant and oxidative damage biomarkers indicated that the temperature manipulations applied in the present study were not severe enough to cause significant oxidative damage to H. scabra, seen by the low production of ROS and absence of oxidative damage in either lipids or DNA. SOD seems to be the most sensitive enzymatic antioxidant in H. scabra in response to thermal stress.
The present study highlights the benefits of a multi-biomarker analysis to better understand and interpret biochemical responses to stress, and in particular thermal stress. Assessing changes in the immune and antioxidant biomarker endpoints, particularly in juvenile H. scabra, are promising tools for monitoring the health status of the organisms. Also, understanding the impacts of temperature stress on these organisms, provide important insight into the possibility of the use of H. scabra, a high-valued species, in global-scale aquaculture from different regions with minimum impact concerning thermal stress.
References
Alves LMF, Nunes M, Marchand P, Le Bizec B, Mendes SL, Correia J, Lemos MFL, Novais SC (2016) Blue sharks (Prionace glauca) as bioindicators of pollution and health in the Atlantic Ocean: Contamination levels and biochemical stress responses. Sci Total Environ 563–564:282–292
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci 90:7915–7922
Battaglene SC, Bell JD (1999) Potential of the tropical sea cucumber, Holothuria scabra, for stock enhancement. In: Howell BR, Moskness E, Svasand T (eds) Stock enhancement and sea ranching. Blackwell Science, Oxford, pp 478–490
Bird RP, Draper AH (1984) Comparative studies on different methods of malondialdehyde determination. Method Enzymol 90:105–110
Blum J, Fridovich I (1985) Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys 240:500–508
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs 9:1761–1805
Box A, Sureda A (2009) Antioxidant responses of bivalve Pinna nobilis colonized with invasive macroalgae Lophodadia lallemandi. Comp Biochem Physiol 149(C):456–460
Bradford MM (1976) Rapid and sensitive method for quantification of microgram quantities of protein utilizing principle of protein-dye binding. Anal-Biochem 72:248–254
Cerenius L, Lee BL, Söderhäll K (2008) The proPOsystem: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Cerutti PA (1985) Prooxidant states and tumor promotion. Science 227:375–381
Cheng W, Chen JC (2000) Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol 10:387–391
Cheng W, Hsiao IS, Hsu CH, Chen JC (2004) Change in water temperature on the immune response of Taiwan abalone Haliotis diversicolor supertexta and its susceptibility to Vibrio parahaemolyticus. Fish Shellfish Immunol 17:235–243
Clairborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Coates CJ, Bradford EL, Krome CA, Nairn J (2012) Effect of temperature on biochemical and cellular properties of captive Limulus polyphemus. Aquaculture 334(337):30–38
Coteur G, Corriere N, Dubois Ph (2004) Environmental factors influencing the immune responses of the common European starfish (Asterias rubens). Fish Shellfish Immunol 16:51–63
Cribb AE, Leeder JS, Spielberg SP (1989) Use of a microplate reader in an assay of glutathione-reductase using 5,50-dithiobis(2-nitrobenzoic acid). Anal Biochem 183:195–196
De Lafontaine Y, Gagne F, Blaise C, Costan G, Gagnon P, Chan HM (2000) Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquat Toxicol 50:51–71
Di Pierro D, Tavazzi B, Lazzarino G, Giardina B (1992) Malondialdehyde is a biochemical marker of peroxidative damage in the isolated reperfused rat heart. Mol Cell Biochem 116:193–196
Dix TA, Aikens J (1993) Mechanisms and biological relevance of lipid peroxidation initiation. Chem Res Toxicol 6:18–62
Dong YW, Dong SL, Tian XL, Wang F, Zhang MZ (2006) Effects of diel temperature fluctuations on growth, oxygen consumption and proximate body composition in the sea cucumber Apostichopus japonicus Selenka. Aquaculture 255:514–521
Dong YW, Dong S, Meng X (2008) Effects of thermal and osmotic stress on growth, osmoregulation and Hsp70 in sea cucumber (Apostichopus japonicus Selenka). Aquaculture 276(1–4):179–186
Dröge W (2003) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Drossos G, Lazou A, Panagopoulos P, Westaby S (1995) Deferoxamine cardioplegia reduces superoxide radical production in human myocardium. Ann Thorac Surg 59:169–172
Farcy E, Serpentini A, Fiévet B, Lebel JM (2007) Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: transcriptional induction in response to thermal stress in hemocyte primary culture. Comp Biochem Physiol B: Biochem Mol Biol 146(4):540–550
Garcia-Arrarás JEG, Dolmatov IY (2010) Echinoderms; potential model systems for studies on muscle regeneration. Curr Pharm Des 16(8):942–955
Gomez-Jimenez S, Uglow RF, Gollas-Galvan T (2000) The effects of cooling and emersion on total haemocyte count and phenoloxidase activity of the spiny lobster Panulirus interruptus. Fish Shellfish Immunol 10:631–635
Hair C, 2012 Sandfish (Holothuria scabra) production and sea-ranching trial in Fiji. In: Hair CA, Pickering TD, Mills DJ (eds.) Asia–Pacific tropical sea cucumber aquaculture. ACIAR Proceedings, vol 136. Australian Centre for International Agricultural Research, Canberra, pp 129–141
Hamel JF, Conand C, Pawson DL, Mercier A (2001) The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): Its biology and exploitation as Beche-de mer. Adv Mar Biol 41:129–223
Hellio C, Bado-Nilles A, Gagnaire B, Renault T, Guyon HT (2007) Demonstration of a true phenoloxidase activity and activation of a ProPO cascade in Pacific oyster, Crassostrea gigas (Thunberg) in vitro. Fish Shellfish Immunol 22:433–440
Howcroft CF, Amorim MJB, Gravato C, Guilhermino L, Soares AMVM (2009) Effects of natural and chemical stressors on Enchytraeus albidus: Can oxidative stress parameters be used as fast screening tools for the assessment of different stress impacts in soils? Environ Int 35:318–324
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs—review. Science 301:929–933
Ji T, Dong Y, Dong Sh (2008) Growth and physiological responses in the sea cucumber Apostichopus japonicas Selenka: aestivation and temperature. Aquaculture 283:180–187
Jiang J, Zhou Z, Dong Y, Guan X, Wang B, Jiang B, Yang A, Chen Z, Gao S, Sun H (2014) Characterization of phenoloxidase from the sea cucumber Apostichopus japonicus. Immunobiology 219:450–456
Kim KY, Lee SY, Cho YS, Bang C, Kim KH, Kim DS, Nam YK (2007) Molecular characterization and mRNA expression during metal exposure and thermal stress of copper/zinc- and manganese superoxide dismutases in disk abalone, Haliotis discus discus. Fish Shellfish Immunol 23(5):1043–1059
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Kühnhold H, Kamyab E, Novais S, Indriana L, Kunzmann A, Slater M, Lemos M (2016) Thermal stress effects on energy resource allocation and oxygen consumption rate in the juvenile sea cucumber, Holothuria scabra (Jaeger, 1833). Aquaculture. doi:10.1016/j.aquaculture.2016.03.018
Laughton AM, Jothy MTS (2011) A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee Apis mellifera. Apidologie 42:140–149
Mathew S, Ashok Kumar K, Anandan R, Viswanathan Nair VG, Devadasan K (2007) Changes in tissue defence system in white spot syndrome virus (WSSV) infected Penaeus monodon. Comp Biochem Physiol C 145:315–320
McCord JM, Fridovich I (1969) Superoxide dismutase. J Biol Chem 244:6049–6055
Menezes S, Soares AMVM, Guilhermino L, Peck MR (2006) Biomarker responses of the estuarine brown shrimp Crangon crangon L. to non-toxic stressors: temperature, salinity and handling stress effects. J Exp Mar Biol Ecol 335:114–122
Motokawa T, Tsuchi A (2003) Dynamic mechanical properties of body wall dermis in various mechanical states and their implications for the behavior of sea cucumber. Biol Bull 205:261–275
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal-tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Olive PL (1988) DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen A 11:487–495. doi:10.1002/em.2850110409
Peakall D (1992) Animal biomarkers as pollution indicators. Chapman & Hall, London, pp 20–37
Purcell SW, Simutoga M (2008) Spatio-temporal and size-dependent variation in the success of releasing cultured sea cucumbers in the wild. Rev Fish Sci 16:204–214
Purcell SW, Hair CA, Mills DJ (2012) Sea cucumber culture, farming and sea ranching in the tropics: progress, problems and opportunities. Aquaculture 681:368–369
Ratcliffe NA, Leonard CM, Rowley AF (1984) Prophenoloxidase activation, non-self recognition and cell co-operation in insect immunity. Science 226:557–559
Rodriguez J, Le Moullac G (2000) State of the art of immunological tools and health control of penaeid shrimp. Aquaculture 191:109–119
Rodriguez AEP, Oliva-Teles T, MesquitaSR Delerue-Matos C, Guimaraes L (2014) Integrated biomarker responses of an estuarine invertebrate to high abiotic stress and decreased metal contamination. Mar Environ Res 101:101–114
Saint-Denis M, Narbonne JF, Arnaud C, Ribera D (2001) Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil: effects of lead acetate. Soil Biol Biochem 33:395–404
Sierra E, Diaz F, Espina S (1999) Energy budget of Ictalurus punctatus exposed to constant and fluctuating temperatures. Riv Ital Acquac 34:71–81
SigmaPlot (1997) SigmaPlot for Windows version 11. SPSS Inc., Chicago
Silva CSE, Novais SC, Lemos MFL, Mendes S, Oliveira AP, Gonçalves EJ, Faria AM (2016) Effects of ocean acidification on the swimming ability, development and biochemical responses of sand smelt larvae. Sci Total Environ 563–564:89–98
Söderhäll K (1981) Fungal cell wall β-1,3-glucans induce clotting and phenoloxidase attachment to foreign surfaces of crayfish haemocyte lysate. Dev Comp Immunol 5:565–573
Söderhäll K, Cerenius L (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Curr Opin Immunol 10:23–28
Söderhäll K, Unestam T (1979) Activation of cray fish serum prophenoloxidase in arthropod immunity. The specificity of cell wall glucan activation and activation by purified fungal glycoproteins. Cun J Microhiol 25:406–414
Sohal RS, Mockett RJ, Orr WC (2002) Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic Biol Med 33:575–586
Spirina IS, Dolmatov IY (2001) Morphology of the Respiratory Trees in the Holothurians Apostichopus japonicus and Cucumaria japonica. Russ J Mar Biol 27(6):367–375
Sritunyalucksana K, Söderhäll K (2000) The proPO and clotting system in crustaceans. Aquaculture 191:53–69
Torres MA, Testa CP, Gaspari C, Masutti MB, Panitz CMN, Curi-Pedrosa R, de Almeida EA, Di Mascio P, Wilhelm D (2002) Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar Pollut Bull 44:923–932
Tseng DY, Ho PL, Huang SY, Cheng SC, Shiu YL, Chiu CS (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol 26:339–344
Tujula N, Radford J, Nair SV, Raftos DA (2001) Effects of tributyltin and other metals on the phenoloxidase activating system of the tunicate Styela plicata. Aquat Toxicol 55:191–201
Uthicke S (2001) Nutrient regeneration by abundant coral reef holothurians. J Exp Mar Biol Ecol 265:153–170
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. J Ecotoxicol Environ Saf 64:178–189
Vargas-Albores F, Hinojosa-Baltazar P, Portillo-Clark G, Magallon-Barajas F (1998) Influence of temperature and salinity on the yellow leg shrimp, Penaeus californiensis Holmes. Aquac Res 29:549–553
Venugopal V (2009) Marine habitat and resources. In: Venugopal V (ed) Marine products for healthcare: functional and bioactive nutraceutical compounds from the ocean. CRC Press Taylor & Francis Group, Boca Raton, pp 23–50
Vigo-Pelfrey C (ed) (1990) Membrane lipid oxidation, vol 1. CRC Press, Boca Raton, p 239
Wang F, Yang H, Gao F, Liu G (2008) Effects of acute temperature or salinity stress on the immune response in sea cucumber, Apostichopus japonicus. Comp Biochem Physiol A(151):491–498
Wilhelm D, Tribess T, Gaspari C, Claudio FD, Torres MA, Magalhaes ARM (2001) Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 203:149–158
Wolkenhauer SM (2008) Burying and feeding activity of adult Holothuria scabra (Echinodermata: Holothuroidea) in a controlled environment. Beche-de-mar info. Bull Secr Pac Community 27:25–28
Wu JL, Wu QP, Peng YP, Zhang JM (2011) Effects of L-Malate on Mitochondrial Oxidoreductases in Liver of Aged Rats. Physiol Res 60:329–336
Zdanovich VV (1999) Some features of growth of the young of Mozambique tilapia, Oreochromis mossambicus, at constant and fluctuating temperatures. Ichthyology 39:100–104
Zhou J, Wang L, Xin Y, Wang WN, He WN, Wang AL, Liu Y (2010) Effect of temperature on antioxidant enzyme gene expression and stress protein response in white shrimp, Litopenaeus vannamei. J Therm Biol 35:284–289
Acknowledgments
This study had the support of the Fundação para a Ciência e a Tecnologia (FCT) Strategic Project UID/MAR/04292/2013 granted to MARE, and from an FCT and Deutscher Akademischer Austauschdienst (DAAD) program for bilateral cooperation funding. Sara Novais was supported by Fundação para a Ciência e Tecnologia through the research Grant SFRH/BPD/94500/2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D.Hume.
E. Kamyab and H. Kühnhold contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kamyab, E., Kühnhold, H., Novais, S.C. et al. Effects of thermal stress on the immune and oxidative stress responses of juvenile sea cucumber Holothuria scabra . J Comp Physiol B 187, 51–61 (2017). https://doi.org/10.1007/s00360-016-1015-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1015-z