Abstract
Insulin-like growth factor-1 (IGF-1) is a neurotrophic factor produced locally in the central nervous system which can promote axonal regeneration, protect motoneurons, and inhibit neuroinflammation. In this study, we used the zebrafish spinal transection model to investigate whether IGF-1 plays an important role in the recovery of motor function. Unlike mammals, zebrafish can regenerate axons and restore mobility in remarkably short period after spinal cord transection. Quantitative real-time PCR and immunofluorescence showed decreased IGF-1 expression in the lesion site. Double immunostaining for IGF-1 and Islet-1 (motoneuron marker)/GFAP (astrocyte marker)/Iba-1 (microglia marker) showed that IGF-1 was mainly expressed in motoneurons and was surrounded by astrocyte and microglia. Following administration of IGF-1 morpholino at the lesion site of spinal-transected zebrafish, swimming test showed retarded recovery of mobility, the number of motoneurons was reduced, and increased immunofluorescence density of microglia was caused. Our data suggested that IGF-1 enhances motoneuron survival and inhibits neuroinflammation after spinal cord transection in zebrafish, which suggested that IGF-1 might be involved in the motor recovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury is a central nervous system (CNS) injury mainly caused by vehicle accidents, falls, and violence (Kjell and Olson 2016) which can result in quadriplegia and paraplegia (Schwab et al. 2006; Jacobs and Nash 2004). Myelin regeneration (Plemel et al. 2014), appropriate neuroinflammatory response (Gensel and Zhang 2015; Stirling et al. 2014; Bollaerts et al. 2017), and neuronal survival (Reimer et al. 2008) play important roles in pathology and repair after spinal transection in rodents and zebrafish.
In mammals (Huebner and Strittmatter 2009), inadequate capability of intrinsic growth of neurons and the creation of extrinsic inhibitory milieu after injury lead to the lack of effective functional recovery in the CNS. In contrast to mammals, adult zebrafish (Becker et al. 1998; Briona et al. 2015) is capable of neuronal proliferation, regeneration, and functional restoration in 6 weeks after completely spinal cord transection. Since zebrafish has profound re-growing ability and genetic tractability (Fleisch et al. 2011), it has been considered as an ideal model to understand the exact molecular and cellular mechanisms of spinal cord regeneration (Fang et al. 2014). Insulin-like growth factor-1 (IGF-1), as a neurotrophic growth factor, is not only beneficial for cell survival and axonal regeneration (Chesik et al. 2008), but also associated with neuroinflammatory response (Labandeira-Garcia et al. 2017).
IGF-1 is a 70-amino acid polypeptide mainly derived from the liver, but it is also locally produced by neurons and glial cells in brain (Rodriguez-Perez et al. 2016; Suh et al. 2013) and spinal cord (Hammarberg et al. 1998) in different disease models. IGF-1 has multiple effects in the CNS, including regulating brain development, synapse formation, myelination (Labandeira-Garcia et al. 2017; Nieto-Estévez et al. 2016; Wrigley et al. 2017). In teleost, IGF-1 regulates neurogenesis in the retina of goldfish by stimulating the proliferation of retinal precursor cells (Otteson et al. 2002; Boucher and Hitchcock 1998), and IGF-1 is required for fin regeneration in zebrafish by reducing apoptosis in the wound epidermis and increasing proliferation of blastema cells (Chablais and Jazwinska 2010). In rodents, IGF-1 treatment protected facial (50%) and lumbar motoneurons (79%) from dying in pmn mutant mice (Jablonka et al. 2011), a model with typical motoneuron degeneration. Increased survival of neurons after traumatic brain injury was observed in IGF-1 overexpressed transgenic mice (Madathil et al. 2013). Lateral cerebral ventricle administration of IGF-1 down-regulated activation of astrocytes and reduced inflammatory protein expression in mice models of depression (Park et al. 2011), which shows the effect of IGF-1 in inhibiting neuroinflammatory response. Previous studies about neuro-regeneration primarily focused on the circulating levels of IGF-1 rather than local levels of IGF-1. The role of IGF-1 produced from injury site in spinal cord regeneration remains unclear. In this study, we used zebrafish to figure out the effect of IGF-1 produced in the lesion site on the recovery of spinal cord transection.
Material and Methods
Animals
Adult zebrafish (wild-type, 5–6 months of age) were obtained from the Shanghai Yinuo Aquatic Technology Company (Shanghai, China). Before the experiment, zebrafish of either sex were allowed to acclimatize for one week in tanks (3.5 l) on a 14-h light/10-h dark rhythm and fed with a commercial diet (GeneBio, Shanghai, China) twice daily. The aquarium water temperature and pH were kept at 28 ± 1 °C and 7.2–7.4, respectively. This research was approved by the Animal Ethics Committee of Jiangnan University. The zebrafish work was performed according to Regulations for the Administration of Affairs Concerning Experimental Animals (approved by the State Council on October 31, 1998 and promulgated by Decree No. 2 of the State Science and Technology Commission on November 14, 1988).
Spinal Transection
Spinal transection was induced as described previously by Ping Fang with slight modifications (Fang et al. 2012). In brief, zebrafish were immersed in 0.033% aminobenzoic acid ethylmethylester (MS222, Sigma, St. Louis, MO, USA) and anesthetized until respiratory movement of the opercula stopped. Then the zebrafish was put on the ice under a stereoscopic microscope. To exposing the spine, a longitudinal incision parallel to the spinal cord was made. Then the spinal cord was completely transected at 4 mm caudal to the brainstem region (Fig. 6a). The sham-operated zebrafish underwent identical to those of the spinal transection animals except the spinal cord was not transected.
Quantitative Real-Time PCR (qRT-PCR) Analysis
At different time points after spinal transection, the spinal cord was collected from 1.5 mm upstream and downstream of the lesion site. EZgene™ Tissue RNA Kit (R6311, Biomiga, San Diego, USA) was used to extract total RNA. Then, the RNA was reverse transcribed into cDNA with PrimeScript™ RT Master Mix (Perfect Real Time) (RR036A, TAKARA, Otsu, Japan) according to the manufacture’s guidelines. PCR was conducted with SYBR Premix Ex Taq™ II (RR820A, Takara, Tokyo, Japan), and all assays were performed in duplicate. The following primers were used: IGF-1, 5′-TCGTCCCCACTCTTGTAAAGC-3′ (forward) and 5′-TGGCGATGGAGCTTGAACAT-3′ (reverse); β-actin, 5′-AATCTTGCGGTATCCACGAGACCA-3′ (forward) and 5′-TCTCCTTCTGCATCCTGTCAGCAA-3′ (reverse). β-Actin served as the internal control gene and a no-template reaction was performed as negative controls. Amplification specificity was confirmed by melting curves. The number of zebrafish was 8 for each group in this assay.
Immunofluorescence and Image Analysis
Spinal cord within 1.5 mm upstream and downstream of the injury site was taken and fixed in 4% paraformaldehyde (4 °C for 12 h). Then the dehydration was performed in a 30% sucrose solution (4 °C for 48 h). Serial sections (10 μm in thickness, longitudinal) through the central canal were prepared. Subsequent antigen retrieval was performed with sodium citrate buffer (95 °C for 40 min). After washed 3 times with phosphate-buffered saline (PBS), the sections were blocked with 5% donkey serum (37 °C for 1 h, SL050, Solarbio, Beijing, China). Then the samples were incubated (4 °C for 12 h) with the following primary antibodies: goat anti-IGF-1 (1:500, ab106836, Abcam, Cambridge, UK); rabbit anti-Islet-1 (1:500, ab209977, Abcam, Cambridge, UK); and rabbit anti-Iba-1 (1:500, 019-19741, Wako, Osaka, Japan). The secondary antibodies used are as follows: CY3-conjugated donkey anti-goat IgG (1:500, A0502, Beyotime, Shanghai, China) and Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (1:500, ab150073, Abcam, Cambridge, UK). Finally, the sections were covered with mounting medium (P0126, Beyotime, Shanghai, China) and imaged with an epifluorescence microscope (Axio Imager Z2, Carl Zeiss, Jena, German). ImageJ was used to analyze the cell counts and immunofluorescence density in 6 randomly chosen sections from each zebrafish. The zebrafish assayed in each group was 3.
Morpholino Treatment
Morpholinos (MO) have high mRNA-binding affinity and exquisite specificity, and they can modify pre-mRNA splicing in the nucleus by targeting splice junctions or splice regulatory sites (Draper et al. 2001; Morcos 2007; Peng et al. 2017). In order to knock down IGF-1 expression, the specific splice-blocking MO (SPL MO) was used. IGF-1 anti-sense MO (5′-ATAATCATGGATGAGCACCTTTGGT-3′) was constructed on exon 3 of IGF-1 pre-mRNA (AGGAC [ACCAAAGgtgctcatccatgattat] gatcctt). Both of the IGF-1 MO and standard control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′) (Gene Tools, Philomath, OR, USA) were dissolved in 100 μl of Danieau solution to get the final concentration of 1 mM. Approximately 500 pl MOs (0.4 mM) was injected into one-cell stage zebrafish embryos (Rosen et al. 2009) to make sure the specificity of IGF-1 anti-sense MO. In the adult spinal transection zebrafish, the gel foam (Upjohn, Kalamazoo, MI, USA) yielded 800 ng of MO was applied in the lesion site (Fig. 6a). Each zebrafish was allowed to survive the surgery for 6 weeks (Fang et al. 2014; Lin et al. 2012). The zebrafish treated with morpholino assayed in each group was 3 for immunofluorescence analysis and 9 for swimming tests.
Analysis of Motor Function
A swim-tracking test was used to evaluate the motor function recovery of zebrafish after MO treatment. Zebrafish were placed in a brightly illuminated tank (20.5 × 9.5 × 10 cm) with aquarium water (5 cm in depth, 28 °C) and recorded from above with a video camera. The swimming path, total distance, and average velocity in 5 min were analyzed by Ethovision software (Noldus, Wageningen, Netherlands). The experiment was conducted at the same time (9:00 a.m.–11:00 a.m.) every week for 6 weeks. The experimenter was blinded to the treatment of the animals. The number of zebrafish assayed was 9 for each group.
Statistical Analysis
Shapiro–Wilk test was used to evaluate normality. For parametric values, differences among two treatment groups were analyzed by the independent samples t test by SPSS 22.0 software (IBM SPSS Statistics, Armonk, New York, USA); the comparison in multiple groups was analyzed by a one-way ANOVA with an LSD post hoc assay. For non-parametric values, differences among two treatment groups were analyzed by Mann–Whitney U test; the comparison in multiple groups was analyzed by Kruskal–Wallis test. Values were expressed as mean ± SEM. P < 0.05 was considered statistically significant between groups (*P < 0.05, **P < 0.01, ***P < 0.001). All experiments were performed in triplicate.
Results
IGF-1 Expression was Down-regulated in the Lesion Site After Spinal Cord Transection
To explore the changes of IGF-1 expression levels in the regeneration process of injured spinal cord, we performed the qRT-PCR and immunofluorescence at 4 h, 12 h, 3 days, 11 days, and 21 days after spinal transection. There were no significant differences between spinal cord-transected group and sham group in IGF-1 mRNA levels at 4 h post injury (hpi) and 12 hpi. Then it was significantly decreased by 80% at 3 days post injury (dpi, P = 0.021) compared with sham injury group and still maintained at a low level until 11 dpi (P = 0.035) and 21 dpi (P = 0.009, Fig. 1). At the protein level, immunofluorescence showed that IGF-1 was expressed in the cytoplasm of cells along the central canal (Fig. 2a). Different from mRNA expression, IGF-1 protein level was significantly decreased at 4 hpi (P = 0.001) when compared with the sham injury group (Fig. 2b).
Time course of insulin-like growth factor-1 (IGF-1) mRNA expression after spinal transection. Quantitative real-time PCR showed the expression of IGF-1 mRNA in the spinal cord tissue within 1.5 mm upstream and downstream of the injury site after spinal transection. Decreased expression was observed at 3 days, 11 days, and 21 days after spinal cord transection compared to the sham injury group (*P < 0.05, **P < 0.01, independent samples t test; n = 8). Values represent mean ± SEM
Time course of insulin-like growth factor-1 (IGF-1) protein expression after spinal transection. a IGF-1 protein expression in spinal cord within 1.5 mm upstream and downstream of the injury site were examined by immunofluorescence at 5 time points after spinal transection. Cytoplasmic expression of IGF-1 protein was confirmed. Decreased IGF-1-positive cells were observed along the central canal at 4 h after spinal transection. *Indicates the central canal, scale bar = 50 μm. b Number of IGF-1-positive cells was quantified with ImageJ software. The lesion-induced decrease of IGF-1 expression achieved significance at 4 h post injury (*P < 0.05, **P < 0.01, ***P < 0.001, Kruskal–Wallis test; n = 3). Values represent mean ± SEM
IGF-1 Colocalized with Motoneurons Along the Central Canal After Spinal Cord Transection
To figure out whether IGF-1 is expressed in motoneurons, double immunostaining was performed for IGF-1 and Islet-1 (a marker for motoneurons) in sham-injured group and spinal cord-transected group (3 dpi). Cytoplasmic expression of IGF-1 in motoneurons along the central canal was found (Fig. 3).
Cytoplasmic expression of insulin-like growth factor-1 (IGF-1) in motoneurons. Double-immunostaining staining of IGF-1 with Islet-1 in longitudinal sections (1.5 mm upstream and downstream from lesion site) showed that IGF-1 was located in the cytoplasmic of motoneurons at 3 days post injury (n = 3). *Indicates the central canal. Scale bar = 50 μm
Increased Immunofluorescence Density of Microglia was Observed After Spinal Transection
To determine the localization between IGF-1 and astrocytes/microglia, double immunostaining for IGF-1 and GFAP/Iba-1 (a marker for astrocytes/microglia) showed that astrocytes/microglia surrounded the IGF-1-positive cells (Figs. 4, 5a). Increased immunofluorescence density of Iba-1 was found at 4 hpi, but did not achieve significance. There was a 2.4-folds increase in spinal cord-transected group at 12 hpi compared with sham injury group. The increased immunofluorescence density induced by spinal cord transection maintained until 21 dpi and showed significant differences with sham injury group (Fig. 5b, 4 h, P = 0.069; 12 h, P = 0.000; 3 days, P = 0.01; 11 days, P = 0.000; 21 days, P = 0.038).
IGF-1-positive motoneurons were surrounded by GFAP-positive astrocytes. Double-immunostaining staining of IGF-1 with GFAP in longitudinal sections (1.5 mm upstream and downstream from lesion site) showed that IGF-1 was surrounded by GFAP-positive astrocytes at 3 days post lesion (n = 3). *Indicates the central canal. Scale bar = 50 μm
Increased fluorescence density of microglia after spinal transection. a Double immunostaining of insulin-like growth factor-1 (IGF-1) with Iba-1 in longitudinal sections (1.5 mm upstream and downstream from lesion site) showed that IGF-1 was surrounded by microglia. *Indicates the central canal, scale bar = 50 μm. b Immunofluorescence density of Iba-1 was quantified with ImageJ software. The lesion-induced increase of immunofluorescence density of microglia achieved significance at 12 h, 3 days, 11 days, and 21 days after spinal transection (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA with an LSD post hoc assay; n = 3). Values represent mean ± SEM
Knockdown of IGF-1 Retarded Motor Recovery
To further investigate the effects of IGF-1 knockdown on motor function recovery, IGF-1 MO was applied to the lesion site immediately after spinal cord transection. In order to verify the specificity of the IGF-1 MO, IGF-1 MO targeting the exon 3/intron 3 splice junction was firstly injected into one-cell stage embryos. RT-PCR was performed using a specific set of primers in exons 3 and 4 for IGF-1 mRNA (Fig. 6b). Compared with IGF-1 MO-injected embryos, the un-spliced fragments on un-injected and control MO-injected embryos are enriched at 24 h post-fertilization (Fig. 6c) and 48 h post-fertilization (Fig. 6d).
The insulin-like growth factor-1 (IGF-1) morpholino (MO) significantly reduced IGF-1 mRNA expression. a Schematic illustration showed the spinal cord transection site and morpholino treatment site. b cDNA was amplified from RNA isolated from IGF-1 knockdown morphants, control MO-injected embryos, and non-injected embryos. RT-PCR was conducted using the primers in exons 3 and 4 for IGF-1. c and d Enrichment of un-spliced fragments was showed on the representative agarose gel. Reduction of un-spliced fragment on IGF-1 MO injected embryos was observed at both 24 h post-fertilization (b) and 48 h post-fertilization (c)
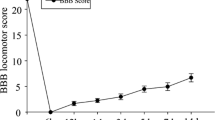
Then we tracked the swim distance and average speed over 5 min to observe the mobility of IGF-1 MO zebrafish after spinal cord transection. Swimming path was recorded by Ethovision software. IGF-1 MO zebrafish showed quite different swim paths 6 weeks post injury (wpi) (Fig. 7a). From 1 to 4 wpi, the swimming ability (mean velocity and total distance moved in 5 min) of IGF-1 MO zebrafish decreased, but no significant difference was achieved. However, IGF-1 MO retarded the motor recovery starting from 5 wpi until 6 wpi. At 5 wpi, the mean velocity (5 wpi, P = 0.025; 6 wpi, P = 0.035) and total distance (5 wpi, P = 0.025; 6 wpi, P = 0.032) moved in 5 min were decreased by 67% in IGF-1 MO group (n = 9) compared with control MO group. Similarly, there was a 65% decrease in IGF-1 MO group (n = 9) at 6 wpi (Fig. 7b, c).
Knockdown of insulin-like growth factor-1 (IGF-1) expression inhibited motor recovery. a Locus diagram of the control morpholino (MO) and IGF-1 MO groups at 6 weeks. b and c Mean velocity and total distance moved in 5 min of zebrafish treated with IGF-1 MO and control MO after spinal cord transection. At 5 and 6 weeks post injury, mean velocity and total distance moved were significantly reduced in IGF-1 MO group compared with control MO group (*P < 0.05, Mann–Whitney U test was used for mean velocity and mean mobility between two groups at 5 weeks, independent samples t test was used for mean velocity and mean mobility between two groups at 6 weeks; n = 9). Values represent mean ± SEM
IGF-1 Knockdown Decreased Motor Neurons
To clarify the effect of IGF-1 MO on the number of motor neurons, double immunostaining for IGF-1 and Islet-1 in IGF-1 MO group showed that IGF-1 was mainly expressed in smaller motor neurons at 3 dpi and 11 dpi compared with control MO group (Fig. 8a). And the number of IGF-1 positive cells in IGF-1 MO group decreased by 21.6% and 22.6%, respectively at 3 dpi (P = 0.039) and 11 dpi (P = 0.017, Fig. 8a, b) compared with control MO group. Simultaneously, Islet-1 positive motoneurons decreased by 25.2% and 27.7%, respectively, at 3 dpi (P = 0.036) and 11 dpi (P = 0.045, Fig. 8a, c).
Islet-1-positive motoneurons decrease after insulin-like growth factor-1 (IGF-1) morpholino (MO) treated. a Double immunostaining of IGF-1 with Islet-1 was performed in IGF-1 MO group and control MO group. *Indicates the central canal, scale bar = 50 μm. b and c The number of IGF-1-positive cells and motoneurons was both decreased in IGF-1 MO group compare with control MO group (*P < 0.05, Mann–Whitney U test; n = 3). Values represent mean ± SEM
Down-regulation of IGF-1 Increased the Immunofluorescence Density of Microglia
To further investigate the relationship between the decrease of IGF-1 and Iba-1+ cells, double immunostaining for IGF-1 and Iba-1 was performed in IGF-1 MO group and control MO group. Decreased IGF-1 positive cells were observed in IGF-1 MO group compared with control MO group at 3 dpi and 11 dpi which indicated successful knockdown of IGF-1 (Fig. 9a). At the same time, immunofluorescence density of Iba-1 in IGF-1 MO group increased by 27.7% and 25.3%, respectively, at 3 dpi (P = 0.026) and 11 dpi (P = 0.008) compared with control MO group, which illustrated that decreased IGF-1 expression resulted in increased the immunofluorescence density of microglia (Fig. 9a, b).
Insulin-like growth factor-1 (IGF-1) morpholino (MO) treatment induced increased immunofluorescence density of microglia. a Double immunostaining of IGF-1 and Iba-1 showed decreased IGF-1 expression in IGF-1 MO group compared with control MO group at 3 days and 11 days after injury. *Indicates the central canal, scale bar = 50 μm. b Increased fluorescence density of Iba-1 in IGF-1 MO group at 3 days and 11 days after injury was observed compared with control MO group (*P < 0.05, **P < 0.01, independent samples t test; n = 3). Values represent mean ± SEM
Discussion
In this study, we investigated the beneficial effects of IGF-1 on spinal-transected zebrafish. We showed that the expression level of IGF-1 was decreased at 3 days after spinal transection. Down-regulation of IGF-1 expression induced by MO treatment inhibited motor recovery of spinal-transected zebrafish. And we also found that IGF-1 MO treatment reduced the number of motoneurons and increased Iba-1+ cells, which indicated the role of IGF-1 in enhancing motoneuron survival and inhibiting neuroinflammation after spinal transection in zebrafish.
Zebrafish are considered as an ideal model for neuro-regeneration and repair. The reasons are as follows: (1) extraordinary ability of axonal regrowth achieves restoration of function by approximately 4–6 weeks after complete spinal cord transection, and (2) there is a remarkable level of conservation (87%) between zebrafish and human genomes. In addition, most molecules involved in the mechanisms preventing successful recovery of motor function in adult mammals have not been explored in zebrafish, which include extrinsic (growth-promoting molecules and/or the surplus of growth-inhibitory molecules) and intrinsic mechanisms (Vajn et al. 2013).
The expression location of IGF-1 varies in different central nervous system diseases and animal models. In rats with unilateral penetrating brain injury, on the uninjured cerebral hemisphere, IGF-1 protein was only expressed in cortical neurons, while on the injured cerebral hemisphere, increased level of IGF-1 protein was not only localized to neurons, but also localized to injury responsive astrocytes and cells of monocyte lineage (Walter et al. 1997). In spinal cord-transected rats, immunohistochemistry showed that IGF-1 was observed in spinal neurons (both motor neurons and sensory neurons), but not astrocytes (Wang et al. 2017). In acute Experimental Autoimmune Encephalomyelitis of mice, IGF-1 immunoreactive neurons were only found in inflamed spinal cords, but not non-inflamed spinal cord (Parvaneh Tafreshi et al. 2017). In our study, IGF-1 was expressed in cytoplasmic of motoneurons and was surrounded by Iba-1+ microglia and GFAP+ astrocytes after spinal cord transection in zebrafish. Thus, we hypothesized that different cell localizations of IGF-1 in these species or disease models may be related to the different repair mechanisms of IGF-1.
We also investigated the changes of IGF-1 expression numerically at the lesion site after spinal cord transection. The level of IGF-1 mRNA began to decrease below basal levels at 3 dpi and persisted until 21 dpi. The decrease in IGF-1 protein only found at 4 hpi reflected by the number of IGF-1 immuno-positive cells. In eukaryotes, only 40% of the variation in proteins can be explained by knowing mRNA abundances, and the remaining 60% of the variation can be explained by post-transcriptional regulation and measurement noise (Vogel and Marcotte 2012). Experiments in mammalian cells found that variation in protein expression levels is primarily determined by regulation of translation (Schwanhausser et al. 2011). Therefore, we hypothesized that the differences in mRNA and protein relative expression levels of IGF-1 might be due to post-transcriptional regulation. Spinal cord transection can directly cause the death of motoneurons, and microglial overactivation induced neural impairments (Wang et al. 2015). In this study, IGF-1 is mainly produced by motoneurons in spinal cord of zebrafish, and the fluorescence density of microglia increased after spinal cord transection, which suggested the possibility of microglia overactivation. The decrease in IGF-1 expression might due to the loss and damage of motoneurons. Reimer et al. found that injury-induced motoneuron proliferation peaks at 2 weeks after lesion and motoneurons migrate to lesion site to compensate for the lost neurons (Reimer et al. 2008), which suggested that the number of motoneurons at the lesion site may increase at 3 weeks after lesion. The proliferation of motoneurons and the mechanisms by which IGF-1 functions still need to be further investigated in the following experiments.
IGF-1 can modulate multiple fundamental cellular processes, such as cell growth, survival, proliferation, and differentiation (Vardatsikos et al. 2009): IGF-1 was reported to protect RCG-5 cells (retinal ganglion cells) from amiodarone-induced apoptosis by stimulating the PI3K/Akt/FoxO3a pathway (Liao et al. 2017); in IGF-1-overexpressed brain-injured mice, increased density of immature neurons, and generation of newborn neurons in the hippocampus were observed (Carlson et al. 2014). In our study, significant decrease of motoneurons was observed in IGF-1 MO-treated group at 3 dpi and 11 dpi, which indicated the neuronal protective effect of IGF-1 in zebrafish. Similarly, cell death reduction of motor neurons was found after adeno-associated virus vector encoding IGF-1 injection into the deep cerebellar nucleus of a spinal muscular atrophy mouse model (Hollis et al. 2009). Therefore, IGF-1 protects motoneurons from dying probably by promoting cell survival and anti-apoptosis, or by stimulating cell proliferation, but the specific mechanism still needs to be further investigated.
Zebrafish have two waves of motoneuron differentiation: primary and secondary motoneurons. Primary motoneurons (PMNs) axons pioneer nerve pathways followed later by axons of secondary motoneurons (SMNs; Myers et al. 1986; Pike et al. 1992). SMNs are born later than PMNs and are more numerous (Lewis and Eisen 2003). Islet-1 has been reported to be expressed in all vertebrate motoneurons and is required for formation of zebrafish primary motoneurons (Hutchinson and Eisen 2006). After IGF-1 MO treatment, Islet-1 is mainly expressed in smaller motor neurons, which indicated that IGF-1 may be involved in the fates of primary motoneurons generation and further mature motoneurons determination directly or by affecting Islet-1. In addition, motor neurons are generated from an olig2-expressing population of PMN-like ependymoradial glial cells in a ventrolateral position at the central canal of zebrafish after lesion (Reimer et al. 2008). IGF-1 regulates multiple cellular processes including cell differentiation and growth (Vardatsikos et al. 2009). After IGF-1 MO treatment, the number of motoneurons decreased and IGF-1 was located in smaller motoneurons, which might be due to IGF-1 being involved in the regeneration of motoneurons and the growth of newborn motoneurons.
In healthy brain, microglia are usually in a resting state but are readily activated when brain occurs to injury, infection, and neuroinflammation. Activated microglia can promote the repair of injured tissues by inducing the secretion of growth factors, removing tissue debris, and resisting harmful pathogens; on the other hand, activated microglia can also release a variety of cytotoxic substances, which have neurotoxic effects on neurons and itself (Kreutzberg 1996). IGF-1 can also function as an anti-inflammatory factor, and it can alleviate the sickness behavior caused by the lateral ventricle injection of proinflammatory cytokines (TNF-α, IL-1β) in mice (Bluthe et al. 2006; Palin et al. 2007). In our study, increased immunofluorescence density of microglia was observed from 12 hpi to 21 dpi in zebrafish. After treatment of IGF-1 MO, increased immunofluorescence density of microglia was also observed. This is probably due to the reduced anti-inflammatory effect of IGF-1. Since IGF-1 was restrictively expressed in motoneurons in zebrafish spinal cord, whether the reduction of IGF-1 in motoneurons will affect microglia activation still needs further investigation.
As an ideal model of spinal cord transection, zebrafish has profound re-growing ability. At 5 weeks and later, the swim distance of the spinal-transected zebrafish recovered to 78% (the peak of recovery in swimming ability) of the sham-operated zebrafish, and lengths of regenerated axons are similar to sham-injured group (Fang et al. 2012). And at 6 weeks, the diameter of the tissue bridge was similar to the spinal cord at the comparable level in un-lesioned zebrafish (Vajn et al. 2014). For years, zebrafish has been used to identify novel genes vital for successful regeneration after spinal cord transection. However, exact molecular and cellular mechanisms of recovery in zebrafish CNS are not fully understood. IGF-1 signals drove axonal outgrowth via AMPK (AMP-activated protein kinase) in dorsal root ganglia neuron cultures (Aghanoori et al. 2019) and contributed to RGC survival and axonal regeneration in adult goldfish retinas through PI3K/Akt system after optic nerve injury. In this study, knockdown of IGF-1 retarded motor recovery (Koriyama et al. 2007), which might due to the beneficial effect of IGF-1 on axon regeneration in spinal-transected zebrafish.
Data Availability
Not applicable.
Abbreviations
- CNS:
-
Central nervous system
- IGF-1:
-
Insulin-like growth factor-1
- MO:
-
Morpholino
- SPL MO:
-
Splice-blocking MO
- qRT-PCR:
-
Quantitative real-time PCR
- PBS:
-
Phosphate-buffered saline
- hpi:
-
Hours post injury
- dpi:
-
Days post injury
- wpi:
-
Weeks post injury
References
Aghanoori M-R, Smith DR, Shariati-Ievari S, Ajisebutu A, Nguyen A, Desmond F, Jesus CHA, Zhou X, Calcutt NA, Aliani M, Fernyhough P (2019) Insulin-like growth factor-1 activates AMPK to augment mitochondrial function and correct neuronal metabolism in sensory neurons in type 1 diabetes. Mol Metab 20:149–165. https://doi.org/10.1016/j.molmet.2018.11.008
Becker T, Bernhardt RR, Reinhard E, Wullimann MF, Tongiorgi E, Schachner M (1998) Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J Neurosci 18(15):5789–5803
Bluthe RM, Kelley KW, Dantzer R (2006) Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun 20(1):57–63. https://doi.org/10.1016/j.bbi.2005.02.003
Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L (2017) Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat Inflamm 2017:9478542. https://doi.org/10.1155/2017/9478542
Boucher SE, Hitchcock PF (1998) Insulin-related growth factors stimulate proliferation of retinal progenitors in the goldfish. J Comp Neurol 394(3):386–394
Briona LK, Poulain FE, Mosimann C, Dorsky RI (2015) Wnt/ss-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev Biol 403(1):15–21. https://doi.org/10.1016/j.ydbio.2015.03.025
Carlson SW, Madathil SK, Sama DM, Gao X, Chen J, Saatman KE (2014) Conditional overexpression of insulin-like growth factor-1 enhances hippocampal neurogenesis and restores immature neuron dendritic processes after traumatic brain injury. J Neuropathol Exp Neurol 73(8):734–746. https://doi.org/10.1097/NEN.0000000000000092
Chablais F, Jazwinska A (2010) IGF signaling between blastema and wound epidermis is required for fin regeneration. Development 137(6):871–879. https://doi.org/10.1242/dev.043885
Chesik D, De Keyser J, Wilczak N (2008) Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci 35(1):81–90. https://doi.org/10.1007/s12031-008-9041-2
Draper BW, Morcos PA, Kimmel CB (2001) Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis 30(3):154–156. https://doi.org/10.1002/gene.1053
Fang P, Lin JF, Pan HC, Shen YQ, Schachner M (2012) A surgery protocol for adult zebrafish spinal cord injury. J Genet Genomics 39(9):481–487. https://doi.org/10.1016/j.jgg.2012.07.010
Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala H, Schachner M, Shen YQ (2014) HMGB1 contributes to regeneration after spinal cord injury in adult zebrafish. Mol Neurobiol 49(1):472–483. https://doi.org/10.1007/s12035-013-8533-4
Fleisch VC, Fraser B, Allison WT (2011) Investigating regeneration and functional integration of CNS neurons: lessons from zebrafish genetics and other fish species. Biochim Biophys Acta 1812(3):364–380. https://doi.org/10.1016/j.bbadis.2010.10.012
Gensel JC, Zhang B (2015) Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res 1619:1–11. https://doi.org/10.1016/j.brainres.2014.12.045
Hammarberg H, Risling M, Hökfelt T, Cullheim S, Piehl F (1998) Expression of insulin-like growth factors and corresponding binding proteins (IGFBP 1–6) in rat spinal cord and peripheral nerve after axonal injuries. J Comp Neurol 400(1):57–72
Hollis ER II, Lu P, Blesch A, Tuszynski MH (2009) IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp Neurol 215(1):53–59. https://doi.org/10.1016/j.expneurol.2008.09.014
Huebner EA, Strittmatter SM (2009) Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ 48:339–351. https://doi.org/10.1007/400_2009_19
Hutchinson SA, Eisen JS (2006) Islet1 and Islet2 have equivalent abilities to promote motoneuron formation and to specify motoneuron subtype identity. Development (Camb Engl) 133(11):2137–2147
Jablonka S, Holtmann B, Sendtner M, Metzger F (2011) Therapeutic effects of PEGylated insulin-like growth factor I in the pmn mouse model of motoneuron disease. Exp Neurol 232(2):261–269. https://doi.org/10.1016/j.expneurol.2011.09.015
Jacobs PL, Nash MS (2004) Exercise recommendations for individuals with spinal cord injury. Sports Med 34(11):727–751. https://doi.org/10.2165/00007256-200434110-00003
Kjell J, Olson L (2016) Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech 9(10):1125–1137. https://doi.org/10.1242/dmm.025833
Koriyama Y, Homma K, Sugitani K, Higuchi Y, Matsukawa T, Murayama D, Kato S (2007) Upregulation of IGF-I in the goldfish retinal ganglion cells during the early stage of optic nerve regeneration. Neurochem Int 50(5):749–756. https://doi.org/10.1016/j.neuint.2007.01.012
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318. https://doi.org/10.1016/0166-2236(96)10049-7
Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI (2017) Insulin-like growth factor-1 and neuroinflammation. Front Aging Neurosci 9:365. https://doi.org/10.3389/fnagi.2017.00365
Lewis KE, Eisen JS (2003) From cells to circuits: development of the zebrafish spinal cord. Prog Neurobiol 69(6):419–449. https://doi.org/10.1016/s0301-0082(03)00052-2
Liao R, Yan F, Zeng Z, Farhan M, Little P, Quirion R, Srivastava LK, Zheng W (2017) Amiodarone-induced retinal neuronal cell apoptosis attenuated by IGF-1 via counter regulation of the PI3k/Akt/FoxO3a pathway. Mol Neurobiol 54(9):6931–6943. https://doi.org/10.1007/s12035-016-0211-x
Lin J-F, Pan H-C, Ma L-P, Shen Y-Q, Schachner M (2012) The cell neural adhesion molecule contactin-2 (TAG-1) is beneficial for functional recovery after spinal cord injury in adult zebrafish. PLoS ONE 7(12):e52376. https://doi.org/10.1371/journal.pone.0052376
Madathil SK, Carlson SW, Brelsfoard JM, Ye P, D’Ercole AJ, Saatman KE (2013) Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS ONE 8(6):e67204. https://doi.org/10.1371/journal.pone.0067204
Morcos PA (2007) Achieving targeted and quantifiable alteration of mRNA splicing with Morpholino oligos. Biochem Biophys Res Commun 358(2):521–527. https://doi.org/10.1016/j.bbrc.2007.04.172
Myers PZ, Eisen JS, Westerfield M (1986) Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci 6(8):2278–2289
Nieto-Estévez V, Defterali Ç, Vicario-Abejón C (2016) IGF-I: a key growth factor that regulates neurogenesis and synaptogenesis from embryonic to adult stages of the brain. Front Neurosci 10:52–52. https://doi.org/10.3389/fnins.2016.00052
Otteson DC, Cirenza PF, Hitchcock PF (2002) Persistent neurogenesis in the teleost retina: evidence for regulation by the growth-hormone/insulin-like growth factor-I axis. Mech Dev 117(1–2):137–149. https://doi.org/10.1016/s0925-4773(02)00188-0
Palin K, Bluthé R-M, McCusker RH, Moos F, Dantzer R, Kelley KW (2007) TNFalpha-induced sickness behavior in mice with functional 55 kD TNF receptors is blocked by central IGF-I. J Neuroimmunol 187(1–2):55–60. https://doi.org/10.1016/j.jneuroim.2007.04.011
Park SE, Dantzer R, Kelley KW, McCusker RH (2011) Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J Neuroinflamm 8:12. https://doi.org/10.1186/1742-2094-8-12
Parvaneh Tafreshi A, Talebi F, Ghorbani S, Bernard C, Noorbakhsh F (2017) Altered expression of IGF-I system in neurons of the inflamed spinal cord during acute experimental autoimmune encephalomyelitis. J Comp Neurol 525(14):3072–3082. https://doi.org/10.1002/cne.24263
Peng SX, Yao L, Cui C, Zhao HD, Liu CJ, Li YH, Wang LF, Huang SB, Shen YQ (2017) Semaphorin4D promotes axon regrowth and swimming ability during recovery following zebrafish spinal cord injury. Neuroscience 351:36–46. https://doi.org/10.1016/j.neuroscience.2017.03.030
Pike SH, Melancon EF, Eisen JS (1992) Path finding by zebrafish motoneurons in the absence of normal pioneer axons. Development 114(4):825–831
Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, Tetzlaff W (2014) Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol 117:54–72. https://doi.org/10.1016/j.pneurobio.2014.02.006
Reimer MM, Sorensen I, Kuscha V, Frank RE, Liu C, Becker CG, Becker T (2008) Motor neuron regeneration in adult zebrafish. J Neurosci 28(34):8510–8516. https://doi.org/10.1523/JNEUROSCI.1189-08.2008
Rodriguez-Perez AI, Borrajo A, Diaz-Ruiz C, Garrido-Gil P, Labandeira-Garcia JL (2016) Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. OncoTarget 7(21):30049–30067. https://doi.org/10.18632/oncotarget.9174
Rosen JN, Sweeney MF, Mably JD (2009) Microinjection of zebrafish embryos to analyze gene function. J Vis Exp. https://doi.org/10.3791/1115
Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ (2006) Experimental strategies to promote spinal cord regeneration—an integrative perspective. Prog Neurobiol 78(2):91–116. https://doi.org/10.1016/j.pneurobio.2005.12.004
Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M (2011) Global quantification of mammalian gene expression control. Nature 473(7347):337–342. https://doi.org/10.1038/nature10098
Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P (2014) Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain J Neurol 137(Pt 3):707–723. https://doi.org/10.1093/brain/awt341
Suh H-S, Zhao M-L, Derico L, Choi N, Lee SC (2013) Insulin-like growth factor 1 and 2 (IGF1, IGF2) expression in human microglia: differential regulation by inflammatory mediators. J Neuroinflamm 10:37–37. https://doi.org/10.1186/1742-2094-10-37
Vajn K, Plunkett JA, Tapanes-Castillo A, Oudega M (2013) Axonal regeneration after spinal cord injury in zebrafish and mammals: differences, similarities, translation. Neurosci Bull 29(4):402–410. https://doi.org/10.1007/s12264-013-1361-8
Vajn K, Suler D, Plunkett JA, Oudega M (2014) Temporal profile of endogenous anatomical repair and functional recovery following spinal cord injury in adult zebrafish. PLoS ONE 9(8):e105857. https://doi.org/10.1371/journal.pone.0105857
Vardatsikos G, Sahu A, Srivastava AK (2009) The insulin-like growth factor family: molecular mechanisms, redox regulation, and clinical implications. Antioxid Redox Signal 11(5):1165–1190. https://doi.org/10.1089/ARS.2008.2161
Vogel C, Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13(4):227–232. https://doi.org/10.1038/nrg3185
Walter HJ, Berry M, Hill DJ, Logan A (1997) Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology 138(7):3024–3034. https://doi.org/10.1210/endo.138.7.5284
Wang F, Cui N, Yang L, Shi L, Li Q, Zhang G, Wu J, Zheng J, Jiao B (2015) Resveratrol rescues the impairments of hippocampal neurons stimulated by microglial over-activation in vitro. Cell Mol Neurobiol 35(7):1003–1015. https://doi.org/10.1007/s10571-015-0195-5
Wang X, Ju S, Chen S, Gao W, Ding J, Wang G, Cao H, Tian H, Li X (2017) Effect of electro-acupuncture on neuroplasticity of spinal cord-transected rats. Med Sci Monit 23:4241–4251. https://doi.org/10.12659/msm.903056
Wrigley S, Arafa D, Tropea D (2017) Insulin-like growth factor 1: at the crossroads of brain development and aging. Front Cell Neurosci 11:14–14. https://doi.org/10.3389/fncel.2017.00014
Acknowledgements
This study was supported by Grants from The National Natural Science Foundation of China (81771384, 81801276), Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX19_1893), Public Health Research Center at Jiangnan University (JUPH201801), and National First-Class Discipline Program of Food Science and Technology (JUFSTR20180101), Chinese Postdoctoral Science Foundation (2018M630512), and Wuxi Municipal Health Commission (1286010241190480).
Author information
Authors and Affiliations
Contributions
The experiment design: LZ, CC, YS, SH, CQ, LY; Spinal transection surgery: LZ, BZ, SH; Quantitative real-time PCR analysis: LZ, BZ, XJ, MS; Immunofluorescence and image analysis: LZ, BZ, SH, ZZ; Morpholino treatment: LZ, BZ, SH, ZZ, MS, LY; Behavioral test: LZ, BZ, YS; Statistical analysis: LZ, FW, ZZ, XJ, CQ, YS; Interpretation and editing of manuscript: LZ, CC, YS.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This research was approved by the Animal Ethics Committee of Jiangnan University. The zebrafish work was performed according to Regulations for the Administration of Affairs Concerning Experimental Animals (approved by the State Council on October 31, 1998 and promulgated by Decree No. 2 of the State Science and Technology Commission on November 14, 1988).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, L., Zhang, B., Huang, S. et al. Insulin-Like Growth Factor-1 Enhances Motoneuron Survival and Inhibits Neuroinflammation After Spinal Cord Transection in Zebrafish. Cell Mol Neurobiol 42, 1373–1384 (2022). https://doi.org/10.1007/s10571-020-01022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-01022-x