Abstract
Amongst the many soluble extracellular factors stimulating intracellular signal transduction pathways and driving cellular processes such as proliferation, differentiation and survival, insulin-like growth factors (IGFs) stand out as indispensable factors for proper oligodendrocyte differentiation and accompanying myelin production. Owing to its potent myelinogenic capacity and its neuroprotective properties, IGFs hold therapeutic potential in demyelinating and neurodengenerative diseases. However, the IGF system is comprised of a complex molecular network involving regulatory binding proteins, proteases, cell surface and extracellular matrix components which orchestrate IGF-specific functions. Thus, the complexity by which these factors are tightly regulated makes a simplistic therapeutic approach towards treating demyelinating conditions unfeasible. In the present review, we address these issues and consider current therapeutic prospects of oligodendrocyte-targeted IGF-based therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several pathologies of the central nervous system (CNS), exhibit oligodendroglial cell damage or loss, an insult which often afflicts intact myelin and its functions. There is considerable interest in therapeutic strategies aimed at CNS tissue repair, a task which, in the case of demyelination, frequently involves the replenishment of oligodendrocyte populations by stimulating proliferation of oligodendrocyte progenitor cells (OPCs) and triggering the differentiation of these cells into mature myelin-producing oligodendrocytes capable of myelinating axons. Coordinating such events would demand a spatial and temporal orchestration of factors stimulating these processes. The insulin-like growth factor (IGF)-1 receptor is expressed in most tissues, granting IGF-1 a broad range of effects throughout human physiology. This wide range of expression indicates potential therapeutic use of IGF-1 for treatment of several disorders. In particular, IGF-1 has pleiotropic functions in nervous tissue with physiological importance in sustaining normal CNS and peripheral nervous system (PNS) functions, supporting its potential application in treating neurological disorders. Although functional deficits of neurological diseases vary, most are pathologically marked by neurodegeneration, a feature that can potentially be impeded by IGF-1 in both sensory and motor neurons of the PNS. Of particular interest are the effects of IGF signaling on oligodendrocytes and their progenitors, as this factor plays an essential role in myelinogenesis during development and exerts survival functions on these cells. In the present review, we will detail the IGF system and its important functions in the CNS, and discuss the effects of IGF signaling on oligodendrocytes. We will also focus on the implications of IGF signaling in CNS pathologies involving demyelination and consider novel strategies, which confront the dilemma of oligodendrocyte targeting.
Extracellular Factors Influence Oligodendrocyte Behavior
Proliferative precursor cells of the oligodendrocyte lineage emerge out of the germinal zones and eventually develop into mature oligodendrocytes with the capacity to produce the multilamellar myelin sheath, which serves to encompass axons of neurons allowing for saltatory conduction. A plethora of factors guide the developmental processes toward maturation of oligodendroglia cueing myelinogenesis. In vitro studies on rodent cells have demonstrated that a variety of factors contribute to specific cellular events of OPCs, which respond to important signals. Specifically, growth factors are known to coordinate many cellular processes such as proliferation, migration, differentiation and survival of OPCs and maturation of oligodendrocytes and myelin production. However, the complexity of these signaling events is demonstrated by individual growth factors, which can generate important signaling events alone, act synergically with additional factors, or counteract/alter the function of other factors. OPCs proliferate in response to platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), neurotrophin 3 (NT3), sonic hedgehog, pituitary adenylyl cyclase-activating peptide, leukemia inhibitory factor, neuregulin and glial growth factor-2, which have all been shown to be mitogenic for this cell type (Adachi et al. 2005; Barres et al. 1993; Bogler et al. 1990; Cohen et al. 1996; Lelievre et al. 2006; Noble et al. 1988; Wang et al. 2007). PDGF is thought to trigger only a limited number of OPC divisions, which can be further stimulated with bFGF, which upregulates PDGF receptor expression and primes oligodendrocyte progenitors for continuous proliferation in response to PDGF (McKinnon et al. 1990). This mechanism is distinguished by the actions of transforming growth factor-β (TGF-β), which interferes with PDGF signaling, reduces proliferation and encourages the process of differentiation (McKinnon et al. 1993). Bone morphogenetic protein-2 also acts to stimulate OPC differentiation by interfering with PDGF signaling (Adachi et al. 2005). In addition to growth factor signaling, other signaling pathways, such as initiated by cell surface integrin receptors, can also alter the role of PDGF receptor signaling from inducing proliferation to enhancing survival in oligodendrocytes (Baron et al. 2003). The extracellular matrix (ECM) glycoprotein tenascin-C has also been shown to be involved in oligodendroglial cell behavior, e.g., demonstrating anti-apoptotic effects that are thought to be associated with PDGF signaling (Garwood et al. 2004). PDGF is not only mitogenic, but also appears to drive the differentiation of neural stem cells (NSCs), which are competent for commitment to the oligodendrocyte lineage, toward O4-positive oligodendrocytes (Hu et al. 2008). PDGF-alpha receptor signaling is also thought to be required for oligodendrogenesis from NSCs of the adult subventricular zone (Jackson et al. 2006). Although this factor is implicated in these initial differentiation processes, PDGF can inhibit myelination, as demonstrated in cocultures of oligodendrocytes with dorsal root ganglion neurons (Wang et al. 2007).
Amongst the factors driving OPC differentiation toward a mature myelin-producing oligodendrocyte are the insulin-like growth factors-1 and -2, which are essential for proper oligodendrocyte maturation and myelin production. In the following sections, we will provide a brief overview of the IGF system including the components involved, mechanisms of regulation, and signaling pathways initiated by these ligands.
The Insulin-like Growth Factor System
The two ligands of insulin-like growth factor system are termed IGF-1 and -2, which are genetically related ubiquitous single-chain polypeptides with similar tertiary structure and amino acid sequence homology. Predominately synthesized in the liver, IGF-1 expression is influenced by hormonal and nutritional factors, primarily regulated by growth hormone, and is one of the major trophic factors in the human circulation (Bichell et al. 1992; Jones and Clemmons 1995). IGF-1 is also synthesized in many other organs, including the brain, where growth hormone does not appear to regulate its production (Aberg et al. 2006). In a vast number of tissues, IGF-1 and -2 serve as potent stimulants of proliferation for many cell types, an action which involves stimulation of multiple signaling pathways. Physiologically, IGF plays a role in promoting acute anabolic effects on protein and carbohydrate metabolism, and longer-term effects on cell replication, survival and differentiation. The biological functions of both IGF-1 and -2 are mediated by a specific cell surface membrane receptor, the IGF-1 (type-1 IGF) receptor, which is transcriptionally regulated by growth factors and tumor suppressors (Jones and Clemmons 1995). After posttranslational modifications, the dimer of an alpha-extracellular and a beta-transmembrane subunit form an alpha-beta half-receptor which binds to another half-receptor to form a mature α2β2-heterotetramer (Rechler and Nissley 1985). The glycosylated α-subunits contain the ligand-binding domain, and β-subunits contain tyrosine kinase domains. Binding of the extracellular ligand to the type 1 IGF receptor stimulates a complex network of intracellular signaling cascades, which ultimately regulate gene transcription. Signal transduction begins with activation of receptor tyrosine kinase domains and results in receptor autophosphorylation. A pivotal role of propagation of the cytoplasmic signal is played by a 185-kDa substrate, termed the insulin-receptor substrate-1/4. Once activated, the receptor phosphorylates IRS-1/4 on multiple tyrosines, which then serve as a large docking protein allowing binding of proteins with src homology 2 domains such as the p85 regulatory subunit of phosphatidyl inositol-3 kinase (PI3-K) and growth factor receptor bound protein 2. Subsequently, IGF-1 is known to initiate at least two signaling cascades; the first involves activation of PI3 kinase and subsequent activation of Akt pathways, which are often associated with survival and inhibition of apoptosis. The second pathway involves the extracellular signal-related kinases, ERK-1 and ERK-2 (MAP kinases), which are translocated to the nucleus where they phosphorylate a variety of transcription factors including c-fos, c-jun and c-myc, which are involved in cellular growth and mitogenesis (for IGF signaling pathways in neuronal cells refer to Feldman et al. 1997).
IGF Regulation
In the circulation and throughout the extracellular space of all tissues, the IGFs are present almost entirely bound to members of a family of high-affinity IGF-binding proteins (IGFBPs). The six known IGFBPs possess an 80% sequence homology and share conserved N- and C-terminal regions that are cystein rich. IGFBPs have been proposed to have four major functions that are essential to coordinate and regulate the biological activities of the IGFs. These are: (1) to act as transport carrier proteins of IGFs, (2) to stabilize and prolong the half-lives of IGFs thereby regulating their metabolic clearance, (3) to provide a means of tissue and cell-type-specific localization, and (4) to directly stimulate or inhibit interactions of the IGFs with their receptors (Jones and Clemmons 1995). In the human circulation, a further component includes a 150-kDa protein known as the acid-labile-subunit, which forms a complex with IGF-1 and IGF binding protein-3 (Baxter et al. 1989). The concentration of this storage form of IGF-1 is under growth-hormone control (Blum et al. 1993).
In addition to these binding components, several IGFBP (tissue)-specific proteases have been identified. These can cleave IGFBPs into fragments, reducing their affinity for the IGFs (Maile and Holly 1999). Hence, the biological functions of the IGFBPs in regulating IGFs are strongly influenced by proteases which can induce tissue-specific IGF release.
Although the IGFBPs share common properties in regulating IGFs, these proteins possess unique individual properties and structural characteristics such as phosphorylation and glycosylation patterns which enable specific functional properties of these proteins. Because of these distinctions, several of these proteins have been shown to demonstrate IGF-independent actions. Through interactions with specific cell surface structures, such as integrins and yet undefined receptors, IGFBPs have been shown to regulate cell cycle, apoptosis and cell migration (Mohan and Baylink 2002).
IGF in the CNS
In the brain, local production is considered the main source of IGFs, as they are not thought to easily cross the human blood–brain barrier (Russo et al. 2005). However, systemic IGF-1 has been demonstrated to access the brain parenchyma through transport mechanisms involving choroid plexus endocytic receptor megalin/low-density lipoprotein receptor-related protein-2 (LRP2), a multicargo transporter (Carro et al. 2005). Glial cells have been shown to be an important source of IGF-1 in the CNS and are thought to produce autocrine and paracrine effects on neighboring cells. During development, expression of IGF-1 is particularly high in neuronal-rich regions of the midbrain, cerebral cortex, hippocampus and olfactory bulb, whereas IGF-2 is most abundant in myelin tissue (Bondy et al. 1992; Garcia-Segura et al. 1991; Rotwein et al. 1988). During foetal and neonatal life, there is high expression of IGF-1 receptors, suggesting an important role for IGFs in developmental processes. Mice carrying null mutations of the genes encoding IGF-1 display reduced brain size, hypomyelination, reduced density of oligodendrocytes and loss of neuron populations (Beck et al. 1995; Liu et al. 1993). By comparison, transgenic mice overexpressing IGF-1 had 55% larger brains with increased myelin content, cell number and size compared to controls (Carson et al. 1993). In accordance with this, transgenic mice overexpressing the IGF inhibitory IGFBP-1 demonstrated decreased percentage of myelinated axons, and the thickness of myelin sheaths were also reduced, a feature that was accompanied by reduction of myelin protein expression (Ye et al. 1995). Albeit, ice-carrying null mutations of the gene encoding for the type 1 IGF receptor die invariably at birth; in utero growth retardation of the brain was demonstrated (Liu et al. 1993). Hence, there is abundant evidence that IGF-1 is an essential factor for proper CNS development and myelin formation.
In addition to its developmental roles, IGFs are of importance in adult life where they exert neurotrophic and neuroprotective effects (Trejo et al. 2004). Roles include the promotion of survival and differentiation of sensory, sympathetic and motor neurons (Oorschot and McLennan 1998; Sendtner 1995). There is also evidence for a strong correlation between reduced levels of circulating IGF-1 and deterioration of cognitive functions, as shown in ageing humans (van Dam and Aleman 2004).
IGF Signaling in Oligodendrocytes Influences Cell Behavior
IGF as a Mitotic Factor for OPCs
Initial in vitro studies revealed the proliferative effects of IGF-1 treatment on oligodendrocytes and their progenitors in cocultures with other glial cells (McMorris et al. 1986; McMorris and Dubois-Dalcq 1988). It has since been shown that IGF-1 serves to enhance oligodendrocyte progenitor proliferation by activating MAPK and Akt pathways (Cui and Almazan 2007). In vitro, synergic effects of FGF-2 and IGF-1 have been reported to enhance the function of cyclin D1, a regulator of cell-cycle progression, in OPCs. Whereas FGF-2 enhanced the expression of cyclin D1, IGF-1 inhibited cyclin D1 degradation and promoted its nuclear localization (Frederick et al. 2007). These cooperative effects were thought to contribute to the enhanced proliferative effect of these growth factors on OPCs.
Differential ablation of the type 1 IGF receptor in either Olig1 [IGF-1R(pre-oligo-KO)] or proteolipid protein [PLP; IGF-1R(oligo-KO)]-expressing cells in mice demonstrated the importance of IGF-1 signaling in cells of oligodendrocyte lineage (Zeger et al. 2007). Especially detrimental effects were demonstrated in IGF-1R(pre-oligo-KO) mice which demonstrated decreased cell number and volume of the corpus callosum and anterior commissure at weeks 2 and 6 of age, respectively. In these mice, it was shown that NG2(+) and CC1(+) cells were greatly reduced. IGF-1R(oligo-KO) mice also showed a reduction in cell number and size in these regions, albeit, reductions were to a lesser degree and at a much later age, namely, 25 week-old mice. Both mice had reduced myelin production. It was postulated that both reduced cell survival and growth were primary factors contributing to cell-number reduction.
IGF-1 Enhances Oligodendrocyte Survival
A cellular action of IGF-1 that is complementary to its stimulation of cell proliferation is its capacity in certain cells to inhibit apoptosis (Kooijman 2006). IGF-1 exerts protective effects on oligodendrocytes and neurons by enhancing cell survival, a mechanism that is thought to be mediated by IGF-induced activation of PI3-K activated pathways. Many in vitro models involving toxic insults have demonstrated the protective effects of IGF-1 on OPCs. Rescue of glutamate-mediated death of late OPCs by IGF-1 was shown to involve inhibition of Bax translocation, cytochrome c release and caspase 3 activation (Wood et al. 2007). In the same study, treatment with IGF-1 rescued OPCs in perinatal white matter after hypoxia–ischemia (Wood et al. 2007).
OPC treatment with tumor necrosis factor (TNF)-alpha results in cell death, which can be effectively protected by IGF-1 treatment (Ye et al. 2007). These IGF-induced survival effects are mediated by PI3K/Akt signaling, which interrupts the mitochondrial apoptotic pathway, e.g., phosphorylation of BAD, blockage of Bax translocation and suppression of caspase-9 and -3 in OPCs (Pang et al. 2007). In vivo, transgenic mice overexpressing TNF-alpha have 20% reduced brain size and 36% reduced number of oligodendrocytes. In cross-bred mice which overexpress both TNF-alpha and IGF-1 mice, IGF-1 was shown to protect myelin and oligodendrocyte loss from TNF-alpha-induced damage (Ye et al. 2007).
Aberg et al. have revealed data suggesting that IGF-1 effects survival of OPCs of the cerebral cortex. After IGF-1 administration in adult hypophysectomized rats, it was shown that the number of BrdU-labeled oligodendrocyte progenitor cells in the cerebral cortex after 6 days did not increase, whereas after 20 days, BrdU-positive OPC population did increase (Aberg et al. 2007). Authors concluded that as IGF-1 did not substantially induce cortical progenitor cell proliferation after 6 days, BrdU-positive cells observed at day 20 must have been derived from increased BrdU-negative OPC populations at an earlier stage, and that this increase reflects IGF-mediated enhanced survival of newborn cells.
IGF-1 Drives Oligodendrocyte Differentiation
Differentiation of oligodendrocytes is a process which involves decreased proliferative capacity, increasing cell process branching and augmentation of myelin protein expression and myelin sheath production. Amongst the array of intrinsic factors which have been implicated in inducing biological events such as those necessary to transform OPCs into a differentiated myelin producing cell, IGF-1 plays a pivotal role as a potent inducer of differentiation. Culture studies have confirmed the maturational effects of IGF-1 on both rat and human oligodendrocytes (Barres et al. 1993; McMorris and Dubois-Dalcq 1988; Mozell and McMorris 1991; Wilson et al. 2003). The well-documented effects of IGF-1 on oligodendrocytes are not only restricted to these cells, but also apply to schwann cells which demonstrate enhanced differentiation, myelin production and increased survival in response to IGF-1 stimulation (Cheng et al. 1999; Ogata et al. 2004). An absence of IGF-1 support, as demonstrated in IGF-1 null mice, instigates a strong reduction of myelin formation and axonal growth during early development, a mechanism that involves a decrease in myelin production (Ye et al. 2002). These effects may result from reduced OPC proliferation, survival and maturation. A further study with IGF-1 null mice revealed a deficit in the numbers of oligodendrocytes in the olfactory bulb, dentate gyrus and striatum (Beck et al. 1995). It has been suggested that a reduction of stem cell proliferation and differentiation in IGF-1 null mice might be the cause of reduced number and maturation of oligodendrocytes (Vicario-Abejon et al. 2003). In upholding this notion, Hsieh et al. showed that IGF-1 stimulates the differentiation of multipotent adult rat hippocampus-derived neural progenitor cells into oligodendrocytes by inhibiting bone morphogenetic protein signaling (Hsieh et al. 2004). In this study, it was also shown that overexpression of IGF-1 in the hippocampus lead to an increase in oligodendrocyte markers.
In another study, a null mutation for IGF-1 in mice demonstrated identical concentration of several myelin-specific proteins and a similar ratio of oligodendrocyte to projection neuron number in adult mice (Cheng et al. 1998). These IGF-1 KO mice only demonstrate a 25% reduction in myelin-associated galactocerebroside and sulfatide. Authors of that study suggested that not IGF-1, but rather axonal factors may provide the determinant of oligodendrocyte survival and myelination. By comparison, Ye et al. also found that IGF-1 KO adult mice have the same number of oligodendrocytes and myelin formation as wild-type mice; however, in developing IGF-1 KO mice, myelin expression and percentage of oligodendrocytes and their progenitors were reduced in all brain regions (Ye et al. 2002). Here, authors suggested other factors than axonal-derived factors guiding myelin normalization in adult animals, as there was a reduction of axonal growth in KO mice. In addition, IGF-2 expression was high in adult IGF-1 KO mice, whereas this ligand generally declines in adult wild-type mice.
The importance of IGF-signaling in myelin production has been demonstrated in the cuprizone model of demyelination. Treatment of mice with cuprizone leads to demyelination in the corpus callosum and superior cerebellar peduncles. When treatment is terminated, remyelination ensues. Cuprizone-induced demyelination in mice deficient of the IGF-1 receptor has demonstrated inadequate remyelination and lack of OPC accumulation at the site of injury; furthermore, OPCs did not proliferate or survive as well as OPCs in the wild-type control mice (Mason et al. 2003). In accordance with these findings, cuprizone insult in transgenic mice which continuously express IGF-1 demonstrated rapid recovery, which was also explained by the lack of apoptosis within the mature oligodendrocyte population (Mason et al. 2000).
Therapeutic Potential of IGF-1 in the CNS
Because of its potency to stimulate oligodendrocyte myelin production, IGF-1 is an attractive candidate for therapeutic application in treating neurodegenerative demyelinating diseases. However, the complex nature of IGF-1 actions in CNS and its regulation is not clearly defined. Stringent ligand regulation by IGFBPs may counterbalance therapeutic efforts to increase ligand levels in the CNS, as IGF-binding proteins neutralize IGFs and are generally upregulated in response to increased IGF signaling, thereby maintaining levels of free biologically available IGF-1. To obtain the desired effects from an IGF-1-based therapy, a rational therapeutic approach must be designed that takes into consideration complex tasks such as transport of ligand into the CNS, regulation by binding proteins and specific cellular targeting. One of the most limiting obstacles preventing beneficial application of IGF-1 in man appears to be the poor penetration of the CNS due to restrictions of the blood–brain barrier (BBB) that prevents free passage of IGF-1 to the damaged site. Although IGF-1 has been shown to cross the BBB of the rat, in human, IGF-1 only poorly crosses the BBB (Russo et al. 2005). Another major issue is the targeting of IGF-1 to the oligodendrocyte to promote remyelination. This is limited, for example, by high expression of type-1 IGF receptors on neurons and other glial cells (Anlar et al. 1999) and levels of IGFBPs which, by regulating IGF actions, might possibly target IGF-1 to other cell types. In the following sections, we will discuss therapeutic value and CNS treatment strategies for IGF-1.
IGF in Demyelinating Disorders
The myelinogenic and neurotrophic effects of IGF-1 suggest its potential usefulness in demyelinating disorders, such as multiple sclerosis (MS). This is demonstrated by a variety of in vivo animal models in which IGF-1 has proven beneficial, such as in cuprizone model of demyelination as described above. In experimental allergic encephalomyelitis (EAE), the animal model of multiple sclerosis, subcutaneous or intravenous IGF-1 application has been shown to reduce disruption of the blood–brain barrier, decrease immune cell infiltration and enhance remyelination (Li et al. 1998; Yao et al. 1995).
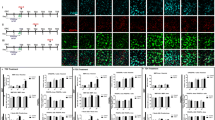
MS is a chronic demyelinating disease in which myelin sheaths are subject to inflammatory insult and consequent breakdown. Ensuing demyelination, axonal damage and subsequent oligodendrocyte cell death are characteristic for this disease (The role and fate of oligodendrocytes in MS is reviewed in Zeis and Schaeren-Wiemers 2008). Remyelination is a natural-occurring regenerative process involving restoration of prelesion cytoarchitecture. However, due to persistent demyelination in chronic MS, remyelination is incomplete (Fressinaud 2005). It is unknown why remyelination fails. A lack of trophic factors cueing a more comprehensive remyelination of denuded axons is one possibility. In our laboratories, we have investigated the IGF system in MS and discovered an upregulation of specific IGF binding proteins on astrocytes, microglia and oligodendrocytes in MS lesions (Chesik et al. 2004; Chesik et al. 2006; Wilczak et al. unpublished results). Our results have shown differential expression patterns of IGFBPs, indicating a coordinate manner of IGF regulation in MS lesions. While astrocytes and microglia upregulate, in particular, IGFBP-2, oligodendrocytes around MS lesions display upregulation of IGFBPs-1 and -6 (Fig. 1 for IGFBP-6, unpublished results). The consequence of a regulation of molecules of the IGF-system in glial cells in MS and its possible role in molding the formation of MS plaques is unclear, but might provide a mechanism reducing IGF signaling in the damaged tissue. When considering tissue repair, this seemingly detrimental mechanism might inhibit remyelination. However, down-regulation of IGF activity may indeed serve other biological purposes, such as to restrict inflammatory processes as IGF-1 is known to stimulate cytokine expression on cells involved in immunological processes. Such effects stress the need for delivery of IGF signaling to the target cell, the oligodendrocyte.
IGFBP-6 expression in oligodendrocytes. To demonstrate IGFBP-6 expression in oligodendrocytes, OLN-93 cells without (a) and with (b) brefeldin-A (BFA) treatment were stained for IGFBP-6 (TRITC-label). BFA, which interrupts secretion processes, allows for intracellular protein accumulation, as shown in (b). IGFBP-6 is indicated by arrowheads. In control human CNS, IGFBP-6 expression has not been detected in oligodendrocytes (c) by immunohistochemistry, whereas this protein is expressed in oligodendrocytes around active lesions in multiple sclerosis patients (d). In c and d, double staining with diamino benzidine (DAB; brown) represents myelin basic protein immunoreactivity, whereas DAB-nickel (blue) represents IGFBP-6 immunoreactivity. Scale bar = 50 μm
Modes of IGF-1 Delivery into the CNS
Transport of IGF-1 from the circulation into the CNS through receptor-mediated transcytosis via type-1 IGF receptors on the endothelium (Reinhardt and Bondy 1994), are not likely to deliver sufficient levels of IGF-1 into the demyelinated lesions of MS and promote myelin repair. Surgically invasive procedures in animals, such as intracerebroventricular administration, are not applicable in humans. Intranasal administration of IGF-1 is a method that provides several advantages, as it is a noninvasive treatment with direct delivery into the CNS. Levels of delivered drugs would be more concentrated, as diluting effects of the circulation are avoided. IGF-1 has been shown to be delivered along an extracellular route from the nasal cavity along olfactory and trigeminal pathways, which was accompanied by activation of signaling pathways in CNS regions that express high levels of type-1 IGF receptors. (Thorne et al. 2004). This study provides evidence for a rapid and direct pathway for protein transport into the CNS after intranasal administration.
Viral methods of gene transfer offer a possibility to overcome the difficulty of attempting chronic delivery of molecules into the CNS. In the superoxide dismutase mutant mouse model of amyotrophic lateral sclerosis, disease progression was reduced after retrograde delivery of recombinant adeno-associated virus injected into muscle, which caused the expression of IGF-1 in motor neurons (Boillee and Cleveland 2004; Kaspar et al. 2003). These beneficial effects were observed even when IGF-1 was delivered at the time of overt disease symptoms. However, sustained and local delivery of IGF-1 into the spinal cord through adeno-associated virus was also achieved in an EAE model, where clinical course of EAE was shown to worsen, and there was no effect on myelination (Genoud et al. 2005).
Utilization of the Entire IGF Axis to Target Oligodendrocytes
By utilizing or manipulating components of the IGF-system, therapeutic obstacles might be circumvented, and targeting of IGF-signaling achieved in specific cell types within the CNS. Such components include regulatory IGF-binding proteins, IGF-binding protein proteases and IGF-receptor species. Strategies aimed at remyelination by means of IGF signaling must evade strict regulation through IGFBPs. This might be achieved by utilizing specific cellular expression patterns for targeting purposes aimed at stimulating type 1 IGF receptors on oligodendrocytes. Because IGFBPs, as ligand carriers, represent a decisive component for cellular outcome, their presence and upregulation in CNS disease and injury are likely to interfere with therapeutic attempts at elevating IGF-1 levels. The question remains as to whether IGFBPs should be considered interfering components of IGF treatment strategies or might possibly serve a biological purpose in repairing damaged tissue.
Biological isoforms of IGF-1 are expressed in the human brain, for example, the des-1–3–IGF-1 isoform which has reduced binding capacity to IGFBPs while retaining a strong affinity for the IGF-1 receptor, making it more potent than IGF-1 (Werther et al. 1998). An other human analog of IGF-1 [(Leu24, 59, 60, Ala31)hIGF-1] has high affinity for IGFBPs, yet has no affinity for the type 1 IGF receptor. Instead, this analog can increase the level of “free” IGF-1 from endogenous pools of IGF bound to IGFBPs. Administration of this analogue after ischemic insult to the rat brain resulted in neuroprotective actions comparable to IGF-1 treatment (Loddick et al. 1998). However, these studies demonstrate a potency which applies to all cells expressing the type 1 IGF receptor and, therefore, disregards targeting approaches. In this context, the high expression of type-1 IGF receptors on neurons, microglia, and astrocytes (De Keyser et al. 1994; Wilczak and De Keyser 1997) would interfere with efforts to target oligodendrocytes.
Other interfering molecules, such as small molecule nonpeptides (e.g., isoquinoline analogues) have been developed, which can display high affinities for IGFBPs, some of which possess specific affinity for individual IGFBPs (Chen et al. 2001; Liu et al. 2001; Zhu et al. 2003). These molecules can be used, e.g., to displace endogenous IGF from complexes with individual IGFBPs. Such a process could elevate levels of free biologically active IGF. Small nonpeptide IGF analogues could possibly bypass the BBB and prove better candidates for entering the CNS. Conceivably, specific expression of IGFBPs in oligodendrocytes, as mentioned above for MS, could be targeted to release IGF in the vicinity of this cell type and activate receptors on oligodendrocytes. (Fig. 2). NBI-31772 is a nonpeptide small molecule that binds to all six IGFBPs and displaces IGF-1 from the complex. The neuroprotective effects of intracerebroventricular administration NBI-31772 has been studied in animal models of cerebral ischemia and have demonstrated reduced brain damage and infarct size (Mackay et al. 2003).
Targeting IGF-1 to oligodendrocytes. In multiple sclerosis, astrocytes express both IGF-1 and IGFBP-2 (BP-2), whereas oligodendrocytes express IGF-1 and IGFBP-6 (BP-6). Complex formation between IGF and IGFBPs generally neutralize IGF actions. Application of small nonpeptide molecules can release IGF-1 from complexes or inhibit complex formation with specific IGFBPs. Treatment strategies might utilize specific expression patterns of IGFBPs in glial cells. As depicted in the cartoon, a small nonpeptide molecule with high affinity to IGFBP-6 could specifically compete for IGFBP-6 binding and release IGF-1 in the vicinity of the oligodendrocyte to act on type 1 IGF receptors
Potential Benefits and Possible Disadvantages of IGF-1-based Therapy for Remyelination
When considering a remyelination-based therapy, we cannot disregard other factors necessary for proper myelin production and those inhibiting remyelination. The microenvironment of MS lesions, e.g., is known to contain factors inhibiting myelin production, such as myelin debris, cytokines and chemokines, and cell–cell and cell–ECM interactions (Zawadzka and Franklin 2007). Even in the presence of an adequate number of myelination-competent oligodendrocytes within the lesion, successful myelination is also dependent on adhesive interactions between oligodendrocytes and axons. Key cellular events necessary for successful remyelination include the recruitment of immature oligodendrocyte progenitor cells by proliferation and migration into demyelinated regions followed by differentiation of these progenitors into mature oligodendrocytes. NG2 (+)/PDGF-alpha receptor (+) OPCs are thought to be responsible for transient remyelination occurring in active MS lesions (Wilson et al. 2006). As these cells are responsible for myelin production, they are the likely target cells of a IGF/myelination-based therapy.
IGFBPs expression and presence in the CNS indicate important biological functions. This is especially true in CNS disease or injury in which IGFBPs are usually found to be upregulated. One of the biological roles of IGFBPs in the CNS may be to regulate the timing of oligodendrocyte differentiation, in which IGF-1 plays an important role. In the case of MS, a prerequisite of proper remyelination of demyelinated lesions is the production of sufficient numbers of OPCs. Whereas IGF-1 is a major stimulator of OPC differentiation and ensuing myelin production, other factors, such as PDGF and bFGF, drive OPC proliferation. Untimely triggering of cell differentiation could have detrimental effects in MS lesions, as IGF-1 might prevent further proliferation of OPCs, thereby limiting the number of these progenitor cells in the plaque vicinity. The expression of IGFBPs in MS lesions may, therefore, regulate the timing of oligodendrocyte differentiation by inhibiting IGF-signaling and allowing OPCs to proliferate for a number of divisions, while preventing premature differentiation induced by IGF-1. In this sense, IGFBPs themselves may indirectly serve as growth stimulators by inhibiting IGF-induced differentiation in a phase when OPCs should proliferate.
In summary, the precise role of the IGF system on processes coordinating oligodendrocyte behavior is uncertain. When considering the vast number of factors involved in spatial and temporal processes of tissue regeneration, elucidating the effects of IGF signaling in pathologies involving demyelination remains challenging.
References
Aberg, N. D., Brywe, K. G., & Isgaard, J. (2006). Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Scientific World Journal, 6, 53–80.
Aberg, N. D., Johansson, U. E., Aberg, M. A., Hellstrom, N. A., Lind, J., Bull, C., et al. (2007). Peripheral infusion of insulin-like growth factor-I increases the number of newborn oligodendrocytes in the cerebral cortex of adult hypophysectomized rats. Endocrinology, 148, 3765–3772.
Adachi, T., Takanaga, H., Kunimoto, M., & Asou, H. (2005). Influence of LIF and BMP-2 on differentiation and development of glial cells in primary cultures of embryonic rat cerebral hemisphere. Journal of Neuroscience Research, 79, 608–615.
Anlar, B., Sullivan, K. A., & Feldman, E. L. (1999). Insulin-like growth factor-I and central nervous system development. Hormone Metabolic Research, 31, 120–125.
Baron, W., Decker, L., Colognato, H., & Ffrench-Constant, C. (2003). Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Current Biology, 13, 151–155.
Barres, B. A., Schmid, R., Sendnter, M., & Raff, M. C. (1993). Multiple extracellular signals are required for long-term oligodendrocyte survival. Development, 118, 283–295.
Baxter, R. C., Martin, J. L., & Beniac, V. A. (1989). High molecular weight insulin-like growth factor binding protein complex. Purification and properties of the acid-labile subunit from human serum. Journal of Biological Chemistry, 264, 11843–11848.
Beck, K. D., Powell-Braxton, L., Widmer, H. R., Valverde, J., & Hefti, F. (1995). Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron, 14, 717–730.
Bichell, D. P., Kikuchi, K., & Rotwein, P. (1992). Growth hormone rapidly activates insulin-like growth factor I gene transcription in vivo. Molecular Endocrinology, 6, 1899–1908.
Blum, W. F., Bertsson-Wikland, K., Rosberg, S., & Ranke, M. B. (1993). Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. Journal of Clinical Endocrinology and Metabolism, 76, 1610–1616.
Bogler, O., Wren, D., Barnett, S. C., Land, H., & Noble, M. (1990). Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proceedings of the National Academy of Science of the United States of America, 87, 6368–6372.
Boillee, S., & Cleveland, D. W. (2004). Gene therapy for ALS delivers. Trends in Neuroscience, 27, 235–238.
Bondy, C., Werner, H., Roberts, C. T., & LeRoith, D. Jr. (1992). Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: Comparison with insulin-like growth factors I and II. Neuroscience, 46, 909–923.
Carro, E., Spuch, C., Trejo, J. L., Antequera, D., & Torres-Aleman, I. (2005). Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. Journal of Neuroscience, 25, 10884–10893.
Carson, M. J., Behringer, R. R., Brinster, R. L., & McMorris, F. A. (1993). Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron, 10, 729–740.
Chen, C., Zhu, Y. F., Liu, X. J., Lu, Z. X., Xie, Q., & Ling, N. (2001). Discovery of a series of nonpeptide small molecules that inhibit the binding of insulin-like growth factor (IGF) to IGF-binding proteins. Journal of Medical Chemistry, 44, 4001–4010.
Cheng, C. M., Joncas, G., Reinhardt, R. R., Farrer, R., Quarles, R., Janssen, J., et al. (1998). Biochemical and morphometric analyses show that myelination in the insulin-like growth factor 1 null brain is proportionate to its neuronal composition. Journal of Neuroscience, 18, 5673–5681.
Cheng, H. L., Russell, J. W., & Feldman, E. L. (1999). IGF-I promotes peripheral nervous system myelination. Annals of New York Academy of Science, 883, 124–130.
Chesik, D., De Keyser, J., Glazenburg, L., & Wilczak, N. (2006). Insulin-like growth factor binding proteins: regulation in chronic active plaques in multiple sclerosis and functional analysis of glial cells. European Journal of Neuroscience, 24, 1645–1652.
Chesik, D., De Keyser, J., & Wilczak, N. (2004). Involvement of insulin-like growth factor binding protein-2 in activated microglia as assessed in post mortem human brain. Neuroscience Letters, 362, 14–16.
Cohen, R. I., Marmur, R., Norton, W. T., Mehler, M. F., & Kessler, J. A. (1996). Nerve growth factor and neurotrophin-3 differentially regulate the proliferation and survival of developing rat brain oligodendrocytes. Journal of Neuroscience, 16, 6433–6442.
Cui, Q. L., & Almazan, G. (2007). IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. Journal of Neurochemistry, 100, 1480–1493.
De Keyser, J., Wilczak, N., & Goossens, A. (1994). Insulin-like growth factor-I receptor densities in human frontal cortex and white matter during aging, in Alzheimer’s disease, and in Huntington’s disease. Neuroscience Letters, 172, 93–96.
Feldman, E. L., Sullivan, K. A., Kim, B., & Russell, J. W. (1997). Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiology of Disease, 4, 201–214.
Frederick, T. J., Min, J., Altieri, S. C., Mitchell, N. E., & Wood, T. L. (2007). Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia, 55, 1011–1022.
Fressinaud, C. (2005). Repeated injuries dramatically affect cells of the oligodendrocyte lineage: effects of PDGF and NT-3 in vitro. 555–566. Glia, 49, 555–66.
Garcia-Segura, L. M., Perez, J., Pons, S., Rejas, M. T., & Torres-Aleman, I. (1991). Localization of insulin-like growth factor I (IGF-I)-like immunoreactivity in the developing and adult rat brain. Brain Research, 560, 167–174.
Garwood, J., Garcion, E., Dobbertin, A., Heck, N., Calco, V., Ffrench-Constant, C., et al. (2004). The extracellular matrix glycoprotein Tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. European Journal of Neuroscience, 20, 2524–2540.
Genoud, S., Maricic, I., Kumar, V., & Gage, F. H. (2005). Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis. Journal of Neuroimmunology, 168, 40–45.
Hsieh, J., Aimone, J. B., Kaspar, B. K., Kuwabara, T., Nakashima, K., & Gage, F. H. (2004). IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. Journal of Cell Biology, 164, 111–122.
Hu, J. G., Fu, S. L., Wang, Y. X., Li, Y., Jiang, X. Y., Wang, X. F., et al. (2008). Platelet-derived growth factor-AA mediates oligodendrocyte lineage differentiation through activation of extracellular signal-regulated kinase signaling pathway. Neuroscience, 151, 138–147.
Jackson, E. L., Garcia-Verdugo, J. M., Gil-Perotin, S., Roy, M., Quinones-Hinojosa, A., VandenBerg, S., et al. (2006). PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron, 51, 187–199.
Jones, J. I., & Clemmons, D. R. (1995). Insulin-like growth factors and their binding proteins: Biological actions. Endocrine Reviews, 16, 3–34.
Kaspar, B. K., Llado, J., Sherkat, N., Rothstein, J. D., & Gage, F. H. (2003). Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science, 301, 839–842.
Kooijman, R. (2006). Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine and Growth Factor Reviews, 17, 305–323.
Lelievre, V., Ghiani, C. A., Seksenyan, A., Gressens, P., & Waschek, J. A. (2006). Growth factor-dependent actions of PACAP on oligodendrocyte progenitor proliferation. Regulatory Peptides, 137, 58–66.
Li, W., Quigley, L., Yao, D. L., Hudson, L. D., Brenner, M., Zhang, B. J., et al. (1998). Chronic relapsing experimental autoimmune encephalomyelitis: effects of insulin-like growth factor-I treatment on clinical deficits, lesion severity, glial responses, and blood brain barrier defects. Journal of Neuropathology and Experimental Neurology, 57, 426–438.
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J., & Efstratiadis, A. (1993). Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell, 75, 59–72.
Liu, X. J., Xie, Q., Zhu, Y. F., Chen, C., & Ling, N. (2001). Identification of a nonpeptide ligand that releases bioactive insulin-like growth factor-I from its binding protein complex. Journal of Biological Chemistry, 276, 32419–32422.
Loddick, S. A., Liu, X. J., Lu, Z. X., Liu, C., Behan, D. P., Chalmers, D. C., et al. (1998). Displacement of insulin-like growth factors from their binding proteins as a potential treatment for stroke. Proceedings of the National Academy of Sciences of the United States of America, 95, 1894–1898.
Mackay, K. B., Loddick, S. A., Naeve, G. S., Vana, A. M., Verge, G. M., & Foster, A. C. (2003). Neuroprotective effects of insulin-like growth factor-binding protein ligand inhibitors in vitro and in vivo. Journal of Cerebral Blood Flow and Metabolism, 23, 1160–1167.
Maile, L. A., & Holly, J. M. (1999). Insulin-like growth factor binding protein (IGFBP) proteolysis: Occurrence, identification, role and regulation. Growth Hormone and Insulin-like Growth Factor Research, 9, 85–95.
Mason, J. L., Xuan, S., Dragatsis, I., Efstratiadis, A., & Goldman, J. E. (2003). Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. Journal of Neuroscience, 23, 7710–7718.
Mason, J. L., Ye, P., Suzuki, K., D’Ercole, A. J., & Matsushima, G. K. (2000). Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. Journal of Neuroscience, 20, 5703–5708.
McKinnon, R. D., Matsui, T., Dubois-Dalcq, M., & Aaronson, S. A. (1990). FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron, 5, 603–614.
McKinnon, R. D., Piras, G., Ida, J. A. Jr., & Dubois-Dalcq, M. (1993). A role for TGF-beta in oligodendrocyte differentiation. Journal of Cell Biology, 121, 1397–1407.
McMorris, F. A., & Dubois-Dalcq, M. (1988). Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. Journal of Neuroscience Research, 21, 199–209.
McMorris, F. A., Smith, T. M., DeSalvo, S., & Furlanetto, R. W. (1986). Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proceedings of the National Academy of Sciences of the United States of America, 83, 822–826.
Mohan, S., & Baylink, D. J. (2002). IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. Journal of Endocrinology, 175, 19–31.
Mozell, R. L., & McMorris, F. A. (1991). Insulin-like growth factor I stimulates oligodendrocyte development and myelination in rat brain aggregate cultures. Journal of Neuroscience Research, 30, 382–390.
Noble, M., Murray, K., Stroobant, P., Waterfield, M. D., & Riddle, P. (1988). Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature, 333, 560–562.
Ogata, T., Iijima, S., Hoshikawa, S., Miura, T., Yamamoto, S., Oda, H., et al. (2004). Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. Journal of Neuroscience, 24, 6724–6732.
Oorschot, D. E., & McLennan, I. S. (1998). The trophic requirements of mature motoneurons. Brain Research, 789, 315–321.
Pang, Y., Zheng, B., Fan, L. W., Rhodes, P. G., & Cai, Z. (2007). IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia, 55, 1099–1107.
Rechler, M. M., & Nissley, S. P. (1985). The nature and regulation of the receptors for insulin-like growth factors. Annual Review of Physiology, 47, 425–442.
Reinhardt, R. R., & Bondy, C. A. (1994). Insulin-like growth factors cross the blood-brain barrier. Endocrinology, 135, 1753–1761.
Rotwein, P., Burgess, S. K., Milbrandt, J. D., & Krause, J. E. (1988). Differential expression of insulin-like growth factor genes in rat central nervous system. Proceedings of the National Academy of Sciences of the United States of America, 85, 265–269.
Russo, V. C., Gluckman, P. D., Feldman, E. L., & Werther, G. A. (2005). The insulin-like growth factor system and its pleiotropic functions in brain. Endocrine Reviews, 26, 916–943.
Sendtner, M. (1995). Molecular biology of neurotrophic factors. Baillieres Clinical Neurology, 4, 575–591.
Thorne, R. G., Pronk, G. J., Padmanabhan, V., & Frey, W. H. (2004). Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience, 127, 481–496.
Trejo, J. L., Carro, E., Lopez-Lopez, C., & Torres-Aleman, I. (2004). Role of serum insulin-like growth factor I in mammalian brain aging. Growth Hormone and Insulin-like Growth Factor Research, 14(Suppl A), S39–S43.
van Dam, P. S., & Aleman, A. (2004). Insulin-like growth factor-I, cognition and brain aging. European Journal of Pharmacology, 490, 87–95.
Vicario-Abejon, C., Yusta-Boyo, M. J., Fernandez-Moreno, C., & de Pablo, F. (2003). Locally born olfactory bulb stem cells proliferate in response to insulin-related factors and require endogenous insulin-like growth factor-I for differentiation into neurons and glia. Journal of Neuroscience, 23, 895–906.
Wang, Z., Colognato, H., & Ffrench-Constant, C. (2007). Contrasting effects of mitogenic growth factors on myelination in neuron-oligodendrocyte co-cultures. Glia, 55, 537–545.
Werther, G. A., Russo, V., Baker, N., & Butler, G. (1998). The role of the insulin-like growth factor system in the developing brain. Hormone Research, 49(Suppl 1), 37–40.
Wilczak, N., & De Keyser, J. (1997). Insulin-like growth factor-I receptors in normal appearing white matter and chronic plaques in multiple sclerosis. Brain Research, 772, 243–246.
Wilson, H. C., Onischke, C., & Raine, C. S. (2003). Human oligodendrocyte precursor cells in vitro: phenotypic analysis and differential response to growth factors. Glia, 44, 153–165.
Wilson, H. C., Scolding, N. J., & Raine, C. S. (2006). Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. Journal of Neuroimmunology, 176, 162–173.
Wood, T. L., Loladze, V., Altieri, S., Gangoli, N., Levison, S. W., Brywe, K. G., et al. (2007). Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Developmental Neuroscience, 29, 302–310.
Yao, D. L., Liu, X., Hudson, L. D., & Webster, H. D. (1995). Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proceedings of the National Acadamy of Science of the United States of America, 92, 6190–6194.
Ye, P., Carson, J., & D’Ercole, A. J. (1995). In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. Journal of Neuroscience, 15, 7344–7356.
Ye, P., Kollias, G., & D’Ercole, A. J. (2007). Insulin-like growth factor-I ameliorates demyelination induced by tumor necrosis factor-alpha in transgenic mice. Journal Neuroscience Research, 85, 712–722.
Ye, P., Li, L., Richards, R. G., DiAugustine, R. P., & D’Ercole, A. J. (2002). Myelination is altered in insulin-like growth factor-I null mutant mice. Journal of Neuroscience, 22, 6041–6051.
Zawadzka, M., & Franklin, R. J. (2007). Myelin regeneration in demyelinating disorders: New developments in biology and clinical pathology. Current Opinions in Neurology, 20, 294–298.
Zeger, M., Popken, G., Zhang, J., Xuan, S., Lu, Q. R., Schwab, M. H., et al. (2007). Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia, 55, 400–11.
Zeis, T., Schaeren-Wiemers, N. (2008). Lame ducks or fierce creatures?-The role of oligodendrocytes in multiple sclerosis. DOI 10.1007/s12031-008-9042-1.
Zhu, Y. F., Wang, X. C., Connors, P., Wilcoxen, K., Gao, Y., Gross, R., et al. (2003). Quinoline-carboxylic acids are potent inhibitors that inhibit the binding of insulin -like growth factor (IGF) to IGF-binding proteins. Bioorganic & Medicinal Chemistry Letters, 13, 1931–1934.
Acknowledgements
Daniel Chesik is a recipient of a grant from the Dutch foundation Stichting Multiple Sclerose Anders to which authors are indebted for supporting studies concerning insuline-like growth factor therapeutic potential in treating multiple sclerosis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chesik, D., De Keyser, J. & Wilczak, N. Insulin-like Growth Factor System Regulates Oligodendroglial Cell Behavior: Therapeutic Potential in CNS. J Mol Neurosci 35, 81–90 (2008). https://doi.org/10.1007/s12031-008-9041-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9041-2