Abstract
Objectives
Our aim was to evaluate the association between salivary gland scintigraphy and the clinical parameters, including histological characteristics of salivary glands, in patients with primary Sjogren’s syndrome (pSS).
Methods
Forty-one pSS patients were included in the study. The patients who had received salivary gland scintigraphy and minor salivary gland biopsy were retrospectively analyzed. Salivary gland scintigraphy was interpreted via semi-quantitative methods obtained by calculating the peak uptake and washout of each gland using regions of interest. All specimens were examined by pathologists for focus scores and leukocyte common antigen (LCA) to determine the degree of inflammatory infiltration.
Results
The mean age of pSS patients was 46.4 years, 82.9% were female, and the mean duration of symptoms was 2.5 years. The focus score was negatively correlated to the mean peak uptake (r = ‒0.396; p = 0.019), mean uptake (r = ‒0.388; p = 0.021), and mean percentage washout (r = ‒0.391; p = 0.02). In addition, the focus score and number of LCA positive cells per mm2 were correlated with the clinical parameters including erythrocyte sedimentation rate, globulin, rheumatoid factor, unstimulated whole saliva, and stimulated whole saliva flow. The number of LCA positive cells per mm2 was negatively correlated to leukocytes and hemoglobin.
Conclusion
Although the diagnostic role of salivary gland biopsy is widely accepted and features in the classification criteria of Sjogren’s syndrome, salivary gland scintigraphy may be an acceptable alternative method especially if a non-invasive test is required.
Key Points • Salivary gland biopsy in patients with primary Sjogren’s syndrome (pSS) showed a significant correlation with several markers of both the parotid and submandibular glands in salivary gland scintigraphy, and with clinical features such as cytopenia, and lower unstimulated whole saliva and stimulated whole saliva flow rates. • Salivary gland scintigraphy might be a good alternative to minor salivary gland biopsy, and the two methods could be complementary to the clinical features in the diagnosis of pSS. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sjogren’s syndrome (SS) is a chronic autoimmune disease characterized by xerophthalmia and xerostomia which are caused by lymphocytic infiltrates of the lachrymal and salivary glands [1]. SS can be classified as either primary SS (pSS) or secondary SS depending on whether it occurs alone or is associated with other autoimmune rheumatic diseases, mainly rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and systemic sclerosis [2]. As with most systemic autoimmune diseases, it has been suggested that the pathogenesis of pSS is due to genetic, hormonal, and environmental factors, although the actual cause remains elusive. It seems that autoantibody production and immune complex formation stimulated by hyperactivity of B-lymphocytes play a central role [3]. Due to the complex pathogenesis and disease heterogeneity of pSS, its diagnosis and treatment are continuously being developed, updated, and improved [4].
More than 10 classification criteria for pSS were proposed between 1965 and 2002 [5,6,7,8,9,10,11]. The American‒European Consensus Group (AECG) classification criteria, which were approved in 2002, were the most widely used until the American College of Rheumatology (ACR) published new classification criteria in 2012 [12, 13]. Even though there are many discrepancies between the two classification systems, they each have a distinct advantage in that the 2002 AECG classification seems to be more specific, while the 2012 ACR classification can detect early forms of the disease [14]. In 2016, the ACR/European League Against Rheumatism (EULAR) published new classification criteria consisting of five objective tests or items that were based on the current ACR and EULAR guidelines and methodologies, with a focus on items that considered data and clinical judgment [15, 16]. The 2016 ACR/EULAR criteria are recognized as the superior tool in pSS diagnosis for its significantly higher sensitivity when compared to several currently available criteria. The introduction of this criteria has increased the need for minor salivary gland (MSG) biopsy [17, 18].
MSG biopsy is an invasive procedure that carries mild complications such as temporary sensory defect, bleeding, and pain, as well as serious complications such as tissue necrosis and persistent impaired sensory defect [19]. For this reason, it may be necessary to replace biopsy, and it has been suggested that a non-invasive and more easily accessible imaging technique be used. Salivary gland scintigraphy was excluded from the 2012 and 2016 classification criteria for pSS due to its low diagnostic specificity [13, 15, 16]. This low diagnostic specificity may have been due to a paucity of the literature on salivary gland scintigraphy for pSS. We believe that salivary gland scintigraphy can be a reliable, objective, and accurate diagnostic method, if it can be proven through objective research data that it does have an association with pSS. Therefore, our aim was to evaluate the association between salivary gland scintigraphy and the clinical parameters of pSS but especially with the histological characteristics of salivary glands in patients with pSS.
Method
Study population
This single-center retrospective study included 41 patients who underwent salivary gland scintigraphy and salivary gland biopsy to confirm the diagnosis, and had a diagnosis of pSS. Patients who visited the department of rheumatology at Ajou University Hospital between January 2016 and December 2021 were recruited. All of the patients with pSS were 18 years of age or older and fulfilled the 2016 ACR/EULAR classification criteria for pSS [15, 16]. Patients with other autoimmune diseases, such as SLE, RA, systemic sclerosis, or immunoglobulin G4-related disease and those who were prescribed muscarinic agonists (pilocarpine and cevimeline) were excluded from the study. Baseline demographic data, clinical data, and laboratory findings related to pSS were reviewed from the relevant medical records. Disease activity was assessed using the EULAR Sjogren’s Syndrome disease activity index (ESSDAI) at the time of diagnosis. The ESSDAI includes the evaluation of 12 domains (cutaneous, respiratory, renal, articular, muscular, peripheral nervous system, central nervous system, hematological, glandular, constitutional, lymphadenopathic, biological), with a total of 123 points, defined as low activity (ESSDAI < 5), moderate activity (5 ≤ ESSDAI ≤ 13), and high disease activity (ESSDAI ≥ 14) [20]. This study protocol was reviewed and approved by the Institutional Review Board of Ajou University Hospital (AJIRB-BMR-MDB-22–031).
Assessment of xerophthalmia and xerostomia
As part of the diagnosis and classification of pSS, the Schirmer test was used for dry eye examination; wetting of 0–5 mm of the Schirmer strip after 5 min, in at least one side, is considered abnormal [15, 16]. As part of the examination for dry mouth, unstimulated whole saliva (UWS) and stimulated whole saliva (SWS) were collected to evaluate saliva secretion rates. UWS was collected for 10 min by the spitting method. Mechanical stimulation (chewing a paraffin gum base) was used to stimulate salivary secretion. The saliva collected for the first 2 min was discarded, then SWS was collected for 5 min. Saliva secretion rates were recorded as mL/min for both UWS and SWS.
Salivary gland scan acquisition and data analysis
Patients were injected with 185 MBq of 99mTc-pertechnetate, and static images (anterior and lateral views) were acquired after 5, 10, and 20 min using a dual-head gamma camera (GE Healthcare, USA) equipped with a low-energy, high-resolution collimator. Then, washout images were acquired 20 min after stimulation of the salivary glands by oral administration of a sialagogue (200 ml of orange juice). Images were analyzed on a Xeleris Workstation (GE Healthcare).
The uptake ratio and percentage of excretion after salivary gland stimulation were assessed semi-quantitatively. Circular regions of interest (ROI) were drawn manually on each parotid and submandibular gland in the anterior view on the 20-min static images. Additionally, a circular ROI was drawn on the skull on a uniform background region (Supplementary Fig. 1). The uptake ratio was calculated by dividing the mean counts of each ROI by that of the background ROI. The percentage of excretion was defined as (counts in the salivary gland at 20 min — counts in the salivary gland after sialagogue administration)/(counts in the salivary gland at 20 min) × 100%.
Histologic evaluation
A hematoxylin and eosin-stained salivary gland biopsy section was evaluated by an expert pathologist (JHH), who was blinded to the clinical data. The extent of the lymphocytic infiltration was represented by the focus score. The presence of more than 50 periductal lymphocytes in 4 mm2 of salivary gland tissue is used as criteria for the definition of a focus [21]. The degree of interstitial fibrosis was assessed blindly for the disease classification and the focus score. Interstitial fibrosis was defined as collagenous fibrosis surrounding the ducts or forming tracts that dissociate the lobules and encase the acini [22]. The slides were scored according to the degree of interstitial fibrosis as follows: 0 = no or minimal fibrosis, 1 = mild fibrosis without acinar replacement, 2 = moderate fibrosis with acinar replacement, 3 = severe fibrosis with acinar replacement and marked gland disruption. The number of germinal center (GC)–like structures within the MSG tissue was also counted. The GC-like structure was defined by the presence of densely aggregated lymphoid cells containing distinguishable dark and light zones.
Immunohistochemistry (IHC)
Immunohistochemical staining for CD45 (Leukocyte Common Antigen, LCA) was performed using BenchMark XT (Ventana Medical Systems, AZ, USA) according to the manufacturer’s instructions. Briefly, 4 μm-thick sections were deparaffinized in xylene and dehydrated in graded ethanol. The antigen retrieval with Cell Conditioning Buffer 1 (CC1) was performed for 40 min. The primary antibody was CD45 (LCA) (2B11&PD7/26, 1:100, Cell Marque, CA, USA) with 1 h of incubation and the secondary anti-HRP antibody was incubated for 32 min. Counterstaining was performed with Hematoxylin II for 10 min and subsequent bluing for 6 min.
Image acquisition and quantitative image analysis
Whole slide images after IHC were digitized in 20 × magnification by Aperio AT2 scanners. Scanned images were analyzed with QuPath version 0.2.3; an open-source software for digital pathology and whole-slide image analysis [23]. The area of the salivary gland only was manually annotated as a ROI in the MSG biopsy digital image. Cells in the ROI were segmented as areas of staining above the background level, by applying optimized segmentation parameters including threshold, median filter radius, sigma, and cell expansion. All images were confirmed by manual inspection by an expert pathologist (JR) for optimal segmentation. The number of infiltrated lymphocytes within the ROIs was quantitatively measured using the number of cells.
Statistical analysis
The Student’s t-test or Mann–Whitney test were used for continuous variables, and the chi-square test or Fisher’s exact test were used for categorical variables. These data are presented as mean ± standard deviation, and for all analyses, a p-value < 0.05 was considered as statistically significant. Spearman’s correlation was conducted to evaluate the relationship between the salivary gland histological data and salivary gland scintigraphy. The Spearman’s correlation coefficient (r) was interpreted as very weak (0.0–0.2), weak (0.2–0.4), moderate (0.4–0.6), strong (0.6–0.8), or very strong (0.8–1.0). We also analyzed the association between the clinical parameters and disease activity with the salivary gland histological data and salivary gland scintigraphy, respectively. All statistical analyses were performed with SPSS software version 25.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
The baseline characteristics of pSS are presented in Table 1. Of the 41 patients included in this study, the mean age was 46.4 ± 10.4 years, 82.9% of the patients were female, and the mean duration of symptoms was 2.5 years. Almost all patients had ocular (97.6%) and oral (95.1%) symptoms, and only 4 patients (9.8%) had enlargement of the parotid glands. The most common extra-glandular symptom was fatigue (85.4%), followed by arthralgia (70.7%), arthritis (26.8%), and purpura (17.1%). The mean ESSDAI score was 4.1 ± 2.2 and the frequency of patients with ESSDAI ≥ 5 was 15 (36.6%). The mean results of the Schirmer test for dry eyes was 4.71 ± 2.31 mm on the left side, and 5.24 ± 2.41 mm on the right side. The mean salivary flow rates of UWS and SWS were 0.2 ± 0.34 mL/min and 0.62 ± 0.62 mL/min, respectively. Regarding the laboratory findings, antinuclear antibody was positive in 32 patients (78%), anti-SSA/Ro was positive in 34 patients (82.9%), anti-SSB/La was positive in 7 patients (17.1%), and rheumatoid factor was positive in 27 patients (65.9%).
Salivary gland scintigraphy and minor salivary gland biopsy
Forty-one patients underwent salivary gland scintigraphy, and the results are summarized in Table 2. The mean values for peak uptake and mean uptake of the parotid and submandibular glands were 5.14 ± 1.63 and 3.35 ± 1.08, respectively. The mean percentage washout of the parotid and submandibular glands was 30.36 ± 14.41.
Table 3 presents the results of the MSG biopsy samples of patients with pSS, depicting the main histological characteristics. The mean number of cell detections was 73,465.7 ± 145,097.8, of which CD45 (LCA) positive cells numbered 16,829.4 ± 53,028.6. The mean number of CD45 (LCA) positive cells per mm2 of MSG was 1506.3 ± 1559.0. The mean focus score was 1.32 ± 1.67, and 27 patients (65.9%) had a focus score ≥ 1. To more accurately assess the relationship between the number of CD45 (LCA) positive cells per mm2 and the focus score per 4 mm2, which further reflects the size of the MSG infiltrate, correlation analyses were performed. There was a positive correlation between the number of CD45 (LCA) positive cells per mm2 of MSG and the focus score per 4 mm2 in pSS patients (r = 0.722, p < 0.001, Supplementary Fig. 2).
Saliva secretion and salivary scintigraphic parameters are positively correlated
To establish whether salivary scintigraphic parameters reflect salivary gland function in pSS, correlation analyses between saliva secretion and salivary scintigraphic parameters were conducted (Table 4). The SWS flow rates were significantly correlated with the mean of peak uptake (r = 0.513; p = 0.012), mean uptake (r = 0.532; p = 0.009), and percentage washout (r = 0.438, p = 0.038) in both the parotid and submandibular glands. In addition, the UWS flow rates had a significant correlation with the peak uptake (right: r = 0.637, p = 0.001 and left: r = 0.523, p = 0.01) and mean uptake (right: r = 0.635, p = 0.001 and left: r = 0.646, p = 0.001) in the submandibular glands only, and the mean percentage washout in both parotid and submandibular glands (r = 0.471, p = 0.048).
Histologic focus score and salivary scintigraphic parameters are negatively correlated
In Table 5, there was a clear trend showing a negative correlation between the histological focus score and salivary scintigraphic parameters. Significantly negative correlations with focus score were the mean of peak uptake (r = − 0.396; p = 0.019), mean uptake (r = − 0.388; p = 0.021), and percentage washout (r = − 0.391; p = 0.02) in both the parotid and submandibular glands. Specifically, the focus score had negative correlations with the peak uptake of the left parotid gland (r = − 0.419; p = 0.012), the mean uptake of the right and left parotid glands (r = − 0.374; p = 0.027 and r = − 0.342; p = 0.044, respectively), and the percentage washout of the right parotid and right submandibular glands (r = − 0.395; p = 0.019 and r = − 0.439; p = 0.008, respectively). Figure 1 shows a comparison of salivary gland scintigraphy in patients with typical histological findings of Sjogren’s syndrome. The presence of a germinal center and high focus scores were significantly associated with decreased uptake and washout of the parotid and salivary glands in salivary scintigraphy. On the other hand, no correlation between the number of CD45 (LCA) positive cells per mm2 and salivary scintigraphic parameters was found in patients with pSS (Supplementary Table 1).
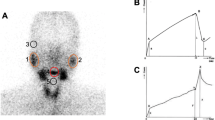
Comparison of salivary gland scintigraphy in patients with typical histological findings of Sjogren’s syndrome. (A) Histological picture showing a germinal center in the minor salivary glands (H&E, 100 ×). Scintigraphic images before (B) and after (C) orange juice stimulation in a patient with pSS. (B) Severely decreased uptake function in both parotid and submandibular glands. (C) Excretory function of both parotid and submandibular glands cannot be evaluated due to nearly non-visualization of uptake activity. (D) Positive minor salivary gland biopsy result with focus score of 2. (E) Severely decreased uptake function in left parotid and both submandibular glands. (F) Decreased excretory function in left parotid gland. H&E, hematoxylin and eosin; RT, right; LT, left
Correlations between histopathologic data and salivary scintigraphic parameters with clinical factors of pSS
We further analyzed the association between the histological findings and salivary scintigraphic parameters with the clinical factors of pSS. As shown in Table 6, the focus score and number of LCA positive cells per mm2 were correlated with the erythrocyte sedimentation rate (ESR) (r = 0.582; p < 0.001 and r = 0.591; p < 0.001, respectively), total protein (r = 0.474; p = 0.003 and r = 0.351; p = 0.03, respectively), globulin (r = 0.521; p = 0.001 and r = 0.506; p = 0.001, respectively), rheumatoid factor (r = 0.533; p < 0.001 and r = 0.608; p < 0.001, respectively), SWS flow rates (r = − 0.491; p = 0.009 and r = − 0.519; p = 0.006, respectively), and UWS flow rates (r = − 0.512; p = 0.006 and r = ‒0.471; p = 0.013, respectively). Only the number of LCA positive cells per mm2 was negatively correlated with leukocytes (r = − 0.37; p = 0.019), and hemoglobin (r = − 0.367; p = 0.02). In addition, the mean percentage washout was negatively correlated with total protein (r = − 0.387; p = 0.029) and globulin (r = − 0.486; p = 0.005).
Discussion
The newly proposed classification criteria (2016 ACR/EULAR criteria) for the diagnosis of pSS included only objective items without scoring the subjective oral and ocular symptoms. Methods to obtain the objective evidence regarding the oral symptoms excluded salivary gland scintigraphy and included MSG biopsy and UWS flow rate [15, 16]. This classification criteria has a different weighting for each factor, and the positive anti-SSA/Ro autoantibody and positive biopsy results are each given a higher weighting of 3 points therefore, MSG biopsy inevitably makes a clear diagnosis in the absence of an anti-SSA/Ro autoantibody. As is widely known, a biopsy procedure is accompanied by the risk of bleeding and infection therefore, alternative methods to biopsy are getting more attention, and ultrasound is currently in the spotlight [24,25,26,27,28]. Nevertheless, the drawback to ultrasound is that the results vary depending on the experience of the person conducting the investigation and the devices used and may therefore serve as a major limitation in the use of ultrasound as an accurate diagnostic test for pSS. Moreover, the scoring system for the diagnostic ultrasound of pSS is complicated with over 30 items and no consensus has been reached on the specific criteria required to classify pSS with ultrasound [29].
Salivary gland scintigraphy using 99 m Tc-pertechnetate is an easy-to-perform and non-invasive method of evaluating salivary gland function. It measures the amount and speed of radioactive isotope uptake and secretion from the four salivary glands separately [30]. Although salivary gland scintigraphy is suitable for detecting subtle salivary gland dysfunction, there are several reasons for the continued decline in its use in the diagnosis of pSS [31]. First, most studies were designed to compared healthy subjects to differentiate the diagnosis of pSS, not patients with xerostomia; thus, the accuracy of salivary gland scintigraphy in the diagnosis of pSS among patients with xerostomia was low [32,33,34]. In addition, the useful and sensitive scintigraphic parameters for the diagnosis of pSS vary depending on the study, and the intensity of xerostomia cannot be quantitatively evaluated [35, 36]. These factors led to a lack of quantitative salivary gland scintigraphy studies that aimed to interpret the progressive decrease in the function of the salivary glands induced by chronic autoimmune-mediated inflammation in relation to scintigraphic parameters, thus raising concerns about using salivary gland scintigraphy as the first tool for diagnosing patients with suspected pSS.
In the present study, we specifically investigated whether salivary gland scintigraphic parameters in patients with pSS reflect the characteristics of biopsy and if the scintigraphic parameters were associated with saliva secretion and the clinical factors of pSS. We showed that salivary flow rates are correlated with the decreased uptake and washout of both submandibular glands in salivary gland scintigraphy. Most of these results were consistent with previous studies [37, 38], and were similar in that the impairment was greater in the submandibular glands than in the parotid glands [33]. Since the parotid gland is known to be the last gland to be affected in patients with pSS, we may have seen greater change in the activity of the submandibular glands as patients in our study were enrolled at the time of diagnosis [39].
The analysis of the salivary gland scintigraphic parameters and the histological characteristics in pSS patients, showed significant correlations between focus scores and scintigraphic parameters and is similar to previous findings. However, most of the existing studies that evaluated the relationship between the pathological features of the salivary glands and the scintigraphic parameters in patients with pSS used an older scoring method, such as the Chisholm and Mason scoring system [40,41,42]. We found that the focus score had a significant correlation with peak uptake, mean uptake, and percentage washout of both the parotid and submandibular glands, and this correlation was more commonly affected by the parotid gland than the submandibular gland. Our finding dissents with the previously reported findings that the impairment of the submandibular gland was seen more often in SWS and UWS flow rates. Inflammatory changes in the parotid gland reflect the severity or duration of the disease therefore, MSG biopsy abnormalities may have been found in patients with parotid gland damage [43]. The focus scores, which are based on the scoring criteria of more than 50 mononuclear lymphoid cells (mainly lymphocytes) being clustered together, may have difficulty in accurately reflecting the size of the periductal lymphocytic infiltration, which is a main histopathological feature of pSS [44]. To compensate for this, we further analyzed the number of LCA positive cells per mm2 measured by CD45 staining. CD45 is important because it is a memory marker expressed in approximately 60‒70% of T lymphocytes. CD4 + T cells are predominant in less severe salivary gland lesions of pSS and B cells accumulate in the later stages of disease, resulting in CD45 infiltrations being dominant in patients with early Sjogren’s syndrome. Unfortunately, there were no significant correlations in our analysis [45]. Given that patients were included at the time of diagnosis in this study and fibrosis was not severe, the CD45 infiltration results are not well explained.
The relationship between the histological findings and clinical factors of pSS was clearly demonstrated; however, no significant results were found between the scintigraphic parameters and clinical factors, except for the UWS and SWS flow rates. The focus score and number of CD45 (LCA) positive cells per mm2 showed a moderate correlation with low saliva flow rates, high ESR, high level of serum globulin and serum protein, and additionally, the number of CD45 (LCA) positive cells per mm2 was associated with low leukocytes and low hemoglobin. As expected, the fibrosis score of the MSG biopsy had a significant negative correlation with the UWS and SWS flow rates (data not shown). To the best of our knowledge, this was the first analysis to present the relationship between the number of CD45 (LCA) positive cells per mm2 and the clinical manifestations of pSS, and significant results were obtained. On the other hand, several recently published studies showed that focus scores and clinical factors had no significant impact, which was in contrast to the results of this study [46,47,48]. These inconsistent results can be explained by the existence of various factors that can affect the clinical symptoms of pSS and disease heterogeneity.
Considering these results, the superiority and accuracy of the MSG biopsy cannot be denied in the diagnosis of pSS and there are published studies that show that the histological findings are not reflected in salivary gland scintigraphy [49, 50]. However, there is a problem with biopsy that not only focus scores but also scales such as Tarpley or Greenspan are used for classification of histopathological findings, and focal lymphocytic sialadenitis and non-specific inflammation are not always distinguished [51]. In addition, in some cases, the MSG biopsy may not be performed due to increased risk, or patient refusal, and even though pSS is highly suspected, the results of MSG biopsy may be normal. The reason for the presence of normal cells in the MSG biopsy may be that too small a sample of tissue was taken and it represented only a portion of the gland, or that autoantibodies against muscarinic receptors that inhibit parasympathetic activation by acetylcholine may lead to functional impairment of the exocrine glands [52]. There is no measurement that can completely replace the histological findings in the work up of pSS, and several diagnostic tests presented in the pSS classification criteria cannot directly replace each other. For this reason, we propose the use of salivary gland scintigraphy as a complementary method to the current classification criteria, when biopsy is not feasible. Furthermore, salivary gland scintigraphy has been used to monitor the disease progression of pSS, measure the therapeutic response, and support therapeutic decisions thus, we believe that contrary to recent trends, salivary gland scintigraphy plays a sufficient role in the management of pSS [53].
The primary strength of our study is that we compared the detailed analyses of histopathology with scintigraphic parameters rather than the existing literature. Nevertheless, there are some limitations. First, this is a retrospective study in a single-center with a relatively small sample size. Potential selection bias could not be avoided and all influencing factors that could affect dry mouth (such as medications, a history of radiation therapy, caffeine consumption, and anxiety) could not be completely controlled. Second, despite the patients being enrolled at the time of diagnosis of pSS, the rate of patients with high disease activity, according to the ESSDAI classification, was small. To address the limitations of this study, further studies are needed to expand the sample size and to include patients with various levels of disease activity.
Conclusion
The diagnostic role of salivary gland biopsy is widely accepted in the classification criteria of Sjogren’s syndrome; however, its replacement with a non-invasive and more easily accessible imaging technique may be necessary. This study demonstrated that salivary gland biopsy in patients with pSS showed a significant correlation with the mean uptake, peak uptake, and percentage washout of both the parotid and submandibular glands in salivary gland scintigraphy, and with clinical features such as cytopenia, high ESR, and lower UWS and SWS flow rates. In our opinion, salivary gland scintigraphy might be a good alternative to MSG biopsy, and the two methods could be complementary to the clinical features in the diagnosis of pSS.
Data availability
All available data are reported in the manuscript and supplementary file.
References
Wang J, Zhou L, Liu B (2020) Update on disease pathogenesis, diagnosis, and management of primary Sjögren’s syndrome. Int J Rheum Dis 23:723–727
Psianou K, Panagoulias I, Papanastasiou AD et al (2018) Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun Rev 17:1053–1064
Cafaro G, Croia C, Argyropoulou OD et al (2019) One year in review 2019: Sjögren’s syndrome. Clin Exp Rheumatol 37(Suppl 118):3–15
Negrini S, Emmi G, Greco M et al (2022) Sjögren’s syndrome: a systemic autoimmune disease. Clin Exp Med 22:9–25
Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ (1965) Sjoegren’s syndrome. A clinical, pathological, and serological study of sixty-two cases. Medicine (Baltimore) 44:187–231
Daniels TE, Silverman S Jr, Michalski JP, Greenspan JS, Sylvester RA, Talal N (1975) The oral component of Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol 39:875–885
Manthorpe R, Frost-Larsen K, Isager H, Prause JU (1981) Sjögren’s syndrome. A review with emphasis on immunological features. Allergy 36:139–153
Fox RI, Robinson CA, Curd JG, Kozin F, Howell FV (1986) Sjögren’s syndrome Proposed criteria for classification. Arthritis Rheum 29:577–585
Skopouli FN, Drosos AA, Papaioannou T, Moutsopoulos HM (1986) Preliminary diagnostic criteria for Sjögren’s syndrome. Scand J Rheumatol Suppl 61:22–25
Vitali C, Bombardieri S, Moutsopoulos HM et al (1993) Preliminary criteria for the classification of Sjögren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 36:340–347
Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K (2004) Revised Japanese criteria for Sjögren’s syndrome (1999): availability and validity. Mod Rheumatol 14:425–434
Vitali C, Bombardieri S, Jonsson R et al (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558
Shiboski SC, Shiboski CH, Criswell L et al (2012) American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 64:475–487
Cornec D, Saraux A, Cochener B et al (2014) Level of agreement between 2002 American-European Consensus Group and 2012 American College of Rheumatology classification criteria for Sjögren’s syndrome and reasons for discrepancies. Arthritis Res Ther 16:R74
Shiboski CH, Shiboski SC, Seror R et al (2017) 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 76:9–16
Shiboski CH, Shiboski SC, Seror R et al (2017) 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 69:35–45
Tsuboi H, Hagiwara S, Asashima H et al (2017) Comparison of performance of the 2016 ACR-EULAR classification criteria for primary Sjögren’s syndrome with other sets of criteria in Japanese patients. Ann Rheum Dis 76:1980–1985
Lee J, Koh JH, Kim JW et al (2018) Performance of the 2016 ACR-EULAR classification criteria for primary Sjogren’s syndrome in a Korean cohort. Rheumatol Int 38:1651–1660
Varoni EM, Villani G, Lombardi N et al (2020) Local complications associated with labial salivary gland biopsy for diagnosis of Sjögren’s syndrome: a retrospective cohort study. J Clin Exp Dent 2(12):e713–e718
Seror R, Ravaud P, Bowman SJ et al (2010) EULAR Sjogren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann Rheum Dis 69:1103–1109
Chisholm DM, Mason DK (1968) Labial salivary gland biopsy in Sjögren’s disease. J Clin Pathol 21:656–660
Costa S, Quintin-Roué I, Lesourd A et al (2015) Reliability of histopathological salivary gland biopsy assessment in Sjögren’s syndrome: a multicentre cohort study. Rheumatology (Oxford) 54:1056–1064
Bankhead P, Loughrey MB, Fernández JA et al (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7:16878
Min HK, Kim SH, Lee KA, et al (2021) Correlation between salivary gland ultrasonography and scintigraphy in primary Sjögren's Syndrome. Rheumatology (Oxford) keab881 (e-pub)
Milic V, Petrovic R, Boricic I et al (2012) Ultrasonography of major salivary glands could be an alternative tool to sialoscintigraphy in the American-European classification criteria for primary Sjogren’s syndrome. Rheumatology (Oxford) 51:1081–1085
Fana V, Dohn UM, Krabbe S, Terslev L (2021) Application of the OMERACT grey-scale ultrasound scoring system for salivary glands in a single-centre cohort of patients with suspected Sjögren’s syndrome. RMD Open 7:e001516
Giovannini I, Lorenzon M, Manfrè V et al (2022) Safety, patient acceptance and diagnostic accuracy of ultrasound core needle biopsy of parotid or submandibular glands in primary Sjögren’s syndrome with suspected salivary gland lymphoma. RMD Open. 8:e001901
Milic VD, Petrovic RR, Boricic IV et al (2009) Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjogren’s syndrome: a comparison with scintigraphy and biopsy. J Rheumatol 36:1495–1500
Delli K, Dijkstra PU, Stel AJ, Bootsma H, Vissink A, Spijkervet FK (2015) Diagnostic properties of ultrasound of major salivary glands in Sjögren’s syndrome: a meta-analysis. Oral Dis 21:792–800
Vinagre F, Santos MJ, Prata A, da Silva JC, Santos AI (2009) Assessment of salivary gland function in Sjögren’s syndrome: the role of salivary gland scintigraphy. Autoimmun Rev 8:672–676
Adams BK, Al Attia HM, Parkar S (2003) Salivary gland scintigraphy in Sjögren’s syndrome: are quantitative indices the answer? Nucl Med Commun 24:1011–1016
Zou Q, Jiao J, Zou MH et al (2012) Semi-quantitative evaluation of salivary gland function in Sjögren’s syndrome using salivary gland scintigraphy. Clin Rheumatol 31:1699–1705
Dugonjić S, Stefanović D, Ethurović B, Spasić-Jokić V, Ajdinović B (2014) Evaluation of diagnostic parameters from parotid and submandibular dynamic salivary glands scintigraphy and unstimulated sialometry in Sjögren’s syndrome. Hell J Nucl Med 17:116–122
Tensing EK, Nordström DC, Solovieva S et al (2003) Salivary gland scintigraphy in Sjögren’s syndrome and patients with sicca symptoms but without Sjögren’s syndrome: the psychological profiles and predictors for salivary gland dysfunction. Ann Rheum Dis 62:964–968
Nadal M, Levy M, Bakhsh A et al (2018) Salivary scintigraphy for Sjögren’s syndrome in patients with xerostomia: a retrospective study. Oral Dis 24:552–560
Kim HA, Yoon SH, Yoon JK et al (2014) Salivary gland scintigraphy in Sjögren’s syndrome. Comparison of the diagnostic performance of visual and semiquantitative analysis. Nuklearmedizin 53:139–145
Kohn WG, Ship JA, Atkinson JC, Patton LL, Fox PC (1992) Salivary gland 99mTc-scintigraphy: a grading scale and correlation with major salivary gland flow rates. J Oral Pathol Med 21:70–74
Löfgren CD, Wickström C, Sonesson M, Lagunas PT, Christersson C (2012) A systematic review of methods to diagnose oral dryness and salivary gland function. BMC Oral Health 12:29
Pijpe J, Kalk WW, Bootsma H, Spijkervet FK, Kallenberg CG, Vissink A (2007) Progression of salivary gland dysfunction in patients with Sjogren’s syndrome. Ann Rheum Dis 66:107–112
Aksoy T, Kiratli PO, Erbas B (2012) Correlations between histopathologic and scintigraphic parameters of salivary glands in patients with Sjögren’s syndrome. Clin Rheumatol 31:1365–1370
Huang J, Wu J, Zhao L et al (2020) Quantitative evaluation of salivary gland scintigraphy in Sjögren’s syndrome: comparison of diagnostic efficacy and relationship with pathological features of the salivary glands. Ann Nucl Med 34:289–298
Güne S, Yilmaz S, Karalezli A, Aktaş A (2010) Quantitative and visual evaluation of salivary and thyroid glands in patients with primary Sjögren’s syndrome using salivary gland scintigraphy: relationship with clinicopathological features of salivary, lacrimal and thyroid glands. Nucl Med Commun 31:666–672
Wise CM, Agudelo CA, Semble EL, Stump TE, Woodruff RD (1988) Comparison of parotid and minor salivary gland biopsy specimens in the diagnosis of Sjögren’s syndrome. Arthritis Rheum 31:662–666
Delli K, Haacke EA, Kroese FG et al (2016) Towards personalised treatment in primary Sjögren’s syndrome: baseline parotid histopathology predicts responsiveness to rituximab treatment. Ann Rheum Dis 75:1933–1938
Verstappen GM, Kroese FGM, Bootsma H (2019) T cells in primary Sjögren’s syndrome: targets for early intervention. Rheumatology (Oxford) 60:3088–3098
Park Y, Lee J, Koh JH et al (2020) Positive histopathologic assessment in salivary glands shows little impact on clinical features of established primary Sjögren’s syndrome in a Korean population. Clin Exp Rheumatol 38(Suppl 126):158–165
Sebastian A, Woytala P, Madej M et al (2021) Is it possible to not perform salivary gland biopsy in targeted patients according to unstimulated salivary flow results in patients with suspected Sjögren’s syndrome? Rheumatol Int 41:1125–1131
Haga HJ (2002) Clinical and immunological factors associated with low lacrimal and salivary flow rate in patients with primary Sjögren’s syndrome. J Rheumatol 29:305–308
Henriksen AM, Nossent HC (2007) Quantitative salivary gland scintigraphy can distinguish patients with primary Sjøgren’s syndrome during the evaluation of sicca symptoms. Clin Rheumatol 26:1837–1841
Kaldeway HP, Ter Borg EJ, van de Garde EMW, Habraken JBA, van Buul MMC (2019) Validation of quantitative salivary gland scintigraphy in relation to the American-European concensus criteria for Sjögren’s syndrome. Nucl Med Commun 40:343–348
Bautista-Vargas M, Vivas AJ, Tobon GJ (2020) Minor salivary gland biopsy: its role in the classification and prognosis of Sjogren’s syndrome. Autoimmun Rev 19:102690
Nakamura T, Matsui M, Uchida K et al (2004) M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 558:561–575
Chen YC, Chen HY, Hsu CH (2021) Recent advances in salivary scintigraphic evaluation of salivary gland function. Diagnostics (Basel). 11:1173
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea [HI16C0992].
Author information
Authors and Affiliations
Contributions
JWK, RJ, JHH, JHK, JYJ, CHS, YSA, and HAK contributed to the study design and data collection, analysis, and interpretation. JWK, RJ, JHH, YSA, and HAK contributed to the data collection and/or data interpretation. All authors revised the manuscript and gave final approval for submission.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the institutional review board of Ajou University Hospital (AJIRB-BMR-MDB-22–031).
Consent to participate
The consent to participate was waived due to the study of retrospective nature.
Consent for publication
The consent for publication was approved.
Disclosures
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, JW., Jin, R., Han, J.H. et al. Correlations between salivary gland scintigraphy and histopathologic data of salivary glands in patients with primary Sjogren’s syndrome. Clin Rheumatol 41, 3083–3093 (2022). https://doi.org/10.1007/s10067-022-06269-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06269-x