Abstract
Abnormal findings on salivary gland scintigraphy (SGS) are part of the classification criteria for Sjøgren’s syndrome (SS), but SGS is operator dependent and poorly standardised. We studied the use of quantitative data on the uptake, concentration and excretion of the four major salivary glands in the evaluation of sicca patients. During an initial clinical evaluation for sicca symptoms (mean duration, 51 months), 24 subjects were classified as either SS (n = 8) or isolated sicca (IS; n = 16). SGS was then performed after i.v. injection of 200 MBq pertecnetat. Digitalised quantitative data on time-to-peak uptake (Tmax), peak tracer distribution (C%) and stimulated excretion (E%) were calculated from time-activity curves and compared between groups and controls (n = 8) and correlated to clinical data. Statistical analysis was performed with non-parametric tests. SS patients had longer Tmax in both parotic glands (18.1 min; p < 0.01)) and both submandibular glands (mean 13.7 min, p < 0.05); whereas Tmax in IS patients was similar as in controls in both parotic (10.4 min; p > 0.2) and submandibular glands (9.4 min; p > 0.4). C% was significantly lower in the parotic glands of both the SS and the IS group compared to the controls (p < 0.01). E% was significantly reduced in SS patients (16.3% for parotic and 17.4% for submandibular glands; p < 0.01); whereas in the IS patients, excretion (32, 2% for parotic and 26, 9% for submandibular glands) was similar from all glands as in the control groups (35, 2% for parotic and 27, 8% for submandibular glands). No correlation was found between these SGS results and age, focus score, erythrocyte sedimentation rate, serum creatinin or immunoglobulin levels. No IS patient progressed to full-blown pSS during the 4 years of follow-up. Quantitative SGS data are useful and objective tools to distinguish patients with SS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xerostomia is one of the main clinical features of Sjøgren’s syndrome (SS). Various methods are available to assess salivary gland involvement in SS, which measure different aspects of salivation and do not correspond well to each other. Salivary flow rate reflects both basal saliva production from all glands and the anatomical and functional integrity of the ductal system. Contrast radiography provides data on the morphology and flow velocity of the salivary duct system during invasive cannulation. Salivary gland biopsy can provide definite evidence of the specific inflammation seen in SS but does not give (functional) information for the major glands as it is taken from the minor labial glands [1–5]. These limitations have become more important in the face of emerging evidence that functional antibodies against muscarinic receptors can independently contribute to defective salivation [6, 7]. Salivary gland scintigraphy (SGS) provides a detailed functional evaluation of salivation as it measures the amount and speed of radioisotope uptake and excretion for all four major salivary glands separately [8]. Although abnormal SGS findings are included as one of the classification criteria for SS [5], SGS is not a very well standardised procedure, and results are reported in a qualitative way and are operator dependent [9, 10]. The use of computer-assisted time-activity curves allows quantisation of salivary gland radioactivity over defined time periods during SGS [11, 12]. This study evaluated the diagnostic value of quantifying salivary gland uptake and secretion in patients with sicca symptoms.
Materials and methods
Subjects
Patients referred for the evaluation of sicca symptoms were included in this cross-sectional study, and the following data were collected: duration and type of sicca symptoms (as defined in [15]) and results of the Schirmer-1 test, Rose bengal dye uptake, labial biopsy, parotid sialography, unstimulated salivary flow, medication and laboratory investigations (erythrocyte sedimentation rate [ESR], total WBC and lymphocyte count, levels of C-reactive protein, haemoglobin, serum creatinin and liver enzymes, anti-Ro/SS-A antibodies [by ELISA], rheumatoid factor [RF; by nephelometry and Waaler] and anti-ds DNA [by immunofluorescence and ELISA]). Data were extracted from patient records by one investigator with the use of a pre-defined data collection form. Twenty-four patients not fulfilling exclusion criteria for SS [16] were subsequently investigated by SGS. Before SGS and based solely on prior collected clinical data, patients were classified as either SS when fulfilling at least four of the preliminary European criteria or as isolated sicca (IS) when fulfilling less than four of these criteria [15]. The control group consisted of eight euthyreoid patients referred for suspected nodular thyroid disease; informed consent for the study was obtained from all participants.
Salivary gland scintigraphy
SGS was performed with a gamma camera (Sophia D57) equipped with a high sensitive collimator (General Purpose). Medication that could influence salivary flow was stopped 2 days before (diuretics in one patient and an anti-cholinergic in another). The head was immobilized in an anterior position relative to the camera; projection was from the anterior with the camera adjusted above the parotid and submandibular glands and the mouth. Immediately after the intravenous injection of 200 MBq 99Tc-pertecnetat, a dynamic acquiring protocol started, and regions of interest were marked manually while carefully avoiding the inclusion of thyroid tissue; images were then acquired every 30 s during a period of up to 40 min representing uptake, concentration and spontaneous secretion of tracer. Subsequently, the mouth was then flushed three times with water to empty the oral cavity for radioactivity, and lemon juice was administered after which imaging continued for another 10 min during the stimulated secretion phase. Data were digitally stored and plotted on separate time-activity curves for each salivary gland after manual selection of the glands. All time-activity curves were also visually analysed to ensure that the correct time points were applied in the curve analysis. The computer software calculated the following variables from the time-activity curve for each gland: (1) time to maximal (Tmax), reflecting the time (in minutes) needed to achieve the maximum concentration; (2) C%, reflecting the percent distribution of total uptake in each different gland; and (3) E%, reflecting the stimulated secretion calculated as the percentage of reduction in concentration from the lemon juice stimulation to the lowest value achieved (E% = (1 min/max) × 100%).
Statistics
Figures represent mean values or numbers (%), unless indicated otherwise. Dichotomous data were analysed with cross tables using Fisher’s exact test, due to small numbers. Analysis of variance was performed for continuous variables. Correlations between continuous variables were analysed by Spearman’s rank test. Data were analysed using SPSS v.11.0. Resulting p-values below 0.05 were considered to indicate statistical significance.
Results
Patient characteristics
Before SGS, eight patients were classified for SS diagnosis; whereas the remaining 16 patients were classified as IS. The mean age of patients was 46 years (range 27–63), and 92% were women with no significant differences between SS and IS patients. The mean symptomatic period before SGS was 51 months (range 9–109). Three IS patients had established RA (one patient) or SLE (two patients). Control patients (84% women; p > 0.2) were younger than sicca patients (38 years; range 20–67; p < 0.05). None of the IS patients progressed to established pSS during the 64 months (range 1–140) of follow-up. Results for routine laboratory findings for SS and IS patients (Table 1) showed significantly higher mean values for ESR, lymphocyte counts and serum IgG levels in the SS group. Four of five SS patients with antibodies against Ro/SSA were also positive for RF, whereas anti-DNA antibodies were not detectable in any patient.
Tc-99m scintigraphy
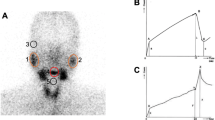
The Tmax tracer uptake was significantly longer in SS patients only; delayed uptake was seen in both parotic glands (mean 18.1 min; p < 0.01)) and both submandibular glands (mean 13.7 min, p < 0.05; Fig. 1a). Uptake was symmetrically delayed in all glands in SS patients (Fig. 1b). Tracer uptake in IS patients was not delayed compared to the controls in both parotic (10.4 and 8.8 min, respectively; p > 0.2) and submandibular glands (9.4 and 8.0 min, respectively; p > 0.4). Peak tracer distribution (C%) was significantly lower in the parotic glands of both the SS (42%) and the IS group (40%) compared to the controls (53%; Fig. 1c; p < 0.01).This reduction was symmetric in IS patients, but asymmetric (significant reduction in the left parotic and submandibular glands only) in SS patients (Fig. 1d). Stimulated excretion (E%) was significantly reduced in SS patients (16.3% for parotic and 17.4% for submandibular glands; p < 0.01); however, in the IS patients, excretion (32, 2% for parotic and 26, 9% for submandibular glands) was similar from all glands as in the control groups (35, 2% for parotic and 27, 8% for submandibular glands; Fig. 1e and f).
Quantitative salivary gland scintigraphy findings in patients with Sjøgren’s syndrome (SS), isolated sicca (IS) and the control group. Tmax tracer uptake in minutes for the parotic and submandibular glands combined (a) and for each separate gland (b). Peak tracer distribution (C%; percentage of total tracer dose) for the parotic and submandibular glands combined (c) and for each separate gland (d). Stimulated excretion of tracer (E%; given as the percent in reduction of maximum concentration) for the parotic and submandibular glands combined (e) and for each separate gland (f). See “Materials and methods” for more details. Asterisks indicate values that are significantly different from the controls
Correlations between SGS data and clinical findings
To determine if SGS findings could be related to specific clinical factors, correlation coefficients were calculated between age, focus score, sialometry, ESR, serum levels of creatinin and immunoglobulins and the results of Tmax, C% and E%. No significant correlations were seen within the separate SS or IS groups or within the whole cohort of sicca patients (all p values greater than 0.05, data not shown).
Discussion
In this study, a reversed parotic/submandibular uptake ratio, a prolonged Tmax tracer uptake and a reduced tracer secretion especially from the parotic glands distinguished SS patients from controls. Although semi-quantitative SGS data have been studied before, this is one of the few studies using absolute measures for isotope uptake and excretion in a controlled fashion. The degree of reduction in speed and peak uptake and secretion in this study agree to a large extent with the data presented in other studies of paired time-activity curves for submandibular and parotid glands in primary SS patients [8, 13, 14]. Some authors found a reduced uptake in the submandibular glands of SS patients; whereas in the present study, parotic uptake was more affected in SS patients. This discrepancy may be because of methodological aspects as the present SS cohort had shorter duration of sicca symptoms (4 years) compared to long standing diseases (mean 11 years) in the other study. Differing sensitivities of the parotic and submandibular glands to insult over time as reported before may also be one of the explanations [15–17]. Our data indicate a near doubling of the Tmax and a reduction by half of the excretion capacity in SS patients compared to the controls. This illustrates how cut-off levels for absolute values in SGS could become a valuable contribution in SS classification/diagnosis but will require larger prospective studies that standardise tracer administration and salivary stimulation [13].
Delayed tracer uptake and secretion in SS patients is thought to be due to a progressive reduction in exocrine function of the major salivary glands resulting from morphological changes induced by chronic immune-mediated inflammation [1, 3, 18, 19]. This line of thought is, however, mainly based on indirect evidence from minor labial gland biopsy findings in SS. However, normal acinar cells are commonly observed in biopsy materials indicating that dryness cannot be solely explained by glandular destruction. Auto antibodies against muscarinic M3 receptors that inhibit parasympathetic neurotransmission may, however, also contribute to impaired glandular function through the disruption of autonomic function without mediating inflammation [6, 7, 20]. Although we lacked the necessary expertise to measure muscarinic receptor auto antibodies, studying the influence of this new class of auto antibodies on salivation may also profit from quantitative SGS testing.
We did not find a significant correlation between SGS results and anti-SSA auto antibodies, which are found in salivary infiltrates [1, 18]. Although this lack of correlation may be well be due to the low numbers in this study, it could again indicate that factors other than inflammation may also be important in reducing salivation in SS. The exact sequence and contribution of inflammation and dysfunction to the development of sicca, symptoms are presently unknown; however, quantitative testing of salivary gland function may provide important information not otherwise obtainable. Although unstimulated sialometry is easily available, it remains an indirect measure of salivary gland function as it depends on the integrity of the ductal system. Sialometric findings have a low specificity for SS as various external factors contribute to large variations in flow in healthy cohorts and also within the same individual [2, 21]. A non-invasive test that could replace labial gland biopsy at the salivary test of choice would be a large step forward in classifying/diagnosing pSS, as it would allow both standardisation and inclusion of a much larger proportion of pSS patients in scientific studies. On the condition that quantitative SGS is developed in a standardized fashion, it could ultimately fulfil that goal [13].
There are several limitations to the interpretation of these results. We did not use the latest criteria from the EU–US consensus, as these criteria were not available during the planning stage of this study. Two patients classified as SS with the use of the preliminary European criteria would not have retained their SS diagnosis with the use of these new criteria set because of the absence of anti-SSA antibodies. In addition, we did not study absolute values for total tracer uptake in our patients. Absolute uptake (i.e. the number of radioactive counts) is a function of salivary gland mass and thus related to body mass. However, these data were not systematically collected in our cohort. Total tracer uptake may be especially interesting in the follow-up of patients with SS as it may reflect the development of gland atrophy. Furthermore, the low number of patients with definite pSS that we were able to recruit and their age difference with controls may have biased our findings. Finally, our control group consisted of patients who were referred for scintigraphy evaluation of suspected nodular thyroid disease. Although all were euthyroid, there have been reports on reduced salivary function in patients with autoimmune thyroiditis [22, 23], and we cannot exclude that subclinical thyroid disease may have influenced our control data. The clinical relevance of distinguishing between IS and pSS lies in the associated risk for extraglandular complications, which occur frequently in pSS but not in IS [1, 2]. This has clear implications for the follow-up and potentially also for the treatment of these patients as confirmed by the lack of progression to pSS in this cohort.
In summary, findings from this controlled pilot study support the use of time activity curves during quantitative SGS as a safe, minimally invasive and objective method to distinguish patients with SS during the evaluation of sicca symptoms. To confirm these results, a cross-sectional study with direct comparison of the diagnostic value of SGS with labial biopsy findings will be needed together with a longitudinal study that investigates the disease course in such cohorts.
References
Anaya JM, Talal N (1999) Sjøgren’s syndrome comes of age. Semin Arthritis Rheum 28(6):355–359
Jonsson R, Haga HJ, Gordon TP (2000) Current concepts on diagnosis, autoantibodies and therapy in Sjøgren’s syndrome. Scand J Rheumatol 29(6):341–348
Kassan SS, Moutsopoulos HM (2004) Clinical manifestations and early diagnosis of Sjøgren syndrome. Arch Intern Med 164(12):1275–1284
Mavragani CP, Tzioufas AG, Moutsopoulos HM (2000) Sjøgren’s syndrome: autoantibodies to cellular antigens. Clinical and molecular aspects. Int Arch Allergy Immunol 123(1):46–57
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE et al (2002) Classification criteria for Sjøgren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis 61(6):554–558
Gao J, Cha S, Jonsson R, Opalko J, Peck AB (2004) Detection of anti-type 3 muscarinic acetylcholine receptor autoantibodies in the sera of Sjøgren’s syndrome patients by use of a transfected cell line assay. Arthritis Rheum 50(8):2615–2621
Li J, Ha YM, Ku NY, Choi SY, Lee SJ, Oh SB et al (2004) Inhibitory effects of autoantibodies on the muscarinic receptors in Sjøgren’s syndrome. Lab Invest 84(11):1430–1438
Hakansson U, Jacobsson L, Lilja B, Manthorpe R, Henriksson V (1994) Salivary gland scintigraphy in subjects with and without symptoms of dry mouth and/or eyes, and in patients with primary Sjøgren’s syndrome. Scand J Rheumatol 23(6):326–333
Schall GL, Larson SM, Anderson LG, Griffith JM (1972) Quantification of parotid gland uptake of pertechnetate using a gamma scintillation camera and a ‘‘region-of-interest’’ system. Am J Roentgenol Radium Ther Nucl Med 115(4):689–697
Schall GL, Di Chiro G (1972) Clinical usefulness of salivary gland scanning. Semin Nucl Med 2(3):270–277
Adams BK, Al Attia HM, Parkar S (2003) Salivary gland scintigraphy in Sjøgren’s syndrome: are quantitative indices the answer? Nucl Med Commun 24(9):1011–1016
Umehara I, Yamada I, Murata Y, Takahashi Y, Okada N, Shibuya H (1999) Quantitative evaluation of salivary gland scintigraphy in Sjøgren’s syndrome. J Nucl Med 40(1):64–69
Nishiyama S, Miyawaki S, Yoshinaga Y (2006) A study to standardize quantitative evaluation of parotid gland scintigraphy in patients with Sjøgren’s syndrome. J Rheumatol 33(12):2470–2474
Shizukuishi K, Nagaoka S, Kinno Y, Saito M, Takahashi N, Kawamoto M, Abe A, Jin L, Inoue T (2003) Scoring analysis of salivary gland scintigraphy in patients with Sjøgren’s syndrome. Ann Nucl Med 17(8):627–631
Lindvall AM, Jonsson R (1986) The salivary gland component of Sjøgren’s syndrome: an evaluation of diagnostic methods. Oral Surg Oral Med Oral Pathol 62(1):32–42
Daniels TE, Powell MR, Sylvester RA, Talal N (1979) An evaluation of salivary scintigraphy in Sjøgren’s syndrome. Arthritis Rheum 22(8):809–814
Sugihara T, Yoshimura Y (1988) Scintigraphic evaluation of the salivary glands in patients with Sjøgren’s syndrome. Int J Oral Maxillofac Surg 17(2):71–75
Jonsson R, Gordon TP, Konttinen YT (2003) Recent advances in understanding molecular mechanisms in the pathogenesis and antibody profile of Sjøgren’s syndrome. Curr Rheumatol Rep 5(4):311–316
Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV (2001) Sialometry and sialochemistry: diagnostic tools for Sjøgren’s syndrome. Ann Rheum Dis 60(12):1110–1116
Wang F, Jackson MW, Maughan V, Cavill D, Smith AJ, Waterman SA et al (2004) Passive transfer of Sjøgren’s syndrome IgG produces the pathophysiology of overactive bladder. Arthritis Rheum 50(11):3637–3645
Vitali C, Bombardieri S, Moutsopoulos HM, Coll J, Gerli R, Hatron PY et al (1996) Assessment of the European classification criteria for Sjøgren’s syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjøgren’s Syndrome. Ann Rheum Dis 55(2):116–121
Chang CP, Shiau YC, Wang JJ, Ho ST, Kao CH (2003) Decreased salivary gland function in patients with autoimmune thyroiditis. Head Neck 25(2):132–137
Changlai SP, Chen WK, Chung C, Chiou SM (2002) Objective evidence of decreased salivary function in patients with autoimmune thyroiditis (chronic thyroiditis, Hashimoto’s thyroiditis). Nucl Med Common 23(10):1029–1033
Acknowledgement
The technical support and advice by Peter van Lom and Professor Johan Sundsfjord at the Department of Nuclear Medicine, University Hospital of North Norway is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henriksen, A.M., Nossent, H.C. Quantitative salivary gland scintigraphy can distinguish patients with primary Sjøgren’s syndrome during the evaluation of sicca symptoms. Clin Rheumatol 26, 1837–1841 (2007). https://doi.org/10.1007/s10067-007-0586-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0586-1