Abstract
This retrospective study is designed to evaluate the diagnostic value of semi-quantitative parameters of salivary gland scintigraphy (SGS) in Sjögren's syndrome (SS) and explore the relationship between SGS parameters and salivary gland histopathologic manifestations. SGS and labial salivary gland biopsy were performed on 95 SS patients and 36 healthy age-matched and sex-matched volunteers. Uptake ratios at 15 min (UR15) and stimulated excretion fraction (EF) of each parotid and submandibular gland were calculated automatically from SGS; histopathologic grades were classified based on focal lymphocytic infiltration, and biopsy focus scores were calculated simultaneously. As a result, the UR15 and EF of each salivary gland in SS patients were significantly lower than that in controls. Multivariate logistic regression equation was: logit p = 6.965 − 1.680 × UR15 of left submandibular gland − 0.040 × EF of left parotid gland − 0.050 × EF of right submandibular gland. The receiver operating characteristic (ROC) curve validated high efficiency of this model with the area under curve of 0.911. And logistic regression analysis on combined original and validation cohorts confirmed its diagnostic value. Moreover, the EF of right parotid gland and both submandibular glands was correlated with histopathologic changes. Consequently, the semi-quantitative parameters of SGS may be helpful for diagnosing SS; UR15 of left submandibular gland, EF of left parotid gland, and EF of right submandibular gland are more powerful predictor of SS. Besides, SGS parameters reflect the degree of salivary gland involvement, severe patients with low parameters would have high histopathologic grades and biopsy focus scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sjögren's syndrome (SS) is an inflammatory autoimmune disease characterized by focal lymphocytic infiltration and the destruction of exocrine glands, especially the salivary and lachrymal glands [1]. Xerostomia is the major manifestation resulting from the dysfunction of salivary glands. As for most patients lacking distinguishing signs and symptoms, evaluating salivary glands function is necessary and important to help in the early diagnosis of SS. Several measurements have been proposed to assess salivary gland functions, such as salivary flow rate, contrast sialography, salivary gland ultrasonography, and labial salivary gland biopsy [2–4]. These methods are either invasive or complex, which are not suitable for usual use. Compared with other methods, salivary gland scintigraphy (SGS) is a noninvasive, sensitive, safe, and objective technique. Not only the location and morphology of salivary glands can be obtained, but also quantitative parameters can be calculated. However, traditional SGS technique took too long and focused on qualitative measuring, such as delayed uptake, reduced concentration, and/or delayed and diminished excretion of the tracer [5]. Even though the procedure and quantitative parameters of SGS were explored widely, so far, there is no consensus on which SGS methods and parameters were more sensitive in evaluating salivary gland function [1, 6, 7]. Further efforts are still necessary to establish a sensitive and quantitative method using SGS in discriminating between SS patients and normal controls.
In this study, we aim to assess the function of salivary glands qualitatively and semi-quantitatively in SS patients and healthy volunteers using modified short time SGS with determined sensitive semi-quantitative parameters. By analyzing these data, we set a logistic regression equation for helping in the diagnosis of SS by using these parameters. Furthermore, the correlation between semi-quantitative parameters of SGS and histopathologic changes of labial biopsy in SS was also investigated.
Materials and methods
Subjects
Ninety-five patients with clinically diagnosed SS in our department were enrolled in this retrospective study. All patients met the classification criteria for SS (2002 American–European Consensus Criteria) [3]. It consisted of 75 primary SS patients and 20 SS patients associated with other autoimmune diseases including rheumatoid arthritis (n = 13) and systemic lupus erythematosus (n = 7). SGS and minor salivary gland biopsy were performed on all patients. Main characteristics of SS patients are summarized in Table 1. Thirty-six healthy volunteers (29 females and seven males, age 12–68 years, median age 36 years) with no evidence of salivary gland dysfunction or no history of autoimmune diseases were also enrolled as a control group. Another 26 SS patients (20 females and six males, age 24–77 years, median age 47 years), 10 healthy subjects (nine females and one male, age 16–63 years, median age 31 years), and 22 patients with no-SS sicca syndrome (20 females and two males, age 15–74 years, median age 47.5 years) were included as validation subjects.
Salivary gland scintigraphy
Patients were in supine position with chin raised. After intravenous injection of 370 MBq 99mTc pertechnetate (99mTcO −4 ), SGS was performed with single-photon emission computed tomography (SPECT) (Millennium VG, GE, USA), using a low-energy, high-sensitivity, parallel-hole collimator at 2 min per frame. The duration of the scan was 30 min with salivary secretion stimulated by oral administration of 0.1 g vitamin C at 15 min. The head was kept stationary during scintigraphy. Images were recorded in 64 × 64 matrix with 2.5 magnification. The energy window around 140 keV photopeak of 99mTc was 15 %.
Images and data analysis
Regions of interest (ROI) of each parotid and submandibular gland were drawn manually, with background ROI painted on the right temporal region. UR15 was defined as (salivary gland count at 15 min − background count )/ background count and EF as (maximum salivary count before stimulation − minimum salivary count after stimulation) / (maximum salivary count before stimulation − background count) × 100 %. In the absence of a gland visualization, a value of zero was given for the UR15 and EF. The images of SGS were analyzed by two experienced nuclear physicians independently.
Labial salivary gland biopsy
The minor salivary glands of the lower lip were routinely sectioned following the informed consent. The biopsy material was fixed in formaldehyde, embedded in paraffin, serially sliced, and stained by hematoxylin–eosin. All slices were carefully examined and then graded by an experienced pathologist. Histopathologic changes were classified from 0 to 4 according to the focal lymphocytic infiltration following Chisholm and Mason's grading criteria (grades 3 and 4 were defined positive) [3, 8]. On the other hand, we calculated the biopsy focus score on the basis of the number of lymphocytic foci every 4 mm2 tissue, which reflected the histopathologic changes quantitatively.
Statistical analysis
All the scintigraphy parameters were expressed as means ± SD. Initially, differences of parameters between patients and controls were compared using Student's t test. Multivariate stepwise binary logistic regression analysis was used to establish a logistic regression model helping in the diagnosis of SS by applying the parameters of SGS (α = 0.05, β = 0.10). ROC analysis was performed and the area under curve (AUC) was calculated to evaluate the diagnostic efficiency of the model. The 26 SS patients and 10 healthy subjects were included as an independent validation group to confirm the predictive efficiency of SGS parameters. And the 22 patients with no-SS xerostomia were used to verify the diagnostic accuracy of the model in patients affected by no-SS sicca syndrome. In addition, the relationship between functional parameters of SGS and histopathologic grades, SGS parameters, and biopsy focus scores was assessed by Spearman rank correlation test. All statistical analyses were employed using statistical product and service solutions version 13.0 and p < 0.05 was considered statistically significant.
Results
Analysis of images in SGS
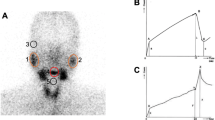
After intravenous injection of 99mTcO −4 , images of parotid and submandibular glands became clear gradually in healthy volunteers and faded rapidly after oral administration of 0.1 g vitamin C at 15 min. Then, imaging agents were excreted into mouth. In contrast, images of patients with SS were not clear or could not be visualized, and the imaging agents are still located in parotid and submandibular glands after oral administration of vitamin C; the changes of oral images were not obvious (Fig. 1).
Comparison of SGS parameters between SS patients and controls
First, we made comparison of SGS parameters between primary SS patients and patients associated with other autoimmune diseases and found that there were no statistical differences in UR15 of each parotid and submandibular gland and EF of each parotid and left submandibular (p > 0.05), other than EF of right submandibular (p = 0.01). Then, the comparison of SGS parameters between patients with SS and healthy volunteers was made and results were shown in Table 2. The UR15 and EF of each parotid and submandibular gland in patients with SS were significantly lower than those in controls (p ≤ 0.001). There was no statistical difference in bilateral scintigraphy parameters (p > 0.05). The UR15 of the submandibular glands was significantly lower compared with parotid glands (p < 0.05), but not in the EF.
Logistic regression model and ROC analysis
We established a binary logistic regression model, determining diagnosis of SS as response variable and parameters of SGS as independent variable. Finally, UR15 of left submandibular gland (X1, p = 0.004), EF of left parotid gland (X2, p = 0.001), and EF of right submandibular gland (X3, p = 0.023) entered the model. The logistic regression equation was logit p = 6.965 − 1.680 × X1 − 0.040 × X2 − 0.050 × X3, with odds ratio (OR) and 95 % confidence interval (CI) of 5.366 (1.711–16.834), 1.041 (1.017–1.066), and 1.051 (1.007–1.097), respectively. We evaluated the efficiency of the logistic regression model using ROC analysis (Fig. 2). The AUC was 0.911 ± 0.024, with 95 % CI (0.863–0.959), which verified the diagnostic value of logistic regression model we established. The validation group is age and sex matched, and there was no statistical difference of SGS parameters between validations and previous subjects. Then, we performed the multivariate logistic analysis in the combined original and validation cohorts and found that X1 (p = 0.002), X2 (p < 0.001), and X3 (p = 0.016) have more sensitive diagnostic value for this combined cohorts, with OR and 95 % CI of 4.839 (1.748–13.394), 1.043 (1.021–1.066), and 1.046 (1.008–1.086), respectively. In the ROC analysis, AUC was 0.911 ± 0.022, with 95 % CI (0.869–0.953). To verify the diagnostic accuracy of the model in SS patients and patients affected by no-SS sicca syndrome, we performed a validation analysis using the 95 SS patients and 22 patients with no-SS sicca syndrome. It showed that only X2 was statistical significant (p = 0.031); but not for X1 and X3.
Correlation between parameters of SGS and histopathologic changes
The relationships between parameters of scintigraphy and minor salivary gland histopathologic changes are demonstrated in Table 3. The EF of right parotid gland and both submandibular glands significantly decreased corresponding to the histopathologic grades (p < 0.05). These indicated that the tracer excretion ability of right parotid gland and both submandibular glands weakened along with higher histopathologic grades. There was no significant correlation between histopathologic grades and UR15 of each salivary gland or EF of left parotid gland (p > 0.05). Furthermore, the minor salivary gland biopsy focus scores of these 95 patients ranged from 0 to 6 with median of 1. The EF of right parotid gland and both submandibular glands was also negatively connected with the biopsy focus score, which reflected histopathologic changes influencing the parameters of scintigraphy to a certain extent.
Discussion
SGS was a safe and sensitive method for assessing functions of salivary glands, and recent report showed that SGS even offered valuable clinical information for prognosis and outcome of the primary SS [9]. SGS has been suggested for diagnosing SS in 2002 American–European criteria with positive qualitatively described as delayed uptake, reduced concentration, and/or delayed and diminished excretion of the tracer [5]. Over the past decades, a variety of different quantitative parameters obtained from SGS such as percent uptake counts, gland to background ratio, time elapsed to peak counts, and magnitude of stimulated salivary excretion have been proposed; however, there has been no consensus on which parameters will be more valuable and sensitive for the diagnosis of SS [1, 6, 7, 10, 11].
Previous researches took more than 40 min for SGS and analyzed peak uptake SGS parameters of salivary glands. However, the time of uptake peak ranged from 15 to 40 min, or even longer, and most traditional SGS took long to make patients uncomfortable. For this reason, we shortened the examination time to 30 min by modifying scintigraphic procedure and calculated UR15 and EF stimulated by vitamin C at 15 min. The UR15 of parotid and submandibular gland reflected the ability of uptaking imaging agent, and EF reflects ability of excreting imaging agent. In our study, semi-quantitative parameters of each parotid and submandibular gland are valuable indications for patients with SS, especially UR15 of left submandibular gland and EF of left parotid gland and right submandibular gland.
The results of our study showed that the parameters of each salivary gland were significantly different between patients and the control group, and no statistical differences were found between primary SS patients and SS patients associated with other autoimmune diseases. The parameters of patients decreased in varying degrees, indicative of dysfunction of parotid and submandibular glands in patients with SS. The results were consistent with previous studies, indicating that our modified SGS is a sensitive method to evaluate salivary function [10, 12, 13]. Further investigation of these parameters by multivariate regression analysis showed that decreased UR15 of left submandibular gland and EF of left parotid and right submandibular were more sensitive parameters for helping in the diagnosis of SS. These asymmetric changes may reflect the uneven nature of the inflammatory process. Adams and Seval also reported these asymmetric changes of salivary glands in patients with SS [14]. The logistic regression model showed that the parameters of submandibular glands contributed more for the diagnosis of SS, which indicated that the dysfunction of submandibular glands was more often in SS. Several researchers reported that the function of submandibular gland was more often affected than parotid gland in SS [15–17]. The mechanism of these asymmetric changes was still unknown. This might attribute to the differences in structure and function of these two salivary glands. Diagnosing SS is a complex procedure. We did not consider that UR15 of left submandibular gland and EF of left parotid gland and right submandibular gland together were enough to diagnose SS. Our results indicated that these parameters were more sensitive in evaluating the function of salivary glands. Patients affected by no-SS sicca syndrome were similar with SS patients for xerostomia, and with the same diminished salivary flow, which might become false positive [18]. However, these two kinds of patients have different pathophysiological changes in salivary glands, which might result in different semi-quantitative parameters. The results of our study show that the equation we formed has the ability to differentiate between these two kinds of patients.
Minor salivary gland biopsy for SS first proposed by Chisholm was believed to be the most sensitive diagnostic approach and has been regarded as “gold standard” in diagnosing SS [8, 19, 20]. The histopathologic hallmark was focal lymphocytic infiltration of the salivary glands, and the grading standard and focus score reflected the degree of inflammatory infiltration which indicated the damages of normal salivary gland formation. Even though labial biopsy provided definite evidence of the specific inflammation in SS, it did not give immediate functional information of salivary glands [21, 22]. In addition, the nature of invasion hampered its wide application. However, evaluating function of salivary glands distinctly was the greatest advantage of SGS. This character attracted interest of some researchers to explore the relationship between SGS parameters and histopathologic grades. There were controversies over association between inflammatory manifestations of labial biopsy and SGS parameters. An early study from Schall suggested that the quantitative parameters of SGS were not correlated with histopathologic grades [5]. However, with the development of SPECT and data analysis system, several authors reported that SGS characteristics connected well with histopathologic changes [1, 19, 21, 23, 24]. Our study demonstrated that the EF of right parotid gland and bilateral submandibular gland was correlated with minor salivary gland inflammation grades and focus scores. Conversely, the UR15 of both parotid gland and submandibular gland was not correlated with the histopathologic grades. This indicated that SGS parameters reflected the degree of salivary gland involvement to some extent, and the excretion function parameters may be more sensitive than uptake parameters in diagnosing SS.
In summary, we believe that the modified semi-quantitative SGS is a safe and reliable method to evaluate salivary glands function in SS patients and can be suitable for clinical use for its noninvasive, sensitive, and objective merits. But it should be noted that this research still had several limitations. First, we just calculated semi-quantitative parameter; absolutely quantitative has not been achieved yet for patients' weight was not being recorded. Secondly, we drew the ROI of parotid and submandibular glands manually which increase the variability. When the images of the salivary glands were not clear enough, we could not draw the ROI accurately, leading to inaccurate parameters. Finally, our results show that there were overlap among SS patients and normal controls for the values of semi-quantitative parameters. The sample size in this study was not big enough and the results should be confirmed by further studies.
Conclusions
Salivary gland scintigraphy is a sensitive and noninvasive method to evaluate the function of salivary glands. Modified salivary gland scintigraphy with sensitive semi-quantitative parameters is valuable and essential for diagnosing Sjögren's syndrome, which is more convenient than traditional technique for its time-saving merit. The UR15 of left submandibular gland and the EF of left parotid gland and right submandibular gland are more sensitive parameters for evaluating the function of salivary glands in patients with SS, and the EF of right parotid gland and both submandibular glands are correlated with histopathologic manifestations.
References
Aung W, Murata Y, Ishida R, Takahashi Y, Okada N, Shibuya H (2001) Study of quantitative oral radioactivity in salivary gland scintigraphy and determination of the clinical stage of Sjögren's syndrome. J Nucl Med 42(1):38–43
Salaffi F, Carotti M, Iagnocco A, Luccioli F, Ramonda R, Sabatini E et al (2008) Ultrasonography of salivary glands in primary Sjögren's syndrome: a comparison with contrast sialography and scintigraphy. Rheumatology 47(8):1244–1249
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE et al (2002) Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61(6):554–558
Milic VD, Petrovic RR, Boricic IV, Marinkovic-Eric J, Radunovic GL, Jeremic PD et al (2009) Diagnostic value of salivary gland ultrasonographic scoring system in primary Sjögren's syndrome: a comparison with scintigraphy and biopsy. J Rheumatol 36(7):1495–1500
Schall GL, Anderson LG, Wolf RO, Herdt JR, Tarpley TM, Cummings NA et al (1971) Xerostomia in Sjögren's syndrome. JAMA 216(13):2109–2116
Adams BK, Al Attia HM, Parkar S (2003) Salivary gland scintigraphy in Sjogren's syndrome: are quantitative indices the answer? Nucl Med Commun 24(9):1011–1016
Shizukuishi K, Nagaoka S, Kinno Y, Saito M, Takahashi N, Kawamoto M et al (2003) Scoring analysis of salivary gland scintigraphy in patients with Sjögren's syndrome. Ann Nucl Med 17(8):627–631
Chisholm DM, Mason DK (1968) Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol 21(5):656–660
Ramos-Casals M, Brito-Zerón P, Perez-De-Lis M, Diaz-Lagares C, Bove A, Soto MJ et al (2010) Clinical and prognostic significance of parotid scintigraphy in 405 patients with primary Sjögren's syndrome. J Rheumatol 37(3):585–590
Aung W, Yamada I, Umehara I, Ohbayashi N, Yoshino N, Shibuya H (2000) Sjögren's syndrome: comparison of assessments with quantitative salivary gland scintigraphy and contrast sialography. J Nucl Med 41(2):257–262
Vivino FB, Hermann GA (2008) Role of nuclear scintigraphy in the characterization and management of the salivary component of Sjögren's syndrome. Rheum Dis Clin N Am 34(4):973–986
Umehara I, Yamada I, Murata Y, Takahashi Y, Okada N, Shibuya H (1999) Quantitative evaluation of salivary gland scintigraphy in Sjorgen's syndrome. J Nucl Med 40(1):64–69
Demangeat R, Didon-Poncelet A, Cherfan J, Demangeat JL (2000) Stimulated salivary pertechnetate clearance revisited: correlation with dynamic scintigraphic indices in Sicca syndrome. Clin Nucl Med 25(11):888–894
Günel SE, Ylmaz S, Karalezli A, Aktas A (2010) Quantitative and visual evaluation of salivary and thyroid glands in patients with primary Sjogren's syndrome using salivary gland scintigraphy: relationship with clinicopathological features of salivary, lacrimal and thyroid glands. Nucl Med Commun 31(7):666–672
Sugihara T, Yoshimura Y (1988) Scintigraphic evaluation of the salivary glands in patients with Sjögren's syndrome. Int J Oral Maxillofac Surg 17(2):71–75
Loutfi I, Nair MK, Ebrahim AK (2003) Salivary gland scintigraphy: the use of semiquantitative analysis for uptake and clearance. J Nucl Med Technol 31(2):81–85
Hermann GA, Vivino FB, Shnier D, Krumm RP, Mayrin V (1999) Diagnostic accuracy of salivary scintigraphic indices in xerostomic populations. Clin Nucl Med 24(3):167–172
Tensing EK, Nordström DC, Solovieva S, Schauman KO, Sippo-Tujunen I, Helve T et al (2003) Salivary gland scintigraphy in Sjögren's syndrome and patients with sicca symptoms but without Sjögren's syndrome: the psychological profiles and predictors for salivary gland dysfunction. Ann Rheum Dis 62(10):964–968
Vitali C, Moutsopoulos HM, Bombardieri S (1994) The European Community Study Group on diagnostic criteria for Sjögren's syndrome. Sensitivity and specificity of tests for ocular and oral involvement in Sjögren's syndrome. Ann Rheum Dis 53(10):637–647
Daniels TE, Whitcher JP (1994) Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Arthritis Rheum 37(6):869–877
Henriksen AM, Nossent HC (2007) Quantitative salivary gland scintigraphy can distinguish patients with primary Sjögren's syndrome during the evaluation of sicca symptoms. Clin Rheumatol 26(11):1837–1841
Jonsson R, Haga HJ, Gordon TP (2000) Current concepts on diagnosis, autoantibodies and therapy in Sjögren's syndrome. Scand J Rheumatol 29(6):341–348
Saito T, Fukuda H, Horikawa M, Ohmori K, Shindoh M, Amemiya A (1997) Salivary gland scintigraphy with 99mTc–pertechnetate in Sjögren's syndrome: relationship to clinicopathologic features of salivary and lacrimal glands. J Oral Pathol Med 26(1):46–50
Arrago J, Rain J, Brocheriou C, Rocher F (1987) Scintigraphy of the salivary glands in Sjögen's syndrome. J Clin Pathol 40(12):1463–1467
Acknowledgments
The study was supported by the Sci-tech Development Program of Guangdong Province (no. 2011B061300050). All the authors would like to thank the SS patients and volunteers for their active cooperation.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, Q., Jiao, J., Zou, MH. et al. Semi-quantitative evaluation of salivary gland function in Sjögren's syndrome using salivary gland scintigraphy. Clin Rheumatol 31, 1699–1705 (2012). https://doi.org/10.1007/s10067-012-2076-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-012-2076-3