Abstract
Objective
The aim of this study was the assessment of semi-quantified salivary gland dynamic scintigraphy (SGdS) parameters independently and in an integrated way in order to predict primary Sjögren’s syndrome (pSS).
Materials and methods
Forty-six consecutive patients (41 females; age 61 ± 11 years) with sicca syndrome were studied by SGdS after injection of 200 MBq of pertechnetate. In sixteen patients, pSS was diagnosed, according to American-European Consensus Group criteria (AECGc).
Semi-quantitative parameters (uptake (UP) and excretion fraction (EF)) were obtained for each gland. ROC curves were used to determine the best cut-off value. The area under the curve (AUC) was used to estimate the accuracy of each semi-quantitative analysis.
To assess the correlation between scintigraphic results and disease severity, semi-quantitative parameters were plotted versus Sjögren’s syndrome disease activity index (ESSDAI). A nomogram was built to perform an integrated evaluation of all the scintigraphic semi-quantitative data.
Results
Both UP and EF of salivary glands were significantly lower in pSS patients compared to those in non-pSS (p < 0.001). ROC curve showed significantly large AUC for both the parameters (p < 0.05).
Parotid UP and submandibular EF, assessed by univariated and multivariate logistic regression, showed a significant and independent correlation with pSS diagnosis (p value <0.05). No correlation was found between SGdS semi-quantitative parameters and ESSDAI. The proposed nomogram accuracy was 87%.
Conclusion
SGdS is an accurate and reproducible tool for the diagnosis of pSS. ESSDAI was not shown to be correlated with SGdS data.
Clinical relevance
SGdS should be the first-line imaging technique in patients with suspected pSS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sjögren’s syndrome (SS) is a chronic, progressive, autoimmune disease, of unknown aetiology, characterized by focal lymphocytic infiltration of exocrine glands with a significant functional impairment, leading to sicca symptoms [1–3]. These symptoms could be also associated with connective tissue disorders, autoimmune diseases (rheumatoid arthritis, systemic sclerosis or systemic lupus erythematosus) or other causes (such as previous head and neck radiotherapy or antidepressant drugs). Therefore, it is important to identify primary Sjögren’s syndrome (pSS) among various aetiologies given that clinical and therapeutic approaches are different [4].
Diagnostic approach in pSS is impervious because the classification criteria, essential to ensure standardization in multicentre studies, show good but not excellent accuracy in clinical settings [5].
Among the various symptoms, xerostomia is an aspecific one. Various methods are available to assess salivary gland involvement, and each one measures different features of salivation: in addition, salivary gland biopsy, performed on minor labial glands, identifies only non-specific lymphocytic infiltration [6].
Salivary gland dynamic scintigraphy (SGdS) has been proposed as a valid and non-invasive tool to evaluate salivary gland involvement in xerostomic patients. It provides a detailed functional assessment of each salivary gland, and it measures various quantitative parameters [6]. Over the past decades, a variety of different quantitative values have been suggested, but no consensus was reached on which parameters will be more accurate for pSS diagnosis [1, 7–10].
The aim of this study was the assessment of semi-quantified salivary gland dynamic scintigraphy (SGdS) parameters independently and in an integrated way in order to predict pSS.
Materials and methods
Patient selection
In this retrospective study, from September 2008 to March 2012, 46 consecutive patients (5 males, 41 females; 61 ± 11 years mean ± DS), referred to our department for suspected pSS and underwent SGdS.
The exclusion criteria were HCV infection, acquired immunodeficiency syndrome (AIDS), sarcoidosis and hyperthyroidism; no subject had a history of lymphoma or head and neck radiation.
In order to confirm pSS diagnosis, one experienced rheumatologist applied the American-European Consensus Group criteria (AECGc) [5]. In sixteen patients, pSS was diagnosed according to AECGc.
The institution ethic committee approved this study, and informed consent for the study was obtained from all the participants.
Imaging technique
Imaging was performed using a gamma camera (Philips Axis) equipped with low-energy and high-resolution parallel-hole collimators (LEHR). Scintigraphy was performed in three steps: syringe counts before injection (first step)—a syringe with 200 MBq pertechnetate 99mTcO4 − was placed at 20 cm from gamma camera head, and the image was acquired during 45 s (matrix 128 × 128, zoom 2, pixel size 2.33 mm, energy window 140 ± 10% keV). In the second step—the patient was placed supine for an anterior head-neck dynamic imaging starting at pertechnetate i.v. administration (40 frames, 45 s per frame, 30 min). After 15 min, salivary secretion was stimulated with 5 ml of lemon juice, administered with a straw avoiding patient’s head movements. Syringe counts after injection—the empty syringe activity was measured as described in the first step.
Image analysis
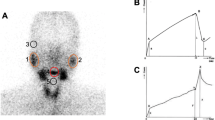
The images were qualitatively assessed by two operators. Afterwards, manual shaped regions of interest (ROIs) were drawn on parotid and submandibular glands on both sides. A rectangular background ROI was placed in the right frontal region (Fig. 1). After background subtraction, data were plotted on separated time-activity curves for each region.
Two SGdS samples processing and corresponding time-activity curves. a SGdS normal pattern of a 54-year-old woman complaining of moderately dry mouth since a month; b SGdS abnormal pattern of a 46-year-old woman affected by sicca symptoms since a year, subsequently classified as pSS, on the basis of AECGc
From time-activity curves, the maximum value before juice administration and the minimum value after juice administration were obtained; from these data, two semi-quantitative parameters were computed according to Eqs. (1) and (2): uptake (UP) and excretion fraction (EF), respectively.
EAI: effective activity injected = syringe counts before injection − syringe counts after injection.
Since no significant difference was found between the right and the left sides for both parotids and submandibulars glands (paired t test), averages of results from the two sides were considered for accuracy purposes of this study.
To assess the inter-operator reproducibility, images were independently analysed by three operators, with different experience (M.M, S.C. and E.P.). The data obtained by each operator for every gland were compared with the corresponding data obtained by the other two operators.
Sjögren’s syndrome disease activity index and SGdS
European League Against Rheumatism (EULAR) promoted and developed the EULAR Sjögren’s syndrome disease activity index (ESSDAI). This model is composed of 12 organ-specific “domains” contributing to disease activity. For each domain, features of disease activity were classified in three or four levels according to their severity [11].
ESSDAI was quantified as the sum of all domain weights. For all pSS patients, ESSDAI was correlated with SGdS results.
Statistical analysis
On the basis of AECGc, results were classified with regards to pSS diagnosis; sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of semi-quantitative results were analysed by ROC curves. The area under the curve (AUC) was calculated to establish accuracy of SGdS data.
On the basis of semi-quantitative data obtained for each major salivary gland by the three operators, the intra-class correlation coefficient (ICC) was calculated.
A multivariate analysis was performed to define the disease probability according to scintigraphy data.
All statistical analyses were performed by using Med Calc vers. 12 (MedCalc Software, Mariakerke, Belgium) and SPSS vers. 19 (IBM Corporation, Armonk, NY, USA). P value <0.05 was considered statistically significant.
Results
Univariate analysis
All semi-quantitative values (both parotid and submandibular UP and EF) were significantly lower in pSS patients compared to those in no-pSS patients. For each semi-quantitative value, ROC-curve AUC was bigger than 0.78, p value <0.05 (Table 1). The best cut-off for each dataset is summarized in Table 1.
Multivariate analysis and integrated evaluation of the salivary gland dynamic scintigraphy
The role of each parameter in a multivariate setting was evaluated by logistic regression (stepwise with constraints 0.05; 0.1). Among the four data, only parotid UP and submandibular EF showed to be independently and significantly correlated with the final diagnosis (p value <0.02 for both).
The logit function that described this correlation is as follows:
In order to estimate the probability of disease, a nomogram was built according to this logistic regression (Fig. 2); values obtained with nomogram were analysed by ROC curve: AUC was 0.91 (p = 0.0001) with sensitivity 75%, specificity 93% and accuracy 87%; best disease probability threshold estimated by the nomogram was 56% (see Fig. 3).
Nomogram, built according to multivariate analysis, which predicts pSS probability on the basis of parotid UP (p.UP) and submandibular EF (s.EF). To calculate pSS probability, locate the patient value of p.UP to the corresponding axis; from this value, draw a line straight upward to the top point axis. Note the value. Repeat the process for s.EF. Sum both values and locate the final sum on the total point axis. Draw a line straight down on the bottom axis to find pSS probability
Inter-operator reproducibility
ICC showed a significant correlation (p < 0.0001) among all the operators with all the coefficients in the interval 0.87–0.96; however in all cases, ICC was higher for gland UP than that for EF (Table 2).
Correlation between salivary gland dynamic scintigraphy and disease activity
No significant correlation has been found between ESSDAI value and SGdS semi-quantitative parameters (UP, EF and nomogram results); R 2 was lower than 0.18 for each data set (Fig. 4).
Discussion
The diagnostic role of SGdS in pSS patients has been studied for years, and several approaches have been proposed [1–4, 6]. Aung et al. and Loufti et al. proposed quantitative and semi-quantitative parameters (uptake ratio, excretion ratio, maximum activity and excretion velocity). Semi-quantitative analysis showed to be able to define the clinical stage of pSS in equivocal cases and was accurate in both diagnosis and follow-up [9, 12]. Also Vinagre et al. confirmed that the use of semi-quantitative values can increase SGdS accuracy [1]. Other authors suggested that a scoring system of dynamic scintigraphy is an objective and reproducible method for evaluating salivary gland function in patients with pSS [6, 7, 13].
On the other hand, several colleagues expressed some concerns about the use of SGdS as a first-choice investigation in patients with suspected pSS [6, 14]; others underlined the low accuracy and the lack of standardized parameters as well as interpretation of SGdS in order to impact the diagnosis and management of pSS [8, 10]. Furthermore, Kim et al. affirmed that qualitative analysis showed higher diagnostic utility than semi-quantitative assessment [15].
Although previous studies have compared pSS patients with healthy volunteers [2, 16], in daily routine, the definition of correct therapeutic approach requires identification of pSS patients among subjects already showing xerostomia. Our work points out the SGdS key role in a group of patients affected by xerostomia.
As already stated, our data do not show any difference between the salivary gland on the two sides. At univariate analysis, each assessed semi-quantitative value (both parotid and submandibular UP and EF) showed to be significantly lower in patients with pSS: these results confirm other previous findings [1, 6, 7, 9, 12, 13].
Furthermore, multivariate analysis showed that only parotid UP and submandibular EF were independently correlated with pSS diagnosis. In fact, our results, in keeping with other papers [12, 16], stress the essential role of excretion fraction, in particular of submandibular glands, in order to detect pSS in its earliest phases, reflecting the particular and prior impairment of these glands. Then, our study showed also the impairment of parotid glands in patients affected by pSS; in particular, quantifying by their uptake, the reduction of these glands is noteworthy, consequently to the parenchyma destruction caused by the lymphocytic infiltration.
Moreover, a nomogram was built to define disease probability (Fig. 2). To calculate pSS probability on this nomogram, the reader should locate the patient value of p.UP to the corresponding axis; from this value, should draw a line straight upward to the top point axis. Then, note the value. Repeat the process for s.EF. Sum both values and locate the final sum on the total point axis. Finally, the reader should draw a line straight down on the bottom axis to find pSS probability.
To date, no nomogram or other statistical instruments of integrated probability have been suggested in order to predict pSS, except a hint in a recent work of Zou [2]. A nomogram is a simple and helpful tool which allows clinicians to define the disease probability. Therefore, it can be helpful in decision-making process.
Lack of standardization among nuclear medicine centres caused concerns about the utility of SGdS.
Standardization of procedures, according to Anjos et al. (measure of injected activity, background subtraction), allows a reproducible estimation of salivary glands UP and EF and allows a comparison among centres as well as longitudinal evaluations of disease [17].
Moreover, the standardization of procedures ensures intra-operator reproducibility. In our settings, results from the three operators showed an excellent agreement despite the operators’ different experience, likely because the learning curve for semi-quantitative analysis is faster than the know-how acquisition for qualitative evaluation.
In our opinion, the lack of correlation between SGdS data and ESSDAI reveals that accuracy of SGdS in predicting pSS is independent from the disease severity, even though we cannot exclude the impact of underpowered statistics.
Limitations of this study were its retrospective nature, the small number of patients and the absence of quantitative evaluation on healthy control group.
Conclusion
The optimal reproducibility of semi-quantitative evaluation and the accuracy of each gland parameter make SGdS an instrument of primary importance in pSS diagnosis. In addition, its excellent accuracy seems to be unaffected by the disease severity. The proposed nomogram shows to be effective in defining the disease probability; its results can be reported by nuclear medicine physicians. Furthermore, SGdS is not a very expensive exam; it delivers low radiation exposure to patients and is quite widespread on both high- and low-income countries.
Therefore, SGdS in sicca syndrome should be always carried out, and, even better, in our opinion, it should be a first-line instrumental investigation.
References
Vinagre F, Santos AI, Santos MJ et al (2008) Salivary gland scintigraphy in the evaluation of patients with sicca complaints. Acta Reumatol Port 33:422–428
Zou Q, Jiao J, Zou M-H et al (2012) Semi-quantitative evaluation of salivary gland function in Sjögren’s syndrome using salivary gland scintigraphy. Clin Rheumatol 31:1699–1705
Klutmann S, Bohuslavizki KH, Kröger S et al (1999) Quantitative salivary gland scintigraphy. J Nucl Med Technol 27:20–26
Ramos-Casals M, Brito-Zerón P, Perez-De-Lis M et al (2010) Clinical and prognostic significance of parotid scintigraphy in 405 patients with primary Sjögren’s syndrome. J Rheumatol 37:585–590
Vitali C, Bombardieri S, Jonsson R et al (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558
Henriksen AM, Nossent HC (2007) Quantitative salivary gland scintigraphy can distinguish patients with primary Sjögren’s syndrome during the evaluation of sicca symptoms. Clin Rheumatol 26:1837–1841
Shizukuishi K, Nagaoka S, Kinno Y et al (2003) Scoring analysis of salivary gland scintigraphy in patients with Sjögren’s syndrome. Ann Nucl Med 17:627–631
Adams BK, Al Attia HM, Parkar S (2003) Salivary gland scintigraphy in Sjögren’s syndrome: are quantitative indices the answer? Nucl Med Commun 24:1011–1016
Aung W, Murata Y, Ishida R et al (2001) Study of quantitative oral radioactivity in salivary gland scintigraphy and determination of the clinical stage of Sjögren’s syndrome. J Nucl Med 42:38–43
Vivino FB, Hermann GA (2008) Role of nuclear scintigraphy in the characterization and management of the salivary component of Sjögren’s syndrome. Rheum Dis Clin N Am 34:973–986
Seror R, Ravaud P, Bowman SJ et al (2010) EULAR Sjögren’s syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjögren’s syndrome. Ann Rheum Dis 69:1103–1109
Loutfi I, Nair MK, Ebrahim AK (2003) Salivary gland scintigraphy: the use of semiquantitative analysis for uptake and clearance. J Nucl Med Technol 31:81–85
Umehara I, Yamada I, Murata Y et al (1999) Quantitative evaluation of salivary gland scintigraphy in Sjorgen’s syndrome. J Nucl Med 40:64–69
Parrago J, Rain J, Brocheriou C et al (1987) Scintigraphy of the salivary glands in Sjögen’s syndrome. J Clin Pathol 40:1463–1467
Kim HA, Yoon SH, Yoon JK et al (2014) Salivary gland scintigraphy in Sjögren’s syndrome. Comparison of the diagnostic performance of visual and semiquantitative analysis. Nuklearmedizin 53:139–145
Güne S, Yilmaz S, Karalezli A et al (2010) Quantitative and visual evaluation of salivary and thyroid glands in patients with primary Sjögren’s syndrome using salivary gland scintigraphy: relationship with clinicopathological features of salivary, lacrimal and thyroid glands. Nucl Med Commun 31:666–672
Anjos DA, Etchebehere EC, Santos AO, et al. (2006) Normal values of [99mTc]pertechnetate uptake and excretion fraction by major salivary glands. Nucl Med Commun 27:395–403.ss
Acknowledgements
The authors would like to thank Prof Alessandro Giordano for his helpful suggestions and the Nuclear Medicine staff for their support as well as Eda Koxhaku for the English editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Financial funding was not required nor obtained: this research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Rights and permissions
About this article
Cite this article
Angusti, T., Pilati, E., Parente, A. et al. Semi-quantitative analysis of salivary gland scintigraphy in Sjögren’s syndrome diagnosis: a first-line tool. Clin Oral Invest 21, 2389–2395 (2017). https://doi.org/10.1007/s00784-016-2034-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-2034-6