Abstract
Multiple sclerosis (MS) is an autoimmune-mediated disease of the central nervous system characterized by inflammation, demyelination and chronic progressive neurodegeneration. Among its broad and unpredictable range of clinical symptoms, cognitive impairment (CI) is a common and disabling feature greatly affecting the patients’ quality of life. Its prevalence is 20% up to 88% with a wide variety depending on the phenotype of MS, with highest frequency and severity in primary progressive MS. Involving different cognitive domains, CI is often associated with depression and other neuropsychiatric symptoms, but usually not correlated with motor and other deficits, suggesting different pathophysiological mechanisms. While no specific neuropathological data for CI in MS are available, modern research has provided evidence that it arises from the disease-specific brain alterations. Multimodal neuroimaging, besides structural changes of cortical and deep subcortical gray and white matter, exhibited dysfunction of fronto-parietal, thalamo-hippocampal, default mode and cognition-related networks, disruption of inter-network connections and involvement of the γ-aminobutyric acid (GABA) system. This provided a conceptual framework to explain how aberrant pathophysiological processes, including oxidative stress, mitochondrial dysfunction, autoimmune reactions and disruption of essential signaling pathways predict/cause specific disorders of cognition. CI in MS is related to multi-regional patterns of cerebral disturbances, although its complex pathogenic mechanisms await further elucidation. This article, based on systematic analysis of PubMed, Google Scholar and Cochrane Library, reviews current epidemiological, clinical, neuroimaging and pathogenetic evidence that could aid early identification of CI in MS and inform about new therapeutic targets and strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating process of the central nervous system, characterized by focal lesions of the gray and white matter progressing to diffuse neurodegeneration. This heterogenous and multifactorial disease, immuno-mediated by activated T-cells with a significant contribution from B-cells is due to a complex interaction of genetic predisposition and environmental factors. MS affects more than 2.5 million people worldwide, involving all ages, but its prevalence is rising due to earlier diagnosis and prolonged survival of the patients, but also true increase in its incidence. According to its natural course, the MS spectrum includes the relapsing-remitting (RRMS/ 70 to 80%), primary progressive (PPMS/15–20%), secondary progressive (SPMS) and progressive-relapsing subtype (PRMS/about 5%), which show different clinical course and neuropathological characteristics (Filippi et al. 2018; Lassmann 2018). Further categories include clinically isolated syndrome (CIS), fulminant subtype with multiple relapses and rapid progression, and benign form with mild disability (Tafti et al. 2024).
Cognitive impairment (CI) as one of the most important determinants of the quality of life (QoL) of MS patients is well established (Bergmann et al. 2023; Nabizadeh et al. 2022). It is observed in all MS phenotypes, with varying degrees (Johnen et al. 2017; Langdon 2011; Piacentini et al. 2023). Cognitive problems that can be present in MS years prior to first apparent progressive symptoms (Charest et al. 2020; Cortese et al. 2016), show a complex spectrum ranging from subtle cognitive decline and mild CI (MCI) to full-blown dementia. A progressively increasing worsening of the overall CI is observed from CIS, moving towards RRMS, PPMS and SPMS (Lugosi et al. 2024). CI involves different cognitive domains, in particular processing speed, executive function (EF) and episodic memory (Benedict et al. 2020; Johnen et al. 2019; Wojcik et al. 2022). Global cognition and memory deteriorate 5 years after disease onset, declining steadily over the next 25 years or later (Lopez-Soley et al. 2021), predicting poorer prognosis and mortality (Cavaco et al. 2022). CI is often associated with depression, fatigue and other neuropsychiatric symptoms, but usually not correlated with motor and other deficits, suggesting different pathophysiological mechanisms. A recent meta-analysis evaluated the relationship between depression and CI in MS (Altieri et al. 2024). Life with either MS and/or CI (and depression) can be challenging, but dealing with both conditions that share various pathogenic and functional mechanisms can be even more so, although both are treatable. A new taxonomy of cognitive phenotypes in MS clarified the type and distribution of cognitive changes (Hancock et al. 2023), paving the way for further investigation of novel fluid and imaging biomarkers, appears promising in facilitating the diagnosis and staging of CI in MS. Stabilization and amelioration of cognitive outcomes is a goal of many ongoing clinical trials for disease-modifying treatment options. This article is based on systematic analysis using PubMed, Google Scholar and Cochrane Library between 2000 and March 2024. Among more than 600 articles, those were selected that met the inclusion criteria of the following sections of this article which will review the connections between MS and cognitive decline, epidemiology of CI in MS and its subtypes, related symptoms and risk factors, available neuroimaging data with focus on the different structural and functional brain lesions, pathomechanisms of CI in MS, cognitive reserve and current treatment options.
Epidemiology of CI in MS
CI is a frequent symptom of MS. Depending on the study cohort, up to 88% of individuals with MS are affected by CI (Drew et al. 2008), with a wide variety, depending on the phenotype of MS, disease severity (Expanded Disability Stats Scale/EDSS score), duration of disease, sex, age at onset, demographic features and risk factors. The overall prevalence of CI in MS at baseline ranges from 11.6% in very early MS (Nourbakhsh et al. 2016) or 22/23% (Broeders et al. 2022; Huiskamp et al. 2023; Johnen et al. 2019; Wilcox et al. 2023; Zhang et al. 2021) to 56% (London et al. 2023), according to others from 43 to 63% (Bobholz and Rao 2003; Caceres et al. 2011; Planche et al. 2016), or between 22% and 70% (Raimo et al. 2023), but there is a considerable variation even among study groups. The average (pooled) prevalence of CI in MS ranges from 30% to about 40% (Amato et al. 2019; Ayromlou et al. 2023; Conti et al. 2021; Damjanovic et al. 2017; Lorefice et al. 2020; Preziosa et al. 2023; Wilcox et al. 2023). An Italian long-term study reported an incidence rate of CI of 96.6/1000 person-year (Patti et al. 2015). In a long-term MS study at Brigham and Women’s Hospital, Boston, 65% had CI (1.5 SD below mean) in ≥ one domain and 75% affecting memory, attention/EF and processing speed, while 78% had CI (3 SD below mean) (Zurawski et al. 2020). A multicenter study from Latin America reported 34% prevalence of CI and 20.9% with significant neuropsychiatric symptoms (Caceres et al. 2014).
The prevalence for pediatric onset MS ranges from 14.1% (Wallach et al. 2020) to 44% (Ruano et al. 2018) and 47.5% (Öztürk et al. 2020), for early onset MS 40–61% (Lanzillo et al. 2016) and 48% for adult onset MS (Ruano et al. 2018), while a systemic review reported a prevalence rate from 0 to 68% for early onset and 4–81% for late onset MS (Fischer et al. 2014). Differences were reported between “benign"and “non-benign” MS (38% vs. 66%) (Bogaardt et al. 2023).
Values varied considerably between different cognitive domains of CI: MCI single-domain 15 to 21.6% and MCI multi-domain 9.19% (De Meo et al. 2021; Mistri et al. 2023b). In a large cohort, at -1.0 SD threshold, 21.6% had single domain, 14.3% bi-domain, and 15.4% multi-domain CI; at -1.5 SD threshold, 14% had single domain, 8.2% bi-domain, and 5% multi-domain impairment (Hancock et al. 2023). In a prospective multicenter study, at baseline, 22% had CI in ≥ 2 cognitive domains with highest frequency in EF and processing speed. At one-year follow-up, only 14% showed CI suggesting effects of retesting (Johnen et al. 2019).
In another multicenter study, 19.5% had mild, and 17.5% severe multi-domain involvement (De Meo et al. 2021), while a recent study reported 31% isolated and 43% multi-domain CI at baseline; at 5 years follow-up, it was present in 69% of MS patients with isolated CI (Bouman et al. 2024). In a small group of RRMS patients, 61.9% were impaired in at least one working memory phenotype (Clough et al. 2022).
Further differences were seen between men and women - CI was more frequent in men (70.1%) than in women (52%); and according to educational level and EDSS score - CI in women with university degree was significantly less common (39.4%) than in those without (66.7%) (Sandi et al. 2017). In another study, 38% females and 42% males were cognitively impaired (Tedone et al. 2023).
There is a remarkably high frequency of CI in older patients with MS (prevalence 77.4% with mean age 59.7 years vs. 42.8% in younger age). The similar profile of CI between older and younger patients suggests that CI is mostly directly related to MS itself and not to comorbid age-related disorders (Branco et al. 2019).
Clinical features and course of CI in MS
General items
Cognitive changes in MS manifest early and can be at least subclinically present years prior to first apparent organic symptoms (Charest et al. 2020). Their onset is insidious and the evolution slowly progressive from CIS (clinically and/or radiologically isolated symptoms), which may show no differences in verbal episodic memory (or other cognitive domains) compared to healthy controls (HCs), although a significant proportion of patients already shows cognitive decline (Lebrun 2015), with impaired mental processing speed being prevalent (Khalil et al. 2011). The CIS group has lower cognitive performance in verbal and nonverbal memory, information processing speed/attention/working memory, executive and visuo-spatial functions compared to HCs (p≤0.04). According to others, the frequency of CI increases dramatically during the first 5 years following a CIS (Reuter et al. 2011). Patients in the inflammatory stage (RRMS) show a previously learned information deficit; memory deficit being one of the most common and severe lesions (Gich et al. 2024). CIS and early stages of MS often show only subtle cognitive decline without manifest diminished scores of essential cognitive domains that may progress to MCI. According to current clinical criteria, it includes amnestic and non-amnestic types (Petersen et al. 2009), which may involve single or multiple cognitive domains.The latter are involved in 5–20% (Hancock et al. 2023). Over 40% of patients with early stages of RRMS experienced delays in cognitive processing that may affect their decision-making ability (Saposnik et al. 2022). However, a number of patients may exhibit predominant cognitive deficits, despite minimal physical disability (Staff et al. 2009).
Pediatric MS patients usually do not differ from healthy pediatric controls on cognitive screens, based on the Brief International Cognitive Assessment for MS (BICAMS) and other brief cognitive assessment tests, but perform better than adults with MS (Krupp et al. 2023), while others reported a faster decline of Symbol Digit Modalities Test (SDMT) scores than adult onset MS (AOMS), and elevated odds of CI in patients with pediatric onset MS (POMS) (McKay et al. 2019). In a German validation study, the modified BICAMS-M turned out to reliably detect cognitive problems in MS patients and to monitor them over time; the SDMT revealed the best predictive value for working ability (Filser et al. 2018). In addition to attention, processing speed and visuospatial skills, the most affected domains in adults, language and intelligence, are also affected in POMS (Ekmekci 2017). Involvement of the cognitive domains of EF, which includes attention and working memory, is most prominent in early MS (Johnen et al. 2019). Juvenile MS presents with a varying spectrum of symptoms, e.g., coordination difficulties and permanent cognitive dysfunction (Prajjwal et al. 2023).
Patients with early RRMS vs. HCs show lower scores in attention/orientation, verbal fluency, working memory, language, visuospatial and general cognitive functions, lowest in memory and fluency, highest in the visuospatial domain (Basci and Tulek 2023). Analysis of visuo-cognitive phenotypes in early MS revealed three oculo-motor constructs (cognitive control, cognitive processing speed and basic visual processing) and four visuo-cognitive phenotypes (early visual changes, efferent-cognitive, cognitive control and afferent-processing speed). This suggests that distinct visual processing deficits in early MS may differently impact cognition (Vagias et al. 2024). The most frequent forms of isolated CI are impaired EF/working memory, followed by processing speed, attention and semantic and episodic memory (Bouman et al. 2024; Gois et al. 2021; Johnen et al. 2019; Kopchak et al. 2021), while procedural and implicit memory appear less affected (Henry and Beatty 2006). Disruptions to working memory serve as one of the mechanisms underpinning disturbances to social cognition in MS (Pennington et al. 2024). According to others, most frequently impaired are EF, inhibition, fluency and working memory (Drew et al. 2008), or visual, spatial and auditory working memory (Clough et al. 2022). The lowest rate of impairment was seen in verbal function domains, highest in the domain of information processing (Bogaardt et al. 2023), while the order of CI was processing speed, visual learning, working memory/attention and EF, the staging of CI predicting neurological disability (Wojcik et al. 2022). Auditory and visual attention and inhibitory control are also significantly impaired (Simani et al. 2022). A systemic analysis showed a variable affection of cognitive domains: visuospatial memory (54–56%), processing speed (27–51%), verbal/episodic memory (29.34%), visuospatial workup (22%), EF (15–18%), and social cognition (frequent) (Cotter et al. 2016). Another study distinguished four cognitive phenotype groups: not impaired (56.3%), processing speed (7.8%), memory (17.2%), processing speed and memory impaired (17.2%) (Leavitt et al. 2018). A latent class analysis of 872 people with MS identified four cognitive phenotypes: (1) only memory difficulties (28.3%); (2) minor memory and language deficits (21.2%); (3) moderate memory, language and attention impairments (18.8%); and (4) severe memory, language, attention, information processing and executive functions difficulties (31.7%). Since less is known about the progressive deterioration of cognition in MS, a taxonomy of distinct subtypes that considers different clustered domains represents a challenge concerning patient-centered outcomes (Podda et al. 2021).
Development of CI
The dynamics and predictors of CI along the disease course in MS are highly variable and, at least in part, poorly understood. The further worsening of cognition depends on the course and phenotype of the disease as well as on the degree of structural and functional brain changes. Longitudinal studies reported an increase of CI prevalence from 29% at baseline to 54% after 5 years (Reuter et al. 2011), or from 19 to 44% (Wilcox et al. 2023). Others reported a threefold increased risk for CI for patients with an EDSS score > 3 (Patti et al. 2015), and 84.2% CI in patients with EDSS score > 5 (Sabanagic-Hajric et al. 2023) or an increase of CI to 88.2% at an average disease duration of 11.8 years (Drew et al. 2008). Conversely, a reduction from 44% CI at baseline to 37.1% after 4 years (Patti et al. 2015) or from baseline 56–38% after 12 months were observed (London et al. 2023). Machine learning techniques used to identify dynamics of cognition detected cognitive deterioration 5 years after disease onset with increases in the scores of global cognition, verbal memory and information processing speed, followed by a decline in global cognition and memory between 5 and 15 years (Lopez-Soley et al. 2021). In 62.2% of persons with MS, cognition deteriorated within 6 years (Damasceno et al. 2020). From 15 to 30 years of disease onset, cognitive decline progressed, more markedly affecting verbal memory and global cognition (Lopez-Soley et al. 2021). Longer disease duration is significantly correlated with worse overall cognitive function, EF and language domain (Sabanagic-Hajric et al. 2023). Subsequently, MS patients face difficulties in everyday activities, particularly when the involved tasks demand a higher load of cognitive effort (Bonnet et al. 2010). Thus, attention deficits are more obvious when multiple tasks are performed simultaneously, the patients being easily distracted (Randolph et al. 2017).
Objective cognitive screening regardless of patients’ or examiners’ perception has been recommended, but the interpretation of a patient’s cognitive changes depends on the methods used (Henry et al. 2023; Jackson et al. 2023). For the frequently used instruments for assessment of cognitive function see Supplementary Table S1. According to a systematic review, the SDMT was the most frequently used test battery (Ezegbe et al. 2023), although some limitations to cognitive evaluation include shortage of time and resources, reduced availability and others (Meca-Lallana et al. 2021). There is preliminary evidence that Adaptive Cognitive Evaluation (ACE), a table-based cognitive assessment battery, can potentially serve as a digital assessment for CI in people with MS (Hsu et al. 2021). According to others, the Attention Network Test-Interaction (ANT-I) and the Test of Everyday Cognitive Ability (TECA) were the most sensitive and specific markers of CI in MS (Eilam-Stock et al. 2021), or the Delis Kaplan Executive Functioning System-Sorting Test and the Stroop Test were the most sensitive tests for differentiating CI between MS and HCs (Raimo et al. 2023; Scarpina and Tagini 2017), whereas the SDMT and the Paced Auditory Serial Addition Test (PASAT) scores do not accurately reflect the cognitive decline in people with RRMS (Castrogiovanni et al. 2023).
Comparison of different methods for assessing cognitive functions that are frequently affected in this disease recommended two standardized regression-based methods and the generalized regression-based method (Henry et al. 2023). However, a recent review emphasized the slow rate of measured changes in cognition in persons with MS and the lack of a gold standard test and consistency in measuring cognitive changes at the population level. It asked for more sensitive testing and follow-up of MS subgroups where cognitive changes follow different trajectories (Ezegbe et al. 2023). Guidelines for monitoring of CI in persons with MS see Fig. 1.
Proposed algorithm for the monitoring of cognitive dysfunction in MS. (modified from Bakirtzis et al. 2018). BICAMS: Brief International Cognitive Assessment for Multiple Sclerosis, MSNQ-I: Multiple Sclerosis Neuropsychological Questionnaire Informant version, SDMT: Symbol Digit Modalities Test, MFIS: Modified Fatigue Impact Scale, FSS: Fatigue Severity Scale, BDI-II: Beck Depression Inventory-II
CI in MS subtypes
Cognitive dysfunctions differ considerably between the subtypes of MS (Table 1). Relapsing-remitting MS (RRMS) patients show predominantly learned information retrieval deficits, while patients in secondary progressive stages do not even correctly appreciate information (Gich et al. 2024). Of note, cognitive problems can be present in both RRMS and primary progressive MS (PPMS) already before apparent organic symptoms; PPMS patients score significantly lower than HCs up to 20 years prior to first progressive symptoms (Cortese et al. 2016). Severe cognitive phenotypes prevail in PPMS, including executive/attention and multidimensional phenotypes (De Meo et al. 2021), PPMS patients displaying more severe decrease in CI than RRMS ones (Johnen et al. 2017). CI was overall higher in PPMS and SPMS than in RRMS (Renner et al. 2020). PPMS patients presented a wide range of deficits in information processing speed, attention, working memory, EF, and verbal episodic memory, whereas the impairments in those with RRMS were limited to information processing speed, and working memory (Ruet et al. 2013). SPMS compared to RRMS are associated with increased frequency in episodic verbal memory, information processing speed, verbal fluency, and working memory (Brochet et al. 2022), working memory accuracy being more impaired in SPMS (Pourmohammadi et al. 2023). These patients were at least two-fold more frequently impaired than late RRMS ones in information processing speed, EF, verbal fluency, verbal episodic memory and visuospatial construction. Numbers of patients with at least one or two deficient cognitive domains were higher in the SPMS group than in patients with late RRMS. Moreover, PPMS patients were more frequently impaired in verbal fluency than late RRMS ones and more often presented at least one impaired cognitive domain (Gois et al. 2021), while both PPMS and SPMS groups differed only for visuospatial construction (p = 0.02) (Planche et al. 2016).
Furthermore, there is an association between the duration of the disease and the frequency and extent of cognitive disorders. SPMS is associated with an increased frequency and severity of CI as compared to RRMS, progressive forms of MS being associated with more severe impairment in certain cognitive areas, such as episodic verbal memory, information processing speed, working memory or verbal fluency, cognitive performance declining over time in SPMS (Brochet et al. 2022). Others, however, stated that PPMS and SPMS subtypes have similar cognitive performance (Mistri et al. 2023b).
Cognition and disease characteristics are also different between adult onset (AO) versus late onset (LO) MS: LOMS patients had significantly shorter disease duration and shorter time to diagnosis. They had similar EDSS scores as AOMS ones but demonstrated significantly more impairment on tasks of visual learning, memory and working memory than AOMS ones, even after accounting for differences in disease duration and more frequent cardiac risk factors (Butler Pagnotti et al. 2022).
Cognitive relapses
Although CI usually develops insidiously and progresses gradually, it can decline abruptly during relapses, although this rapid decline has been documented only in the past few years (Benedict et al. 2014). The presence of such a clinically meaningful event is substantiated by a decline in neuropsychological testing, observed or reported cognitive change, and a subset of patients presenting a gadolinium-enhancing MRI lesion (Meli et al. 2020). The relevance of isolated cognitive relapses (ICRs), i.e., those occurring in the absence of new sensorimotor symptoms, remains poorly characterized. They are not associated with changes in mood, fatigue levels or cognitive performance self-evaluation. ICRs can be associated with a significantly reduced cognitive performance at the follow-up evaluation (Pardini et al. 2014), but improvement follows relapse (Benedict and Zivadinov 2011), although recovery is incomplete (Benedict et al. 2021). In a recent 3-year prospective cohort study, 63.6.% met ICR criteria, while 28.4% were classified as “relapse with cognitive decline” (Morrow et al. 2023). Such short-lasting cognitive declines should be clarified by neuropsychological tests, and other causes, like depression, fatigue or changes in living situations, should be excluded.
In conclusion, clinical features of CI that manifest early in MS are highly variable, involving several cognitive domains, and increasing with progression of the disease. Their type and severity differ between pediatric and adult onset MS and between its various phenotypes, PPMS showing more frequent, more severe and progressive decrease of CI than RRMS. Cognitive relapses show short-lasting rapid decline of neurocognitive functions in the absence of new organic symptoms, usually followed by improvement of cognitive functions.
Risk factors of CI in MS
Major risk factors for CI on MS are genetic susceptibility, older age at disease onset, higher EDSS scores, lower physical activity in childhood, lower premorbid IQ, lower educational level and comorbid neuropsychiatric illness (Amato et al. 2019; Benedict and Zivadinov 2011; Bilgin et al. 2023a, b). Over 100 common genetic risk variants have been identified, but most carriers do not develop MS. Increased genetic susceptibility to MS may affect brain structures with smaller gray matter (GM) volume over the whole brain and cognition without manifest disease, pointing to a “hidden burden” of MS (Ikram et al. 2017).
As for risk scores, CI was related with lower physical activity in childhood/adolescence but the only significant predictors of CI were older age and lower premorbid IQ (Amato et al. 2019). Furthermore, physical disabilty, smoking and depression negatively impacted cognitive performance, whereas presence of higher education had a favorable influence on cognition in MS patients (Kopchak et al. 2021). The role of the APOE genotype for CI in MS is a matter of discussion. Older studies stated that its presence was associated with neither CI nor impairment in neuropsychological tests (Portaccio et al. 2009), and most of the reports did not find a significant association between APOE genostatus and cognitive outcomes in patients with MS. A meta-analysis stated that APOE ε4 may have a domain-specific association with CI in MS patients who had more delayed responses to stimuli, while the rate of impaired visuospatial perception was lower in these patients (Naseri et al. 2022). Others found that APOE ε4-positive MS patients are most likely to have at least one impaired cognitive test (Mashayekhi et al. 2022). Another review stated that APOE ε4 patients had more delayed responses to stimuli, while the rate of impaired visuospatial perception was lower in these patients. Based on the current evidence, there is doubt about the clinical significance of the association of APOE ε4 genotype and cognitive outcomes in MS (Naseri et al. 2022).
Lifestyle and cardiovascular risk are associated with verbal learning dysfunctions (Reia et al. 2021); dyslipidemia, triglyceride and cholesterol are negatively correlated with the cognitive functioning score in MS patients (Andaloro et al. 2022). Available evidence suggests a link between increased serum LDL cholesterol and total cholesterol and cognitive dsfunction in MS (Hernández-Ledesma et al. 2020; Noori et al. 2019; Sanaie et al. 2024). Moreover, impaired glucose and insulin metabolism can exacerbate cognitive decline. Greater verbal memory and spatial comprehension were observed in MS patients with insulin resistance (Ayromlou et al. 2023). Lower serum 25-hydroxy-vitamin D levels are also associated with worse cognitive function in MS (Spiezia et al. 2023). Cognitive dysfunction in pediatric MS has been related to glymphatic system dysfunction associated with brain focal lesions (Margoni et al. 2024).
The presence of cardiovascular comorbidities and especially dyslipidemia was shown to increase the rate of cognitive decline in MS patients (Giannopapas et al. 2023). On the other hand, orthostatic hypotension prevalence is also correlated to the severity of CI, particularly with a lower performance rate of EF (Bocti et al. 2017). Among older veterans with MS, the risk of CI progressing to dementia was higher compared to matched controls even after controlling for comorbidities, which should be considered in caring for older MS patients (Fleming et al. 2024). The cognitive profiles of MS and Alzheimer disease (AD) are distinct. In contrast to AD, MS is associated with impairment of memory consolidation, although there may be some overlap between cognitive deficits related to MS and amnestic MCI with decreased semantic fluency. Anyway, specific biomarkers should be used to confirm or rule out AD pathology (Roy et al. 2018).
CI in MS and associated symptoms
MS patients often experience a typical cluster of symptoms in association with CI, such as depression (35–50%), anxiety (34–57%) and fatigue (70–75%). Neurocognitive disorders, depression and anxiety are common in MS, with substantial impact on patients’ QoL (Benedict et al. 2020; Kalb et al. 2018; NICE-Guideline 2022). Such manifestation may occur in the earliest phases of the disease and become more frequent with disease progression and more severe disability (Margoni et al. 2023). CI in MS is occasionally associated with depression (Golan et al. 2018; Wallis et al. 2020), while others found no relations between cognitive alterations and depression (Chang et al. 2023a, b; Ruiz-Sánchez et al. 2022; Siegert and Abernethy 2005; Yigit et al. 2021). This seems rather surprising given the mounting evidence for the neuropsychological deficits accompanying depression (Shenal et al. 2003). Depression mediates effects of both perceived and objective cognitive functioning (Crouch et al. 2022). A recent meta-analysis found a significant relationship between depression and global cognition, attention, processing speed (p < 0.01) and working memory (p = 0.037), suggesting that patients with MS and higher levels of depressive symptomatology may have more difficulties in several aspects of cognition, especially those needed to retain, respond and progress information and for adequate stimulation in processing relevant information (Altieri et al. 2024).
CI, depression and fatigue are common among people with MS, amounting to 78% and 55%, respectively (Bogaardt et al. 2023). Of note, persons with depression and fatigue increasingly complain about cognitive deficits which often cannot be confirmed by objective tests. Others found that depressive symptoms and disability explained 12-23.7% of cognitive performance (Artemiadis et al. 2020). Alongside the well known defects in information processing speed, EF and episodic memory, recent evidence also highlighted socio-cognitive impairment in MS, such as emotion-recognition deficits, which may result from more fundamental cognitive dysfunction (Degraeve et al. 2024). Disruption of working memory appears to serve as one of the mechanisms underpinning disturbances of social cognition (Pennington et al. 2024). There is a close relationship of motor impairment with cognitive and emotional alterations. While anxiety is related to walking resistance, none was found between depression, CI and balance or walking ability (Cuerda-Ballester et al. 2023).
On the other hand, CI is significantly associated with motor incoordination, balance impairment, gait abnormality, increased fall risk and upper extremity incoordination (Alharthi and Almurdi 2023) as well as with gait speed; an increase in stance phase may imply that detection of a decrease in gait speed and an increase in stance phase time can predict progression of CI (Chang et al. 2023a). Patients with CI have a higher falling rate and, therefore should be closely monitored for the risk of falling (Bilgin et al. 2023a, b). All these factors associated with disease burden will ultimately influence QoL of patients with MS (Bergmann et al. 2023).
Restless legs syndrome, including worse symptoms at falling asleep and during the day while active, might be concomitant with worse cognitive function, particularly processing speed and memory (Cederberg et al. 2022).
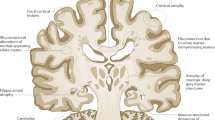
Neuroimaging data
Gray matter lesions / general
The CIS group with lower cognitive performance in verbal and nonverbal memory, information processing speed/attention/EF and visuo-spatial functions compared to HC that were present in 18–37% of patients depending on the criteria used, showed reduced brain atrophy predominantly in fronto-temporal regions and thalamus. Cognitive performance was not associated with structural brain changes except for the association between worse visuo-spatial performance and lower white matter (WM) volume. Lower cognitive performance may be present in up to one-third of patients with CIS, but unlike those with MS, variability in their degree of cognitive performance may lead to a lack of consistent association with structural brain changes (Hyncicová et al. 2017).
Markers of cerebral atrophy have emerged as more important correlates of cognitive decline than lesion volume (Bermel and Bakshi 2006). Verbal memory score correlated to regional cortical thinning in the insula, whereas verbal memory scores correlated to parietal thinning (Tillema et al. 2016). Comparison of the cognitive status in single-patient evaluation showed that it accurately reflected the measures of brain atrophy with a sensitivity up to 90%. CI patients showed lower volume of the entire brain, GM, limbic lobe, insula and cerebellum, compared to cognitively normal patients (Ziccardi et al. 2022). A longitudinal study found strong correlations between the BICAMS subtests and whole brain and GM volumes as well as significant changes in the global brain volumes from baseline to follow-up, with clear differences between patients defined as cognitively impaired at both baseline and follow-up (Skorve et al. 2023). Multiple regression analysis found a significant association of severity of CI with lower volumes of cortex, thalamus, putamen, pallidum and hippocampus, indicating that decreased volumes of several subcortical GM structures, in particular of thalamus, contribute to cognitive dysfunction in MS, mainly influencing EF (Lorefice et al. 2020). In particular, atrophy of the putamen could contribute to impaired cognitive speed in MS, especially in later disease stages after a transition to SPMS (Akaishi et al. 2024). Compared with whole brain GM, deep GM lesion volumes and some local components correlated more closely with clinical outcomes (Colato et al. 2021). Volumes of total brain, cortex and deep GM derived from 3D-FLAIR and 3D-T1 images showed close relationship with cognitive dysfunction in MS (Noteboom et al. 2023). In older people with MS, in addition to global and thalamic degenerative changes, the presence of CI was explained by the thickness of multiple cortical regions (Jakimovski et al. 2023). A meta-analysis of T1- and T2-weighted lesions provided strong evidence of their correlation with cognitive function in MS patients (Nabizadeh et al. 2024). Both the thalamic volume, diffusion tensor imaging (DTI) measures and functional activity determine the degree of CI (Amin and Ontaneda 2021; Gouveia et al. 2017; Schoonheim et al. 2015). There is an independent association between thalamic volume and processing speed and memory performance (Wilcox et al. 2023), and global cognitive status correlated with atrophy of frontal-temporal-connected thalamic subregions (Bisecco et al. 2021).
Hippocampal and deep GM nuclei atrophy are key factors associated with CI in MS (Damjanovic et al. 2017). Hippocampal volume was related to fornix diffusion measures and to those of episodic/visual-spatial memory and information processing speed, highlighting the role of the hippocampus in cognitive dysfunction (Koenig et al. 2014). Hippocampal volume was lower in MS patients compared to HCs, and hippocampal activation that increased in cognitively preserved, decreased in CI ones (Hulst et al. 2015). Fornix, thalamus and hippocampus displayed atrophy and/or abnormal diffusion metrics, with the fornix showing the most severe changes. Lower fractional anisotropy (FA) and higher diffusivity in fornix, lower hippocampus FA and lower thalamus volume were strongly correlated with CI, giving evidence for microstructural abnormalities and extensive atrophy of the fornix and interconnected deep GM in MS with cognitive deficits (Valdés Cabrera et al. 2022). Isolated CI was explained by a model of cortical volume and FA, memory by a model with cortical and hippocampal volume, while EF/ working memory were not associated with any MRI measure (Bouman et al. 2024).
MRI studies are important in identifying the distinct cognitive phenotypes in MS. Severe cognitive phenotypes prevailed in patients with PPMS. Patients with mild verbal memory/semantic fluency exhibited decreased mean hippocampus volume; those with multi-domain phenotype had decreased mean cortical GM volume, those with severe executive/attention had higher T2-hyperintense lesion volume, and those with severe multi-domain phenotype had extensive brain damage, with decreased volume in all brain structures except the pallidum, caudate nucleus and amygdala (De Meo et al. 2021).
Volumetric analysis of the cerebellum and its lobules in MS showed a reduction of cerebellar GM and WM volumes, with widespread cortical thinning. These changes were negatively correlated with information processing speed and visuospatial memory. This inverse association may represent a compensatory mechanism activated in MS engaging additional high-level cortical areas interconnected with the damaged cerebellum, in order to cope with the cognitive demands (Iliadou et al. 2022). No significant differences in cognitive (or motor) symptoms emerged between MS patients showing dentate nuclei hyperintensity and those without hyperintensity. Gadolinium retention in the dentate nuclei was not associated with long-term cognitive (or motor) outcome (Scaravilli et al. 2023).
GM changes in MS phenotypes
Patients with RRMS and CI showed higher cortical lesions and decreased brain volume compared with cognitively unimpaired patients. Multivariate analysis revealed that age, cortical lesion volume and neocortical GM volume were independent predictors of CI index, indicating that the burden of cortical lesions and tissue loss are among the major structural changes associated with CI in RRMS (Calabrese et al. 2009). PPMS and SPMS have similar neuropsychological performance. Cognitive dysfunction in both phenotypes are related to distinct patterns of structural MS abnormalities and involvement of different WM tracts, whereas resting state functional connectivities did not contribute to explaining their global cognitive dysfunctioning (Mistri et al. 2023a). An earlier study showed no differences in deep GM atrophy between PPMS and RRMS, while faster cognitive decline was observed in PPMS compared to RRMS. These results indicate that cortical atrophy and cognitive decline accelerate together during the course of MS and that substrates of cognitive decline shifts from worsening pathology in stable RRMS to deep GM atrophy in converting RRMS and to accelerated cortical atrophy in PPMS (Eijlers et al. 2019a).
A recent study of a group of MS cases with predominance of RRMS, which were less often associated with CI, aimed to analyze the relationship between advanced MRI sequences and physical disability and cognitive functioning. It showed that GM volume was associated with age and physical disability, T2 lesion load with physical disability, but there was no significant correlation between CI and cerebral volume. PPMS, associated with greater risk of cognitive dysfunction, also showed no correlations between CI and cerebral volumes. These results that differ from other MRI studies could be due to the fact that in this group only 22% showed cognitive dysfunction (normally ranging from 40 to 60%) and predominance of RRMS, which is normally associated with less cognitive damage (Peño et al. 2023).
At one-year follow-up, decreased volumes of the left amygdala and right orbitofrontal cortex were identified as possible predictors of worse performance in verbal and visuo-spatial memory (Meira et al. 2023). A 6-year follow-up study of patients with RRMS showed positive correlation of semantic fluency and word comprehension tests with cortical and deep GM volumes, while higher EDSS on follow-up correlated negatively with baseline bilateral pallidum, right caudate and putamen and right accumbens volumes (Kania et al. 2024). A 5-year longitudinal study of multi-modal MRI neuroimaging signatures in a cohort of MS patients predicted cognitive decline with high accuracy and can be used in future trials to personalise the management of cognitive decline in MS (Al-Iedani et al. 2024). A 20-year study confirmed that CI patients showed higher focal cortical pathology at diagnosis compared to cognitively unimpaired subjects and that volumes of both cortical and WM lesions emerged as the metrics most associated with long-term CI. The number of cortical lesions (especially severe ones) was strongly associated with severe long-term CI (p < 0.001), more than the number of WM lesions. Since the latter had a low predictive value and a poor applicability of lesion volume estimation in the daily clinical context, the evaluation of number of cortical lesions was suggested to represent a reliable prognostic marker of CI (Ziccardi et al. 2023).
Recently, a radiomics model based on clinical data and cortical damages was developed that may have a great potential to identify efficiently the MS patients with CI for clinical cognitive assessment (Yan et al. 2024).
White matter lesions
CI in MS has been related to myelin loss, and different neuroimaging methods have been used to quantify myelin and to relate it to cognitive dysfunction; among them DTI, magnetization transfer ratio (MTR), and more recently, PET with 11C-PIB. MTR and DTI (FA) differed in patients with and without CI. DTI (FA) and DT (axial diffusion) were associated with condition and psychomotor speed for PPMS, and of 11C-PIB uptake and MTR for RRMS in the thalamus and corpus callosum, indicating that lower myelin content is associated with worse cognitive status (Campanholo et al. 2022).
Increased myelin heterogeneity in normal appearing WM was associated with decreased cognitive processing speed performance. These correlations were highly significant. Conversely, myelin heterogeneity was not associated with SDMT scores in controls in any regions of interest (Abel et al. 2020a, b). Radial diffusivity of WM was strongly related to CI, although its strong association with both cognition and whole brain lesion volume suggests that it is a surrogate marker for general decline rather than for specific cognitive functions in MS (Baijot et al. 2022). RRMS patients show abnormal diffusion kurtosis and susceptibility characteristics in the U-fiber region and these tissue abnormalities are correlated with cognitive deficits and underlying pathophysiological mechanisms (Luo et al. 2024). Higher FA values in various WM tracts, e.g., the left corticothalamic tract, left arcuate fascicle and superior cerebellar peduncle, contribute to CI in RRMS (Elkhooly et al. 2023).
Longitudinal fiber-specific WM damage was correlated with cognitive decline in MS. At baseline, all fiber-specific measures (fiber density, microstructural diffuse axonal damage, fiber cross section, etc.) were significantly worse in MS compared to HCs. For both fiber density and cross-section, a similar pattern was observed in SPMS followed by PPMS and RRMS. These lesions were more pronounced in severe CI. Microstructural damage was most pronounced in cingulum, macrostructural lesions in the corticospinal tract, cingulum and superior longitudinal fasciculus. Over time, WM alterations worsened in PPMS, WM atrophy progression mainly affecting the corticospinal tract and microstructural axonal damage worsening in cingulum and superior longitudinal fasciculus. Longitudinal deterioration of WM damage was most marked in PPMS, indicating that WM degeneration is important to characterize this phenotype (Koubiyr et al. 2024). Patients with SPMS had higher total, periventricular and non-periventricular WM lesion load than those with PPMS, as well as greater involvement of frontal, occipital horns, trigones, third ventricle, basal ganglia, parietal and occipital lobes (Comi et al. 1995).
A multiparametric study showed that the best predictors of CI were WM lesions in the right longitudinal fasciculus (100%), left anterior thalamic radiation (93%), left posterior corona radiata (78%), left medial lemniscus (74%), left inferior longitudinal fasciculus (70%), left optic radiation (69%), right middle cerebellar peduncle (60%), and right optic radiation (53%), decreased FA in splenium of the corpus callosum (64%), left optic radiation (53%), body of the corpus callosum (52%) and fornix (51%), but also atrophy of the left precuneus, right cerebellum, caudate nucleus, left thalamus and supplementary motor area (Conti et al. 2021). A 17-year longitudinal study confirmed the strong association of corpus callosum atrophy with CI in MS (Granberg et al. 2015), and a recent study discussed the model of a “callosal disconnection syndrome” and its possible contribution to social-cognitive dysfunctions in MS (Degraeve et al. 2023).
An important question is the association between myelin damage in tissue that appears completely normal on standard imaging, but can be detected by myelin water imaging with cognitive performance in MS. Myelin water was measured via the T2 relaxation signal in the cingulum, superior longitudinal fasciculus and corpus callosum, and significant associations were observed between water measures and neuropsychological test scores for all three regions, while no significant associations were found in controls. These findings suggest that myelin water measure is associated with cognitive performance in MS (Abel et al. 2020b).
Combined gray and white matter lesions
Structural MRI revealed higher WM lesion load in MS-CI compared to HCs and a more severe atrophy of GM regions connected to cognition (Louapre et al. 2014). In addition to GM atrophy of the parietal regions, left thalamus and right hippocampus, they showed atrophy of several WM tracts, mainly located in posterior brain and widespread WM diffusivity abnormalities. The latter changes in cognitive-relevant WM tracts followed by atrophy of cognitive-relevant GM regions explained global CI, while variable patterns of normal appearing white matter (NAWM) and GM damage were associated with deficits in selected cognitive domains (Preziosa et al. 2016). Lower WM volume, lower FA and higher diffusivity in both cortex and NAWM are seen in MS patients with CI; they also had higher T2 WM lesion volume, lower normalized brain and GM volume, and more severe diffusivity abnormalities in WM lesions, and NAWM, while cortical measures did not differ between CI and cognitive preserved patients. This indicated that “diffuse” GM and NAWM damage and WM lesions, rather than intrinsic cortical damage explained CI in MS (Preziosa et al. 2017). More recently, the same group showed that MS-CI patients had higher WM lesion volume, lower normalized brain, cortical and thalamic volume and WM volume, as well as lower NAWM FA, higher NAWM, normal appearing cortex and thalamic mean diffusivity (MD), lower NAWM intracellular volume fraction (ICV), lower WM orientation dispersion index (ODI), and higher NAWM ODI. The best predictors of CI were NAWM FA (100%), thalamic volume (96%), normalized brain volume (84.7%), thalamic MD (43%), normal appearing cortex volume (40.6%), WM lesion volume (23.2%) and WM lesion ODI (17.9%). These results suggested that neuro-axonal damage and loss of microarchitecture integrity in focal WM lesions and GM contribute to CI in MS (Preziosa et al. 2023).
Regional GM and WM damage associated with CI differed according to sex. In both sexes, a higher T2-hyperintense lesion prevalence in cognitively-relevant WM tracts was significantly correlated with worse cognitive performance, with stronger association in females than males in global cognition. In both sexes, worse cognitive performance was associated with widespread NAWM FA, with stronger associations in females in global cognition and verbal memory. Furthermore, a disconnection syndrome due to focal WM lesions and diffuse NAWM microstructural abnormalities seemed to be more relevant in female MS-CI patients (Tedone et al. 2023).
CI in benign MS is associated with WM damage (reduced FA) of parietal regions, GM atrophy and increased functional connectivity (FC) in fronto-temporo-parietal regions, T2 lesions of the corpus callosum, reduced posterior corona radiata, and caudate nucleus atrophy (Riccitelli et al. 2020).
Chronic inflammation in MS, associated with a more severe disease course, has been related to paramagnetic rim lesions (PRL) and choroid plexus (CP) enlargement. Compared to HCs, patients with CI showed significantly higher T2-hyperintense WM lesion volume, lower normalized brain, thalamic, hippocampal, caudate and cortical volumes, and higher normalized choroid plexus volume. PRLs and CP enlargement, therefore, may contribute to the pathophysiology of CI in MS (Preziosa et al. 2024).
MS-CI and cerebral blood flow
Cognitive dysfunction in MS may partially stem from inadequate cerebral blood flow (CBF), although the available results are controversial. Cerebral vasoreactivity was lower in patients with CI than in cognitively normal persons, indicating that CI in MS may be mediated through decreased cerebral vasoreactivity (Metzger et al. 2018). RRMS patients showed hypoperfusion of Brodman areas and lobes. Reduced CBF was seen mainly in the frontal lobe and related prefrontal areas in both hemispheres, but with left hemisphere predominance. Executive dysfunction was associated with robust CBF deficits in frontal and prefrontal cortex in RRMS patients (Messinis et al. 2019). RRMS patients showed reduced hemodynamic activation of the executive/attention network in the hippocampus, pallidum and anterior cingulate cortex (Wagner et al. 2022). Other studies suggested significant association between arterial stiffness and cognitive processing speed (Zheng et al. 2023), while other transcranial Doppler measures in patients with MS showed resilience to exercise-induced acute changes in pulsatility index of the middle cerebral artery (MCA) despite transient carotid stiffening, potentially via reductions in MCA conductance. This suggests that change in cognitive performance under aerobic exercise are not directly related to transient cerebrovascular responses (Lefferts et al. 2021). Association between lower cognitive performance and lower total CBF was observed in MS-CI patients (Jakimovski et al. 2020). They exhibited significant variability of the prefrontal cerebral metabolic rate of oxygen, which may be a sensitive measure of MS-related cognitive decline (Zuppichini et al. 2023), whereas there is no evidence of an association between the presence and severity of chronic cerebrospinal venous insufficiency with CI (and depression) (Benedict et al. 2013). On the other hand, recent studies suggested that intracerebral venous susceptibility may be an indicator of oxygen metabolism and cognitive function in RRMS (Sawan et al. 2024). FC abnormalities in brain networks in MS are accompanied by altered local CBF (Jandric et al. 2021).
Brain network disturbances
Previous studies have demonstrated extensive functional network disturbances in MS, showing less efficient brain networks. MS subjects with a more randomly organized GM network show worse cognitive functioning, suggesting that single-subject GM graphs may capture neuronal dysfunction (Rimkus et al. 2019). Recent studies indicated that the dynamic properties of the brain network show a strong correlation with cognitive function, and the relevance for CI in MS of structural and functional connectivities, reflecting common pathogenic mechanisms in WM and GM has been recently explored by novel MRI analysis methods (Zhang et al. 2021). In CI, both the default mode network (DMN) and frontoparietal network (FPN) showed increased FC with the rest of the brain compared to HCs, with no change within- or between-network connectivity (Meijer et al. 2017), whereas others found reduced resting state (RS) FC in sensorimotor, cognitive, thalamic and cerebellar networks correlated with more severe CI (Rocca et al. 2018). FC was significantly decreased in the anterior and posterior DMNs and increased in the bilateral FPNs (Jandric et al. 2021). A systemic review in RS fMRI connectivity changes found patterns of both high and low FC related to poor cognitive performance. There was no clear link to increased FC during early stages of MS and reduced FC in later stages, as predicted by common models of MS pathology, indicating substantial heterogeneity in study methodology (Jandric et al. 2022). Patients with early MS showed reduced functional network dynamics at baseline. Longitudinal changes showed longer time spent in a state of low FC and more connectivity disturbance within- and between-network FC (Koubiyr et al. 2022). MS-CI patients exhibited a more unstable network reconfiguration compared to preserved ones, i.e., brain regions switched between subnetworks more often, which was related to structural damage (Broeders et al. 2022).
MS-CI patients have reduced RS FC between subcortical and default mode networks, suggesting that slow inter-network connectivity contributes to cognitive dysfunction (d’Ambrosio et al. 2020). Reduced dynamics were demonstrated in DMN, FPN and visual networks and loss of interplay between them (Eijlers et al. 2019b). Others described disconnection in the DMN and attention networks due to extensive WM lesion (Louapre et al. 2014), structural brain connectivity being disturbed due to widespread impairment of WM connections and GM structures (Llufriu et al. 2016). Others found significant deficit in each of the five cognitive networks: DMN, attention, verbal memory, memory, and visuospatial working memory due to significant reduction of GM and thalamus volumes (Nejad-Davarani et al. 2016). MS patients recruit the ventral striatum, caudate nucleus and ventromedial prefrontal cortex in response to task performance, suggesting that they also recruit cortico-striatal regions during feedback-based learning. Decreased local FC within the basal ganglia plays a role in cognitive fatigue in MS, while increased global FC between the basal ganglia and the cortex may serve as compensatory mechanism (Langley et al. 2023). People with MS also recruit cortico-striatal regions and alternative connections within the striatum to assist with learning (Cagna et al. 2023).
In RRMS patients with mild disease, thalamic atrophy and thalamo-cortical connection damage lead to slower cognitive processes, and WM damage at specific fasciculi to episodic memory impairment (Bernabéu-Sanz et al. 2021). Thalamic involvement was correlated with abnormalities of cortico-thalamic connections at various levels (Bisecco et al. 2015). Hippocampal-thalamic-prefrontal disruption affects cognitive performance in early RRMS due to both WM and GM involvement, affecting functional pathways related to cognition (Kern et al. 2014). Poor cognitive performance in patients with RRMS and very mild disability were connected with increased FC but decreased structural connectivity in the DMN that was correlated with more than one cognitive domain rather than one specific network for each domain (Has Silemek et al. 2020). RRMS showed altered FC lateralisation patterns of the DMN with a significant leftward shift, while diminished dorsal attention network laterality was associated with increased FA asymmetry in the superior longitudinal fasciculus (Veréb et al. 2022). FC and presence of structural abnormalities in the hippocampus in RRMS were correlated with the degree of CI and extent of disability (Gu et al. 2022). Damage to the memory circuit associated with lesion volume of the hippocampus, parahippocampus, fornix and cingulate causing disruption of multiple connections were associated with memory dysfunction in MS (Kletenik et al. 2023). RRMS patients showed an association between the inter-network connectivity in various components of DMN and verbal memory deficits (Zhang et al. 2024).
Microstructural abnormalities and extensive atrophy of the fornix and interconnected GM are associated with CI in MS (Valdés Cabrera et al. 2022). The extent of disruption of microstructural disorganization in the main limbic pathways impacts the extent of CI (Keser et al. 2017), and there is a consistent trend between CI index and mean diffusivity and FA in the amygdalothalamic tracts/basolateral limbic circuit (Keser et al. 2018). Cognitively worsened MS patients in a 3-year longitudinal study showed decreased RS FC in the right hippocampus of the working memory network and in the right insula of the DMN due to GM atrophy progression in cognitively relevant brain regions combined with disorders of the relevant networks (Azzimonti et al. 2023).
MS-CI shows FC differences compared to HC that involve the cerebellum, visual and language-associated areas, hippocampus and basal ganglia; SDMT scores were correlated with FC between cerebellum and lateral occipital cortex (Carter et al. 2023). Cerebellar damage and connectivity changes were most prominent in SPMS related to worse CI (Schoonheim et al. 2021). Abnormal patterns of neuronal activity and synchronization between networks involved in the control of cognitive macrodomains underlie cognitive dysfunction in PPMS, and cortical lesions may play a role in variability and FC abnormalities (Petracca et al. 2017). CI patients with pediatric MS had decreased recruitment of several areas located in parietal and occipital lobes and cerebellum, and increased deactivation of the anterior cingulate cortex due to structural damage of WM tracts connecting these regions (De Meo et al. 2017).
In conclusion, neuroimaging findings, in addition to classical MS pathology, revealed structural changes (atrophy) of GM and WM in fronto-temporal lobes, corpus callosum, and hippocampus, as well as in subcortical regions, with extensive microstructural lesions in WM and subcortical structures being correlated with various changes of cognitive phenotypes. Myelin damage in NAWM is frequently associated with decreased cognitive functions. CBF and metabolic rate are reduced in frontal and prefrontal areas. More than structural changes, severe alterations of essential brain networks (DMN, SN, FTN) and inter-network disruptions are essential for the development and severity of cognitive dysfunctions. In addition, disruptions in the cortico-thalamic and prefronto-hippocampal pathways and disconnections within neurocognitive networks induce CI (and depression).
Neurophysiological analysis
EEG can be used as a tool for detecting cortical involvement during MS and its correlation with CI (Jamoussi et al. 2023). In early MS, nodal centrality was higher bi-parietally (theta-band) but markedly lower left temporally (upper alpha- and beta-band). Lower cognitive performance correlated to decreased centrality over left temporal (lower alpha-band) and right temporal (beta-band), indicating functional disconnection of the temporal region associated with CI in MS (Hardmeier et al. 2012). Comparative MRI and event-related potential (ERP) studies showed a relationship between P300 latency and lower hippocampal and amygdala volumes, while the amplitude of P300 was associated with lower cortical volume (Lorefice et al. 2021). The degree of cortical plasticity correlated with cognitive performance in RRMS, synaptic plasticity was significantly reduced in patients with CI (Balloff et al. 2022).
Magnetoencephalography (MEG) showed different correlation patterns between RS FC and verbal fluency and cognitive fatigue, both of which involved a shift from the posterior DMN to the language network. It demonstrated that MS induces distinct changes in the RS functional brain architecture that relate to specific cognitive alterations (Sjøgård et al. 2021). At 5-year follow-up, a more integrated beta-band network and a less integrated delta-band network predicted cognitive decline independent of structural damage (Nauta et al. 2021). Lower global integration was found in the alpha- and beta-bands, but higher global integration in the theta-band, indicating that the network analysis was able to detect changes in MS patients with CI (Tewarie et al. 2014). Increased alpha 1 and theta power was associated with impaired cognition and differed from HC, slowing of neuronal activity in bilateral parietotemporal cortical areas and thalamus being strongly related to CI (Schoonhoven et al. 2019). CI had higher power in low-frequency bands and lower power in high bands compared to HC, indicating neuronal slowing (Simon et al. 2023). Furthermore, a correlation between 1/f spectral slope and working memory functioning in the prefrontal and parietal cortex was observed (Akbarian et al. 2023). Long-range structure-function coupling was stronger in MS-CI compared to HC, but more research is needed to explore this measure as a biomarker in MS (Kulik et al. 2022).
Cognitive reserve and resilience in MS
Higher education, occupational attainment, leisure activities, modifications in lifestyle, physical activity, etc., have been considered proxies of cognitive reserve (CR), which is considered as a modulator of a more favorable trajectory, less CI and disability and a better QoL, intellectual enrichment protecting MS patients from CI, thereby extending the CR hypothesis to the MS course (Sumowski et al. 2012). On the other hand, the protective influence of higher CR against disease related cognitive deficits was discussed (Sumowski et al. 2009a, b).
Patients with MS can successfully build CR through multiple routes, including formal education and cognitive enrichment, which in a US study were more strongly associated with cognitive performance than were years of education. Cognitive enrichment was not associated with cognitive performance among participants with high education level. In contrast, among those with low education quality, cognitive enrichment was strongly associated with cognitive performance, suggesting that higher engagement in cognitively enriching activities provided protection similar to high educational level. Thus, persons can successfully build CR through multiple routes, including formal education or informal cognitive enrichment (Grant et al. 2023). On the other hand, cognitively resilient MS patients showed higher estimated intellectual ability and reportedly less anxiety and subclinical depressive symptoms. They also showed a trend toward more reported compensatory strategy than the not cognitively resilient ones (Randolph et al. 2022). A meta-analysis reported effect sizes (ES) of CR being associated with better cognitive task performance in verbal and spatial memory, attention, processing speed, verbal fluency and inhibitory control. These relations were significant except for verbal fluency. Metaregression analysis revealed older age and female sex increased the ES for attention and verbal memory outcome (Santangelo et al. 2019a). CR significantly moderated the relationship between brain damage and verbal fluency, but did not influence the relationship between EDSS and cognitive performance (Santangelo et al. 2019b). A moderating effect of CR was observed in the relationship between EDSS score and specific cognitive domains (processing efficacy, visuospatial learning, and memory), while in persons with high level of CR, there was no relationship between cognitive domains and EDSS, which supports the protective role of CR in MS-related cognitive dysfunction (Alexandra et al. 2023; Artemiadis et al. 2020; Maffezzini et al. 2023). The fact that at least one-third of MS patients are not overtly impaired despite significant radiographic brain tissue damage argues for protective factors (brain reserve, CR) that requires further clarification. It was suggested that the reported correlations between neuroimaging findings and cognitive function do not imply causality (Paul 2016). On the other hand, CR seems to have a marginally significant effect on brain structural connectivity, observed in patients with more severe CI. It protects them from cognitive decline regardless of the cognitive status yet once CI has set in; brain damage and aging are also influencing cognitive performance (Lopez-Soley et al. 2020). Higher education moderates the negative effects of WM lesion burden and third ventricle width (suggestive of thalamic atrophy) on cognitive performance (Pinter et al. 2014).
Regression analysis showed that MS patients with higher CR index have a significantly reduced FC within the salient network (SN), the dorsal anterior insula and occipital regions. This can have implications for how CR may modulate the susceptibility to cognitive dysfunction in MS (Bizzo et al. 2021). A study aimed to explore longitudinal reorganization of brain networks over 2 years by combining DTI, rsfMRI, MEG and a comprehensive neuropsychological battery, despite a general loss in structural connectivity in RRMS showed preserved hub connectivity over time in DMN. These results indicated that cognitive stability despite ongoing neurodegeneration might indicate a resilience mechanism of DMN mimicking a physiological reorganization observed in healthy aging (Has Silemek et al. 2023).
A recent systematic review of studies which investigated CR for MS indicated that accounting for MS symptoms may impact findings concerning the protective nature of CR. Therefore, establishing greater consistency and rigor across CR research in MS will be crucial to achieve a more accurate understanding of CR in MS (Stein et al. 2023).
In conclusion, CR is suggested to play a role in the presentation of CI, but it is not protective against general cognitive decline, although there is evidence that it protected several cognitive domains from further decline. CR has been documented by changes in some specific connectivities, but more exact studies concerning the protective nature of CR are warranted.
Pathogenic mechanisms
Many pathogenic mechanisms including chronic oxidative and nitrosative stress, neuroinflammation, peripheral inflammation, neuroendocrine and mitochondrial dysfunction, immune cell activation that involve novel pathways of programmed cell death, and neurodegenerative processes, leading to demyelination, axonal damage and both structural and connectivity brain alterations have been considered to contribute to the comorbidity between MS and CI (Maiese 2023). Neuroinflammation is an important factor contributing to CI in MS patients (Bruno et al. 2023; de Araújo Boleti et al. 2020). The presence of activated T- and B-cells and the subsequent bidirectional interaction leading to activated microglia is considered to be essential for MS-specific pathology. The presence of activated microglia is important for the interplay between MS-related cognitive structures and neuroinflammation (Barros and Fernandes 2021). Upregulation of miRNAs also appears to be involved in microglial toxicity and T-cell pathology. The pro-inflammatory activity of effector cytokines of the NLR family pyrin domain containing 3 (NLRP3) inflammasome supports the hypothesis that it is actively involved in the development of inflammatory and autoimmune mechanisms (Maciak et al. 2023).
Molecular mechanisms related to the gut-brain axis that mediate dysbiosis, intestinal barrier dysfunction and disruption of the blood-brain barrier integrity, neuroinflammation, suggested that the gut microbiota is part of the pathogenesis of MS-related CI (Ghadiri et al. 2022; Thirion et al. 2023).
The neuropsychological status depends on the thalamus and hippocampus metabolic processes related to the γ-aminobutyric (GABA) system. In the thalamus, GABA ratios (GABA/total choline and creatine, GABA/n-acetyl-aspartate) were significantly lower in RRMS than in HCs. Both total choline and myoinositol-ratios correlated with lower ADM test scores, indicating that neurocognitive performance in RRMS is associated with metabolic abnormalities of the thalamus (Kantorová et al. 2022). Other studies showed increased GABA receptor density in deep GM, while the concentrations of GABA and glutamate did not differ between cognitively impaired, preserved and control groups (Huiskamp et al. 2023). Furthermore, RRMS patients exhibited reduced hippocampal glutathione (GSH) and glutamate (Glu) levels, significantly related to worse verbal and visuospatial memory. Hippocampal Glu/GSH ratio significantly correlated with processing speed, thus being a potential marker for CI in RRMS (Li et al. 2024). RRMS patients demonstrated significantly reduced GABA and Glu concentrations and aberrant FC involving cognitive-related networks compared with HCs. Decremented hippocampal GABA levels mediated the association between inter-network FC in components of DMN and verbal memory deficits. These findings are in favor of the essential function of GABAergic system abnormalities in reducing network connectivities related to CI in MS (Zhang et al. 2024).
These and other findings suggest (neuro)immune and metabolic mechanisms linked to cognitive disorders in MS. However, its pathogenesis remains partially unclear, even though genetic, immune-inflammatory and neuroendocrine factors might be seen to play an essential role in addition to brain structural and connectivity alterations.
Biomarkers for CI in MS
Biomarkers for CI in MS could aid in both diagnostic and prognostic evaluation and in the development of new cognitive enhancing treatments. Promising biomarkers for CI in the preclinical stage of MS are: (1) description of the cognitive profile in several subdomains applying the SDMT criteria or comparable validated test (at least once a year); (2) EEG markers, e.g., increased alpha1- and delta-power and slowing of neuronal activity in parietotemporal cortex; (3) prolonged visual evoked potential (VEP) (Covey et al. 2021); (4) measurement of CSF and/or plasma neurofilament light-chain (NfL), GFAP and Aβ-42 levels; (5) assessment of serum glial cell line-derived neurotrophic factor (GDNF), (6) assessment of levels of CD8+, TNF-α, IL-6, Treg, IFN-γ, and other inflammatory-related markers, and BACE1 or BC200 levels in CSF or plasma; (7) blood concentrations of metallic nanoparticles (iron and zinc); (8) plasma levels of sirtuin 1 and 3 (metabolism modifying factor); (9) MRI findings detecting local brain atrophy using VBM and other technologies, WMH burden and functional brain network changes; (10) combined MRI imaging and EEG examination detecting widespread structural and EEG abnormalities; (11) combination of serum NfL, MRI lesion volume and GM volume (88.7–90.8% accuracy) (Brummer et al. 2022); (12) detection of olfactory deficits and microstructural changes in the anterior olfactory structure; (13) detection of retinal thinning and quantifying the peripapillary retinal nerve fibers by optical coherence tomography (OCT), a sensitive method for quantifying retinal neurons and axonal structures (Alba-Arbalat et al. 2023; Dreyer-Alster et al. 2022). The use of a multimodal marker, e.g., the combination of GM volume and serum NfL, could be valuable as indicator for cognitive changes in early stages of MS or prognostic indicators at follow-up as well as predictors for treatment results. However, fluid and imaging (bio)markers reflect different aspects of neurodegeneration and cannot be used interchangeable as markers for cognitive functioning in MS (van Dam et al. 2023). Central vestibular dysfunctions also correlate with cognitive dysfunctions in MS (Cochrane et al. 2021).
Fluid biomarkers
Serum and CSF biomarkers predict early cognitive decline. Adjusted NfL scores at baseline and 3 months, CSF NfL baseline values and CSF Aβ-42, combined with a clinical score (BREMSO) can acutely predict early cognitive decline in RRMS patients at the time of diagnosis (Tiu et al. 2022). CSF NfL is higher in MS patients with CI and impaired information processing speed and verbal fluency (Gaetani et al. 2019). High levels of serum NfL but not glial fibrillary acidic protein (GFAP) are associated with CI and predict cognitive decline in MS patients at high risk for developing an underlying progressive pathology (Barro et al. 2023). Another study confirmed that NfL levels in serum and third ventricle width are associated with an impairment of cognitive function (Oset et al. 2023). Others stated that NfL, chitinases and vitamin D in serum and CSF are promising biomarkers to monitor cognitive decline (Rademacher et al. 2023). In a real-world setting of early RRMS, kappa free light chain (KFLC) predicted cognitive decline (Rosenstein et al. 2023). Brain-derived neurotrophic factor (BDNF) and NfL levels measured at the time of diagnosis are inversely correlated with cognitive performance in MS, suggesting that CSF biomarkers linked to different pathophysiological processes reflect neuropsychological impairment in the earliest stages of the disease. Combining different CSF markers can facilitate recognition of cognition in MS (Yalachkov et al. 2022), and combining blood NfL and imaging measure improved the accuracy of predicting CI, highlighting the clinical utility of cross-modal biomarkers (Brummer et al. 2022; Williams et al. 2022).
Other biomarkers correlated with CI in RRMS and SPMS are increased plasma levels of sirtuins 1 and 3, as metabolic and epigenetic modifying factors indicating mitochondrial dysfunction, correlated with disability and CI (Foolad et al. 2023). Recent studies showed that the blood concentration of metallic nanoparticles, particularly iron (and zinc) is a promising biomarker for monitoring CI in MS (de Oliveira et al. 2023). Furthermore, CSF mediators related to B- and T-cell immunity and chemotaxis differentiate cognitively normal and MCI from those with severe CI. CXCL13 was the only molecule that differentiated severe CI from MCI (Pitteri et al. 2022). Other studies indicated that axonal damage biomarkers (NfL), and in particular tau protein seem to reflect CI in early stages of MS, although CSF tau was a week predictor of slowed information processing speed (IPS) and CI (Virgilio et al. 2022).
CSF levels of β-site amyloid precursor protein cleaving enzyme 1 (BACE1) and a group of pro-and anti-inflammatory molecules, including interleukin (IL-4, IL-17, IL-13, IL-9) and interferon-γ have a role in different key mechanisms such as oxidative stress and inflammation, influencing cognitive disorders and disability progression in MS (Bruno et al. 2023), suggesting that the two long non-coding RNAs, BACE1 and BC200, have a significant impact on the cognitive function in MS (Kamal et al. 2023).
Management of CI in MS
No specific treatment seems to be effective in CI related to MS, but given its devastating effects, appropriate strategies to ameliorate cognitive deficits in MS may reduce the negative impact of the disease on their lives, such as disability, loss of employment and poor QoL (O’Brien et al. 2008). The impact of disease-modifying therapies (DMTs) is still a matter of debate. Theoretically, they could exert a beneficial effect by means of reducing neuroinflammation and neurodegeneration/brain atrophy, which are established correlates of cognitive dysfunction, but a recent meta-analysis of clinical trials pointed out the difficulties associated with assessing of DMTs’ effects on cognitive dysfunctions in MS (Kania et al. 2023). Different hight-efficacy DMTs of RRMS have varying effects on disability and whole brain volume (Koudriavtseva and Mainero 2016), but the association between regional brain volume and treatment efficacy is currently unclear. A 2-year cohort study in Japanese patients showed improvement of mid-term prognosis by reducing disease activity and regional brain volume loss (Yokote et al. 2022). The commonly reported rate of adverse events was generally similar among DMTs, although natalizumab and ocrelizumab were shown to be the safest ones (Sladowska et al. 2022). Brain volume loss, one of the components of disability worsening, has been reduced by several forms of DMT and was associated with marked impact on disability worsening (Chylinska et al. 2023). Brain volume atrophy after initiation of immunotherapy was comparable to the results of other studies, suggesting that it is associated with disease deterioration in MS (Nold et al. 2023).
The possibilities for treatment and management of CI in MS are summarized in Table 2.
Prevention
It has been suggested that cognitive disease-induced CI can be compensated the more the higher CR has been increased by mental activity (Stein et al. 2023). It has been shown that negative effect of structural brain damage and brain atrophy, a well-established MRI marker in MS clinical trials, could be reduced through higher CR (Pinter et al. 2014; Sumowski et al. 2009b). Therefore, it is important to motivate people to remain at work, activate social contacts, change their lifestyle physical activity (PA) for yielding improvements in cognitive processing, learning and memory (Motl et al. 2024). PA represents a promising behavioral approach for managing cognitive dysfunction in MS. Higher peak 30-min steps-based metrics was associated with better performance in cognitive processing speed, verbal learning and memory, daily steps only with processing speed, highlighting the importance of PA intensity for cognition in MS (Zheng et al. 2024). Home-based neurofunctional exercise is a safe and feasible approach for improving some aspects of ambulation in persons with MS and CI (Mardaniyan Ghahfarrokhi et al. 2022). Internet-delivered lifestyle physical activity intervention provided evidence for improving cognitive processing speed and mitigating its negative impact on other outcomes (Motl et al. 2024).
Pharmacological therapy
Recent data on the latest advances in pharmacological management of CI in MS, including DMTs which indirectly may or may not influence it, are encouraging and inspiring for future studies. Overall, there is preliminary evidence of a beneficial effect of DMTs on cognition, particularly for high-efficacy DMTs. For symptomatic treatment, dalfampridine appears to be the only drug with robust evidence of a positive effect on cognition, but further studies should investigate the possible positive effects of multimodal interventions on cognition (Bellinvia et al. 2023). A metaanalysis of 41 studies in more than 7,000 MS patients revealed a small to moderately positive effect on cognitive test performance of DMTs in general, but no statistical differences between any single DMT and β-interferon (Landmeyer et al. 2020), although another study showed that early administration of high-efficacy DMTs is associated with greater cognitive improvements than delayed commencement (Labiano-Fontcuberta et al. 2022).
A prospective 4-year, multicenter observational, open-label, single-arm study of natalizumab treatment in patients with early RRMS showed improvement in cognitive processing speed, disability and patient-reported outcome (Perumal et al. 2023). Extended interval dosing natalizumab showed improvement of CI in RRMS, but a prospective observational study is warranted (McManus et al. 2022). For patients with SPMS, rituximab, a genetically engineered chimeric mouse/human monoclonal antibody representing a glycosylated immunoglobulin with human IgG1 content regions, showed a significant effect on a number of subgroups of minimal assessment of cognitive function in multiple sclerosis (MACFIMS) test (Salehizadeh et al. 2022). A meta-analysis of the frequently reported drugs for MS-induced CI, classified into five main groups - acetylcholine esterase inhibitors, CNS stimulants, fampridine, herbal remedies, and miscellaneous - did not affect cognitive function in any of the tasks (SDMT, PASAT, controlled oral word association test (COWAT), California verbal learning test (CVLT), etc.) (p > 0.05). However, in a subgroup analysis, significant improvement was seen after treatment with fampridine, whereas the majority of currently proposed therapeutic agents had no beneficial effects in MS-induced CI (Motavalli et al. 2020).
Existing literature on co-occurrence of cannabis use and MS lacks high quality evidence to recommend for or against cannabis and cannabinoid therapies for patients with MS on cognitive effects. Existing data suggest that cognition may be differently impacted depending on the type of product, the duration of use, and the indication (Landrigan et al. 2022).
Transcranial current stimulation
Over the last two decades, non-invasive brain stimulation, especially transcranial direct current stimulation (tDCS) has increasingly been used to modulate brain functions in various pathological conditions. Since the efficacy in MS patients remained questionable with the results of existing studies being conflicting, a meta-analysis showed promising benefits in ameliorating fatigue and cognitive symptoms in patients where other treatment results remained limited (Hiew et al. 2022). A few studies examined the effects of anodal tDCS along with cognitive training on cognitive performance in MS patients. A study comparing tDCS alone and paired with or without cognitive training as compared to sham appeared to be promising. Although the cognitive training group experienced an immediate improvement in attention and inhibitory control, the difference however was not significant at follow-up (Simani et al. 2022). A randomized controlled trial showed that transcranial alternating current stimulation (tACS) is a well-tolerated, non-pharmacological intervention, with a single session showing marginally significant improvement of SDMT score, while the effects of repeated tACS on cognitive function merit further research (Hsu et al. 2023).
Cognition enhancing strategies
Since improvement of cognition is important for many persons with MS (Langdon 2011), strategies are accepted only when improvement can be proved. Cognitive training in MS may stimulate preexisting neural reserves or recruit neural activity as “compensatory scaffolding”, prompting neuroplastic reorganization to meet everyday demands. Such strategies may include playing an instrument, reading music training and other cognitive interventions. An updated evaluation regarding the efficacy of cognitive interventions in MCI is needed given improvements in adherence to MCI diagnostic criteria in subject selection, better defined interventions, as well as identification of moderator variables which may influence treatment. A meta-analytic review on the efficacy of cognitive interventions in individuals with MCI versus randomized controls showed that interventions with memory and multi-domain forms of content appear to be particularly helpful; they may facilitate partial activation of compensatory scaffolding and neuroplastic reorganization. The positive benefit of these strategies may also reflect transfer effects indicative of compensatory network activation and the multiple-path involved in memory and other cognitive processes (Sherman et al. 2017). Cognitive therapy with Cogmed Working Memory Training (CWMT) has improved attention/working memory in MS patients in Canada (Blair et al. 2021), as has restorative cognitive training in Spain (Esbrí et al. 2023), while, despite the high importance of cognitive care, there is little consistency in training/assessment of CI across services for people with MS in Ireland (Hynes et al. 2022). Specific differences in the management of CI in MS among local health care systems (e.g., Europe vs. North America) could not be identified according to the available sources. Aerobic exercise represents a cost-effective, widely available, natural and self-administered treatment with no adverse side effects that may be effective for memory impairment in MS (Leavitt et al. 2014).
Cognitive rehabilitation
Cognitive rehabilitation and exercise training have been identified as possible candidates for treating MS-related CI (DeLuca et al. 2020). A Cochrane database review demonstrated improvement of verbal and visual memory, information processing speed and QoL by cognitive rehabilitation (Taylor et al. 2021). Home-based integral cognitive rehabilitation program (ICRP) aimed at potentiation of restorative and compensatory mechanisms, can improve cognitive function and prevent the deterioration of cognitive deficits by limiting the progression of cognitive dysfunction in MS (Sharbafshaaer et al. 2022). Rehabilitation programs of the National Health System increased either cognitive or motor performance or both performances. Persons with MS seem to benefit from a combined approach more than from cognitive or motor rehabilitation alone (Argento et al. 2023). Cognition rehabilitation therapy offers cognitive function and memory benefits, for which a multidisciplinary care team and regular reassessment are recommended to manage changing symptoms and ensure continuity of care (Iodice et al. 2023). Both cognitive rehabilitation therapy (RT) and mindfulness-based cognitive therapy (MBCT) alleviated cognitive complaints in MS patients immediately after treatment completion, but these benefits do not persist. In the long term, RT showed benefits on personal cognitive goals and MBCT on processing speed, indicating a specific contribution of available cognitive treatments (Nauta et al. 2023). Patients who most likely benefit from restorative cognitive rehabilitation may exhibit impairment within the domain of interest as well as having lower cognitive burden overall (Ziccardi et al. 2024). The availability of advanced home-based cognitive rehabilitation mechanisms is fundamental for supporting standardized cognitive rehabilitation protocols. A computerized MS-specific CR system has given promising results in cognitive rehabilitation (Gaspari et al. 2023). A combined cognitive rehabilitation plus aerobic exercise, however, did not seem to improve processing speed in people with progressive MS, although the sham interventions were not inactive (Feinstein et al. 2023). Cognitive rehabilitation increases the knowledge of the disease, confidence and strength in everyday life, which leads to emotional and social improvement and positive effects on QoL (Klein et al. 2019).
Neuroimaging of effects of treatment strategies
Treatment effect on cognition was strongly associated with effects on brain atrophy but not with effects on active MRI lesions (Sormani et al. 2023). A study comparing feedback-based learning ability in MS with cortico-striatal function and connectivity showed recruitment of cortico-striatal regions and displayed stronger FC between the ventral striatum and task-relevant regions (left angular and right superior temporal gyrus), indicating that people with MS may recruit alternative connections with the striatum (Cagna et al. 2023). Neurofeedback training was associated with increased FA and FC within the sensorimotor and salience networks (Pinter et al. 2021), and computer-aided cognitive rehabilitation with increased FC in posterior cingulate and inferior parietal cortex of the DMN (Bonavita et al. 2015). Brain network function predicted connectivity goal achievement after MBCT and CRT, and processing speed improvements after these methods. Aerobic exercise resulted in 16.5% increase in hippocampal volume and 53.7% increase in memory, as well as increased hippocampal resting-state FC. Improvements were specific, with no comparable changes in overall cerebral GM or non-hippocampal subcortical GM structures (thalamus, caudate nucleus) (Leavitt et al. 2014). Lower WM tract disruption in a network of region-pairs centered on precuneus and posterior cingulate (DNM regions) predicted greater response to restorative cognitive rehabilitation from the interaction between structural network disruption and FC in the DMN (Fuchs et al. 2020). Persons with MS with neuronal slowing and hyperconnectivity were most prone to show treatment response, making network function a promising tool for personal treatment recommendations (Nauta et al. 2024).
Conclusions and outlook
MS, an autoimmune-mediated disease, causing central nervous system demyelination and neurodegeneration, is commonly associated with cognitive deterioration, the prevalence of which is highly variable. Manifesting early, with insidious onset, it develops progressively as a disabling feature affecting the patients’ QoL. Major risk factors of CI are genetic and premorbid burden, lifestyle and co-morbidities. Involving different cognitive domains, CI is often associated with depression and other neurosychiatric symptoms, but usually not correlated with sensorimotor symptoms, suggesting different pathogenesis. The phenotypes of MS show different prevalence and severity of CI that show higher frequency and severity in PPMS compared to RRMS. While specific neuropathological findings related to CI in MS are missing, neuroimaging studies have shown general brain atrophy with predominant affection of (pre)fronto-temporal cortices, corpus callosum and limbic areas, with microstructural changes of NAWM and extensive damage to specific WM areas. These and other GM and WM lesions are associated with cortico-subcortical (fronto-striatal and thalamo-cortical) disconnections and disruption of essential neuromodulatory networks (DMN, salience, FPN, and others), all of which are involved in the pathogenesis of CI and related symptoms. Cognitive reserve is suggested to play a modifying role in the presentation of CI, protecting several cognitive domains from further decline without definite prohibition of general cognitive decline,
The pathogenesis of CI in MS is related to shared mechanisms including oxidative and nitrosative stress, neuroinflammation, peripheral immune processes, micro-RNA lesions, neuroendocrine dysfunctions (HPA and GABA system), and other mechanisms, such as disorders of the gut-brain dysbiosis, that may occur prior to development of structural brain lesions.
Biomarkers for CI in MS are neuropsychological validation, plasma and CSF NfL, GDNF and GFAP, inflammatory markers, neuroimaging and EEG abnormalities, with focus on multimodal markers as indicators for CI already in early stages of MS.
Since CI and other neuropsychiatric symptoms including depression show an excessive variability between MS patients, they are still under-assessed and under-treated, although there are multiple possibilities for effective prevention and management of CI (and other neuropsychiatric symptoms). In addition to DMT of MS, they include pharmacotherapy with natalizumab or rituximab, transcranial current stimulation, cognitive enhancing strategies, cognitive rehabilitation, mindfulness-based interventions, neurofeedback training and physical activity as well as changes in lifestyle. Although some of these treatment modalities await validation and statistical confirmation, for every single MS patient, an individual combination of these and other treatment modalities is recommended in order to improve their quality of life.
Abbreviations
- AD:
-
Alzheimer disease
- BICAMS:
-
Brief International Cognitive Assessment for multiple sclerosis
- CBF:
-
Cerebral blood flow
- CI:
-
Cognitive impairment
- CIS:
-
Clinically isolated syndrome
- DMN:
-
Default mode network
- DMTs:
-
Disease-modifying therapies
- DTI:
-
Diffusion tensor imaging
- EDSS:
-
Expanded Disability Stats Scale
- EF:
-
Executive function
- FC:
-
Functional connectivity
- FPN:
-
Frontoparietal network
- GABA:
-
γ-Aminobutyric acid
- GM:
-
Gray matter
- HCs:
-
Healthy controls
- MCI:
-
Mild cognitive impairment
- MS:
-
Multiple sclerosis
- NAWM:
-
Normal appearing white matter
- NfL:
-
Neurofilament light-chain
- PPMS:
-
Primary progressive MS
- PRMS:
-
Progressive-relapsing subtype MS
- QoL:
-
Quality of life
- RRMS:
-
Relapsing-remitting MS
- RS:
-
Resting state
- SD:
-
Standard deviation
- SDMT:
-
Symbol Digit Modalities Test
- SPMS:
-
Secondary progressive MS
- WM:
-
White matter
References
Abel S, Vavasour I, Lee LE, Johnson P, Ackermans N, Chan J, Dvorak A, Schabas A, Wiggermann V, Tam R, Kuan AJ, Morrow SA, Wilken J, Laule C, Rauscher A, Bhan V, Sayao AL, Devonshire V, Li DK, Carruthers R, Traboulsee A, Kolind SH (2020a) Myelin damage in normal appearing white matter contributes to impaired cognitive processing speed in multiple sclerosis. J Neuroimaging 30:205–211
Abel S, Vavasour I, Lee LE, Johnson P, Ristow S, Ackermans N, Chan J, Cross H, Laule C, Dvorak A, Schabas A, Hernández-Torres E, Tam R, Kuan AJ, Morrow SA, Wilken J, Rauscher A, Bhan V, Sayao AL, Devonshire V, Li DKB, Carruthers R, Traboulsee A, Kolind SH (2020b) Associations between findings from myelin water imaging and cognitive performance among individuals with multiple sclerosis. JAMA Netw Open 3:e2014220
Akaishi T, Fujimori J, Nakashima I (2024) Basal ganglia atrophy and impaired cognitive processing speed in multiple sclerosis. Cureus 16:e52603
Akbarian F, Rossi C, Costers L, D’Hooghe MB, D’Haeseleer M, Nagels G, Van Schependom J (2023) The spectral slope as a marker of excitation/inhibition ratio and cognitive functioning in multiple sclerosis. Hum Brain Mapp 44:5784–5794
Al-Iedani O, Lea S, Alshehri A, Maltby VE, Saugbjerg B, Ramadan S, Lea R, Lechner-Scott J (2024) Multi-modal neuroimaging signatures predict cognitive decline in multiple sclerosis: a 5-year longitudinal study. Mult Scler Relat Disord 81:105379
Alba-Arbalat S, Solana E, Lopez-Soley E, Camos-Carreras A, Martinez-Heras E, Vivó F, Pulido-Valdeolivas I, Andorra M, Sepulveda M, Cabrera JM, Fonseca E, Calvi A, Alcubierre R, Dotti-Boada M, Saiz A, Martinez-Lapiscina EH, Villoslada P, Blanco Y, Sanchez-Dalmau B, Llufriu S (2023) Predictive value of retinal atrophy for cognitive decline across disease duration in multiple sclerosis. J Neurol Neurosurg Psychiatry 95:419–425
Alexandra T, Kim C, Estefania B, Elaine R, Pierre D, Isabelle R (2023) Cognitive reserve as a moderating factor between EDSS and cognition in multiple sclerosis. Mult Scler Relat Disord 70:104482
Alharthi HM, Almurdi MM (2023) Association between cognitive impairment and motor dysfunction among patients with multiple sclerosis: a cross-sectional study. Eur J Med Res 28:110
Altieri M, Cerciello F, Gallo A, Santangelo G (2024) The relationship between depression and cognitive performance in multiple sclerosis: a meta-analysis. Clin Neuropsychol 38:21–41
Altieri M, Fratino M, Maestrini I, Dionisi C, Annecca R, Vicenzini E, Di Piero V (2020) Cognitive performance in relapsing-remitting multiple sclerosis: at risk or impaired? Dement Geriatr Cogn Disord 49:539–543
Amato MP, Prestipino E, Bellinvia A, Niccolai C, Razzolini L, Pastò L, Fratangelo R, Tudisco L, Fonderico M, Mattiolo PL, Goretti B, Zimatore GB, Losignore NA, Portaccio E, Lolli F (2019) Cognitive impairment in multiple sclerosis: an exploratory analysis of environmental and lifestyle risk factors. PLoS ONE 14:e0222929
Amin M, Ontaneda D (2021) Thalamic injury and cognition in multiple sclerosis. Front Neurol 11:623914
Andaloro A, Russo M, Pastura C, Sessa E, Calatozzo P, Maggio MG, Bramanti P (2022) Is there a correlation between dyslipidemia and cognitive impairment in patients with multiple sclerosis? Int J Neurosci 132:201–206
Argento O, Piacentini C, Bossa M, Caltagirone C, Santamato A, Saraceni V, Nocentini U (2023) Motor, cognitive, and combined rehabilitation approaches on MS patients’ cognitive impairment. Neurol Sci 44:1109–1118
Artemiadis A, Bakirtzis C, Ifantopoulou P, Zis P, Bargiotas P, Grigoriadis N, Hadjigeorgiou G (2020) The role of cognitive reserve in multiple sclerosis: a cross-sectional study in 526 patients. Mult Scler Relat Disord 41:102047
Ayromlou H, Hosseini S, Khalili M, Ayromlou S, Khamudchiyan S, Farajdokht F, Hassannezhad S, Amiri Moghadam S (2023) Insulin resistance is associated with cognitive dysfunction in multiple sclerosis patients: a cross-sectional study. J Neuroendocrinol 35:e13288
Azzimonti M, Preziosa P, Pagani E, Valsasina P, Tedone N, Vizzino C, Rocca MA, Filippi M (2023) Functional and structural brain MRI changes associated with cognitive worsening in multiple sclerosis: a 3-year longitudinal study. J Neurol 270:4296–4308
Baijot J, Van Laethem D, Denissen S, Costers L, Cambron M, D’Haeseleer M, D’Hooghe MB, Vanbinst AM, De Mey J, Nagels G, Van Schependom J (2022) Radial diffusivity reflects general decline rather than specific cognitive deterioration in multiple sclerosis. Sci Rep 12:21771
Bakirtzis C, Ioannidis P, Messinis L, Nasios G, Konstantinopoulou E, Papathanasopoulos P, Grigoriadis N (2018) The rationale for monitoring cognitive function in multiple sclerosis: practical issues for clinicians. Open Neurol J 12:31–40
Balloff C, Penner IK, Ma M, Georgiades I, Scala L, Troullinakis N, Graf J, Kremer D, Aktas O, Hartung HP, Meuth SG, Schnitzler A, Groiss SJ, Albrecht P (2022) The degree of cortical plasticity correlates with cognitive performance in patients with multiple sclerosis. Brain Stimul 15:403–413
Barro C, Healy BC, Saxena S, Glanz BI, Paul A, Polgar-Turcsanyi M, Guttmann CR, Bakshi R, Weiner HL, Chitnis T (2023) Serum NfL but not GFAP predicts cognitive decline in active progressive multiple sclerosis patients. Mult Scler 29:206–211
Barros C, Fernandes A (2021) Linking cognitive impairment to neuroinflammation in multiple sclerosis using neuroimaging tools. Mult Scler Relat Disord 47:102622
Basci D, Tulek Z (2023) Assessment of cognitive function and its predictors in patients with multiple sclerosis: a case-control study. Neurol Sci 44:1009–1016
Bellinvia A, Portaccio E, Amato MP (2023) Current advances in the pharmacological prevention and management of cognitive dysfunction in multiple sclerosis. Expert Opin Pharmacother 24:435–451
Benedict RH, Zivadinov R (2011) Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 7:332–342
Benedict RH, Weinstock-Guttmam B, Marr K, Valnarov V, Kennedy C, Carl E, Brooks C, Hojnacki D, Zivadinov R (2013) Chronic cerebrospinal venous insufficiency is not associated with cognitive impairment in multiple sclerosis. BMC Med 11:167
Benedict RH, Morrow S, Rodgers J, Hojnacki D, Bucello MA, Zivadinov R, Weinstock-Guttman B (2014) Characterizing cognitive function during relapse in multiple sclerosis. Mult Scler 20:1745–1752
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19:860–871
Benedict RH, Pol J, Yasin F, Hojnacki D, Kolb C, Eckert S, Tacca B, Drake A, Wojcik C, Morrow SA, Jakimovski D, Fuchs TA, Dwyer MG, Zivadinov R, Weinstock-Guttman B (2021) Recovery of cognitive function after relapse in multiple sclerosis. Mult Scler 27:71–78
Bergmann C, Becker S, Watts A, Sullivan C, Wilken J, Golan D, Zarif M, Bumstead B, Buhse M, Kaczmarek O, Covey TJ, Doniger GM, Penner IK, Hancock LM, Bogaardt H, Barrera MA, Morrow S, Gudesblatt M (2023) Multiple sclerosis and quality of life: the role of cognitive impairment on quality of life in people with multiple sclerosis. Mult Scler Relat Disord 79:104966
Bermel RA, Bakshi R (2006) The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol 5:158–170
Bernabéu-Sanz Á, Morales S, Naranjo V, Sempere ÁP (2021) Contribution of gray matter atrophy and white matter damage to cognitive impairment in mildly disabled relapsing-remitting multiple sclerosis patients. Diagnostics (Basel) 11:578
Bilgin YOU, Koskderelioglu A, Gedizlioglu M (2023a) Correction to: fall risk is related to cognitive functioning in ambulatory multiple sclerosis patients. Neurol Sci 44:3369
Bilgin YOU, Koskderelioglu A, Gedizlioglu M (2023b) Fall risk is related to cognitive functioning in ambulatory multiple sclerosis patients. Neurol Sci 44:3233–3242
Bisecco A, Rocca MA, Pagani E, Mancini L, Enzinger C, Gallo A, Vrenken H, Stromillo ML, Copetti M, Thomas DL, Fazekas F, Tedeschi G, Barkhof F, Stefano ND, Filippi M (2015) Connectivity-based parcellation of the thalamus in multiple sclerosis and its implications for cognitive impairment: a multicenter study. Hum Brain Mapp 36:2809–2825
Bisecco A, Capuano R, Caiazzo G, d’Ambrosio A, Docimo R, Cirillo M, Russo A, Altieri M, Bonavita S, Rocca MA, Filippi M, Tedeschi G, Gallo A (2021) Regional changes in thalamic shape and volume are related to cognitive performance in multiple sclerosis. Mult Scler 27:134–138
Bizzo BC, Arruda-Sanchez T, Tobyne SM, Bireley JD, Lev MH, Gasparetto EL, Klawiter EC (2021) Anterior insular resting-state functional connectivity is related to cognitive reserve in multiple sclerosis. J Neuroimaging 31:98–102
Blair M, Goveas D, Safi A, Marshall C, Rosehart H, Orenczuk S, Morrow SA (2021) Does cognitive training improve attention/working memory in persons with MS? A pilot study using the Cogmed Working Memory Training program. Mult Scler Relat Disord 49:102770
Bobholz JA, Rao SM (2003) Cognitive dysfunction in multiple sclerosis: a review of recent developments. Curr Opin Neurol 16:283–288
Bocti C, Pépin F, Tétreault M, Cossette P, Langlois F, Imbeault H, Duval N, Lacombe G, Fulop T (2017) Orthostatic hypotension associated with executive dysfunction in mild cognitive impairment. J Neurol Sci 382:79–83
Bogaardt H, Golan D, Barrera MA, Attrill S, Kaczmarek O, Zarif M, Bumstead B, Buhse M, Wilken J, Doniger GM, Hancock LM, Penner IK, Halper J, Morrow SA, Covey TJ, Gudesblatt M (2023) Cognitive impairment, fatigue and depression in multiple sclerosis: is there a difference between benign and non-benign MS? Mult Scler Relat Disord 73:104630
Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d’Ambrosio A, Docimo R, Bisecco A, Lavorgna L, Corbo D, Cirillo S, Gallo A, Esposito F, Tedeschi G (2015) Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol 262:91–100
Bonnet MC, Allard M, Dilharreguy B, Deloire M, Petry KG, Brochet B (2010) Cognitive compensation failure in multiple sclerosis. Neurology 75:1241–1248
Bouman PM, van Dam MA, Jonkman LE, Steenwijk MD, Schoonheim MM, Geurts JJG, Hulst HE (2024) Isolated cognitive impairment in people with multiple sclerosis: frequency, MRI patterns and its development over time. J Neurol 271:2159–2168
Branco M, Ruano L, Portaccio E, Goretti B, Niccolai C, Patti F, Chisari C, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Bellomi F, Simone M, Viterbo RG, Amato MP (2019) Aging with multiple sclerosis: prevalence and profile of cognitive impairment. Neurol Sci 40:1651–1657
Brochet B, Clavelou P, Defer G, De Seze J, Louapre C, Magnin E, Ruet A, Thomas-Anterion C, Vermersch P (2022) Cognitive impairment in secondary progressive multiple sclerosis: effect of disease duration, age, and progressive phenotype. Brain Sci 12:183
Broeders TAA, Douw L, Eijlers AJC, Dekker I, Uitdehaag BMJ, Barkhof F, Hulst HE, Vinkers CH, Geurts JJG, Schoonheim MM (2022) A more unstable resting-state functional network in cognitively declining multiple sclerosis. Brain Commun 4:fcac095
Brummer T, Muthuraman M, Steffen F, Uphaus T, Minch L, Person M, Zipp F, Groppa S, Bittner S, Fleischer V (2022) Improved prediction of early cognitive impairment in multiple sclerosis combining blood and imaging biomarkers. Brain Commun 4:fcac153
Bruno A, Dolcetti E, Azzolini F, Buttari F, Gilio L, Iezzi E, Galifi G, Borrelli A, Furlan R, Finardi A, Carbone F, De Vito F, Musella A, Guadalupi L, Mandolesi G, Matarese G, Centonze D, Stampanoni Bassi M (2023) BACE1 influences clinical manifestations and central inflammation in relapsing remitting multiple sclerosis. Mult Scler Relat Disord 71:104528
Butler Pagnotti R, Hua LH, Miller JB (2022) Cognition and disease characteristics in adult onset versus late onset multiple sclerosis. Mult Scler 28:933–941
Caceres F, Vanotti S, Rao S (2011) Epidemiological characteristics of cognitive impairment of multiple sclerosis patients in a latin American country. J Clin Exp Neuropsychol 33:1094–1098
Caceres F, Vanotti S, Benedict RH (2014) Cognitive and neuropsychiatric disorders among multiple sclerosis patients from Latin America: results of the RELACCEM study. Mult Scler Relat Disord 3:335–340
Cagna CJ, Ceceli AO, Sandry J, Bhanji JP, Tricomi E, Dobryakova E (2023) Altered functional connectivity during performance feedback processing in multiple sclerosis. Neuroimage Clin 37:103287
Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M (2009) Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol 66:1144–1150
Campanholo KR, Pitombeira MS, Rimkus CM, Mendes MF, Apóstolos-Pereira SL, Busatto Filho G, Callegaro D, Buchpiguel CA, Duran F, De Paula Faria D (2022) Myelin imaging measures as predictors of cognitive impairment in MS patients: a hybrid PET-MRI study. Mult Scler Relat Disord 57:103331
Campbell J, Langdon D, Cercignani M, Rashid W (2016) A randomised controlled trial of efficacy of cognitive rehabilitation in multiple sclerosis: a cognitive, behavioural, and MRI study. Neural Plast 2016:4292585
Carter SL, Patel R, Fisk JD, Figley CR, Marrie RA, Mazerolle EL, Uddin MN, Wong K, Graff LA, Bolton JM, Marriott JJ, Bernstein CN, Kornelsen J (2023) Differences in resting state functional connectivity relative to multiple sclerosis and impaired information processing speed. Front Neurol 14:1250894
Castrogiovanni N, Mostert J, Repovic P, Bowen JD, Uitdehaag BMJ, Strijbis EMM, Cutter GR, Koch MW (2023) Longitudinal changes in cognitive test scores in patients with relapsing-remitting multiple sclerosis: an analysis of the DECIDE dataset. Neurology 101:e1–e11
Cavaco S, Ferreira I, Moreira I, Santos E, Samões R, Sousa AP, Pinheiro J, Teixeira-Pinto A, da Martins A (2022) Cognitive dysfunction and mortality in multiple sclerosis: long-term retrospective review. Mult Scler 28:1382–1391
Cederberg KLJ, Mathison B, Schuetz ML, Motl RW (2022) Restless legs syndrome severity and cognitive function in adults with multiple sclerosis: an exploratory pilot study. Int J MS Care 24:154–161
Chang MC, Lee BJ, Yang D, Kim CR, Park D, Kim S (2023a) The association between cognition and gait disturbance in central nervous system demyelinating disorder with mild disability. BMC Neurol 23:177
Chang YT, Kearns PKA, Carson A, Gillespie DC, Meijboom R, Kampaite A, Valdés Hernández MDC, Weaver C, Stenson A, MacDougall N, O’Riordan J, Macleod MA, Carod-Artal FJ, Connick P, Waldman AD, Chandran S, Foley P (2023b) Network analysis characterizes key associations between subjective fatigue and specific depressive symptoms in early relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 69:104429
Charest K, Tremblay A, Langlois R, Roger É, Duquette P, Rouleau I (2020) Detecting subtle cognitive impairment in multiple sclerosis with the montreal cognitive assessment. Can J Neurol Sci 47:620–626
Charvet LE, Yang J, Shaw MT, Sherman K, Haider L, Xu J, Krupp LB (2017) Cognitive function in multiple sclerosis improves with telerehabilitation: results from a randomized controlled trial. PLoS ONE 12:e0177177
Chiaravalloti ND, Deluca J (2002) Self-generation as a means of maximizing learning in multiple sclerosis: an application of the generation effect. Arch Phys Med Rehabil 83:1070–1079
Chylinska M, Komendzinski J, Wyszomirski A, Karaszewski B (2023) Brain atrophy as an outcome of disease-modifying therapy for remitting-relapsing multiple sclerosis. Mult Scler Int 2023:4130557
Clough M, Bartholomew J, White OB, Fielding J (2022) Working memory phenotypes in early multiple sclerosis: appraisal of phenotype frequency, progression and test sensitivity. J Clin Med 11:2936
Cochrane GD, Christy JB, Sandroff BM, Motl RW (2021) Cognitive and central vestibular functions correlate in people with multiple sclerosis. Neurorehabil Neural Repair 35:1030–1038
Colato E, Stutters J, Tur C, Narayanan S, Arnold DL, Gandini Wheeler-Kingshott CAM, Barkhof F, Ciccarelli O, Chard DT, Eshaghi A (2021) Predicting disability progression and cognitive worsening in multiple sclerosis using patterns of grey matter volumes. J Neurol Neurosurg Psychiatry 92:995–1006
Comi G, Filippi M, Martinelli V, Campi A, Rodegher M, Alberoni M, Sirabian G, Canal N (1995) Brain MRI correlates of cognitive impairment in primary and secondary progressive multiple sclerosis. J Neurol Sci 132:222–227
Conti L, Preziosa P, Meani A, Pagani E, Valsasina P, Marchesi O, Vizzino C, Rocca MA, Filippi M (2021) Unraveling the substrates of cognitive impairment in multiple sclerosis: a multiparametric structural and functional magnetic resonance imaging study. Eur J Neurol 28:3749–3759
Cortese M, Riise T, Bjørnevik K, Bhan A, Farbu E, Grytten N, Hogenesch I, Midgard R, Smith Simonsen C, Telstad W, Ascherio A, Myhr KM (2016) Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol 80:616–624
Cotter J, Firth J, Enzinger C, Kontopantelis E, Yung AR, Elliott R, Drake RJ (2016) Social cognition in multiple sclerosis: a systematic review and meta-analysis. Neurology 87:1727–1736
Covey TJ, Golan D, Doniger GM, Sergott R, Zarif M, Srinivasan J, Bumstead B, Wilken J, Buhse M, Mebrahtu S, Gudesblatt M (2021) Visual evoked potential latency predicts cognitive function in people with multiple sclerosis. J Neurol 268:4311–4320
Crouch TA, Reas HE, Quach CM, Erickson TM (2022) Does depression in multiple sclerosis mediate effects of cognitive functioning on quality of life? Qual Life Res 31:497–506
Cuerda-Ballester M, Martínez-Rubio D, García-Pardo MP, Proaño B, Cubero L, Calvo-Capilla A, de la Sancho-Cantus D, Rubia Ortí JE (2023) Relationship of motor impairment with cognitive and emotional alterations in patients with multiple sclerosis. Int J Environ Res Public Health 20:1387
d’Ambrosio A, Valsasina P, Gallo A, De Stefano N, Pareto D, Barkhof F, Ciccarelli O, Enzinger C, Tedeschi G, Stromillo ML, Arévalo MJ, Hulst HE, Muhlert N, Koini M, Filippi M, Rocca MA (2020) Reduced dynamics of functional connectivity and cognitive impairment in multiple sclerosis. Mult Scler 26:476–488
Damasceno A, Pimentel-Silva LR, Damasceno BP, Cendes F (2020) Cognitive trajectories in relapsing-remitting multiple sclerosis: a longitudinal 6-year study. Mult Scler 26:1740–1751
Damjanovic D, Valsasina P, Rocca MA, Stromillo ML, Gallo A, Enzinger C, Hulst HE, Rovira A, Muhlert N, De Stefano N, Bisecco A, Fazekas F, Arévalo MJ, Yousry TA, Filippi M (2017) Hippocampal and deep gray matter nuclei atrophy is relevant for explaining cognitive impairment in MS: a multicenter study. AJNR Am J Neuroradiol 38:18–24
de Araújo Boleti AP, de Oliveira Flores TM, Moreno SE, Anjos LD, Mortari MR, Migliolo L (2020) Neuroinflammation: an overview of neurodegenerative and metabolic diseases and of biotechnological studies. Neurochem Int 136:104714
De Meo E, Moiola L, Ghezzi A, Veggiotti P, Capra R, Amato MP, Pagani E, Fiorino A, Pippolo L, Pera MC, Comi G, Falini A, Filippi M, Rocca MA (2017) MRI substrates of sustained attention system and cognitive impairment in pediatric MS patients. Neurology 89:1265–1273
De Meo E, Portaccio E, Giorgio A, Ruano L, Goretti B, Niccolai C, Patti F, Chisari CG, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Stampatori C, Simone M, Viterbo RG, Bonacchi R, Rocca MA, De Stefano N, Filippi M, Amato MP (2021) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78:414–425
de Oliveira M, Santinelli FB, Lisboa-Filho PN, Barbieri FA (2023) The blood concentration of metallic nanoparticles is related to cognitive performance in people with multiple sclerosis: an exploratory analysis. Biomedicines 11:1819
Degraeve B, Sequeira H, Mecheri H, Lenne B (2023) Corpus callosum damage to account for cognitive, affective, and social-cognitive dysfunctions in multiple sclerosis: a model of callosal disconnection syndrome? Mult Scler 29:160–168
Degraeve B, Henry A, Lenne B (2024) Relationship between emotion recognition and cognition in multiple sclerosis: a meta-analysis protocol. BMJ Neurol Open 6:e000471
DeLuca J, Chiaravalloti ND, Sandroff BM (2020) Treatment and management of cognitive dysfunction in patients with multiple sclerosis. Nat Rev Neurol 16:319–332
Drew M, Tippett LJ, Starkey NJ, Isler RB (2008) Executive dysfunction and cognitive impairment in a large community-based sample with multiple sclerosis from New Zealand: a descriptive study. Arch Clin Neuropsychol 23:1–19
Dreyer-Alster S, Gal A, Achiron A (2022) Optical coherence tomography is associated with cognitive impairment in multiple sclerosis. J Neuroophthalmol 42:e14–e21
Eijlers AJC, Dekker I, Steenwijk MD, Meijer KA, Hulst HE, Pouwels PJW, Uitdehaag BMJ, Barkhof F, Vrenken H, Schoonheim MM, Geurts JJG (2019a) Cortical atrophy accelerates as cognitive decline worsens in multiple sclerosis. Neurology 93:e1348–e1359
Eijlers AJC, Wink AM, Meijer KA, Douw L, Geurts JJG, Schoonheim MM (2019b) Reduced network dynamics on functional MRI signals cognitive impairment in multiple sclerosis. Radiology 292:449–457
Eilam-Stock T, Shaw MT, Krupp LB, Charvet LE (2021) Early neuropsychological markers of cognitive involvement in multiple sclerosis. J Neurol Sci 423:117349
Ekmekci O (2017) Pediatric multiple sclerosis and cognition: a review of clinical, neuropsychologic, and neuroradiologic features. Behav Neurol 2017:1463570
Elkhooly M, Bao F, Raghib M, Millis S, Bernitsas E (2023) Role of white matter in cognitive impairment among relapsing remitting multiple sclerosis patients. Mult Scler Relat Disord 79:105030
Esbrí SF, Sebastián Tirado A, Zaragoza Mezquita M, Sanchis Segura C, Forn C (2023) Pre-training working memory/information processing capabilities and brain atrophy limit the improving effects of cognitive training. Mult Scler J Exp Transl Clin 9:20552173231196990
Ezegbe C, Zarghami A, van der Mei I, Alty J, Honan C, Taylor B (2023) Instruments measuring change in cognitive function in multiple sclerosis: a systematic review. Brain Behav 13:e3009
Feinstein A, Amato MP, Brichetto G, Chataway J, Chiaravalloti ND, Cutter G, Dalgas U, DeLuca J, Farrell R, Feys P, Filippi M, Freeman J, Inglese M, Meza C, Motl RW, Rocca MA, Sandroff BM, Salter A (2023) Cognitive rehabilitation and aerobic exercise for cognitive impairment in people with progressive multiple sclerosis (CogEx): a randomised, blinded, sham-controlled trial. Lancet Neurol 22:912–924
Feys P, Moumdjian L, Van Halewyck F, Wens I, Eijnde BO, Van Wijmeersch B, Popescu V, Van Asch P (2019) Effects of an individual 12-week community-located start-to-run program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler 25:92–103
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA (2018) Multiple sclerosis. Nat Rev Dis Primers 4:43
Filser M, Schreiber H, Pöttgen J, Ullrich S, Lang M, Penner IK (2018) The brief International Cognitive Assessment in multiple sclerosis (BICAMS): results from the German validation study. J Neurol 265:2587–2593
Fischer M, Kunkel A, Bublak P, Faiss JH, Hoffmann F, Sailer M, Schwab M, Zettl UK, Köhler W (2014) How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? J Neurol Sci 343:91–99
Fleming NH, Bahorik A, Xia F, Yaffe K (2024) Risk of dementia in older veterans with multiple sclerosis. Mult Scler Relat Disord 82:105372
Foolad F, Samadi-Bahrami Z, Khodagholi F, Nabavi SM, Moore GRW, Javan M (2023) Sirtuins and metabolism biomarkers in relapsing-remitting and secondary progressive multiple sclerosis: a correlation study with clinical outcomes and cognitive impairments. Mol Neurobiol 61:3442–3460
Fuchs TA, Ziccardi S, Benedict RHB, Bartnik A, Kuceyeski A, Charvet LE, Oship D, Weinstock-Guttman B, Wojcik C, Hojnacki D, Kolb C, Escobar J, Campbell R, Tran HD, Bergsland N, Jakimovski D, Zivadinov R, Dwyer MG (2020) Functional connectivity and structural disruption in the default-mode network predicts cognitive rehabilitation outcomes in multiple sclerosis. J Neuroimaging 30:523–530
Gaetani L, Salvadori N, Lisetti V, Eusebi P, Mancini A, Gentili L, Borrelli A, Portaccio E, Sarchielli P, Blennow K, Zetterberg H, Parnetti L, Calabresi P, Di Filippo M (2019) Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J Neurol 266:2157–2163
Gaspari M, Zini F, Stecchi S (2023) Enhancing cognitive rehabilitation in multiple sclerosis with a disease-specific tool. Disabil Rehabil Assist Technol 18:313–326
Genova HM, Lancaster K, Myszko Z, Morecraft J, Leddy J, Smith A, Chiaravalloti N, Lengenfelder J (2022) Emotional processing intervention (EMOPRINT): a blinded randomized control trial to treat facial affect recognition deficits in multiple sclerosis. Mult Scler Relat Disord 59:103536
Ghadiri F, Ebadi Z, Asadollahzadeh E, Naser Moghadasi A (2022) Gut microbiome in multiple sclerosis-related cognitive impairment. Mult Scler Relat Disord 67:104165
Giannopapas V, Stavrogianni K, Christouli N, Kitsos D, Sideri E, Bakalidou D, Voumvourakis K, Papagiannopoulou G, Tzartos J, Paraskevas G, Tsivgoulis G, Giannopoulos S (2023) Do cardiovascular disease comorbidities affect the cognitive function of multiple sclerosis patients? J Clin Neurosci 112:20–24
Gich J, Freixanet J, García R, Vilanova JC, Genís D, Silva Y, Montalban X, Ramió-Torrentà L (2015) A randomized, controlled, single-blind, 6-month pilot study to evaluate the efficacy of MS-Line! A cognitive rehabilitation programme for patients with multiple sclerosis. Mult Scler 21:1332–1343
Gich J, Salavedra-Pont J, Coll-Martinez C, Quintana E, Álvarez-Bravo G, Robles-Cedeño R, Buxó M, Contreras-Rodriguez O, Ramió-Torrentà L (2024) The nature of memory impairment in multiple sclerosis: understanding different patterns over the course of the disease. Front Psychol 14:1269794
Gois LCP, Pimentel-Silva LR, Damasceno BP, Damasceno A (2021) Associations between cognitive and clinical disability across MS subtypes: the role of the underlying brain damage. Mult Scler Relat Disord 48:102701
Golan D, Doniger GM, Wissemann K, Zarif M, Bumstead B, Buhse M, Fafard L, Lavi I, Wilken J, Gudesblatt M (2018) The impact of subjective cognitive fatigue and depression on cognitive function in patients with multiple sclerosis. Mult Scler 24:196–204
Gouveia A, Dias SP, Santos T, Rocha H, Coelho CR, Ruano L, Galego O, Diogo MC, Seixas D, Sá MJ, Batista S (2017) Cognitive impairment and magnetic resonance imaging correlates in primary progressive multiple sclerosis. Acta Neurol Scand 136:109–115
Granberg T, Martola J, Bergendal G, Shams S, Damangir S, Aspelin P, Fredrikson S, Kristoffersen-Wiberg M (2015) Corpus callosum atrophy is strongly associated with cognitive impairment in multiple sclerosis: results of a 17-year longitudinal study. Mult Scler 21:1151–1158
Grant JG, Rapport LJ, Darling R, Waldron-Perrine B, Lumley MA, Whitfield KE, Bernitsas E (2023) Cognitive enrichment and education quality moderate cognitive dysfunction in black and white adults with multiple sclerosis. Mult Scler Relat Disord 78:104916
Gu XQ, Liu Y, Gu JB, Li LF, Fu LL, Han XM (2022) Correlations between hippocampal functional connectivity, structural changes, and clinical data in patients with relapsing-remitting multiple sclerosis: a case-control study using multimodal magnetic resonance imaging. Neural Regen Res 17:1115–1124
Hancock LM, Galioto R, Samsonov A, Busch RM, Hermann B, Matias-Guiu JA (2023) A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: the International classification of Cognitive disorders in MS (IC-CoDiMS). Mult Scler 29:615–627
Hardmeier M, Schoonheim MM, Geurts JJ, Hillebrand A, Polman CH, Barkhof F, Stam CJ (2012) Cognitive dysfunction in early multiple sclerosis: altered centrality derived from resting-state functional connectivity using magneto-encephalography. PLoS ONE 7:e42087
Has Silemek AC, Fischer L, Pöttgen J, Penner IK, Engel AK, Heesen C, Gold SM, Stellmann JP (2020) Functional and structural connectivity substrates of cognitive performance in relapsing remitting multiple sclerosis with mild disability. Neuroimage Clin 25:102177
Has Silemek AC, Nolte G, Pöttgen J, Engel AK, Heesen C, Gold SM, Stellmann JP (2023) Topological reorganization of brain network might contribute to the resilience of cognitive functioning in mildly disabled relapsing remitting multiple sclerosis. J Neurosci Res 101:143–161
Henry JD, Beatty WW (2006) Verbal fluency deficits in multiple sclerosis. Neuropsychologia 44:1166–1174
Henry A, Stefaniak N, Schmid F, Kwiatkowski A, Hautecoeur P, Lenne B (2023) Assessing cognitive changes in multiple sclerosis: criteria for a reliable decision. J Clin Exp Neuropsychol 45:321–344
Hernández-Ledesma AL, Rodríguez-Méndez AJ, Gallardo-Vidal LS, García-Gasca T, Alatorre-Cruz JM, García-Solís P, López Reyes J, Solís-Saínz JC (2020) Lipid profile: causal relationship on cognitive performance in multiple sclerosis? Mol Biol Rep 47:9667–9676
Hiew S, Nguemeni C, Zeller D (2022) Efficacy of transcranial direct current stimulation in people with multiple sclerosis: a review. Eur J Neurol 29:648–664
Hsu WY, Rowles W, Anguera JA, Anderson A, Younger JW, Friedman S, Gazzaley A, Bove R (2021) Assessing cognitive function in multiple sclerosis with digital tools: observational study. J Med Internet Res 23:e25748
Hsu WY, Zanto T, Park JE, Gazzaley A, Bove RM (2023) Effects of transcranial alternating current stimulation on cognitive function in people with multiple sclerosis: a randomized controlled trial. Mult Scler Relat Disord 80:105090
Huiskamp M, Yaqub M, van Lingen MR, Pouwels PJW, de Ruiter LRJ, Killestein J, Schwarte LA, Golla SSV, van Berckel BNM, Boellaard R, Geurts JJG, Hulst HE (2023) Cognitive performance in multiple sclerosis: what is the role of the gamma-aminobutyric acid system? Brain Commun 5:fcad140
Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BM, Barkhof F, Geurts JJ (2015) Memory impairment in multiple sclerosis: relevance of hippocampal activation and hippocampal connectivity. Mult Scler 21:1705–1712
Hyncicová E, Vyhnálek M, Kalina A, Martinkovic L, Nikolai T, Lisý J, Hort J, Meluzínová E, Laczó J (2017) Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J Neurol 264:482–493
Hynes SM, O’Keeffe F, Bane E, Oglesby MH, Dwyer CP, Joyce R, Klein OA (2022) Assessment and management of cognitive and psychosocial difficulties for people with multiple sclerosis in Ireland: a national survey of clinical practice. Int J Clin Pract 2022:3232076
Ikram MA, Vernooij MW, Roshchupkin GV, Hofman A, van Duijn CM, Uitterlinden AG, Niessen WJ, Hintzen RQ, Adams HH (2017) Genetic susceptibility to multiple sclerosis: brain structure and cognitive function in the general population. Mult Scler 23:1697–1706
Iliadou P, Bakirtzis C, Ioannidis P, Possin K, Zygouris S, Sintila SA, Grigoriadis N, Aretouli E (2022) Neuropsychological correlates of cerebellar volumes in multiple sclerosis: an MRI volumetric analysis study. J Integr Neurosci 21:13
Iodice R, Aceto G, Ruggiero L, Cassano E, Manganelli F, Dubbioso R (2023) A review of current rehabilitation practices and their benefits in patients with multiple sclerosis. Mult Scler Relat Disord 69:104460
Jackson DA, Nicholson R, Bergmann C, Wilken J, Kaczmarek O, Bumstead B, Buhse M, Zarif M, Penner IK, Hancock LM, Golan D, Doniger GM, Bogaardt H, Barrera M, Covey TJ, Gudesblatt M (2023) Cognitive impairment in people with multiple sclerosis: perception vs. performance - factors that drive perception of impairment differ for patients and clinicians. Mult Scler Relat Disord 69:104410
Jakimovski D, Benedict RH, Marr K, Gandhi S, Bergsland N, Weinstock-Guttman B, Zivadinov R (2020) Lower total cerebral arterial flow contributes to cognitive performance in multiple sclerosis patients. Mult Scler 26:201–209
Jakimovski D, Zivadinov R, Weinstock Z, Fuchs TA, Bartnik A, Dwyer MG, Bergsland N, Weinstock-Guttman B, Benedict RHB (2023) Cortical thickness and cognition in older people with multiple sclerosis. J Neurol 270:5223–5234
Jamoussi H, Ali NB, Missaoui Y, Cherif A, Oudia N, Anane N, Ftouhi L, Mahmoud MB, Fray S, Fredj M (2023) Cognitive impairment in multiple sclerosis: utility of electroencephalography. Mult Scler Relat Disord 70:104502
Jandric D, Lipp I, Paling D, Rog D, Castellazzi G, Haroon H, Parkes L, Parker GJM, Tomassini V, Muhlert N (2021) Mechanisms of network changes in cognitive impairment in multiple sclerosis. Neurology 97:e1886–e1897
Jandric D, Doshi A, Scott R, Paling D, Rog D, Chataway J, Schoonheim MM, Parker G, Muhlert N (2022) A systematic review of resting-state functional MRI connectivity changes and cognitive impairment in multiple sclerosis. Brain Connect 12:112–133
Johnen A, Landmeyer NC, Bürkner PC, Wiendl H, Meuth SG, Holling H (2017) Distinct cognitive impairments in different disease courses of multiple sclerosis-A systematic review and meta-analysis. Neurosci Biobehav Rev 83:568–578
Johnen A, Bürkner PC, Landmeyer NC, Ambrosius B, Calabrese P, Motte J, Hessler N, Antony G, König IR, Klotz L, Hoshi MM, Aly L, Groppa S, Luessi F, Paul F, Tackenberg B, Bergh FT, Kümpfel T, Tumani H, Stangel M, Weber F, Bayas A, Wildemann B, Heesen C, Zettl UK, Zipp F, Hemmer B, Meuth SG, Gold R, Wiendl H, Salmen A (2019) Can we predict cognitive decline after initial diagnosis of multiple sclerosis? Results from the German National early MS cohort (KKNMS). J Neurol 266:386–397
Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, Gingold J, Goverover Y, Halper J, Harris C, Kostich L, Krupp L, Lathi E, LaRocca N, Thrower B, DeLuca J (2018) Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler 24:1665–1680
Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E, Giesser B, Halper J, Karpatkin H, Keller J, Ng AV, Pilutti LA, Rohrig A, Van Asch P, Zackowski K, Motl RW (2020) Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler 26:1459–1469
Kamal A, Swellam M, Shalaby NM, Darwish MK, El-Nahrery EM (2023) Long non-coding RNAs BACE1-AS and BC200 in multiple sclerosis and their relation to cognitive function: a gene expression analysis. Brain Res 1814:148424
Kania K, Ambrosius W, Kozubski W, Kalinowska-Lyszczarz A (2023) The impact of disease modifying therapies on cognitive functions typically impaired in multiple sclerosis patients: a clinician’s review. Front Neurol 14:1222574
Kania K, Pawlak MA, Forycka M, Wilkosc-Debczynska M, Michalak S, Lukaszewska A, Wyciszkiewicz A, Wypych A, Serafin Z, Marcinkowska J, Kozubski W, Kalinowska-Lyszczarz A (2024) Predicting clinical progression and cognitive decline in patients with relapsing-remitting multiple sclerosis: a 6-year follow-up study. Neurol Neurochir Pol 58:176–184
Kantorová E, Hnilicová P, Bogner W, Grendár M, Grossmann J, Kovácová S, Hecková E, Strasser B, Cierny D, Zelenák K, Kurca E (2022) Neurocognitive performance in relapsing-remitting multiple sclerosis patients is associated with metabolic abnormalities of the thalamus but not the hippocampus- GABA-edited 1H MRS study. Neurol Res 44:57–64
Kern KC, Gold SM, Lee B, Montag M, Horsfall J, O’Connor MF, Sicotte NL (2014) Thalamic-hippocampal-prefrontal disruption in relapsing-remitting multiple sclerosis. Neuroimage Clin 8:440–447
Keser Z, Hasan KM, Mwangi B, Gabr RE, Steinberg JL, Wilken J, Wolinsky JS, Nelson FM (2017) Limbic pathway correlates of cognitive impairment in multiple sclerosis. J Neuroimaging 27:37–42
Keser Z, Kamali A, Younes K, Schulz PE, Nelson FM, Hasan KM (2018) Yakovlev’s basolateral limbic circuit in multiple sclerosis related cognitive impairment. J Neuroimaging 28:596–600
Khalil M, Enzinger C, Langkammer C, Petrovic K, Loitfelder M, Tscherner M, Jehna M, Bachmaier G, Wallner-Blazek M, Ropele S, Schmidt R, Fuchs S, Fazekas F (2011) Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult Scler 17:173–180
Klein OA, Drummond A, Mhizha-Murira JR, Mansford L, dasNair R (2019) Effectiveness of cognitive rehabilitation for people with multiple sclerosis: a meta-synthesis of patient perspectives. Neuropsychol Rehabil 29:491–512
Kletenik I, Cohen AL, Glanz BI, Ferguson MA, Tauhid S, Li J, Drew W, Polgar-Turcsanyi M, Palotai M, Siddiqi SH, Marshall GA, Chitnis T, Guttmann CRG, Bakshi R, Fox MD (2023) Multiple sclerosis lesions that impair memory map to a connected memory circuit. J Neurol 270:5211–5222
Koenig KA, Sakaie KE, Lowe MJ, Lin J, Stone L, Bermel RA, Beall EB, Rao SM, Trapp BD, Phillips MD (2014) Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magn Reson Imaging 32:354–358
Kopchak OO, Odintsova TA, Pulyk OR (2021) Cognitive functions in multiple sclerosis patients depending on the different risk factors presence. Wiad Lek 74:2444–2451
Koubiyr I, Broeders TA, Deloire M, Brochet B, Tourdias T, Geurts JJ, Schoonheim MM, Ruet A (2022) Altered functional brain states predict cognitive decline 5 years after a clinically isolated syndrome. Mult Scler 28:1973–1982
Koubiyr I, Krijnen EA, Eijlers AJC, Dekker I, Hulst HE, Uitdehaag BMJ, Barkhof F, Geurts JJG, Schoonheim MM (2024) Longitudinal fibre-specific white matter damage predicts cognitive decline in multiple sclerosis. Brain Commun 6:fcae018
Koudriavtseva T, Mainero C (2016) Brain atrophy as a measure of neuroprotective drug effects in multiple sclerosis: influence of inflammation. Front Hum Neurosci 10:226
Krupp LB, Waubant E, Waltz M, Casper TC, Belman A, Wheeler Y, Ness J, Graves J, Gorman M, Benson L, Mar S, Goyal M, Schreiner T, Weinstock-Guttman B, Rodriguez M, Tillema JM, Lotze T, Aaen G, Rensel M, Rose J, Chitinis T, George A, Charvet LE (2023) A new look at cognitive functioning in pediatric MS. Mult Scler 29:140–149
Kulik SD, Nauta IM, Tewarie P, Koubiyr I, van Dellen E, Ruet A, Meijer KA, de Jong BA, Stam CJ, Hillebrand A, Geurts JJG, Douw L, Schoonheim MM (2022) Structure-function coupling as a correlate and potential biomarker of cognitive impairment in multiple sclerosis. Netw Neurosci 6:339–356
Labiano-Fontcuberta A, Costa-Frossard L, Sainz de la Maza S, Rodríguez-Jorge F, Chico-García JL, Monreal E (2022) The effect of timing of high-efficacy therapy on processing speed performance in multiple sclerosis. Mult Scler Relat Disord 64:103959
Landmeyer NC, Bürkner PC, Wiendl H, Ruck T, Hartung HP, Holling H, Meuth SG, Johnen A (2020) Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: a meta-analysis. Neurology 94:e2373–e2383
Landrigan J, Bessenyei K, Leitner D, Yakovenko I, Fisk JD, Prentice JL (2022) A systematic review of the effects of cannabis on cognition in people with multiple sclerosis. Mult Scler Relat Disord 57:103338
Langdon DW (2011) Cognition in multiple sclerosis. Curr Opin Neurol 24:244–249
Langley C, Masuda N, Godwin S, De Marco G, Smith AD, Jones R, Bruce J, Thai NJ (2023) Dysfunction of basal ganglia functional connectivity associated with subjective and cognitive fatigue in multiple sclerosis. Front Neurosci 17:1194859
Lanzillo R, Chiodi A, Carotenuto A, Magri V, Napolitano A, Liuzzi R, Costabile T, Rainone N, Freda MF, Valerio P, Brescia Morra V (2016) Quality of life and cognitive functions in early onset multiple sclerosis. Eur J Paediatr Neurol 20:158–163
Lassmann H (2018) Multiple sclerosis pathology. Cold Spring Harb Perspect Med 8:a028936
Leavitt VM, Cirnigliaro C, Cohen A, Farag A, Brooks M, Wecht JM, Wylie GR, Chiaravalloti ND, DeLuca J, Sumowski JF (2014) Aerobic exercise increases hippocampal volume and improves memory in multiple sclerosis: preliminary findings. Neurocase 20:695–697
Leavitt VM, Tosto G, Riley CS (2018) Cognitive phenotypes in multiple sclerosis. J Neurol 265:562–566
Lebrun C (2015) The radiologically isolated syndrome. Rev Neurol (Paris) 171:698–706
Lefferts WK, Rosenberg AJ, Schroeder EC, Grigoriadis G, Sandroff BM, Motl RW, Baynard T (2021) Assessment of cerebrovascular dynamics and cognitive function with acute aerobic exercise in persons with multiple sclerosis. Int J MS Care 23:162–169
Li F, Zong W, Xin C, Ren F, Li N, Li H, Li X, Wu L, Dai Z, Chen W, Li M, Gao F, Wang G (2024) Unlocking the link: how hippocampal glutathione-glutamate coupling predicts cognitive impairment in multiple sclerosis patients. Cereb Cortex 34:bhad400
Llufriu S, Martinez-Heras E, Solana E, Sola-Valls N, Sepulveda M, Blanco Y, Martinez-Lapiscina EH, Andorra M, Villoslada P, Prats-Galino A, Saiz A (2016) Structural networks involved in attention and executive functions in multiple sclerosis. Neuroimage Clin 13:288–296
London F, De Haan A, Benyahia Z, Landenne G, Duprez T, van Pesch V, El Sankari S (2023) Cognitive trajectories in relapsing-remitting multiple sclerosis: evidence of multiple evolutionary trends. Mult Scler Relat Disord 77:104848
Lopez-Soley E, Solana E, Martínez-Heras EE, Andorra M, Radua J, Prats-Uribe A, Montejo C, Sola-Valls N, Sepulveda M, Pulido-Valdeolivas I, Blanco Y, Martinez-Lapiscina EH, Saiz A, Llufriu S (2020) Impact of cognitive reserve and structural connectivity on cognitive performance in multiple sclerosis. Front Neurol 11:581700
Lopez-Soley E, Martinez-Heras E, Andorra M, Solanes A, Radua J, Montejo C, Alba-Arbalat S, Sola-Valls N, Pulido-Valdeolivas I, Sepulveda M, Romero-Pinel L, Munteis E, Martínez-Rodríguez JE, Blanco Y, Martinez-Lapiscina EH, Villoslada P, Saiz A, Solana E, Llufriu S (2021) Dynamics and predictors of cognitive impairment along the disease course in multiple sclerosis. J Pers Med 11:1107
Lorefice L, Carta E, Frau J, Contu F, Casaglia E, Coghe G, Barracciu MA, Cocco E, Fenu G (2020) The impact of deep grey matter volume on cognition in multiple sclerosis. Mult Scler Relat Disord 45:102351
Lorefice L, Fenu G, Mammoliti R, Carta E, Sechi V, Frau J, Coghe G, Canalis L, Barracciu MA, Marrosu G, Marrosu MG, Cocco E (2021) Event-related potentials and deep grey matter atrophy in multiple sclerosis: exploring the possible associations with cognition. Mult Scler Relat Disord 49:102785
Lott S, Koszalinski R, Hurt M, Harris R (2022) Looking beyond physical disability: cognitive impairment in persons with multiple sclerosis. Rehabil Nurs 47:179–186
Louapre C, Perlbarg V, García-Lorenzo D, Urbanski M, Benali H, Assouad R, Galanaud D, Freeman L, Bodini B, Papeix C, Tourbah A, Lubetzki C, Lehéricy S, Stankoff B (2014) Brain networks disconnection in early multiple sclerosis cognitive deficits: an anatomofunctional study. Hum Brain Mapp 35:4706–4717
Lugosi K, Engh MA, Huszár Z, Hegyi P, Mátrai P, Csukly G, Molnár Z, Horváth K, Mátis D, Mezei Z (2024) Domain-specific cognitive impairment in multiple sclerosis: a systematic review and meta-analysis. Ann Clin Transl Neurol 11:564–576
Luo D, Peng Y, Zhu Q, Zheng Q, Luo Q, Han Y, Chen X, Li Y (2024) U-fiber diffusion kurtosis and susceptibility characteristics in relapsing-remitting multiple sclerosis may be related to cognitive deficits and neurodegeneration. Eur Radiol 34:1422–1433
Maciak K, Dziedzic A, Saluk J (2023) Possible role of the NLRP3 inflammasome and the gut-brain axis in multiple sclerosis-related depression. FASEB J 37:e22687
Maffezzini S, Pucci V, Riccardi A, Montemurro S, Puthenparampil M, Perini P, Rinaldi F, Gallo P, Arcara G, Mondini S (2023) Clinical profiles in multiple sclerosis: cognitive reserve and motor impairment along disease duration. Behav Sci (Basel) 13:708
Maiese K (2023) Cognitive impairment in multiple sclerosis. Bioeng (Basel) 10:871
Mardaniyan Ghahfarrokhi M, Banitalebi E, Faramarzi M, Motl R (2022) Feasibility and efficacy of home-based neurofunctional exercise vs. resistance exercise programs for ambulatory disability of multiple sclerosis patients with cognitive impairment. Mult Scler Relat Disord 58:103400
Margoni M, Preziosa P, Rocca MA, Filippi M (2023) Depressive symptoms, anxiety and cognitive impairment: emerging evidence in multiple sclerosis. Transl Psychiatry 13:264
Margoni M, Pagani E, Meani A, Preziosa P, Mistri D, Gueye M, Moiola L, Filippi M, Rocca MA (2024) Cognitive impairment is related to glymphatic system dysfunction in pediatric multiple sclerosis. Ann Neurol: https://doi.org/10.1002/ana.26911
Mashayekhi F, Sadigh-Eteghad S, Naseri A, Asadi M, Abbasi Garravnd N, Talebi M (2022) ApoE4-positive multiple sclerosis patients are more likely to have cognitive impairment: a cross-sectional study. Neurol Sci 43:1189–1196
McKay KA, Manouchehrinia A, Berrigan L, Fisk JD, Olsson T, Hillert J (2019) Long-term cognitive outcomes in patients with pediatric-onset vs adult-onset multiple sclerosis. JAMA Neurol 76:1028–1034
McManus EJ, Clark KM, Frampton C, Macniven JAB, Schepel J (2022) Extended interval dosing natalizumab and impact on neuropsychological deficits in relapsing-remitting multiple sclerosis. Mult Scler J Exp Transl Clin 8:20552173211070752
Meca-Lallana V, Gascón-Giménez F, Ginestal-López RC, Higueras Y, Téllez-Lara N, Carreres-Polo J, Eichau-Madueño S, Romero-Imbroda J, Vidal-Jordana Á, Pérez-Miralles F (2021) Cognitive impairment in multiple sclerosis: diagnosis and monitoring. Neurol Sci 42:5183–5193
Meijer KA, Eijlers AJC, Douw L, Uitdehaag BMJ, Barkhof F, Geurts JJG, Schoonheim MM (2017) Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology 88:2107–2114
Meira T, Coelho A, Onat S, Ruano L, Cerqueira JJ (2023) One-year regional brain volume changes as potential predictors of cognitive function in multiple sclerosis: a pilot study. Ir J Med Sci 193:957–965
Meli R, Roccatagliata L, Capello E, Bruschi N, Uccelli A, Mancardi G, Inglese M, Pardini M (2020) Ecological impact of isolated cognitive relapses in MS. Mult Scler 26:114–117
Messinis L, Apostolopoulos D, Spiridonidis T, Nasios G, Giazkoulidou A, Tsolaki M, Panagiotopoulos V (2019) Robust regional cerebral blood flow perfusion deficits in relapsing-remitting multiple sclerosis patients with executive function impairment. Hell J Nucl Med 22:147–159
Metzger A, Le Bars E, Deverdun J, Molino F, Maréchal B, Picot MC, Ayrignac X, Carra C, Bauchet L, Krainik A, Labauge P, de Menjot N (2018) Is impaired cerebral vasoreactivity an early marker of cognitive decline in multiple sclerosis patients? Eur Radiol 28:1204–1214
Migliore S, Ghazaryan A, Simonelli I, Pasqualetti P, Squitieri F, Curcio G, Landi D, Palmieri MG, Moffa F, Filippi MM, Vernieri F (2017) Cognitive impairment in relapsing-remitting multiple sclerosis patients with very mild clinical disability. Behav Neurol 2017:7404289
Mistri D, Cacciaguerra L, Valsasina P, Pagani E, Filippi M, Rocca MA (2023a) Cognitive function in primary and secondary progressive multiple sclerosis: a multiparametric magnetic resonance imaging study. Eur J Neurol 30:2801–2810
Mistri D, Tedone N, Biondi D, Vizzino C, Pagani E, Rocca MA, Filippi M (2023b) Cognitive phenotypes in multiple sclerosis: mapping the spectrum of impairment. J Neurol 271:1571–1583
Morrow SA, Weinstock ZL, Mirmosayyeb O, Conway D, Fuchs T, Jaworski MG, Eckert S, Hojnacki DH, Dwyer MG, Zivadinov R, Weinstock-Guttman B, Benedict RHB (2023) Detecting isolated cognitive relapses in persons with MS. Mult Scler 29:1786–1794
Motavalli A, Majdi A, Hosseini L, Talebi M, Mahmoudi J, Hosseini SH, Sadigh-Eteghad S (2020) Pharmacotherapy in multiple sclerosis-induced cognitive impairment: a systematic review and meta-analysis. Mult Scler Relat Disord 46:102478
Motl RW, Sandroff BM, Benedict RHB, Aldunate R, Cutter G, Barron E (2024) Internet-delivered lifestyle physical activity intervention for cognitive processing speed in multiple sclerosis. Contemp Clin Trials 138:107446
Nabizadeh F, Balabandian M, Rostami MR, Owji M, Sahraian MA, Bidadian M, Ghadiri F, Rezaeimanesh N, Moghadasi AN (2022) Association of cognitive impairment and quality of life in patients with multiple sclerosis: a cross-sectional study. Curr J Neurol 21:144–150
Nabizadeh F, Pirahesh K, Azami M, Moradkhani A, Sardaripour A, Ramezannezhad E (2024) T1 and T2 weighted lesions and cognition in multiple sclerosis: a systematic review and meta-analysis. J Clin Neurosci 119:1–7
Naseri A, Baghernezhad K, Seyedi-Sahebari S, Alhoseini SA, Gholipour-Khalili E, Zafarani F, Talebi M (2022) The association of apolipoprotein E (ApoE) genotype and cognitive outcomes in multiple sclerosis; a systematic review and meta-analysis. Mult Scler Relat Disord 65:104011
Nauta IM, Kulik SD, Breedt LC, Eijlers AJ, Strijbis EM, Bertens D, Tewarie P, Hillebrand A, Stam CJ, Uitdehaag BM, Geurts JJ, Douw L, de Jong BA, Schoonheim MM (2021) Functional brain network organization measured with magnetoencephalography predicts cognitive decline in multiple sclerosis. Mult Scler 27:1727–1737
Nauta IM, Bertens D, Fasotti L, Fieldhouse J, Uitdehaag BMJ, Kessels RPC, Speckens AEM, de Jong BA (2023) Cognitive rehabilitation and mindfulness reduce cognitive complaints in multiple sclerosis (REMIND-MS): a randomized controlled trial. Mult Scler Relat Disord 71:104529
Nauta IM, Kessels RPC, Bertens D, Stam CJ, Strijbis EEM, Hillebrand A, Fasotti L, Uitdehaag BMJ, Hulst HE, Speckens AEM, Schoonheim MM, de Jong BA (2024) Neurophysiological brain function predicts response to cognitive rehabilitation and mindfulness in multiple sclerosis: a randomized trial. J Neurol 271:1649–1662
Nejad-Davarani SP, Chopp M, Peltier S, Li L, Davoodi-Bojd E, Lu M, Bagher-Ebadian H, Budaj J, Gallagher D, Ding Y, Hearshen D, Jiang Q, Cerghet M (2016) Resting state fMRI connectivity analysis as a tool for detection of abnormalities in five different cognitive networks of the brain in multiple sclerosis patients. Clin Case Rep Rev 2:464–471
NICE-Guideline (2022) Multiple sclerosis in adults: management. https://www.nice.org.uk/guidance/ng220/resources/multiple-sclerosis-in-adults-management-pdf-66143828948677.
Nold AK, Wittayer M, Weber CE, Platten M, Gass A, Eisele P (2023) Short-term brain atrophy evolution after initiation of immunotherapy in a real-world multiple sclerosis cohort. J Neuroimaging 33:904–908
Noori H, Gheini MR, Rezaeimanesh N, Saeedi R, Rezaei Aliabadi H, Sahraian MA, Naser Moghadasi A (2019) The correlation between dyslipidemia and cognitive impairment in multiple sclerosis patients. Mult Scler Relat Disord 36:101415
Noteboom S, van Nederpelt DR, Bajrami A, Moraal B, Caan MWA, Barkhof F, Calabrese M, Vrenken H, Strijbis EMM, Steenwijk MD, Schoonheim MM (2023) Feasibility of detecting atrophy relevant for disability and cognition in multiple sclerosis using 3D-FLAIR. J Neurol 270:5201–5210
Nourbakhsh B, Nunan-Saah J, Maghzi AH, Julian LJ, Spain R, Jin C, Lazar A, Pelletier D, Waubant E (2016) Longitudinal associations between MRI and cognitive changes in very early MS. Mult Scler Relat Disord 5:47–52
O’Brien AR, Chiaravalloti N, Goverover Y, Deluca J (2008) Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: a review of the literature. Arch Phys Med Rehabil 89:761–769
Oset M, Domowicz M, Wildner P, Siger M, Karlinska I, Stasiolek M, Swiderek-Matysiak M (2023) Predictive value of brain atrophy, serum biomarkers and information processing speed for early disease progression in multiple sclerosis. Front Neurol 14:1223220
Ozakbas S, Turkoglu R, Tamam Y, Terzi M, Taskapilioglu O, Yucesan C, Baser HL, Gencer M, Akil E, Sen S, Turan OF, Sorgun MH, Yigit P, Turkes N (2018) Prevalence of and risk factors for cognitive impairment in patients with relapsing-remitting multiple sclerosis: multi-center, controlled trial. Mult Scler Relat Disord 22:70–76
Ozkul C, Guclu-Gunduz A, Eldemir K, Apaydin Y, Yazici G, Irkec C (2020) Combined exercise training improves cognitive functions in multiple sclerosis patients with cognitive impairment: a single-blinded randomized controlled trial. Mult Scler Relat Disord 45:102419
Öztürk Z, Gücüyener K, Soysal S, Konuskan GD, Konuskan B, Dikmen AU, Anlar B (2020) Cognitive functions in pediatric multiple sclerosis: 2-years follow-up. Neurol Res 42:159–163
Pardini M, Uccelli A, Grafman J, Yaldizli Ö, Mancardi G, Roccatagliata L (2014) Isolated cognitive relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry 85:1035–1037
Patti F, Nicoletti A, Messina S, Bruno E, Fermo SL, Quattrocchi G, Chisari CG, Maimone D, Cilia S, Zappia M (2015) Prevalence and incidence of cognitive impairment in multiple sclerosis: a population-based survey in Catania, Sicily. J Neurol 262:923–930
Paul F (2016) Pathology and MRI: exploring cognitive impairment in MS. Acta Neurol Scand 134 Suppl 200:24–33
Pennington CR, Oxtoby MC, Shaw DJ (2024) Social cognitive disruptions in multiple sclerosis: the role of executive (dys)function. Neuropsychology 38:157–168
Peño LIC, De Miguel S, de Torres CL, Ortiz L, Moreno ME, Rodeño MJG, Carpio BO, Muñoz RT, Montoya JS, Sepúlveda BPD, De Antonio Sanz MÁS, Ayuso E, Salaices SA MG (2023) Brain atrophy and physical and cognitive disability in multiple sclerosis. Basic Clin Neurosci 14:311–316
Pérez-Martín MY, González-Platas M, Eguía-Del Río P, Croissier-Elías C, Jiménez Sosa A (2017) Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr Dis Treat 13:245–252
Perrenoud M, Blosch L, Du Pasquier R, Pot C (2023) [Cognitive impairment in multiple sclerosis: when to think about it?]. Rev Med Suisse 19:791–793
Perumal J, Balabanov R, Su R, Chang R, Balcer LJ, Galetta SL, Avila RL, Rutledge D, Fox RJ (2023) Improvements in cognitive processing speed, disability, and patient-reported outcomes in patients with early relapsing-remitting multiple sclerosis treated with natalizumab: results of a 4-year, real-world, open-label study. CNS Drugs 37:275–289
Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR Jr (2009) Mild cognitive impairment: ten years later. Arch Neurol 66:1447–1455
Petracca M, Saiote C, Bender HA, Arias F, Farrell C, Magioncalda P, Martino M, Miller A, Northoff G, Lublin F, Inglese M (2017) Synchronization and variability imbalance underlie cognitive impairment in primary-progressive multiple sclerosis. Sci Rep 7:46411
Piacentini C, Argento O, Nocentini U (2023) Cognitive impairment in multiple sclerosis: classic knowledge and recent acquisitions. Arq Neuropsiquiatr 81:585–596
Pinkston JB, Kablinger A, Alekseeva N (2007) Multiple sclerosis and behavior. Int Rev Neurobiol 79:323–339
Pinter D, Sumowski J, DeLuca J, Fazekas F, Pichler A, Khalil M, Langkammer C, Fuchs S, Enzinger C (2014) Higher education moderates the effect of T2 lesion load and third ventricle width on cognition in multiple sclerosis. PLoS ONE 9:e87567
Pinter D, Kober SE, Fruhwirth V, Berger L, Damulina A, Khalil M, Neuper C, Wood G, Enzinger C (2021) MRI correlates of cognitive improvement after home-based EEG neurofeedback training in patients with multiple sclerosis: a pilot study. J Neurol 268:3808–3816
Pitteri M, Magliozzi R, Nicholas R, Ziccardi S, Pisani AI, Pezzini F, Marastoni D, Calabrese M (2022) Cerebrospinal fluid inflammatory profile of cognitive impairment in newly diagnosed multiple sclerosis patients. Mult Scler 28:768–777
Planche V, Gibelin M, Cregut D, Pereira B, Clavelou P (2016) Cognitive impairment in a population-based study of patients with multiple sclerosis: differences between late relapsing-remitting, secondary progressive and primary progressive multiple sclerosis. Eur J Neurol 23:282–289
Podda J, Ponzio M, Pedullà L, Monti Bragadin M, Battaglia MA, Zaratin P, Brichetto G, Tacchino A (2021) Predominant cognitive phenotypes in multiple sclerosis: insights from patient-centered outcomes. Mult Scler Relat Disord 51:102919
Portaccio E, Goretti B, Zipoli V, Nacmias B, Stromillo ML, Bartolozzi ML, Siracusa G, Guidi L, Federico A, Sorbi S, De Stefano N, Amato MP (2009) APOE-epsilon4 is not associated with cognitive impairment in relapsing-remitting multiple sclerosis. Mult Scler 15:1489–1494
Pourmohammadi A, Motahharynia A, Shaygannejad V, Ashtari F, Adibi I, Sanayei M (2023) Working memory dysfunction differs between secondary progressive and relapsing multiple sclerosis: effects of clinical phenotype, age, disease duration, and disability. Mult Scler Relat Disord 69:104411
Prajjwal P, Marsool MDM, Natarajan B, Inban P, Gadam S, Sowndarya D, John J, Abbas R, Vaja H, Marsool ADM, Amir Hussin O (2023) Juvenile multiple sclerosis: addressing epidemiology, diagnosis, therapeutic, and prognostic updates along with cognitive dysfunction and quality of life. Ann Med Surg (Lond) 85:4433–4441
Preziosa P, Rocca MA, Pagani E, Stromillo ML, Enzinger C, Gallo A, Hulst HE, Atzori M, Pareto D, Riccitelli GC, Copetti M, De Stefano N, Fazekas F, Bisecco A, Barkhof F, Yousry TA, Arévalo MJ, Filippi M (2016) Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: a Multicenter Study. Hum Brain Mapp 37:1627–1644
Preziosa P, Pagani E, Morelli ME, Copetti M, Martinelli V, Pirro F, Falini A, Comi G, Filippi M, Rocca MA (2017) DT MRI microstructural cortical lesion damage does not explain cognitive impairment in MS. Mult Scler 23:1918–1928
Preziosa P, Pagani E, Meani A, Marchesi O, Conti L, Falini A, Rocca MA, Filippi M (2023) NODDI, diffusion tensor microstructural abnormalities and atrophy of brain white matter and gray matter contribute to cognitive impairment in multiple sclerosis. J Neurol 270:810–823
Preziosa P, Pagani E, Meani A, Storelli L, Margoni M, Yudin Y, Tedone N, Biondi D, Rubin M, Rocca MA, Filippi M (2024) Chronic active lesions and larger choroid plexus explain cognition and fatigue in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 11:e200205
Pusswald G, Mildner C, Zebenholzer K, Auff E, Lehrner J (2014) A neuropsychological rehabilitation program for patients with multiple sclerosis based on the model of the ICF. NeuroRehabilitation 35:519–527
Rademacher TD, Meuth SG, Wiendl H, Johnen A, Landmeyer NC (2023) Molecular biomarkers and cognitive impairment in multiple sclerosis: state of the field, limitations, and future direction - A systematic review and meta-analysis. Neurosci Biobehav Rev 146:105035
Raimo S, Giorgini R, Gaita M, Costanzo A, Spitaleri D, Palermo L, Liuzza MT, Santangelo G (2023) Sensitivity of conventional cognitive tests in multiple sclerosis: application of item response theory. Mult Scler Relat Disord 69:104440
Randolph JJ, Randolph JS, Wishart HA (2017) Association between cognitive complaints and vulnerability to environmental distraction in multiple sclerosis. Arch Clin Neuropsychol 32:21–28
Randolph JJ, Randolph JS, Wishart HA (2022) Subgroup analysis of individuals with multiple sclerosis showing cognitive resilience. Arch Clin Neuropsychol 37:302–308
Reia A, Petruzzo M, Falco F, Costabile T, Conenna M, Carotenuto A, Petracca M, Servillo G, Lanzillo R, Brescia Morra V, Moccia M (2021) A retrospective exploratory analysis on cardiovascular risk and cognitive dysfunction in multiple sclerosis. Brain Sci 11:502
Renner A, Baetge SJ, Filser M, Ullrich S, Lassek C, Penner IK (2020) Characterizing cognitive deficits and potential predictors in multiple sclerosis: a large nationwide study applying brief International Cognitive Assessment for multiple sclerosis in standard clinical care. J Neuropsychol 14:347–369
Reuter F, Zaaraoui W, Crespy L, Faivre A, Rico A, Malikova I, Soulier E, Viout P, Ranjeva JP, Pelletier J, Audoin B (2011) Frequency of cognitive impairment dramatically increases during the first 5 years of multiple sclerosis. J Neurol Neurosurg Psychiatry 82:1157–1159
Riccitelli GC, Pagani E, Meani A, Valsasina P, Preziosa P, Filippi M, Rocca MA (2020) Cognitive impairment in benign multiple sclerosis: a multiparametric structural and functional MRI study. J Neurol 267:3508–3517
Rimkus CM, Schoonheim MM, Steenwijk MD, Vrenken H, Eijlers AJ, Killestein J, Wattjes MP, Leite CC, Barkhof F, Tijms BM (2019) Gray Matter networks and cognitive impairment in multiple sclerosis. Mult Scler 25:382–391
Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC, Martinelli V, Martinelli-Boneschi F, Falini A, Comi G, Filippi M (2018) Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult Scler 24:459–471
Rosenstein I, Axelsson M, Novakova L, Rasch S, Blennow K, Zetterberg H, Lycke J (2023) High levels of kappa free light chain synthesis predict cognitive decline in relapsing-remitting multiple sclerosis. Front Immunol 14:1106028
Roy S, Drake A, Snyder S, Cline B, Khan A, Fuchs T, Zivadinov R, Weinstock-Guttman B, Szigeti K, Benedict RHB (2018) Preliminary investigation of cognitive function in aged multiple sclerosis patients: challenges in detecting comorbid Alzheimer’s disease. Mult Scler Relat Disord 22:52–56
Ruano L, Portaccio E, Goretti B, Niccolai C, Severo M, Patti F, Cilia S, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Stampatori C, Trojano M, Viterbo RG, Amato MP (2017) Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler 23:1258–1267
Ruano L, Branco M, Portaccio E, Goretti B, Niccolai C, Patti F, Chisari C, Gallo P, Grossi P, Ghezzi A, Roscio M, Mattioli F, Stampatori C, Simone M, Viterbo RG, Amato MP (2018) Patients with paediatric-onset multiple sclerosis are at higher risk of cognitive impairment in adulthood: an Italian collaborative study. Mult Scler 24:1234–1242
Ruet A, Deloire M, Charré-Morin J, Hamel D, Brochet B (2013) Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology 80:1501–1508
Ruiz-Sánchez FJ, do, Rosário Martins M, Soares S, Romero-Morales C, López-López D, Gómez-Salgado J, Jiménez-Cebrián AM (2022) Impact of multiple sclerosis and its association with depression: an analytical case-control investigation. Healthcare (Basel) 10:2218
Sabanagic-Hajric S, Memic-Serdarevic A, Sulejmanpasic G, Salihovic-Besirovic D, Kurtovic A, Bajramagic N, Mehmedika-Suljic E (2023) Cognitive imapirment in multiple sclerosis: relation to dysability, duration and type of disease. Mater Sociomed 35:23–27
Salehizadeh S, Saeedi R, Sahraian MA, Rezaei Aliabadi H, Hashemi SN, Eskandarieh S, Gheini MR, Shahmirzaei S, Owji M, Naser Moghadasi A (2022) Effect of Rituximab on the cognitive impairment in patients with secondary progressive multiple sclerosis. Casp J Intern Med 13:484–489
Sanaie S, Koohi N, Mosaddeghi-Heris R, Rezai S, Movagharnia E, Karimi H, Moghaddamziabari S, Hamzehzadeh S, Gholipour-Khalili E, Talebi M, Naseri A (2024) Serum lipids and cognitive outcomes in multiple sclerosis; a systematic review and meta-analysis. Mult Scler Relat Disord 85:105530
Sandi D, Biernacki T, Szekeres D, Füvesi J, Kincses ZT, Rózsa C, Mátyás K, Kása K, Matolcsi J, Zboznovits D, Burány Z, Langane É, Vécsei L, Bencsik K (2017) Prevalence of cognitive impairment among Hungarian patients with relapsing-remitting multiple sclerosis and clinically isolated syndrome. Mult Scler Relat Disord 17:57–62
Sandroff BM, DeLuca J (2020) Will behavioral treatments for cognitive impairment in multiple sclerosis become standards-of-care? Int J Psychophysiol 154:67–79
Sandroff BM, Motl RW, Deluca J (2017) The influence of cognitive impairment on the fitness-cognition relationship in multiple sclerosis. Med Sci Sports Exerc 49:1184–1189
Santangelo G, Altieri M, Enzinger C, Gallo A, Trojano L (2019a) Cognitive reserve and neuropsychological performance in multiple sclerosis: a meta-analysis. Neuropsychology 33:379–390
Santangelo G, Altieri M, Gallo A, Trojano L (2019b) Does cognitive reserve play any role in multiple sclerosis? A meta-analytic study. Mult Scler Relat Disord 30:265–276
Saposnik G, Andhavarapu S, Sainz de la Maza S, Castillo-Triviño T, Borges M, Barón BP, Sotoca J, Alonso A, Caminero AB, Borrega L, Sánchez-Menoyo JL, Barrero-Hernández FJ, Calles C, Brieva L, Blasco MR, García-Soto JD, Del Campo-Amigo M, Navarro-Cantó L, Agüera E, Garcés M, Carmona O, Gabaldón-Torres L, Forero L, Hervás M, García-Arcelay E, Terzaghi M, Gómez-Ballesteros R, Maurino J (2022) Delayed cognitive processing and treatment status quo bias in early-stage multiple sclerosis. Mult Scler Relat Disord 68:104138
Sawan H, Li C, Buch S, Bernitsas E, Haacke EM, Ge Y, Chen Y (2024) Reduced oxygen extraction fraction in deep cerebral veins associated with cognitive impairment in multiple sclerosis (PREPRINT). https://doi.org/10.1101/2024.1101.1110.24301049. medRxiv 2024.01.10.24301049
Scaravilli A, Tranfa M, Pontillo G, Falco F, Criscuolo C, Moccia M, Monti S, Lanzillo R, Brescia Morra V, Palma G, Petracca M, Tedeschi E, Elefante A, Brunetti A, Cocozza S (2023) MR imaging signs of gadolinium retention are not associated with long-term motor and cognitive outcomes in multiple sclerosis. AJNR Am J Neuroradiol 44:396–402
Scarpina F, Tagini S (2017) The Stroop Color and Word Test. Front Psychol 8:557
Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, Barkhof F, Geurts JJ (2015) Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 84:776–783
Schoonheim MM, Douw L, Broeders TA, Eijlers AJ, Meijer KA, Geurts JJ (2021) The cerebellum and its network: disrupted static and dynamic functional connectivity patterns and cognitive impairment in multiple sclerosis. Mult Scler 27:2031–2039
Schoonhoven DN, Fraschini M, Tewarie P, Uitdehaag BM, Eijlers AJ, Geurts JJ, Hillebrand A, Schoonheim MM, Stam CJ, Strijbis EM (2019) Resting-state MEG measurement of functional activation as a biomarker for cognitive decline in MS. Mult Scler 25:1896–1906
Sharbafshaaer M, Trojsi F, Bonavita S, Azimi A (2022) Integrated cognitive rehabilitation home-based protocol to improve cognitive functions in multiple sclerosis patients: a randomized controlled study. J Clin Med 11:3560
Shenal BV, Harrison DW, Demaree HA (2003) The neuropsychology of depression: a literature review and preliminary model. Neuropsychol Rev 13:33–42
Sherman DS, Mauser J, Nuno M, Sherzai D (2017) The efficacy of cognitive intervention in mild cognitive impairment (MCI): a meta-analysis of outcomes on neuropsychological measures. Neuropsychol Rev 27:440–484
Siegert RJ, Abernethy DA (2005) Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 76:469–475
Simani L, Roozbeh M, Shojaei M, Ramezani M, Gharehgozli K, Rostami M (2022) The effectiveness of anodal tDCS and cognitive training on cognitive functions in multiple sclerosis; a randomized, double-blind, parallel-group study. Mult Scler Relat Disord 68:104392
Simon S, Nauta IM, Hillebrand A, Schoonheim MM, Uitdehaag BM, van Dam M, Hulst HE, Klein M, Stam CJ, de Jong BA, Strijbis EM (2023) Neurophysiological MEG markers of cognitive impairment and performance validity in multiple sclerosis. Mult Scler 29:1001–1011
Simpson R, Mair FS, Mercer SW (2017) Mindfulness-based stress reduction for people with multiple sclerosis - a feasibility randomised controlled trial. BMC Neurol 17:94
Sjøgård M, Wens V, Van Schependom J, Costers L, D’Hooghe M, D’Haeseleer M, Woolrich M, Goldman S, Nagels G, De Tiège X (2021) Brain dysconnectivity relates to disability and cognitive impairment in multiple sclerosis. Hum Brain Mapp 42:626–643
Skorve E, Lundervold AJ, Torkildsen Ø, Riemer F, Grüner R, Myhr KM (2023) Brief international cognitive assessment for MS (BICAMS) and global brain volumes in early stages of MS - A longitudinal correlation study. Mult Scler Relat Disord 69:104398
Sladowska K, Kawalec P, Holko P, Osiecka O (2022) Comparative safety of high-efficacy disease-modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Neurol Sci 43:5479–5500
Sormani MP, Schiavetti I, Ponzano M, Colato E, De Stefano N (2023) Treatment effect on brain atrophy correlates with treatment effect on cognition in multiple sclerosis. Ann Neurol 94:925–932
Spiezia AL, Falco F, Manganelli A, Carotenuto A, Petracca M, Novarella F, Iacovazzo C, Servillo G, Lanzillo R, Brescia Morra V, Moccia M (2023) Low serum 25-hydroxy-vitamin D levels are associated with cognitive impairment in multiple sclerosis. Mult Scler Relat Disord 79:105044
Staff NP, Lucchinetti CF, Keegan BM (2009) Multiple sclerosis with predominant, severe cognitive impairment. Arch Neurol 66:1139–1143
Stein C, O’Keeffe F, Strahan O, McGuigan C, Bramham J (2023) Systematic review of cognitive reserve in multiple sclerosis: accounting for physical disability, fatigue, depression, and anxiety. Mult Scler Relat Disord 79:105017
Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8:448–460
Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R (2005) Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex 15:394–402
Sumowski JF, Chiaravalloti N, DeLuca J (2009a) Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol 31:913–926
Sumowski JF, Chiaravalloti N, Wylie G, Deluca J (2009b) Cognitive reserve moderates the negative effect of brain atrophy on cognitive efficiency in multiple sclerosis. J Int Neuropsychol Soc 15:606–612
Sumowski JF, Chiaravalloti N, Deluca J (2010) Retrieval practice improves memory in multiple sclerosis: clinical application of the testing effect. Neuropsychology 24:267–272
Sumowski JF, Chiaravalloti N, Leavitt VM, Deluca J (2012) Cognitive reserve in secondary progressive multiple sclerosis. Mult Scler 18:1454–1458
Tafti D, Ehsan M, Xixis KL (2024) Multiple sclerosis. StatPearls [Internet], 2022/09/07 Edition. StatPearls Publishing Treasure Island (FL). https://pubmed.ncbi.nlm.nih.gov/29763024/
Taylor LA, Mhizha-Murira JR, Smith L, Potter KJ, Wong D, Evangelou N, Lincoln NB, das Nair R (2021) Memory rehabilitation for people with multiple sclerosis. Cochrane Database Syst Rev 10:CD008754
Tedone N, Preziosa P, Meani A, Pagani E, Vizzino C, Filippi M, Rocca MA (2023) Regional white matter and gray matter damage and cognitive performances in multiple sclerosis according to sex. Mol Psychiatry 28:1783–1792
Tewarie P, Hillebrand A, Schoonheim MM, van Dijk BW, Geurts JJ, Barkhof F, Polman CH, Stam CJ (2014) Functional brain network analysis using minimum spanning trees in multiple sclerosis: an MEG source-space study. NeuroImage 88:308–318
Thirion F, Sellebjerg F, Fan Y, Lyu L, Hansen TH, Pons N, Levenez F, Quinquis B, Stankevic E, Søndergaard HB, Dantoft TM, Poulsen CS, Forslund SK, Vestergaard H, Hansen T, Brix S, Oturai A, Sørensen PS, Ehrlich SD, Pedersen O (2023) The gut microbiota in multiple sclerosis varies with disease activity. Genome Med 15:1
Tillema JM, Hulst HE, Rocca MA, Vrenken H, Steenwijk MD, Damjanovic D, Enzinger C, Ropele S, Tedeschi G, Gallo A, Ciccarelli O, Rovira A, Montalban X, de Stefano N, Stromillo ML, Filippi M, Barkhof F (2016) Regional cortical thinning in multiple sclerosis and its relation with cognitive impairment: a multicenter study. Mult Scler 22:901–909
Tiu VE, Popescu BO, Enache II, Tiu C, Terecoasa E, Panea CA (2022) Serum and CSF biomarkers predict active early cognitive decline rather than established cognitive impairment at the moment of RRMS diagnosis. Diagnostics (Basel) 12:2571
Vagias H, Byrne ML, Millist L, White O, Clough M, Fielding J (2024) Visuo-cognitive phenotypes in early multiple sclerosis: a multisystem model of visual processing. J Clin Med 13:649
Valdés Cabrera D, Smyth P, Blevins G, Emery D, Beaulieu C (2022) Diffusion imaging of fornix and interconnected limbic deep grey matter is linked to cognitive impairment in multiple sclerosis. Eur J Neurosci 55:277–294
van Dam M, de Jong BA, Willemse EAJ, Nauta IM, Huiskamp M, Klein M, Moraal B, de Geus-Driessen S, Geurts JJG, Uitdehaag BMJ, Teunissen CE, Hulst HE (2023) A multimodal marker for cognitive functioning in multiple sclerosis: the role of NfL, GFAP and conventional MRI in predicting cognitive functioning in a prospective clinical cohort. J Neurol 270:3851–3861
Veréb D, Kovács MA, Kocsis K, Tóth E, Bozsik B, Király A, Kincses B, Faragó P, Fricska-Nagy Z, Bencsik K, Klivényi P, Kincses ZT, Szabó N (2022) Functional connectivity lateralisation shift of resting state networks is linked to visuospatial memory and white matter microstructure in relapsing-remitting multiple sclerosis. Brain Topogr 35:268–275
Virgilio E, Vecchio D, Crespi I, Puricelli C, Barbero P, Galli G, Cantello R, Dianzani U, Comi C (2022) Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. J Neurol 269:3249–3257
Wagner B, Härig CL, Walter B, Sommer J, Sammer G, Berghoff M (2022) Is there reduced hemodynamic brain activation in multiple sclerosis even with undisturbed cognition? Int J Mol Sci 24:112
Wallach AI, Waltz M, Casper TC, Aaen G, Belman A, Benson L, Chitnis T, Gorman M, Graves J, Harris Y, Lotze TE, Mar S, Moodley M, Ness JM, Rensel M, Rodriguez M, Rose JW, Schreiner T, Tillema JM, Waubant E, Weinstock-Guttman B, Charvet LE, Krupp LB (2020) Cognitive processing speed in pediatric-onset multiple sclerosis: baseline characteristics of impairment and prediction of decline. Mult Scler 26:1938–1947
Wallis O, Bol Y, Köhler S, van Heugten C (2020) Anxiety in multiple sclerosis is related to depressive symptoms and cognitive complaints. Acta Neurol Scand 141:212–218
Wilcox O, Amin M, Hancock L, Nakamura K, Lace J, Ontaneda D, Galioto R (2023) Associations between cognitive impairment and neuroimaging in patients with multiple sclerosis. Arch Clin Neuropsychol 39:196–203
Williams T, Tur C, Eshaghi A, Doshi A, Chan D, Binks S, Wellington H, Heslegrave A, Zetterberg H, Chataway J (2022) Serum neurofilament light and MRI predictors of cognitive decline in patients with secondary progressive multiple sclerosis: analysis from the MS-STAT randomised controlled trial. Mult Scler 28:1913–1926
Wojcik C, Fuchs TA, Tran H, Dwyer MG, Jakimovski D, Unverdi M, Weinstock-Guttman B, Zivadinov R, Eshaghi A, Benedict RH (2022) Staging and stratifying cognitive dysfunction in multiple sclerosis. Mult Scler 28:463–471
Yalachkov Y, Anschütz V, Jakob J, Schaller-Paule MA, Schäfer JH, Reiländer A, Friedauer L, Behrens M, Steffen F, Bittner S, Foerch C (2022) Brain-derived neurotrophic factor and neurofilament light chain in cerebrospinal fluid are inversely correlated with cognition in multiple sclerosis at the time of diagnosis. Mult Scler Relat Disord 63:103822
Yan Z, Yuan S, Zhu Q, Wang X, Shi Z, Zhang Y, Liu J, Feng J, Wei Y, Yin F, Chen S, Li Y (2024) Radiomics models based on cortical damages for identification of multiple sclerosis with cognitive impairment. Mult Scler Relat Disord 81:105348
Yigit P, Acikgoz A, Mehdiyev Z, Dayi A, Ozakbas S (2021) The relationship between cognition, depression, fatigue, and disability in patients with multiple sclerosis. Ir J Med Sci 190:1129–1136
Yokote H, Miyazaki Y, Toru S, Nishida Y, Hattori T, Niino M, Sanjo N, Yokota T (2022) High-efficacy therapy reduces subcortical grey matter volume loss in Japanese patients with relapse-onset multiple sclerosis: a 2-year cohort study. Mult Scler Relat Disord 67:104077
Zhang J, Cortese R, De Stefano N, Giorgio A (2021) Structural and functional connectivity substrates of cognitive impairment in multiple sclerosis. Front Neurol 12:671894
Zhang C, Zhang K, Hu X, Cai X, Chen Y, Gao F, Wang G (2024) Regional GABA levels modulate abnormal resting-state network functional connectivity and cognitive impairment in multiple sclerosis. Cereb Cortex 34:bhad535
Zheng P, Pilutti LA, DuBose NG, Motl RW (2023) Vascular function and cognition in persons with multiple sclerosis: preliminary examination. Mult Scler Relat Disord 71:104578
Zheng P, Sandroff BM, Motl RW (2024) Free-living ambulatory physical activity and cognitive function in multiple sclerosis: the significance of step rate vs. step volume. J Neurol 271:1638–1648
Ziccardi S, Pizzini FB, Guandalini M, Tamanti A, Cristofori C, Calabrese M (2022) Making visible the invisible: automatically measured global and regional brain volume is associated with cognitive impairment and fatigue in multiple sclerosis. Bioeng (Basel) 10:41
Ziccardi S, Pisani AI, Schiavi GM, Guandalini M, Crescenzo F, Colombi A, Peloso A, Tamanti A, Bertolazzo M, Marastoni D, Calabrese M (2023) Cortical lesions at diagnosis predict long-term cognitive impairment in multiple sclerosis: a 20-year study. Eur J Neurol 30:1378–1388
Ziccardi S, Fuchs T, Dwyer MG, Zivadinov R, Hulst HE, Calabrese M, Benedict RH (2024) Cognitive phenotypes predict response to restorative cognitive rehabilitation in multiple sclerosis. Mult Scler 30:448–452
Zuppichini MD, Sivakolundu DK, West KL, Okuda DT, Rypma B (2023) Investigating the link between regional oxygen metabolism and cognitive speed in multiple sclerosis: implications for fatigue. Mult Scler Relat Disord 80:105074
Zurawski J, Healy BC, Ratajska A, Barker L, Glanz BI, Houtchens M (2020) Identification of a predominant cognitive phenotype in patients with multiple sclerosis. Eur J Neurol 27:1083–1088
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl for secretarial and editorial work.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jellinger, K.A. Cognitive impairment in multiple sclerosis: from phenomenology to neurobiological mechanisms. J Neural Transm 131, 871–899 (2024). https://doi.org/10.1007/s00702-024-02786-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-024-02786-y