Abstract
Background
Available criteria for cognitive phenotypes in multiple sclerosis (MS) do not consider the severity of impairment.
Objectives
To identify cognitive phenotypes with varying degrees of impairment in MS patients and describe their demographic, clinical and MRI characteristics.
Methods
Two hundred and forty-three MS patients and 158 healthy controls underwent neuropsychological tests to assess memory, attention, and executive function. For each domain, mild impairment was defined as performing 1.5 standard deviations below the normative mean on two tests, while the threshold for significant impairment was 2 standard deviations. Patients were classified into cognitive phenotypes based on severity of the impairment (mild/significant) and number of domains affected (one/more).
Results
Five cognitive phenotypes emerged: Preserved cognition (PC; 56%), Mild Single-Domain Impairment (MSD; 15%), Mild Multi-Domain Impairment (MMD; 9%), Significant Single-Domain Impairment (SSD; 12%), Significant Multi-Domain Impairment (SMD; 8%). Compared with PC, MSD patients were older, had longer disease duration (DD) and higher T2-hyperintense lesion volume (LV; all p ≤ 0.02); MMD patients were older, had longer DD, higher disability, higher T2 LV and lower thalamic volume (all p ≤ 0.01); SSD patients had longer DD and lower gray matter cortical volume, thalamic, caudate, putamen and accumbens volumes (all p ≤ 0.04); and SMD patients were older, had longer DD, higher disability and more extensive structural damage in all brain regions explored (all p ≤ 0.03), except white matter and amygdala volumes.
Conclusions
We identified five cognitive phenotypes with graded levels of impairment. These phenotypes were characterized by distinct demographic, clinical and MRI features, indicating potential variations in the neural substrates of dysfunction throughout disease stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment is a highly prevalent and debilitating symptom in patients with multiple sclerosis (MS) [1]. Up to 65% [2, 3] of MS patients exhibit deficits in at least one cognitive domain. Speed of information processing [2, 3], complex aspects of attention [2], episodic memory [2, 3], working-memory [2] and executive function [2,3,4] are commonly affected. Deficits in visuospatial processing and social cognition have also been reported, but less frequently [3, 5]. Linguistic abilities, semantic memory and attention span are usually spared [2, 3].
Cognitive impairment is present in all disease stages and clinical phenotypes although patients with progressive MS (PMS) (i.e., primary and secondary progressive MS) generally have more severe deficits than those with relapsing–remitting (RR) MS [3]. Cognitive dysfunction may be subtle, particularly in the early phases, and the trajectory of cognitive worsening is characterized by great inter-patient variability [2]. The sequence of decline across cognitive domains is unclear. Previous research suggested that verbal fluency [6, 7] and verbal memory [6,7,8] are likely the first cognitive functions to be impaired, followed by a decrease in information processing speed [6, 7], visuospatial memory [6,7,8], and executive function [7]. It is worth mentioning that in one study [9] examining the staging of cognitive dysfunction in MS, a specific sequence of impairments was identified, with processing speed, visual learning, verbal learning, working memory/attention, and executive functions being affected in that order. However, deficits in information processing speed, executive function, and non-verbal memory can be present since the early phases of the disease [10,11,12], highlighting the need for a comprehensive neuropsychological assessment even when neurological impairment is minimal and brain imaging does not reveal extensive lesions [12].
In the context of MS, cognitive performance is often dichotomized into two categories, impaired or non-impaired, however, this oversimplification does not fully capture the complexity and variability of cognitive dysfunction in the disease. Recent studies have attempted to better characterize the heterogeneity of cognitive deficits in MS by identifying groups of patients who share similar cognitive profiles termed “cognitive phenotypes”. Two investigations [7, 13] adopted an empirical approach by using latent profile analysis to define patterns of cognitive performance examining neuropsychological data. Through this method, multiple profiles of impairment characterized by distinct demographic, clinical and MRI features have been extracted [7, 13]. Despite appealing, this methodology does not generate operational criteria that would be applicable by clinicians and researchers. To overcome this issue, other studies [14, 15] used an a priori classification approach whereby cognitive phenotypes are designated by specific impairment criteria. Hancock et al. [14] defined impairment as the presence of at least two test scores that fell below a cut-off value (< 1.0 or < 1.5 standard deviations [SD] below the normative mean) within a given cognitive domain. The authors then categorized MS patients into phenotypes based on the number of impaired domains (“intact”, “single domain”, “bi-domain”, and “multi-domain”). Leavitt et al. [15] established cognitive phenotypes according to memory and processing speed capabilities. Individuals who scored 1.0 SD below the normative value on at least one memory measure were classified as memory-impaired, while those who scored 1.0 SD below the normative mean on two processing speed measures were classified as processing speed-impaired. These criteria were then used to classify MS patients into one of four cognitive phenotypes: “not impaired”, “memory-impaired”, “processing speed-impaired”, or “memory and processing speed-impaired”.
These studies provided two sets of operational criteria which allow to identify four cognitive phenotypes ranging from preserved cognition to multi-domain impairment [14, 15]. However, these methods allow MS patients to be classified based only on the number of affected domains, without considering the severity of impairment. Furthermore, they did not include MRI measures. MRI studies have greatly helped to identify the neuroanatomical substrates of cognitive dysfunction including the presence, extent and location of white matter (WM) lesions [1], gray matter (GM) atrophy [16], normal-appearing WM damage [16] and abnormal functional activation patterns [17].
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) [18] was the first major attempt to bring cohesion in the field of neurocognitive disorders. The Neurocognitive Disorders Work Group for DSM-5 described specific cognitive domains for assessment (learning and memory, complex attention, executive function, language, perceptual-motor function, and social cognition) and developed a taxonomy of cognitive disorders that lies on a continuum of cognitive impairment from normality to significant impairment [19]. Nonetheless, the utilization of these terms has yet to significantly permeate the field of MS research literature.
The one exception is a recent study [20] that explored the prevalence of Mild and Major Neurocognitive Disorder (NCD) in 210 MS patients. According to the DSM-5 criteria, 66.7% of the sample was diagnosed with Mild NCD, while 20.5% had Major NCD. The distinction between the two diagnostic categories was based on the degree of cognitive decline and the loss of independence in daily activities due to cognitive dysfunction. However, clear cut-off thresholds for distinguishing between moderate and severe cognitive impairments were not established, highlighting the need for valid and applicable criteria.
Against this background, aim of this study was to apply the classification criteria proposed by the Neurocognitive Disorders Work Group for DSM-5 [19], amended for MS [14, 21], to assess mild and significant impairments in learning and memory, complex attention and executive function in a relatively large cohort of MS patients. We then identified unique cognitive phenotypes according to the degree of impairment (i.e., mild vs significant) and the number of affected domains (i.e., single-domain vs multidomain). To gain additional insights into the mechanisms underlying the distinction between the main cognitive phenotypes, we also explored their main demographic, clinical and structural MRI features, including T2 lesion volume (LV) and atrophy.
Methods
Study population
We enrolled 243 MS patients and 158 age-, sex-, education-matched healthy controls (HC). Inclusion criteria were: (1) age ≥ 18 years; (2) Italian native speaking; (3) no major systemic, psychiatric or neurological diseases (other than MS); and (4) a score ≤ 9 on the Montgomery-Åsberg Depression Rating Scale (MADRS) [22] (i.e., no depressive symptoms). MS patients had also to be relapse- and steroid-free for at least 3 months before cognitive assessment and have a stable disease-modifying treatment from at least 6 months.
Clinical and neuropsychological assessment
All participants were administered the Brief Repeatable Battery of Neuropsychological Tests [23] and the computerized version of the Wisconsin Card Sorting Test (WCST) [24] to assess cognitive function across three domains [19]: learning and memory (Selective Reminding Test- [SRT] long-term storage [lts], [23] SRT-consistent long-term retrieval [cltr], [23] SRT-delayed recall, [23] 10/36 Spatial Recall Test [SPART] [23] and SPART-delayed recall [23]), complex attention (Paced Auditory Serial Addition Test [PASAT] 3″ [23], PASAT 2″ [23] and Symbol Digit Modalities Test [SDMT] [23]) and executive function (Word List Generation [WLG] [23] and number of perseverative errors and number of perseverative responses on the WCST [24]). Age-, sex-, and education-adjusted scores were calculated based on normative data, and z-scores were obtained for each neuropsychological measure [23, 24]. According to the traditional dichotomous classification of cognitive dysfunction, participants who scored below the 5th percentile of the normative sample on tests assessing at least two different cognitive domains were classified as cognitively impaired [25].
On the same day, MS patients underwent a neurological examination with Expanded Disability Status Scale (EDSS) [26] score rating and definition of clinical phenotype (RR or progressive) [27]. Patients with a RR phenotype were further divided into early- (disease duration < 5 years) and late-RRMS (disease duration ≥ 5 years) [28].
Cognitive phenotyping
Each cognitive domain was considered mildly impaired if a minimum of two cognitive tests in that domain were more than 1.5 SD below the normative mean [19, 21, 29], whereas if two or more cognitive tests fell below 2 SD the domain was defined significantly impaired [19, 21, 29]. The number of impaired cognitive domains (i.e., none, one or more than one) and the severity of the impairment (i.e., not impaired, mild or significant) were examined to determine cognitive phenotypes.
Five phenotypes were considered: (1) mild single-domain impairment (MSD), defined as having mild impairment in one of the three cognitive domains; (2) mild multi-domain impairment (MMD), defined as having mild impairment in two or more of the three cognitive domains; (3) significant single-domain impairment (SSD), defined as having significant impairment in one of the three cognitive domains; (4) significant multi-domain impairment (SMD), defined as having significant impairment in two or more of the three cognitive domains; and (5) preserved cognition (PC) included patients with no impairment in any of the three cognitive domains.
The same criteria were also applied to HCs in order to assess the probability of false positive results.
Cognitive phenotypes were generated for participants who completed at least two tests in each cognitive domain (78 HC and 243 MS).
MRI acquisition and analysis
Within 2 days of clinical and neuropsychological assessment, 210 MS patients and 139 HCs underwent brain MRI scans for brain T2-hyperintense WM lesions and atrophy quantification using two 3.0 T scanners (Scanner 1, Achieva, Philips Medical Systems, Eindhoven, The Netherlands: 92 MS patients and 70 age-, sex-, education-matched HCs; Scanner 2, Ingenia, Philips Medical Systems, Eindhoven, The Netherlands: 118 MS patients and 69 age-, sex-, education-matched HCs). By using Scanner 1, we acquired dual-echo turbo spin echo (repetition time [TR] = 2599 ms; echo time [TE] = 16–80 ms; flip angle = 90°; matrix = 256 × 256; field of view [FOV] = 240 × 240 mm2; echo train length [ETL] = 6; 44 contiguous axial slices, 3 mm thick) and 3D T1-weighted fast field echo (TR = 25; TE = 4.6 ms; flip angle = 30°; matrix = 256 × 256; FOV = 230 mm2; 220 contiguous axial slices, 0.8 mm thick).
Images acquired using Scanner 2 included: (1) 3D fluid attenuated inversion recovery (FLAIR) (TR = 4800 ms; TE = 270 ms; TI = 1650 ms; matrix size = 256 × 256; FOV = 256 × 256 mm2; ETL = 167; 192 contiguous sagittal slices, 1 mm thick); (2) 3D T2-weighted sequence (TR = 2500 ms; TE = 330 ms; matrix size = 256 × 256; FOV = 256 × 256 mm2; ETL = 117; 192 contiguous sagittal slices, 1 mm thick); and (3) 3D T1-weighted turbo field echo (TR = 7 ms; TE = 3.2 ms; TI = 1000 ms; flip angle = 8°; matrix size = 256 × 256; FOV = 256 × 256 mm2; 204 contiguous sagittal slices, 1 mm thick).
For MS patients acquired on Scanner 1, T2-hyperintense WM lesions were manually identified on dual-echo scans by an experienced observer, and LV was quantified using a semi-automatic local thresholding segmentation technique (Jim 7.0, Xinapse Systems Ltd, Colchester, UK). For patients acquired on Scanner 2, T2-hyperintense WM lesions were identified using a fully automated approach based on a cascade of two 3D patch-wise convolutional neural networks, using 3D fluid-attenuated inversion recovery and 3D T1-weighted MRI sequences as input images [30], and total T2-hyperintense LV was calculated after a visual check of the results of the automated method. On both scanners, normalized brain, WM and cortical GM volumes (NBV, NWMV and NCGMV, respectively) were calculated using FSL SIENAx software on lesion-filled [31] 3D T1-weighted images. The normalized volumes of bilateral hippocampus, thalamus, caudate, putamen, pallidum, amygdala and accumbens were measured using the FIRST tool [32].
Statistical analysis
All statistical analyses were performed with R-4.2.2 and SPSS, version 26.0 (IBM, Armonk, NY, USA).
Demographic and neuropsychological variables were compared between MS patients and HC using the Chi-square test, Mann–Whitney U test or two-sample t test. Demographic and clinical features were compared between cognitive phenotypes using the Chi-square test, Mann–Whitney U test or linear models as appropriate. Benjamini–Hochberg false discovery rate (FDR) correction was applied to adjust for multiple tests.
For easier interpretation of MRI measures, normalized brain volumes were converted to z-scores by subtracting the mean and dividing by the SD of the HCs, separately for each scanner. Brain T2 LVs were log-transformed and converted to z-scores according to the overall distribution in MS patients. MRI variables were compared between HC and cognitive phenotypes and between cognitive phenotypes using age- and sex-adjusted linear models. FDR correction was applied to adjust for multiple tests.
Results
Demographic, clinical and neuropsychological features
Table 1 summarizes the main demographic, clinical and neuropsychological characteristics of the study population. MS patients had higher MADRS scores than HCs (p = 0.006), without reaching the cut-off to be classified as depressed.
Patients with MS exhibited higher rates of cognitive impairment (p < 0.001) and poorer performance than controls in learning and memory (z SRT-lts, z SRT-cltr, z SRT-recall, z SPART and z SPART-recall), complex attention (z SDMT, z PASAT 3″ and z PASAT 2″) and executive function (z WLG and z WCST perseverative errors) (all p ≤ 0.004) (Table 1). Z WCST preservative responses revealed no significant differences (Table 1).
Supplementary Table 1 summarizes the main demographic and clinical features of MS patients grouped according to disease clinical phenotype: 175 patients were diagnosed with RRMS (72 early-RRMS and 103 late-RRMS), and 68 with PMS. Demographic and clinical features of the subgroups of participants acquired on Scanner 1 and Scanner 2 are reported in Supplementary Table 2.
Cognitive phenotypes
In the whole sample of patients with MS, 18% (44 patients) had mild and 13% (32 patients) had significant memory and learning impairment. Mild and significant complex attention impairment was found in 7% (17 patients) and 14% (34 patients), respectively. Five% (11 patients) of MS patients had mild executive function impairment and 7% (17 patients) had significant executive function impairment.
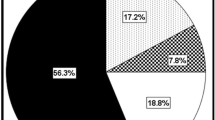
Percent representation of the cognitive phenotypes is summarized in Fig. 1. Of the whole MS sample, 56% (136 patients) were classified as PC. Fifteen % (37 patients) met the criteria for MSD; 9% (22 patients) met the criteria for MMD; 12% (29 patients) met the criteria for SSD; 8% (19 patients) met the criteria for SMD. Five HCs (6%) met the criteria for MSD (Fig. 1b). Similarly, of the subsample of 210 MS patients who underwent brain MRI, 57% (119 patients) were classified as PC, 14% (30 patients) as MSD, 9% (20 patients) as MMD, 13% (27 patients) as SSD and 7% (14 patients) as SMD.
Figure 2 shows cognitive phenotype distribution according to MS phenotype. Compared with late-RRMS (52%, p = 0.02) and PMS (43%, p = 0.001), early-RRMS had higher percentage of patients with PC (73%). In early-RRMS patients, the most common type of impairment was the MSD phenotype (15%). In late-RRMS patients, the most common profile of impairment was the SSD phenotype (19%). PMS patients had the highest proportion of patients with SMD (18%).
a Pie chart representation of percentage of cognitive phenotypes in each multiple sclerosis clinical phenotype. b Stacked bar graph showing the percentage distribution of multiple sclerosis’ clinical phenotypes across cognitive phenotypes. RRMS relapsing–remitting multiple sclerosis, PMS progressive multiple sclerosis
Demographic and clinical characteristics of cognitive phenotypes
Table 2 summarizes demographic and clinical variables across cognitive phenotypes. Patients with PC were younger than patients with MSD (p = 0.001), MMD (p = 0.01), and SMD (p = 0.003); had higher years of education than patient with SSD (p = 0.01); had the shortest disease duration (all p ≤ 0.01); had less severe disability than patients with MMD (p = 0.01), and SMD (p < 0.001); and had the lowest frequency of cognitive dysfunction according to the dichotomous classification of cognitive impairment (p < 0.001).
Figure 2b shows the distribution of MS clinical phenotypes within each cognitive phenotype. The PC phenotype had the highest proportion of early-RRMS (39%), late-RRMS accounted for 40% of the cases and PMS represented 21% of the total number. In the MSD phenotype, 30% were early-RRMS, 35% late-RRMS and 35% PMS. In the MMD phenotype, late-RRMS and PMS were 45% and 41% respectively. The majority of patients with SSD were late-RRMS (69%). The SMD phenotype had the highest proportion of PMS (63%) and the lowest percentage of early-RRMS (5%).
MRI features of cognitive phenotypes
Table 3 summarizes lesional and atrophy findings of the five cognitive phenotypes. Two hundred ten of 243 MS patients (86%) and 139 of 158 healthy controls (88%) underwent MRI, representing 87% of the study population.
Compared with HCs, patients with PC had lower volumes in all the investigated brain structures (all p ≤ 0.005), except for the amygdala, whereas patients with MSD had lower NBV and normalized volume of hippocampus, thalamus, caudate, putamen and accumbens (all p ≤ 0.03). Patients with MMD, SSD and SMD showed reduced volumes across all the analysed structures (all p ≤ 0.01).
Compared with patients with PC, those with MSD exhibited higher T2 LV (p = 0.02), those with MMD showed higher T2 LV (p = 0.007) and lower thalamic volume (p = 0.008), those with SSD were characterized by lower NCGMV and normalized volume of thalamus, caudate, putamen and accumbens (all p ≤ 0.04), those with SMD were characterized by higher T2 LV and lower volumes in all the analysed brain structures (all p ≤ 0.008), except for NWMV and amygdala.
Compared with patients with PC, those with mild learning and memory impairment exhibited higher T2 LV (p < 0.001), while those with significant learning and memory impairment exhibited higher T2 LV and lower volumes in all the examined brain structures (all p ≤ 0.01), except for NWMV (Supplementary Table 3).
Compared with patients with PC, those with mild complex attention impairment showed higher T2 LV and lower NBV, NWMV, thalamic, caudate and putamen volume (all p ≤ 0.02). In comparison to patients with PC, those with significant complex attention impairment were characterized by higher T2 LV and lower volumes across all the examined brain structures (all p ≤ 0.03), except for NWMV and pallidum (Supplementary Table 4).
Compared with patients with PC, those with mild executive function impairment showed no differences, while those with significant impairment had higher T2 LV (p = 0.01) and lower NWMV (p = 0.01) (Supplementary Table 5).
Discussion
In this study, we used the criteria proposed by the Neurocognitive Disorders Work Group for DSM-5 [19], adapted for MS [14, 29], to detect mild and significant impairments in learning and memory, complex attention, and executive function in a large sample of patients with MS. Based on the severity of the dysfunction and the number of affected domains, we identified five distinct cognitive phenotypes, representing a graded degree of impairment: 56% of patients fell into the PC phenotype, 15% in the MSD phenotype, 9% in the MMD phenotype, 12% in the SSD phenotype and 8% in the SMD phenotype. Overall, 44% of MS patients had cognitive impairments of varying degrees, consistent with previous cross-sectional studies reporting impairment rates ranging from 34 to 65% [3]. In MS, cognition has traditionally been dichotomized (impaired vs preserved), neglecting the heterogeneity of cognitive manifestations among these patients. Recent attempts to develop new taxonomies of cognitive phenotypes using specific impairment criteria [14, 15] represent an advance in characterizing the patterns of impairment in this condition. However, the application of non-stringent cut-off criteria [29] (i.e., test performance 1 SD below the normative mean) [14, 15] and the lack of MRI data [14, 15] have limited their impact in the MS research literature and their applicability in the clinical setting. In this study, we attempted to overcome these limitations by applying strict impairment criteria and integrating the analysis with MRI data. As recommended by the Neurocognitive Disorders Work Group for DSM-5 [19], in each cognitive domain, significant impairment was defined as performing 2 SD below the normative value. Mild impairment, however, was defined using the cut-off 1.5 SD rather than 1 SD, as evidence from multiple studies suggests a possible increase in false-positive diagnoses of cognitive impairment when using the 1 SD threshold [14, 29]. In addition, a domain was considered impaired if two tests within that domain fell below the designated cut-off [14]. By doing so, we were able to distinguish five cognitively homogeneous groups of patients, each characterized by unique demographic and clinical features. MRI analysis allowed the identification of distinct neuroanatomical substrates associated with each profile of impairment and provided biological evidence to support the proposed classification system.
The first phenotype, PC, comprised patients who exhibited a pattern of average functioning across cognitive domains. This phenotype was prevalent among patients with early-RRMS and included younger patients with shorter disease duration compared to the other cognitive phenotypes. Despite preserved cognitive abilities, these patients showed reduced normalized brain volumes compared to HC in all regions examined except the amygdala. The presence of structural brain damage in PC patients may seem counterintuitive. However, due to the early stage of the disease, structural damage may be relatively limited [7, 33]. In addition, individuals in this group are likely to benefit from several protective factors that mitigate the pathological effects of MS. Adaptive brain plasticity and functional reorganization may allow for structural damage to be compensated and cognitive function to be maintained [1]. Furthermore, it is possible that patients with higher cognitive reserve, which contributes to the preservation of cognitive abilities, fell into the PC phenotype [13, 34].
The second phenotype included patients with mild impairment in a single cognitive domain. Compared to PC patients, those with MSD were older and had longer disease duration. In line with this finding, large cross-sectional studies have shown that age and disease duration are often associated and that cognitive function tends to decline as disease progresses [35, 36]. This group was characterized by a higher T2 LV compared to the PC phenotype, consistent with research linking higher lesion burden to poorer performance on measures of memory [37], processing speed [38], sustained attention [37] and executive function [37, 38]. The relationship between T2 LV and cognitive impairment is not entirely straightforward [1]. However, a high T2 lesion burden may have contributed to the disruption of critical WM pathways leading to mild cognitive deficit [39].
The third cognitive phenotype included patients with mild impairment in multiple domains. Compared to the PC group, these patients had a more severe clinical profile characterized by older age, longer disease duration, greater physical disability, and a higher prevalence of PMS. As mentioned above, the presence of cognitive deficits may be associated with more advanced disease stage [35, 36, 40]. Consistent with this, patients with mild impairment in multiple domains had higher T2 LV and more severe thalamic atrophy compared to PC ones. The thalamus plays a central role in several cognitive processes, ranging from learning and memory to flexible adaption [41]. Its extensive connections with different brain regions makes it vulnerable to degeneration due to demyelination and axonal loss in brain WM [42]. Previous MRI studies have consistently demonstrated a robust correlation between thalamic atrophy with a wide range of clinical manifestations including cognitive decline, motor deficits and fatigue in patients with MS [1, 43, 44].
The fourth phenotype, SSD, included patients with both a longer disease duration and lower level of education in comparison to those with PC. Educational attainment is considered an indicator of cognitive reserve, i.e., the ability of the brain to mitigate or compensate for the effects of a disease burden on cognitive functions [45]. Thus, it is reasonable to speculate that individuals in this group might have had a comparatively lower cognitive reserve, making them more vulnerable to the detrimental effects of MS on cognition. The relative frequency of patients with late-RRMS was highest within the SSD phenotype (69%), suggesting that cognitive problems in the later stages of RRMS are confined to a specific cognitive function rather than affecting multiple cognitive domains. Cortical atrophy and reduced volumes of the thalamus, caudate, putamen and accumbens emerged as the distinctive MRI features of this cognitive phenotype. The central location of deep GM structures and close proximity to the lesion-prone periventricular WM make them susceptible to atrophy, contributing to cognitive deficits from the early disease stages [46]. Interestingly, a longitudinal investigation suggested that the pathologic substrates of cognitive dysfunction may gradually shift from WM lesions and deep GM atrophy, in the inflammatory RRMS phase, towards cortical atrophy in the more advanced stage of the disease [46].
The fifth phenotype involved a subset of patients with significant impairments in multiple cognitive domains. In terms of their clinical features, these patients had longer disease duration and a higher degree of physical disability compared to patients with PC. Moreover, a significant proportion (63%) of patients with SMD were diagnosed with a progressive form of MS, supporting the notion that cognitive dysfunction is more widespread and pronounced in PMS than in RRMS [40]. The clinical and cognitive profile of this phenotype is consistent with the extensive brain atrophy observed on MRI affecting all tissue compartments examined except NWMV and amygdala.
In line with previous research [3], in our sample of MS patients, memory and complex attention were the most commonly affected cognitive areas, while executive function showed lower frequency of impairment. The pairwise comparisons of MRI metrics revealed that patients with mild and significant impairments in specific cognitive domains exhibited distinct patterns of brain structural changes. More in details, compared to PC patients, those with mild learning and memory deficits showed a higher T2 LV, suggesting an association between mild memory impairment and increased lesion burden [47]. On the other hand, patients with significant learning and memory dysfunction showed higher T2 LV and decreased volumes in all brain regions studied except NWMV, indicating more widespread neurodegenerative processes that may contribute to the severity of this cognitive deficit [48]. Similar patterns were observed in the complex attention domain, with higher T2 LV and decreased volumes across multiple brain regions in the mild impairment subgroup, and a greater volume loss in all brain structures examined, except NWMV and pallidum, in the severe impairment subgroup. These results suggest that severe impairment in complex attention may be associated with broader neurodegenerative changes affecting various cognitively relevant brain networks [49]. Finally, patients with significant executive function impairment exhibited higher T2 LV and lower NWMV, suggesting that damage to WM tracts may contribute to executive dysfunction in MS patients [4]. Clearly, the relatively small number of patients presenting this deficit may have limited the statistical power of this latter analysis.
Notably, 21% of patients exhibited deficits in complex attention (7% mild and 14% significant impairment), while this cognitive area was preserved in a large proportion of impaired patients. This finding has significant implications for standard cognitive screening procedures and highlights the limitations of using a single measure, such as SDMT, to identify cognitive impairment at the individual level [50].
This study has a few limitations. First, the cross-sectional design does not allow for the examination of both the stability and temporal changes of cognitive phenotypes over time. Second, the neuropsychological tests used focused on specific cognitive domains, potentially overlooking deficits in other cognitive areas, such as visuospatial processing and social cognition, which have been reported in MS, even if with lower prevalence [3, 5]. Moreover, cognitive tests such as the SDMT [51] and PASAT [52] may tap into cognitive abilities outside the designated cognitive domain, potentially limiting the validity of the proposed classification system. However, due to their psychometric properties and established norms, these standardized tests continue to be widely used in clinical and research settings [23, 53]. Third, despite the use of a standardized neuropsychological battery, our results may have been influenced by the inclusion of a broader range of memory and learning measures compared to other cognitive domains. Additionally, the fact that PASAT 3″ and PASAT 2″ are closely related tasks may have contributed to potential overdiagnosis of complex attention deficits. Future studies should evaluate the applicability of the proposed criteria to different neuropsychological batteries and cohorts of MS patients as was done with the IC-CoDiMS criteria [14]. Fourth, most participants, though not all, underwent MRI at the time of cognitive assessment. Nevertheless, their distribution into cognitive phenotypes closely resembled that of the entire sample. Finally, the subgroup analysis for each cognitive phenotype might have limited statistical power, particularly for less frequent phenotypes.
In conclusion, this study presents a novel approach to identify five distinct cognitive phenotypes in MS that is applicable in research and clinical settings. These phenotypes allow for a more nuanced understanding of cognitive manifestations in MS and can serve as a basis for personalized approaches to cognitive assessment and intervention. Further research is needed to examine their longitudinal trajectory and predictive value in relation to disease progression and clinical outcome.
Data availability
The dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
References
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, Rovira A et al (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14(3):302–317. https://doi.org/10.1016/S1474-4422(14)70250-9
Amato MP, Portaccio E, Goretti B, Zipoli V, Hakiki B, Giannini M, Pastò L et al (2010) Cognitive impairment in early stages of multiple sclerosis. Neurol Sci 31(S2):211–214. https://doi.org/10.1007/s10072-010-0376-4
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19(10):860–871. https://doi.org/10.1016/s1474-4422(20)30277-5
Marchesi O, Bonacchi R, Valsasina P, Preziosa P, Pagani E, Cacciaguerra L, Meani A et al (2021) Functional and structural MRI correlates of executive functions in multiple sclerosis. Mult Scler J 28(5):742–756. https://doi.org/10.1177/13524585211033184
Chalah MA, Ayache SS (2017) Deficits in social cognition: an unveiled signature of multiple sclerosis. J Int Neuropsychol Soc 23(3):266–286. https://doi.org/10.1017/s1355617716001156
Achiron A, Polliack M, Rao SM, Barak Y, Lavie M, Appelboim N, Harel Y (2005) Cognitive patterns and progression in multiple sclerosis: construction and validation of percentile curves. J Neurol Neurosurg Psychiatry 76(5):744–749. https://doi.org/10.1136/jnnp.2004.045518
De Meo E, Portaccio E, Giorgio A, Ruano L, Goretti B, Niccolai C, Patti F et al (2021) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78(4):414. https://doi.org/10.1001/jamaneurol.2020.4920
Amato MP, Ponziani G, Siracusa G, Sorbi S (2001) Cognitive dysfunction in early-onset multiple sclerosis: a reappraisal after 10 years. Arch Neurol 58(10):1602–1606. https://doi.org/10.1001/archneur.58.10.1602
Wojcik C, Fuchs TA, Tran H, Dwyer MG, Jakimovski D, Unverdi M, Weinstock-Guttman B et al (2022) Staging and stratifying cognitive dysfunction in multiple sclerosis. Mult Scler 28(3):463–471. https://doi.org/10.1177/13524585211011390
Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B (2011) MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 76(13):1161–1167. https://doi.org/10.1212/WNL.0b013e318212a8be
Feuillet L, Reuter F, Audoin B, Malikova I, Barrau K, Cherif AA, Pelletier J (2007) Early cognitive impairment in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 13(1):124–127. https://doi.org/10.1177/1352458506071196
Schulz D, Kopp B, Kunkel A, Faiss JH (2006) Cognition in the early stage of multiple sclerosis. J Neurol 253(8):1002–1010. https://doi.org/10.1007/s00415-006-0145-8
Podda J, Ponzio M, Pedullà L, Monti Bragadin M, Battaglia MA, Zaratin P, Brichetto G et al (2021) Predominant cognitive phenotypes in multiple sclerosis: Insights from patient-centered outcomes. Multiple Scler Relat Disord 51:102919. https://doi.org/10.1016/j.msard.2021.102919
Hancock LM, Galioto R, Samsonov A, Busch RM, Hermann B, Matias-Guiu JA (2022) A proposed new taxonomy of cognitive phenotypes in multiple sclerosis: The International Classification of Cognitive Disorders in MS (IC-CoDiMS). Mult Scler J. https://doi.org/10.1177/13524585221127941
Leavitt VM, Tosto G, Riley CS (2018) Cognitive phenotypes in multiple sclerosis. J Neurol 265(3):562–566. https://doi.org/10.1007/s00415-018-8747-5
Preziosa P, Rocca MA, Pagani E, Stromillo ML, Enzinger C, Gallo A, Hulst HE et al (2016) Structural MRI correlates of cognitive impairment in patients with multiple sclerosis. Hum Brain Mapp 37(4):1627–1644. https://doi.org/10.1002/hbm.23125
Rocca MA, Valsasina P, Leavitt VM, Rodegher M, Radaelli M, Riccitelli GC, Martinelli V et al (2017) Functional network connectivity abnormalities in multiple sclerosis: correlations with disability and cognitive impairment. Mult Scler J 24(4):459–471. https://doi.org/10.1177/1352458517699875
Diagnostic and statistical manual of mental disorders: DSM-5™. 5th edition. DSM-5. 2013, American Psychiatric Publishing, a division of American Psychiatric Association, Washington, DC
Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, Petersen RC (2014) Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol 10(11):634–642. https://doi.org/10.1038/nrneurol.2014.181
Hancock LM, Hermann B, Schoonheim MM, Hetzel SJ, Brochet B, DeLuca J (2022) Comparing diagnostic criteria for the diagnosis of neurocognitive disorders in multiple sclerosis. Mult Scler Relat Disord 58:103479. https://doi.org/10.1016/j.msard.2021.103479
Eijlers AJC, Meijer KA, Wassenaar TM, Steenwijk MD, Uitdehaag BMJ, Barkhof F, Wink AM et al (2017) Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology 88(10):952–960. https://doi.org/10.1212/wnl.0000000000003689
Hawley CJ, Gale TM, Sivakumaran T (2002) Defining remission by cut off score on the MADRS: selecting the optimal value. J Affect Disord 72(2):177–184. https://doi.org/10.1016/s0165-0327(01)00451-7
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L, De Caro MF, Patti F et al (2006) The Rao’s Brief Repeatable Battery and Stroop Test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12(6):787–793. https://doi.org/10.1177/1352458506070933
Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin card sorting test manual: revised and expanded. Psychological Assessment Resources Inc, Odessa
Amato M, Morra V, Falautano M, Ghezzi A, Goretti B, Patti F, Riccardi A et al (2018) Cognitive assessment in multiple sclerosis-an Italian consensus. Neurol Sci 39(8):1317–1324. https://doi.org/10.1007/s10072-018-3427-x
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452. https://doi.org/10.1212/wnl.33.11.1444
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Debernard L, Melzer TR, Van Stockum S, Graham C, Wheeler-Kingshott CA, Dalrymple-Alford JC, Miller DH et al (2013) Reduced grey matter perfusion without volume loss in early relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 85(5):544–551. https://doi.org/10.1136/jnnp-2013-305612
Fischer M, Kunkel A, Bublak P, Faiss JH, Hoffmann F, Sailer M, Schwab M et al (2014) How reliable is the classification of cognitive impairment across different criteria in early and late stages of multiple sclerosis? J Neurol Sci 343(1–2):91–99. https://doi.org/10.1016/j.jns.2014.05.042
Valverde S, Cabezas M, Roura E, Gonzalez-Villa S, Pareto D, Vilanova JC, Ramio-Torrenta L et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168. https://doi.org/10.1016/j.neuroimage.2017.04.034
Battaglini M, Jenkinson M, De Stefano N (2012) Evaluating and reducing the impact of white matter lesions on brain volume measurements. Hum Brain Mapp 33(9):2062–2071. https://doi.org/10.1002/hbm.21344
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56(3):907–922. https://doi.org/10.1016/j.neuroimage.2011.02.046
Eshaghi A, Marinescu RV, Young AL, Firth NC, Prados F, Jorge Cardoso M, Tur C et al (2018) Progression of regional grey matter atrophy in multiple sclerosis. Brain 141(6):1665–1677. https://doi.org/10.1093/brain/awy088
Rocca MA, Riccitelli GC, Meani A, Pagani E, Del Sette P, Martinelli V, Comi G et al (2018) Cognitive reserve, cognition, and regional brain damage in MS: a 2-year longitudinal study. Mult Scler J 25(3):372–381. https://doi.org/10.1177/1352458517750767
Aktas O, Achiron A, Chapman J, Magalashvili D, Dolev M, Lavie M, Bercovich E et al (2013) Modeling of cognitive impairment by disease duration in multiple sclerosis: a cross-sectional study. PLoS One 8(8):e71058. https://doi.org/10.1371/journal.pone.0071058
Ruano L, Portaccio E, Goretti B, Niccolai C, Severo M, Patti F, Cilia S et al (2017) Age and disability drive cognitive impairment in multiple sclerosis across disease subtypes. Mult Scler J 23(9):1258–1267. https://doi.org/10.1177/1352458516674367
Sacco R, Bisecco A, Corbo D, Della Corte M, d’Ambrosio A, Docimo R, Gallo A et al (2015) Cognitive impairment and memory disorders in relapsing–remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol 262(7):1691–1697. https://doi.org/10.1007/s00415-015-7763-y
Summers M, Swanton J, Fernando K, Dalton C, Miller DH, Cipolotti L, Ron MA (2008) Cognitive impairment in multiple sclerosis can be predicted by imaging early in the disease. J Neurol Neurosurg Psychiatry 79(8):955–958. https://doi.org/10.1136/jnnp.2007.138685
Geurts JJG, Schoonheim MM, Douw L, Steenwijk MD, Meijer KA (2020) Long-range connections are more severely damaged and relevant for cognition in multiple sclerosis. Brain 143(1):150–160. https://doi.org/10.1093/brain/awz355
Brochet B, Ruet A (2019) Cognitive impairment in multiple sclerosis with regards to disease duration and clinical phenotypes. Front Neurol. https://doi.org/10.3389/fneur.2019.00261
Saalmann YB, Kastner S (2015) The cognitive thalamus. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2015.00039
Houtchens MK, Benedict RHB, Killiany R, Sharma J, Jaisani Z, Singh B, Weinstock-Guttman B et al (2007) Thalamic atrophy and cognition in multiple sclerosis. Neurology 69(12):1213–1223. https://doi.org/10.1212/01.wnl.0000276992.17011.b5
Bisecco A, Capuano R, Caiazzo G, d’Ambrosio A, Docimo R, Cirillo M, Russo A et al (2019) Regional changes in thalamic shape and volume are related to cognitive performance in multiple sclerosis. Mult Scler J 27(1):134–138. https://doi.org/10.1177/1352458519892552
Minagar A, Barnett MH, Benedict RHB, Pelletier D, Pirko I, Sahraian MA, Frohman E et al (2013) The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 80(2):210–219. https://doi.org/10.1212/WNL.0b013e31827b910b
Pettigrew C, Soldan A (2019) Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-019-0917-z
Eijlers AJC, Dekker I, Steenwijk MD, Meijer KA, Hulst HE, Pouwels PJW, Uitdehaag BMJ et al (2019) Cortical atrophy accelerates as cognitive decline worsens in multiple sclerosis. Neurology 93(14):e1348–e1359. https://doi.org/10.1212/wnl.0000000000008198
Randolph JJ, Wishart HA, Saykin AJ, McDonald BC, Schuschu KR, Macdonald JW, Mamourian AC et al (2005) FLAIR lesion volume in multiple sclerosis: relation to processing speed and verbal memory. J Int Neuropsychol Soc. https://doi.org/10.1017/s1355617705050253
Preziosa P, Pagani E, Mesaros S, Riccitelli GC, Dackovic J, Drulovic J, Filippi M et al (2017) Progression of regional atrophy in the left hemisphere contributes to clinical and cognitive deterioration in multiple sclerosis: a 5-year study. Hum Brain Mapp 38(11):5648–5665. https://doi.org/10.1002/hbm.23755
Azzimonti M, Preziosa P, Pagani E, Valsasina P, Tedone N, Vizzino C, Rocca MA et al (2023) Functional and structural brain MRI changes associated with cognitive worsening in multiple sclerosis: a 3-year longitudinal study. J Neurol. https://doi.org/10.1007/s00415-023-11778-z
Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, Gingold J et al (2018) Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler 24(13):1665–1680. https://doi.org/10.1177/1352458518803785
Sandry J, Simonet DV, Brandstadter R, Krieger S, Katz Sand I, Graney RA, Buchanan AV et al (2021) The symbol digit modalities test (SDMT) is sensitive but non-specific in MS: lexical access speed, memory, and information processing speed independently contribute to SDMT performance. Mult Scler Relat Disord 51:102950. https://doi.org/10.1016/j.msard.2021.102950
Tombaugh T (2006) A comprehensive review of the paced auditory serial addition test (PASAT). Arch Clin Neuropsychol 21(1):53–76. https://doi.org/10.1016/j.acn.2005.07.006
Benedict RHB, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R (2017) Validity of the symbol digit modalities test as a cognition performance outcome measure for multiple sclerosis. Mult Scler J 23(5):721–733. https://doi.org/10.1177/1352458517690821
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
DM, DB, NT, CV have no competing interests to declare that are relevant to the content of this article. EP received speakers’ honoraria from Biogen Idec. MF is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. MAR received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders.
Ethical statement
Approval was obtained from the institutional ethical standards committee on human experimentation of IRCCS Ospedale San Rafaele for any experiments using human subjects (protocol number 2015-33). Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mistri, D., Tedone, N., Biondi, D. et al. Cognitive phenotypes in multiple sclerosis: mapping the spectrum of impairment. J Neurol 271, 1571–1583 (2024). https://doi.org/10.1007/s00415-023-12102-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12102-5