Abstract
Background
The structural changes associated with cognitive performance in older people with multiple sclerosis (PwMS; age ≥ 50 years old) remain unknown.
Objective
To determine the relationship between whole-brain (WBV), thalamus as the largest deep gray matter nuclei, and cortex-specific volume measurements with both cognitive impairment and numerical performance in older PwMS. The main hypothesis is that cognitive impairment (CI) in older PwMS is explained by cortical thinning in addition to global and thalamic neurodegenerative changes.
Methods
A total of 101 older PwMS underwent cognitive and neuroimaging assessment. Cognitive assessment included tests established as sensitive in MS samples (Minimal Assessment of Cognitive Function in MS; MACFIMS), as well as those tests often utilized in Alzheimer’s dementia studies (Wechsler’s Memory Scale, Boston Naming Test, Visual Motor Integration and language). Cognitive impairment (CI) was based on -1.5 standard deviations in at least 2 cognitive domains (executive function, learning and memory, spatial processing, processing speed and working memory and language) when compared to healthy controls. WBV and thalamic volume were calculated using SIENAX/FIRST and cortical thickness using FreeSurfer. Differences in cortical thickness between CI and cognitively preserved (CP) were determined using age, sex, education, depression and WBV-adjusted analysis of covariance (ANCOVA). The relationship between domain-specific cognitive performance and cortical thickness was analyzed by linear regression models adjusted for age, sex, education, depression, WBV and thalamic volume. Benjamini-Hochberg-adjusted p-values lower than 0.05 were considered significant.
Results
The average age of the study population was 62.6 (5.9) years old. After adjustment, CI PwMS had significantly thinner left fusiform (p = 0.0003), left inferior (p = 0.0032), left transverse (p = 0.0013), and bilateral superior temporal gyri (p = 0.002 and p = 0.0011) when compared to CP PwMS. After adjusting for age, sex, education, depression WBV, and thalamic volume, CI status was additionally predicted by the thickness of the left fusiform (p = 0.001) and left cuneus gyri (p = 0.004). After the adjustment, SDMT scores were additionally associated with left fusiform gyrus (p < 0.001) whereas letter-based verbal fluency performance with left pars opercularis gyrus (p < 0.001).
Conclusion
In addition to global and thalamic neurodegenerative changes, the presence of CI in older PwMS is additionally explained by the thickness of multiple cortical regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a neuroinflammatory, demyelinating, and neurodegenerative disease characterized by chronic accrual of neurological disability commonly affecting people aged 18–50 years old [1]. In addition to physical limitations, many people with multiple sclerosis (PwMS) also exhibit insidious cognitive decline that can negatively impact employment status and quality of life [2]. Cognitive impairment (CI) affects 30–60% of PwMS, with higher percentages reported in older PwMS with longer disease duration, progressive MS phenotype and more severe MS pathology [2].
Over the past 30 years, advances in pharmacological and rehabilitative treatment of PwMS have drastically improved overall MS life expectancy, slowed disability worsening, and prolonged quality of life maintenance well into the expected retirement years [3]. These improvements to care resulted in a substantial epidemiological shift from a young (18–50 years old) PwMS population to an older, comorbid, and more frail population of older people with MS (> 50 years old, PwMS) [3]. For example, the peak prevalence age of PwMS in the US was 30–39 years in 1976, whereas the latest epidemiological queries (performed in 2010) resulted in a peak age of 55–64 years old [4].
Because aging is intrinsically associated with questions regarding changes to cognitive functioning, it is challenging to determine whether cognitive declines in older PwMS result from normal aging, disease progression, or some combination of the two. While it is known that processing speed and memory typically represent the most common cognitive deficits in MS, several studies showed that other domains (such as language) may be more relevant in older PwMS [5, 6]. These changes were initially recognized by Müller et al., where aging secondary-progressive MS (SPMS) patients had verbal fluency deficits similar to those that can be seen in amnestic mild cognitive impairment (aMCI), a prodromal stage of Alzheimer’s disease (AD) [7]. Similar comparisons also demonstrate significant deficits in cognitive processing speed and categorical verbal fluency of older PwMS when compared to aging healthy controls (HCs) [8]. Lastly, our group has utilized a comprehensive panel of MS and AD-specific tests to fully characterize the domain-specific cognitive performance in more than 100 older PwMS and showed deficits in processing speed and verbal fluency [9]. These findings have since been replicated in the literature in other MS samples [10,11,12].
Aging in MS is also associated with greater neurodegenerative processes resulting in increased brain atrophy when compared to healthy individuals and younger PwMS [13, 14]. Studies suggest that brain structures have a differential vulnerability to pathological atrophy, which can vary throughout the lifespan and MS disease, resulting in different ratios of age-associated brain loss versus MS-specific atrophy [14, 15]. For example, the thalamus is highly vulnerable to atrophy during early disease while the cerebral cortex is predominantly affected in long-standing and older PwMS [15,16,17]. It is well established in the literature that early changes in the thalamic structures are highly correlated with MS cognitive processing speed [18, 19]. Such relationships between cortical thickness and cognitive performance of older PwMS have not yet been established.
The main hypothesis of this study is that cognitive impairment in older PwMS may be additionally explained by cortical thinning in addition to global and thalamic atrophy. For this purpose, we examined 101 older PwMS (age > 50 years old) using a comprehensive neuropsychological battery and correlated their performance with cortical thickness and deep gray matter (DGM) MRI measures. Since such analyses were not previously reported in the literature, we considered all gyri equally and corrected for false discovery rate. We also directly compared the cortical thicknesses between CI and cognitively preserved (CP) PwMS and between PwMS with and without isolated cognitive domain impairments. Findings from such analyses could determine the age-specific vulnerable regions that could serve as future exploratory neurodegenerative outcomes in neuroprotective and cognitive rehabilitative studies for older PwMS.
Materials and methods
Study population
All study participants were enrolled at the Jacobs MS Center for Treatment and Research, University at Buffalo and participated as research participants in the cardiovascular, environmental and genetic risk factors in MS (CEG-MS) study [9]. The inclusion criteria for the older PwMS were: (1) MS diagnosis based on 2010 and 2017-revised McDonald criteria based on their time of recruitment [20, 21], (2) age of at least 50 years old, (3) availability of MRI examination and neuropsychological assessment within 6 months of each other and (4) availability of MS-specific and dementia-specific neuropsychological assessment. The exclusion criteria were: (1) diagnosis of additional major neurological disorder, (2) major depressive disorder, (3) use of psychoactive agents that could affect the cognitive performance, (4) vision acuity worse than 20/70 that could affect the performance on multiple vision-dependent cognitive tests and (5) clinically-determined relapse or received intravenous corticosteroid therapy within the previous 30 days of the MRI acquisition.
The inclusion criteria for the HCs were: (1) age of at least 50 years old and (2) availability of MS and AD-specific neuropsychological assessment for comparative purposes and calculation of normative values. The exclusion criteria for the HCs were: (1) presence of current or history of a major neurological disorder, (2) diagnosis of major depressive disorder, (3) use of psychoactive agents that could affect the cognitive performance, (4) vision acuity worse than 20/70 that could affect the performance on multiple vision-dependent cognitive tests. The study population has been previously published elsewhere [9]. The discrepancy in PwMS between the two publications is due to the lack of MRI examination in 3 PwMS [9].
Neurostatus-certifed examiners calculated the Expanded Disability Status Scale (EDSS) for all patients [22]. Additional clinical data regarding disease duration, years of formal education and the type of disease-modifying treatment (DMT) were collected using standardized questionnaires. Disease phenotype was classified according to the 2013 Lublin criteria using clinical presentation and medical history. PwMS diagnosed with primary-progressive MS (PPMS) and secondary-progressive MS (SPMS) were merged into one progressive MS (PMS) group [23]. Motor performance was measured using the Timed 25-Foot Walk Test (T25FWT) and 9-Hole Peg Test (9HPT) for dysfunction of the lower and upper extremities, respectively [24, 25].
Neuropsychological assessment
Neuropsychological testing of the older PwMS was performed by trained technicians under the supervision of a board-certified neuropsychologist. The specific neuropsychological tests, their contribution to specific cognitive domains and their references are outlined in Supplement Table 1. Cognitive reserve was estimated with years of formal education. Patient-reported depressive and fatigue symptoms were quantified with the Beck Depression Inventory – Fast Screen (BDI-FS) and Fatigue Severity Scale (FSS) questionnaires, respectively. The majority of cognitive assessment was performed using the Minimal Assessment of Cognitive Function in MS (MACFIMS) battery, which evaluates cognitive processing speed [Paced Auditory Serial Addition Test – 3 s (PASAT-3) and Symbol Digit Modalities Test (SDMT)], learning and memory [California Verbal Learning Test–second edition (CVLT-II) and Brief Visuospatial Memory Test-Revised (BVMT-R)], language [Controlled Oral Word Association Test (COWAT)] and executive function [Delis-Kaplan Executive Function System (D-KEFS)]. Additional AD-specific cognitive tests were included: Logical Memory from Wechsler Memory Scale-Revised (WMS-R), Boston Naming Test (BNT), Beery Visual-Motor Integration (VMI), and clock drawing test. In all tests, higher scores indicate better performance.
The older PwMS were considered CI when they scored -1.5 standard deviations (SD) in at least two out of five cognitive domains (executive control, learning and memory, spatial processing, processing speed and working memory, and language) when compared to age-matched healthy control data [9]. Based on common classification from the literature, PwMS with performance comparable to the HCs or with an isolated impairment in one domain were initially considered as CP. Moreover, comparisons between PwMS with and without isolated impairment within the specific domains of processing speed and language were performed. The definition of impairment in one specific cognitive domain was also based on performance in at least one domain-specific test of – 1.5 SD when compared to the healthy control data.
MRI acquisition and analyses
All MS patients were scanned on the same 3 T Signa Excite HD 12.0 Twin Speed MRI scanner (GE, Milwaukee, WI, USA) using an 8-channel head and neck coil. The two main sequences utilized for this study were: (1) high-resolution 3D T1-weighted fast spoiled gradient-echo with magnetization-prepared inversion recovery (IR-FSPGR) with repetition time (TR)/echo time (TE)/ inversion time (TI) of 5.9/2.8/900 ms, field of view (FOV) of 25.6 X 19.2 cm2 (256 × 256 matrix), flip angle of 10 degrees and 180 1 mm slices without gaps, (2) 2D T2-Fluid attenuated inversion recovery (FLAIR) scans with TR/TE/TI of 8500/120/2100 ms, FOV of 25.6 X 19.2 cm2 (256 × 256 matrix), flip angle of 90 degrees, echo train length (ETL) of 24 and 48 3 mm slices without gaps.
Semi-automated quantification of white matter (WM) lesion volume (T2-FLAIR hyperintensities) were assessed using Java Image Manipulation software (JIM, Xinapse systems, Essex, UK) by an experienced operator. The image analyses were blinded from the clinical and neuropsychological status. Volumes of whole-brain (WBV), WM, and gray matter (GM) were derived using cross-sectional Structural Image Evaluation, using Normalisation, of Atrophy (SIENAX, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/SIENA), normalized for head-size. Lesion filling was performed prior to segmentation to minimize tissue misclassification. Normalized DGM volume and specific volumes of the thalamus, caudate, putamen, globus pallidus, and hippocampus were obtained with FMRIB’s Integrated Registration and Segmentation Tool (FIRST, https://fsl.fmrib.ox.ac.uk/fsl, version 6.0). Lastly, FreeSurfer (version 6.0, http://surfer.nmr.mgh.harvard.edu) was used to parcellate the cortex into 68 different regions (34 per hemisphere) based on the Desikan-Killiany atlas and cortical thickness was obtained [26]. Full description of each gyrus is available elsewhere [27]. Outputs from all MRI analyses were visually inspected and corrections were made if necessary (e.g. editing brain masks, adding control points) during the FreeSurfer analysis.
Statistical analyses
All statistical analyses were performed in SPSS version 24.0 (IBM, Armonk, NY, USA). The data distribution was assessed using visual inspection of the histograms and Q-Q plots. Normally distributed data is shown as mean and SD whereas data without normal distribution are reported using median and interquartile range (IQR).
The demographic, clinical and neuropsychological measures between the older PwMS and HCs were compared using χ2 test, Student’s t-test and Mann–Whitney U test, as appropriate. Differences in MRI measures and cognitive measures between the CP and CI PwMS were determined using analysis of covariance (ANCOVA) adjusted for age, sex, years of education, patient-reported depression (BDI-FS), whole brain volume and thalamic volume. Cohen’s d was used to determine the effect size for the comparisons.
All MRI comparisons between the CP vs. CI PwMS groups were initially corrected for age, years of education, patient-reported depression (BDI-FS), WBV, and thalamic volume. Based on the hypothesis, thalamic volume was included in the corrections to determine the relationship between cortical thickness and cognitive performance after excluding the effect of already established thalamic associations with cognitive performance in MS. The multiple comparisons of cortical thickness in all 34 cortical regions on both sides (total of 68 comparisons) between the groups and their per-domain classifications were further adjusted using Benjamini–Hochberg procedure. Only p-values that survived multiple comparison adjustment were considered as statistically significant. To ensure that no single subject was driving our comparison findings, bootstrapping using 1000 Monte Carlo simulations was performed [28]. The same comparisons were performed between pwRRMS and pwPMS. For comparisons of general demographic, clinical and neuropsychological data, a p-value lower than 0.05 was considered statistically significant.
The relationship between cognitive performance in domain-specific tests and cortical thickness was assessed using linear regression models that were adjusted for age, sex, education, BDI-FS scores, WBV and thalamic volume. Step-wise criteria to determine the best predictors of cognitive performance used entry and exit tolerance of 0.01 and 0.05, respectively. R2 and standardized β from the regression models were reported. Additional logistic regression models and receiver operating characteristic (ROC) curves were used to determine the best measures to accurately predict the presence of CI in the older PwMS. In both the comparison and regression analyses, the FSS and disease duration were not included as covariates due to their collinearity (high variance inflation factor; VIF) with BDI-FS and age, respectively.
Results
Demographic and clinical characteristics of older PwMS
The demographic and clinical characteristics of the total PwMS population, HCs, the CI PwMS and CP PwMS are shown in Table 1. The PwMS were on average 62.6 (SD = 5.9) years old, 75.2% were female and had an average disease duration of 21.2 (SD = 12.6) years. The differences between PwRRMS and PwPMS groups are shown in Supplement Table 2. The overall fraction of cognitive impairment in PwMS was 36.6% with only a numerically greater fraction of CI in the PwPMS (40.5% vs. 34.4%, p = 0.536). There were no demographic differences between the CI and CP PwMS in age (62.5 vs. 62.7 years old, p = 0.84), disease duration (23.9 vs. 19.7 years, p = 0.101), years of education (14.7 vs. 15.1 years, p = 0.375), patient-reported depression (BDI-FS median 1.0 vs. 1.0 p = 0.824) and fatigue (FSS median 5.2 vs. 4.7, p = 0.15). The CI PwMS were more disabled when compared to the CP PwMS in EDSS (median 5.5 vs. 3.25, p = 0.003), T25FWT (median 9.1 s vs. 6.1 s, p = 0.011) and 9HPT (27.2 s vs. 23.5 s, p = 0.001).
Neuropsychological performance of the older PwMS
The neuropsychological performance of the HCs vs. older PwMS and between the CI vs. CP PwMS are described in detail in Table 2. The biggest differences in cognitive performance between HCs and PwMS per effect size were in SDMT (55.4 vs 43.9, Cohen’s d = 1.059, p < 0.001) and categorical verbal fluency (45.9 vs. 37.1, Cohen’s d = 0.967, p < 0.001). As expected, CI PwMS had significantly worse performance in all cognitive tests when compared to CP PwMS (p < 0.002). The biggest effect sizes were seen in processing speed (SDMT, Cohen’s d = 1.384; PASAT-3, Cohen’s d = 1.218), verbal and visuospatial memory (CVLT-II, Cohen’s d = 1.455 and BVMT-R, Cohen’s d = 1.454) and language (COWAT, Cohen’s d = 1.239 and 1.336 for letter and categorical verbal fluency). Of note, only 61.4% of the PwMS had data available for the executive function (D-KEFS test).The differences in cognitive performance between PwRRMS and PwPMS are shown in Supplement Table 3.
Cortical parcellation and neuroimaging characterization of the older PwMS
The global neuroinflammatory and neurodegenerative characteristics of the older PwMS population are shown in Table 3. After adjusting for multiple comparisons, CI PwMS had significantly lower WBV (1374.1 mL vs. 1427.6 mL, p < 0.001), GM volume (682.5 mL vs. 724.8 mL, p < 0.001) and putamen volume (9.2 mL vs. 10.2 mL, p = 0.018) when compared to CP PwMS.
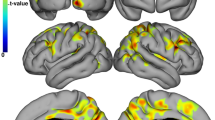
Of note, FreeSurfer failed in 9 PwMS and the median time between the MRI and neuropsychological examination was 2 months. The sample size in each table hereafter accounts for these failures. The comparisons regarding the cortical thickness between CI and CP PwMS are shown in Table 4. After correction for age, sex education, BDI-FS scores, WBV and thalamic volume, the CI PwMS had significantly thinner left fusiform gyrus (p = 0.0003, partial η [2] = 0.149), left inferior (p = 0.0032, partial η2 = 0.101), superior (p = 0.001, partial η2 = 0.128) and transverse temporal gyri (p = 0.0013, partial η2 = 0.119) and the right superior frontal (p = 0.002, partial η2 = 0.11) and superior temporal gyri (p = 0.0011, partial η2 = 0.121), when compared to the CP PwMS group (all surviving after Benjamini–Hochberg multiple comparison correction). (Table 4) No changes were noted after bootstrapping.
In an age, sex, education, BDI-FS, WBV and thalamic volume-adjusted logistic regression model, CI status was predicted using the thickness of the left fusiform (p = 0.001) and left cuneus gyri (p = 0.004). It successfully predicted CI in PwMS with 77.8% accuracy. The ROC curve predicting CI PwMS is also shown in Fig. 1. The area under the curve (AUC) was 0.863 (95% confidence intervals from 0.786 to 0.94).
ROC curve predicting cognitive impairment using demographic, clinical and neuroimaging measures. ROC receiver operating characteristic curve. The predictive measure utilized data from patient age, education, patient-reported depression (BDI-FS), whole brain volume, thalamic volume and thickness of the left fusiform and left cuneus gyr
Detailed analyses of the global and cortical thickness between older PwRRMS and older PwPMS groups are shown in Supplement Tables 3 and 4.There were no differences in cortical thickness in any investigated gyrus between the older PwPMS and PwRRMS.
Relationship between domain-specific cognitive performance scores and neuroimaging characteristics
The differences in cortex-specific MRI measures between older PwMS with isolated impairment within the domains of cognitive processing speed/working memory and language when compared to older PwMS without the respective domain impairment are shown in Supplement Table 5 and Supplement Table 6, respectively. There were no demographic differences between the 29 PwMS with cognitive processing speed impairment and the remaining 72 PwMS with no impairment in age (63.1 vs. 63.6 years old, p = 0.697), education (14.7 vs. 15.7 years, p = 0.076), disease duration (22.8 vs. 20.3 years, p = 0.453), BDI-FS (2.0 vs. 1.0, p = 0.272) and FSS (4.7 vs. 4.9, p = 0.499).
After adjusting for age, sex, years of education, BDI-FS, WBV and thalamic volume, PwMS with isolated impairment in the cognitive processing speed had multiple significantly thinner cortical gyri including bilateral fusiform (p = 0.0002, partial η2 = 0.152 and p = 0.002, partial η2 = 0.11 for left and right respectively), bilateral inferior temporal gyrus (p = 0.0005, partial η2 = 0.138 and p = 0.014, partial η2 = 0.07), bilateral superior frontal/temporal gyri (p < 0.0091, smallest partial η2 = 0.08) and bilateral transverse temporal gyri (p = 0.0007 and p = 0.0076 for left and right, respectively, smallest partial η2 = 0.083).
A smaller number of cortical regions were significantly thinner in the PwMS with impairment in the language domain when compared to those without. After correction for age, sex, years of education, WBV and thalamic volume, the language-impaired PwMS had thinner left fusiform gyrus (p = 0.0032, partial η2 = 0.101), left pars opercularis (p = 0.0024, partial η2 = 0.107), left precentral (p = 0.0025, partial η2 = 0.106), left superior (p = 0.0019, partial η2 = 0.111) and transverse temporal gyri (p = 0.0008, partial η2 = 0.127) and the left insula (p = 0.0005, partial η2 = 0.135) as well as thinner right post-central gyrus (p = 0.045, partial η2 = 0.094) when compared to language-intact PwMS.
Additional linear regression analyses determined the best predictors of cognitive processing speed performance (as measured by SDMT scores) and verbal fluency performances (as measured by letter-based and categorical-based COWAT scores) after adjusting for age, sex, education, BDI-FS, WBV and thalamic volume. In addition to the significant effects from the WBV (standardized β = 0.31, p = 0.009), SDMT performance was significantly associated with left fusiform gyrus thickness (standardized β = 0.507 p < 0.001) with R2 increase from 0.214 to 0.294. Letter-based verbal fluency performance was significantly associated with WBV (standardized β = 0.312, p = 0.009), and left pars opercularis gyrus thickness (BA44) (standardized β = 0.438, p < 0.001) with R2 increase from 0.121 to 0.279. Similarly, categorical fluency performance was significantly associated with WBV (standardized β = 0.26, p = 0.036), and left pars opercularis gyrus (BA44) thickness (standardized β = 0.371, p = 0.001) with R2 increase from 0.094 to 0.204.
Discussion
The findings of this study are multifold. As initially hypothesized, CI older PwMS had significantly thinner cortical gyri when compared to CP PwMS. In particular, the thickness of the fusiform gyrus and pars opercularis was associated with cognitive processing speed and verbal fluency performance. These findings were significant despite correcting for WBV and DGM-based measures.
Over a period of 5 years, a study by Eijlers et al. suggested that cognitive decline in older PMS is driven by significantly greater cortical atrophy [29]. In comparison, cognitive decline in the younger RRMS group was associated with lesion accrual rate whereas cognitive decline in the transitioning group (pwMS that transition from RRMS to PMS over follow-up) was associated with DGM atrophy [29]. Further, no differences were observed in DGM atrophy rates between the younger RRMS and older PMS groups [29]. As an extension to their work, we suggest that CI in older PwMS is driven by asymmetrically left-sided cortical pathology and localized to the fusiform gyrus, the inferior, transverse and superior temporal gyri. With the proposition that the cortical atrophy in long-standing MS may happen through a distinct and non-random pattern, a similar pattern of domain-specific cognitive impairments may follow suit [17].
A recent analysis based on progressive and older PwMS from the lipoic acid trial (NCT03161028) demonstrated that poorer verbal and visual memory were associated with lower mean cortical thickness [30]. This study has the most comparable demographic and clinical characteristics to our population, with patients having an average age of 59.2 years old and 22.4 years of disease duration [30]. While this study did not publish gyri-specific correlations, the same directionality of findings is highly encouraging. Although the relationship between verbal fluency and specific cortical gyri thickness has not been investigated in an older MS sample, recent studies of younger MS populations do report an association with global and WM-based MRI outcomes [31]. Based on our literature search, Amato et al. were the first to find an association between total neocortical atrophy and verbal fluency performance (the study included 28 considerably younger RRMS PwMS when compared to our population) [32]. A more recent study with a much larger cohort of 1,212 PwMS (average age of 41 years old) demonstrated that mild verbal memory and verbal fluency impairments were the most common cognitive phenotype (29.9%) and were associated with decreased hippocampal volume [11]. Other studies do suggest associations between damage within the ventral and dorsal pathway streams with categorical and letter-based verbal fluency, respectively. [33] Resting-state functional connectivity analysis in PwMS suggested that deviations in the default mode network and language networks are associated with poorer verbal fluency and such analysis can provide greater explanatory power when compared to only structural measures [34, 35]. Our study expands on the literature, pinpointing cortical anatomical changes within Brodman’s areas 37, 41/42 and 44.
The findings regarding the role of the fusiform gyrus, the pars opercularis and the superior/transversal temporal gyrus associations with the language/verbal fluency impairment can be substantiated by other studies linking these regions language-related tasks. First, data from functional MRI and event-related potentials performed during cerebral processing of unseen masked words (thinking but not vocalizing the target word) has significantly implicated the fusiform gyrus in the unconscious process of word priming [36]. In comparison to visible words which activate left-lateralized parietal cortex, anterior cingulate, fusiform, inferior prefrontal, precentral cortex and supplementary motor areas (previously established as word reading network), masked words were sizably localized mainly in the left fusiform gyrus and precentral sulcus [36]. The older PwMS with verbal fluency deficits had significant thinning in exactly these two left-sided areas. The left fusiform gyrus has been significantly associated with auditory word recognition and potentially implicated in the initiation of a semantic category decision [37]. Even in highly specific pathological states such as semantic dementia (a variant of frontotemporal lobar degeneration), the level of semantic impairment is mainly associated with hypometabolism of the fusiform cortex [38]. In particular, the left anterior fusiform cortex has been associated with expressive verbal tasks whereas the right fusiform cortex with non-verbal tests of semantic knowledge [38].
Together with pars orbitalis and pars triangularis, the pars opercularis is commonly considered a building block of Broca’s area and main site of speech production [39]. In normal aging, worse verbal fluency performance is associated with thinner pars opercularis [40]. Moreover, findings from a recent case-controlled study of people with primary progressive aphasia isolated the damage within the frontal aslant tract (pathway connecting the pars opercularis from Broca’s region with the anterior cingulate and supplementary motor area) and suggested it as the main underlying factor of verbal fluency and semantic processing [41]. Lastly, the superior and transversal temporal gyri, areas primarily associated with the auditory cortex, are shown to activate during covert (inner) speech and influence the generation of verbal speech [42].
The findings of this study should also be interpreted in comparison to the findings in younger pwMS that are published in the literature. A study by Nygaard et al. investigated the cortical features in 61 PwMS (average age 34.2 years old) and demonstrated that depression, fatigue and verbal memory were correlated regionally with smaller cortical surface and volume [43]. Interestingly, the cortices indicated in their study did coincide with our analysis, having the fusiform, orbital frontal, and temporal gyri as the most implicated [43]. The laterality of greater cortical thinning favoring the left hemisphere has also been shown in a large phase 3 trial (CombiRx trial with 250 PwMS and average age of 38.2 years old) [44]. In this analysis, the EDSS scores and disease duration were significantly associated with the thickness coinciding with the same left-sided gyri such as the middle and superior and middle temporal, pars opercularis, fusiform and paracentral [44]. Similar findings were also seen in a recent study that suggested multiple cognitive tasks from the 5 domains are associated with cortical regions of the left hemisphere, predominantly correlating with the temporal, gyrus frontalis and insular gyri [45].
Another future analysis should investigate the difference in cortical thinning versus central atrophy (including the DGM/thalamus) between aging adult-onset MS and late-onset MS (disease onset > 50 years old). Such an analysis could provide insights regarding the brain vulnerability to early MS pathophysiological processes in different age circumstances. In a previous study, late-onset MS demonstrated significantly faster lateral ventricular expansion when compared to age-matched adult-onset MS, suggesting that the central brain structures may be most vulnerable to pathology during the early onset stage regardless of the age of the PwMS [46]. Lastly, our analysis demonstrated a lack of cortical thickness differences between older PwRRMSand older PwPMS. Based on these findings, cortical thinning and the emergence of specific cognitive impairments may be related to an interaction between age and disease duration-related pathology when compared to the phenotype classification.
The current study has several limitations. First, it is cross-sectional in nature and cannot assess directionality between cognitive performance and cortical thickness. Future longitudinal studies should determine the temporal and spatial course of cortical thickness and its relationship with cognitive phenotypical changes. The cognitive and neurodegenerative changes in these age groups typically follow an exponential trajectory and could be misrepresented by conventional linear-based statistics. Given that our analysis includes up to 68 different cortical regions (34 regions on each hemisphere), spurious relationships may be only true in our specific sample and not representative of the disease changes in general. However, we used strict correction for multiple comparisons which was necessary in this exploratory study without a single hypothesis of what cortical regions would be involved.
Another limitation is the lack of amyloid PET or another biomarker that may have screened for AD in this older age MS cohort. Distinguishing between cognitive impairment in older pwMS and early AD or its prodromal stage called aMCI is an important clinical problem that we are trying to address. We and others have proposed that rapid forgetting and semantic processing deficits are akin to AD and may signal this comorbidity. The ultimate test of course, will be to verify this hypothesis using valid biomarker that has not been done in this study. We have recently reported on an older PwMS that was deemed as potentially having a dual diagnosis of MS and AD where amyloid-based PET scan demonstrated minimal focal uptake within the occipital lobe [6]. While the inclusion criteria for the study was 50 years old or greater, the median age of the entire population was 63 years and less than 25% of the PwMS were younger than 58 years old. As the MS population continues to age, the age limit for such analyses should increase. Another limitation is the fact that only a portion of PwMS were assessed for executive function performance and the proportion of CI PwMS may have been slightly underrepresented. Lastly, while we did not perform the MRI analysis in the HCs, the literature does demonstrate significant differences in all inflammatory and neurodegenerative MRI measures between older PwMS and age-matched HCs [47].
In conclusion, after correcting for global neurodegenerative changes, CI older PwMS have significantly thinner cortical regions when compared to CP older PwMS. The associations between cognitive performance and anatomical changes in older PwMS may shift from DGM-based pathology to cortical-centric neurodegenerative processes.
References
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. The Lancet 391:1622–1636
Benedict RHB, Amato MP, DeLuca J, Geurts JJG (2020) Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol 19:860–871
Vaughn CB, Jakimovski D, Kavak KS et al (2019) Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol 15:329–342
Wallin MT, Culpepper WJ, Campbell JD et al (2019) The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 92:e1029–e1040
Roy S, Drake A, Snyder S et al (2018) Preliminary investigation of cognitive function in aged multiple sclerosis patients: Challenges in detecting comorbid Alzheimer’s disease. Mult Scler Relat Disord 22:52–56
Jakimovski D, Szigeti K, Jaworski M 3rd et al (2020) Differential Diagnosis of Cognitive Decline in Elderly Individuals With Multiple Sclerosis. Cogn Behav Neurol 33:294–300
Müller S, Saur R, Greve B et al (2012) Recognition performance differentiates between elderly patients in the long term course of secondary progressive multiple sclerosis and amnestic mild cognitive impairment. Mult Scler J 19:799–805
Roth AK, Denney DR, Burns JM, Lynch SG (2018) Cognition in older patients with multiple sclerosis compared to patients with amnestic mild cognitive impairment and healthy older adults. Neuropsychology 32:654–663
Jakimovski D, Weinstock-Guttman B, Roy S et al (2019) Cognitive profiles of aging in multiple sclerosis. Front Aging Neurosci 11:105
Lebkuecher AL, Chiaravalloti ND, Strober LB (2021) The role of language ability in verbal fluency of individuals with multiple sclerosis. Mult Scler Relat Disord. https://doi.org/10.1016/j.msard.2021.102846
De Meo E, Portaccio E, Giorgio A et al (2021) Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurol 78:414–425
Barois E, Sagawa Y, Yilmaz S, Magnin E, Decavel P (2021) What (more) can verbal fluency tell us about multiple sclerosis? Ann Phys Rehabil Med 64:101394
Ghione E, Bergsland N, Dwyer MG et al (2019) Aging and brain atrophy in multiple sclerosis. J Neuroimaging 29:527–535
Azevedo CJ, Cen SY, Jaberzadeh A, Zheng L, Hauser SL, Pelletier D (2019) Contribution of normal aging to brain atrophy in MS. Neurol Neuroimmunol Neuroinflamm 6:e616
Azevedo CJ, Cen SY, Khadka S et al (2018) Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann Neurol 83:223–234
Zivadinov R, Havrdová E, Bergsland N et al (2013) Thalamic atrophy is associated with development of clinically definite multiple sclerosis. Radiology 268:831–841
Steenwijk MD, Geurts JJ, Daams M et al (2016) Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain 139:115–126
Bergsland N, Zivadinov R, Dwyer MG, Weinstock-Guttman B, Benedict RH (2016) Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler 22:1327–1336
Amin M, Ontaneda D (2020) Thalamic injury and cognition in multiple sclerosis. Front Neurol 11:623914
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology 17:162–173
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Lublin FD, Reingold SC, Cohen JA et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286
Motl RW, Cohen JA, Benedict R et al (2017) Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler 23:704–710
Feys P, Lamers I, Francis G et al (2017) The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler 23:711–720
Desikan RS, Ségonne F, Fischl B et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Klein A, Tourville J (2012) 101 labeled brain images and a consistent human cortical labeling protocol. Front Neurosci 6:171
Efron B, Tibshirani RJ (1994) An introduction to the bootstrap: CRC press
Eijlers AJC, Dekker I, Steenwijk MD et al (2019) Cortical atrophy accelerates as cognitive decline worsens in multiple sclerosis. Neurology 93:e1348–e1359
Spain RI, Hildebrand A, Waslo CS et al (2023) Processing speed and memory test performance are associated with different brain region volumes in Veterans and others with progressive multiple sclerosis. Front Neurol 14:1188124
Rocca MA, Riccitelli GC, Meani A et al (2019) Cognitive reserve, cognition, and regional brain damage in MS: A 2 -year longitudinal study. Mult Scler 25:372–381
Amato MP, Portaccio E, Goretti B et al (2007) Association of neocortical volume changes with cognitive deterioration in relapsing-remitting multiple sclerosis. Arch Neurol 64:1157–1161
Blecher T, Miron S, Schneider GG, Achiron A, Ben-Shachar M (2019) Association between white matter microstructure and verbal fluency in patients with multiple sclerosis. Front Psychol 10:1607
Sjøgård M, Wens V, Van Schependom J et al (2021) Brain dysconnectivity relates to disability and cognitive impairment in multiple sclerosis. Hum Brain Mapp 42:626–643
Koenig KA, Beall EB, Sakaie KE et al (2021) Evaluation of a connectivity-based imaging metric that reflects functional decline in Multiple Sclerosis. PLoS One 16:e0251338
Dehaene S, Naccache L, Cohen L et al (2001) Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4:752–758
Balsamo LM, Xu B, Gaillard WD (2006) Language lateralization and the role of the fusiform gyrus in semantic processing in young children. Neuroimage 31:1306–1314
Mion M, Patterson K, Acosta-Cabronero J et al (2010) What the left and right anterior fusiform gyri tell us about semantic memory. Brain 133:3256–3268
Tomaiuolo F, MacDonald JD, Caramanos Z et al (1999) Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci 11:3033–3046
Vonk JMJ, Rizvi B, Lao PJ et al (2019) Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb Cortex 29:2694–2700
Catani M, Mesulam MM, Jakobsen E et al (2013) A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136:2619–2628
Shergill SS, Brammer MJ, Fukuda R et al (2002) Modulation of activity in temporal cortex during generation of inner speech. Hum Brain Mapp 16:219–227
Nygaard GO, Walhovd KB, Sowa P et al (2015) Cortical thickness and surface area relate to specific symptoms in early relapsing-remitting multiple sclerosis. Mult Scler 21:402–414
Narayana PA, Govindarajan KA, Goel P et al (2012) Regional cortical thickness in relapsing remitting multiple sclerosis: a multi-center study. Neuroimage Clin 2:120–131
Stellmann J-P, Wanke N, Maarouf A et al (2021) Cognitive performance shows domain specific associations with regional cortical thickness in multiple sclerosis. NeuroImage Clin 30:102606
Jakimovski D, Dujmic D, Hagemeier J et al (2020) Late onset multiple sclerosis is associated with more severe ventricle expansion. Mult Scler Relat Disord 46:102588
Jakimovski D, Bergsland N, Dwyer MG et al (2020) Long-standing multiple sclerosis neurodegeneration: volumetric magnetic resonance imaging comparison to Parkinson’s disease, mild cognitive impairment, Alzheimer’s disease, and elderly healthy controls. Neurobiol Aging 90:84–92
Funding
This study was supported by a grant from the Consortium of Multiple Sclerosis Centers (CMSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Zachary Weinstock, Niels Bergsland, and Alexander Bartnik have nothing to disclose. Dejan Jakimovski serves as Associate Editor of Clinical Neurology and Neurosurgery and compensated by Elsevier B.V. Tom Fuchs receives research support from the European Committee for Treatment and Research in Multiple Sclerosis and received consulting fees for Click Therapeutics. Robert Zivadinov has received personal compensation from Bristol Myers Squibb, EMD Serono, Sanofi, Janssen, 415 Capital, MAPI Pharma and Novartis for speaking and consultant fees. He received financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Octave, Mapi Pharma, CorEvitas, Protembis and V-WAVE Medical. Michael G. Dwyer received personal compensation from Keystone Heart for speaking and consultant fees. He received financial support for research activities from Bristol Myers Squibb, Novartis and Keystone Heart. Bianca Weinstock-Guttman received honoraria as a speaker and/or as a consultant for Biogen Idec, Teva Pharmaceuticals, EMD Serono, Genzyme, Sanofi, Genentech, Novartis, Celgene/BMS, Janssen and Horizon Dr Weinstock-Guttman received research funds from Biogen Idec, EMD Serono, Genzyme, Genentech, Sanofi, Novartis. Ralph HB Benedict received honoraria, speaking, or consulting fees from BMS, EMD Serono, Roche, Novartis, and Roche has received research support from Biogen, BMS, Novartis, National Institutes of Health and National Multiple Sclerosis Society. He has received royalties from Psychological Assessment resources, Inc.
Ethical standards
The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was approved by the University at Buffalo Institutional Review Board (IRB) and the requirement for informed consent for these retrospective analyses was waived. All study participants signed written consent when they were enrolled in the CEG-MS study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jakimovski, D., Zivadinov, R., Weinstock, Z. et al. Cortical thickness and cognition in older people with multiple sclerosis. J Neurol 270, 5223–5234 (2023). https://doi.org/10.1007/s00415-023-11945-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11945-2