Abstract
Background
Randomized studies could not demonstrate significant outcome benefit after single-incision laparoscopic cholecystectomy compared to classic four-port laparoscopic cholecystectomy (CLC). The new robotic single-site platform might offer potential benefits on local inflammation and postoperative pain due to its technological advantages. This prospective randomized double-blind trial compared the short-term outcomes between single-incision robotic cholecystectomy (SIRC) and CLC.
Methods
Two groups of 30 eligible patients were randomized for SIRC or CLC. During the first postoperative week, patients and study monitors were blinded to the type of procedure performed by four dressing tapes applied on the abdomen. Pain was assessed at 6 h and on day 1, 7 and 30 after surgery, along with a 1–10 cosmetic score.
Results
No significant difference in postoperative pain occurred in the two groups at any time point nor for any of the abdominal sites. Nineteen (63 %) SIRC patients reported early postoperative pain in extra-umbilical sites. Intraoperative complications which might influence postoperative pain, such as minor bleeding and bile spillage, were similar in both groups and no conversions occurred. The cosmetic score 1 month postoperatively was higher for SIRC (p < 0.001). Two SIRC patients had wound infection, one of which developed an incisional hernia.
Conclusions
SIRC does not offer any significant reduction of postoperative pain compared to CLC. SIRC patients unaware of their type of operation still report pain in extra-umbilical sites like after CLC. The cosmetic advantage of SIRC should be balanced against an increased risk of incisional hernias and higher costs.
Trial registration number
ACTRN12614000119695 (http://www.anzctr.org.au).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The development of single-incision laparoscopic surgery (SILS) is the result of the ongoing trend to minimize the invasiveness of laparoscopy. Cholecystectomy is the most commonly performed single-incision procedure, and continuous technical refinements along with many dedicated devices have been described since the original report of the technique in 1997 [1]. A SILS approach to this procedure has been proven feasible, although it increases the complexity of the surgery and, mostly for this reason, is currently far from becoming the standard practice in cholecystectomy. Indeed, the hypothetical widespread application of this procedure has raised understandable concern on safety, particularly outside the ideal patient, with an expected increase in bile duct injuries [2, 3]. Several prospective randomized trials and one meta-analysis have failed to show any distinct reduction of postoperative pain after SILS compared to classic four-port cholecystectomy (CLC), though a cosmetic advantage remains, given the lack of scarring with the trans-umbilical approach [3, 4].
Recently, novel single-site instruments and accessories for the daVinci Si Surgical System (Intuitive Surgical, Inc. Sunnyvale, CA) have been developed [5, 6]. These have allowed users to significantly shorten the learning curve typical of the laparoscopic single-incision approach [7], likely, in turn, to increase the safety of SILS and expand its adoption. Beyond its potential advantages in terms of reducing the complexity of the SILS approach, the single-incision robotic cholecystectomy (SIRC) might provide additional benefit in further reducing the already minimal pain of classic 4-port cholecystectomy. In fact, the higher stability of the system, which does not require trans-abdominal stitching of the gallbladder to expose the hilum and which allows smoother tissue dissection with a three-dimensional high-definition view, has the potential to reduce to a minimum intraoperative bile spillage and to limit blood loss and local trauma to the gallbladder fossa. This should decrease local inflammation and thus the amount of postoperative pain.
A prospective randomized trial was designed to test this hypothesis, by comparing short-term outcomes between SIRC and CLC. Since patients’ expectations from new technology could influence pain perception, our participants were blinded for 1 week to the type of surgery.
Materials and methods
This study (ANZCTR number: ACTRN12614000119695) was conducted between September 2011 and May 2013 at the Fondazione IRCCS Policlinico San Matteo of Pavia, Italy, having received prior approval from the local Institutional Review Board. Every patient gave written consent to enter the study after having been informed about the nature of the two proposed interventional techniques and the general design of the study. Inclusion criteria were as follows: (a) diagnosis of gallbladder lithiasis or polyps with no evidence of choledocholithiasis, (b) age between 18 and 80 years old, (c) body mass index (BMI) under 30 kg/m2, (d) ability to adhere to the protocol and to give written informed consent. Exclusion criteria were as follows: (a) evidence of acute cholecystitis or stones in the common duct as assessed by liver function tests and abdominal ultrasound, (b) gallbladder stone larger than 3 cm, (c) previous abdominal surgery through a midline or a right subcostal laparotomic incision, (d) ongoing pregnancy, (e) liver cirrhosis, (f) American Society of Anesthesiologists (ASA) physical status category higher than II and (g) known allergy to the analgesic drugs adopted in the study protocol. Allocation of patients to one of two groups, SIRC or CLC, was done using a computer-generated randomization list, with randomly permuted in blocks of varying size. Concealment was attained by using sealed envelopes.
All the operations were scheduled and performed early in the morning by one of four surgeons with prior experience with both SIRC and CLC. Patients were allowed to eat a soft diet on the same evening and a regular breakfast on the day after. Patients were discharged 24 h postoperatively if satisfactorily recovered at that time point.
Operative technique
For CLC, after pneumoperitoneum creation with Veress needle, a 12-mm trocar was placed in the umbilicus. Three additional 5-mm trocars were then inserted, under endoscopic view, in the left subcostal region (LSC) along the pararectal line, in the right subcostal region (RSC) at the midclavicular line, and in the right iliac fossa (RIF). The gallbladder was retrieved with an endo-bag through the umbilical incision, closed with a single absorbable fascial suture. For SIRC, a vertical skin incision of approximately 2 cm was made through the umbilicus, the fascia and the peritoneum to accommodate the single-site robotic port. Docking of the robot, preparation of the whole system and removal of the gallbladder from the liver bed then proceeded as previously described [8]. The fascia defect was finally closed with 4–6 interrupted absorbable sutures. All skin wounds were approximated with a subcuticular suture in both groups.
Pain management
Standard analgesia was administered intravenously (tramadol 100 mg + ketorolac 30 mg) in every patient approximately 30 min before the estimated end of the intervention. Also, a 10 ml dose of ropivacaine 7.5 mg/7 ml were injected in surgical sites at closure time in both groups. No subphrenic injection of local anesthetic was done intraoperatively.
Postoperative analgesic therapy consisted of i.v. paracetamol 1 g administered only on patient’s demand up to a maximum of one dose every 8 h as a standard protocol regardless of the technique.
Intra-operative data collection
During surgery a specific form was filled in for each patient by the designated nurse with the aim to collect all intraoperative information. These included patients’ personal and anthropometric data, allocation to SIRC or CLC, operative times, adverse events (bleeding from the gallbladder fossa, gallbladder damage and consequent bile spillage), need for conversion and reasons for, different analgesic administration from the standardized dose or drugs. Operative times were recorded as follows: “total operative time”, from induction of anesthesia to awakening; “docking time”, from Veress needle insertion to the end of robot docking in SIRC group and trocars positioning in CLC; “dissection time”, from first gallbladder retraction to its complete detachment from liver bed; “closure time”, after specimen extraction to wound medication.
Blinding and postoperative data collection
The type of procedure performed was known only to the operating team. To blind the patient and the study monitor to this data, four pieces of dressing tape, soaked with povidone-iodine solution to avoid postoperative visual clues from wound secretions, were applied on the four abdominal port sites in both groups at the end of surgery. Analgesics’ consumption during the following 24 h was recorded and a standard 10-point visual analog scale (VAS) was used for patients’ self-evaluation. A designated nurse monitor assessed pain intensity at 6 h postoperatively, and at 1, 7 and 30 days after surgery. At every time point, different VAS scores were obtained for each of the four abdominal areas covered with dressing tape, and for shoulder tip (ST) pain. Dressing tapes were removed by the nurse monitor at the scheduled 7 day check. Cosmetic results were evaluated 1 month after surgery using a patient satisfaction score from 0 to 10, (0 = no satisfaction, 10 = complete satisfaction). In practice, patients were asked to compare the actual photograph of their own scar(s) with a preoperative picture. Finally, every trial’s participant was called back and checked again 15 months after the end of the study to assess the occurrence of incisional hernia.
Study endpoints
For postoperative pain evaluation, patients were stratified according to the VAS score summed up for the four abdominal sites as follows: “mild pain” if the VAS sum was <16, “moderate to severe pain” if VAS sum was ≥16 (with VAS ≥ 4 on each site).
Based on the hypothesis that SIRC might improve postoperative pain perception, the primary endpoint of the study was to evaluate the reduction by 50 % of SIRC patients with moderate to severe pain at 24 h after surgery compared to the CLC group. For this aim, the proportion of patients with VAS sum ≥16 was compared between the groups. Secondary endpoints included changes over time of the median VAS sum at different time points (6 h, 24 h, 7 and 30 days postoperatively) and cosmetic outcome of the surgical scars assessed as previously described. Further objectives were investigated as tertiary endpoints of the study: operative times, intra and postoperative morbidity, rate of incisional hernia.
Statistical analysis
Sample size was calculated with the aim of assessing the primary endpoint. Considering the VAS sum of the four abdominal sites recorded at 24 h after surgery, based on previous experiences a moderate to severe pain (VAS ≥ 16) is expected in 90 % of CLC patients [9–12]. If a 50 % reduction of this pain level is hypothesized in SIRC group, 28 patients should be enrolled in each arm of the study to maintain a 5 % alpha-error (two-tailed test) with a power of 90 %. Assuming a drop-out rate of 7 %, 60 patients were enrolled in the study (30 patients for each group). Calculation was made by the use of nQuery Advisor 4 software (Statistical Solutions, Cork, Ireland).
Data were described within each treatment group as mean and standard deviation (SD) or median and 25th–75th percentiles if continuous, and as counts and percent if categorical. Accordingly, they were compared either with the Student’s t test, the Mann–Whitney U test or the Fisher exact test, respectively. Differences in means, medians or proportions were computed together with their 95 % confidence interval (CI). Median regression with bootstrapped standard errors was used to compare changes over time of the summated pain score.
All analyses were intention-to-treat. Patients who skipped one of the outpatient checks or violated blinding were excluded from the analysis. Stata 13 (StataCorp, College Station, TX, USA) was used for computation. A p value < 0.05 was considered statistically significant.
Results
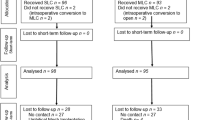
One hundred thirty-three consecutive patients assessed for eligibility were selected and then randomized to one of the two arms of the study until the target of 60 cases (30 per arm) suitable for the analysis was reached. The trial’s flow chart is depicted in Fig. 1.
The groups were comparable in terms of age, sex and BMI. Median pain intensity, cumulative of the 5 considered surgical sites (umbilicus, RIF, RSC, LSC, ST), at each time point after SIRC and CLC is shown in Fig. 2. Figure 3 shows site-specific pain scores at 24-h after surgery in the two groups.
Primary endpoint
The hypothesis of pain reduction by 50 % in SIRC patients with moderate to severe pain on postoperative day 1, stated as primary endpoint, was not verified by the available data. In fact, the proportion of that subgroup of patients was similar in both SIRC and CLC (10 vs. 7 %, p = 1.00; 95 % CI −0.10 to 0.17). Even supplementary analysis for further assessment of the primary endpoint confirmed this result (see Table 1).
Secondary endpoints
Significant changes over time in pain perception expressed as median VAS sum recorded at each of the four postoperative time point were not observed between SIRC and CLC (p = 0.60; see Table 1).
Cosmetic results showed a statistically significant increased satisfaction for SIRC at 1 month after surgery, with 22 (73.3 %) out of 30 patients scoring 9 or 10 versus only 5 out of 30 in the CLC group. The median cosmetic score was 9.0 (range 8–10) versus 8.0 (range 7–8) (p < 0.001), as depicted in Table 1.
Tertiary endpoints
Total operative time for SIRC was longer compared to CLC (98 min ± 34 vs. 87 min ± 30), although not statistically significant. For the other time intervals (docking, dissection and closure time), a significant difference was found in favor of CLC (Table 1).
No major adverse event occurred in either group and no patient was converted. Intraoperative bile spillage and minor bleeding, mostly due to liver damage at the gallbladder fossa, were more common though not significant during CLC, occurring in 5 (16 %) and 2 (6 %) patients, respectively, as opposed to 4 (13 %) and 3 (10 %) with SIRC patients.
Two SIRC patients (6 %) developed a wound infection versus none in the CLC group (p = 0.49). One of the affected patients (3 %) eventually required incisional hernia repair 6 months later.
Outcomes for further variables
Analgesic consumption resulted comparable in the two groups during hospital stay (p = 0.60 at 6 h; p = 0.70 at 24 h) and also after discharge, according to the information retrieved by the nurse monitor at 7 (p = 0.10) and 30 (p = 0.40) days outpatient checks.
Median hospital stay was 1.2 (range 1–3) days in both groups. Median follow-up was 32.0 (25th–75th percentile: 22.4–30.1) months in SIRC and 36.8 (25th–75th percentile: 26.9–39.5) months in CRC (p = 0.60).
Discussion
The introduction of the single-site robotic platform addressed many of the technical limitations of single-incision laparoscopic cholecystectomy, such as ergonomics, internal and external instrument clashing, frequent changing of difficult-to-insert curved instruments and image instability. This new technique is easily mastered by surgeons with previous experience in robotics, as opposed to the steep learning curve of SILS [7, 8]. The robotic technology also eliminates the need for gallbladder retraction by a transabdominal suture through the fundus, frequently done in the SILS procedure, or by the placement of a subcostal mini-grasper [13]. Mechanical conflict between camera and instruments is common in SILS [14]; therefore, inadvertent trauma to the gallbladder and liver bed is not rare resulting in a higher incidence of intraoperative bile spillage and blood loss compared to CLC [2, 3], potentially responsible for local inflammation and postoperative pain. Thus, in SILS, the expected reduction of postoperative pain attributable to the single access point could be counterbalanced by an increased visceral component of pain itself. In addition, the greater use of traction around the SILS port [15] and the increased manipulation on the umbilical wound edges [16] might accentuate postoperative umbilical pain. For all these reasons SIRC, unlike SILS, might improve the early postoperative outcome, particularly with pain diminution. To address this question, site-specific pain evaluation was adopted in our study, where we blinded patients and the study monitor to the surgical procedure, in order to obviate the possible influence of technology-driven expectations. This method has already been recognized as a crucial point by two prospective randomized trials [17, 18] which showed similar results between SILS and CLC either in terms of pain scores, at any time points, or recovery time. Luna et al. [19] have also shown that the inflammatory reaction to surgery, as measured by IL-6 and CPR levels, was similar after SILS and CLC. In our study, too, there was no significant difference in median pain intensity (as measured by the VAS score) between SIRC and CLC at any postoperative time point (Fig. 2). We specifically hypothesized, as primary endpoint of this trial, that SIRC patients might perceive significantly less pain in the early postoperative course than CLC ones due to the technical advantages provided by the robotic single-site platform. Anyway, our data failed to confirm this assumption. In fact, no relevant difference was found when SIRC and CLC were compared in terms of different pain scores at 24 h after surgery, even when separately comparing each abdominal site at this time point. In CLC a 12-mm trocar was used to facilitate specimen’s extraction; anyway, although the umbilical wound was always larger in SIRC patients, no significant difference in umbilical pain was seen at any time in the two groups. Even more surprisingly 19 (63 %) SIRC patients, unaware of their type of operation, still reported early postoperative pain in extra-umbilical sites (Fig. 3). Whether this is attributable to a non-specific generalized reaction to the CO2 pneumoperitoneum used or rather a reverse-placebo effect due to the presence of dressing tapes, would be hard to prove. With the reverse-placebo or “nocebo” phenomenon [21], the affective state associated with an expectation of pain, such as the presence of dressing tapes in our patients, causes effective pain in the expectant [22]. Regardless of the underlying cause, though, it should be noted that the amount of pain after CLC is already so minimal that the hope of further containment as a consequence of technical refinement might be an illusion.
In the present study robotic procedures required longer, although not statistically significant, total operative time than conventional laparoscopic technique. This was mainly due to the extra time needed to robot docking (23 min ± 7) and to the closure of the umbilical wound (19 min ± 5), which were both significantly longer compared to the corresponding CLC data (p < 0.001). The pure surgical dissection time was also longer for SIRC (56 min ± 26 versus 44 min ± 16; p = 0.035). In CLC the time needed for specimen retrieval without enlarging the 12-mm umbilical wound was not specifically recorded since it had no relevance for the analysis. However, this explains the gap between total operative time and the other time intervals in this group.
Complication rate was similarly low in both techniques, confirming SIRC’s high standard of safety, at least in selected subjects. Fewer intraoperative bile spillages and minor bleedings occurred in SIRC, although with no statistical significance, in line with its high degree of precision in tissue dissection [20]. Nevertheless, this observation could not be reconciled with the amount of postoperative pain declared by patients at 24 h after surgery, affecting either the right hypochondrium or shoulder tip (Fig. 3).
Our 10-point cosmetic score system showed a statistically significant increased satisfaction for SIRC patients at 1 month. Using the same methodology, Marks and colleagues also reported a significant cosmetic advantage with SILS at 7, 14, 30 and 90 days after surgery [17]. Brown, however, showed a loss of significance at 1 month between SILS and CLC, suggesting that the fear of a poor cosmetic result after CLC tends to vanish when the patient realizes the actual impact of the 4-port approach [18]. Probably, there is a tendency on patient’s side in overestimating the cosmetic advantage of SILS over conventional laparoscopy. Nonetheless, the undeniable attractive power of almost no-scar surgery should be carefully balanced here against its primary potential drawback. The strongest area of concern with SILS and SIRC relates to an increased risk of incisional hernia after a larger trans-umbilical incision. Garg reported a higher rate of umbilical wound seroma in SILS patients [3]. In a systematic review of the medical literature encompassing 3989 SILS procedures, Pollard found a 2.5 % incidence of minor postoperative complications, the majority being wound-related [15]. A recent prospective trial showed a 1.2 % port-site hernia rate after conventional laparoscopy as opposed to 8.4 % after SILS [9]. We observed wound infection in 6 % of our SIRC patients, one of whom subsequently required the repair of an incisional hernia (3 %). Contrarily, no wound infection was observed in CLC patients. Although not statistically relevant, these data support the current concern for the larger umbilical incision.
Our trial has some intrinsic limitations. First, for sample size calculation we assumed a given decrease in postoperative pain at 24 h after surgery in the robotically treated group compared to CLC patients. Despite our series is small, we cannot reasonably presume that a higher number of randomized selected subjects would behave differently, as already shown in larger trials with similar results [9, 10].
Second, pain after abdominal surgery is a complex phenomenon, hardly ascribable to well-defined mechanisms. Residual pneumoperitoneum, intraoperative blood loss or bile spillage might contribute to ongoing diffuse abdominal pain at 24 h, thus impairing site-specific pain analysis at that time point. However, it is unlikely that a later evaluation of pain (i.e., at 48 or 72 h) could be more informative to assess the benefit of one technique, SIRC or CLC, over the other.
Third, it could be questioned that dressing tapes were not removed after discharge. Despite patients’ blindness could not be objectively verified, the quite high proportion of losts to follow-up (21 % overall) was mostly due to violation of the protocol rules which led to the exclusion of almost two-thirds of this share of patients, as shown in Fig. 1. We can therefore speculate that those eventually included in the analysis were reliable subjects as to the blinding method used in this trial.
There is no objective evidence demonstrating that robotic surgery is a less painful technique than conventional laparoscopy. In the setting of single-site cholecystectomy, it is at least presumable that a lesser mechanical component weighs on the amount of pain compared with SILS thanks to the advantages of robotic technology. However, our trial failed to demonstrate this expected benefit despite comparison was not made with a standard single-incision technique. A third arm including either robotic four-ports cholecystectomy or SILS cholecystectomy should be considered in future trials to specifically assess the influence of each technique on postoperative pain.
Finally, there is little doubt that the cost of SIRC is considerably higher than that of CLC. Even excluding the purchase and maintenance costs of the robot from the economic analysis [23], and considering only the disposable instruments needed for a single-site robotic cholecystectomy, this cost was found to be as high as $1268 [24]. Taking into account how frequently cholecystectomies are scheduled at most hospitals, we believe that the expense of a routine single-site robotic approach to gallstone disease, outside clinical trials, will strongly limit its wider adoption. Anyway, a proper cost analysis was not addressed in this study.
In conclusion, our randomized double-blind trial showed that both SIRC and CLC produced similar levels of postoperative pain. The advantages of robotic technology applied to the single-site platform could not be able to decrease pain perception after surgery as one might expect. On the other hand, patients operated on with SIRC reported an increased satisfaction rate in terms of cosmesis at 1 month than those treated with conventional laparoscopy. However, during follow-up two SIRC patients developed incisional hernia on the access site for which surgical repair was needed. Therefore, hospitals that can afford SIRC as an alternative to CLC for their patients should consider that the potential cosmetic superiority offered by this technology might be outweighed by an increased number of incisional hernias.
References
Navarra G, Pozza E, Occhionorelli S, Carcoforo P, Donini I (1997) One-wound laparoscopic cholecystectomy. Br J Surg 84(5):695
Joseph M, Phillips MR, Farrell TM, Rupp C (2012) Single incision laparoscopic cholecystectomy is associated with a higher bile duct injury rate: a review and a word of caution. Ann Surg 256(1):1–6
Garg P, Thakur JD, Singh I, Nain N, Mittal G, Gupta V (2012) A prospective controlled trial comparing single-incision and conventional laparoscopic cholecystectomy: caution before damage control. Surg Laparosc Endosc Percutan Tech. 22(3):220–225
Pisanu A, Reccia I, Porceddu G, Uccheddu A (2012) Meta-analysis of prospective randomized studies comparing single-incision laparoscopic cholecystectomy (SILC) and conventional multiport laparoscopic cholecystectomy (CMLC). J Gastrointest Surg. 16(9):1790–1801
Haber GP, White MA, Autorino R, Escobar PF, Kroh MD, Chalikonda S, Khanna R, Forest S, Yang B, Altunrende F, Stein RJ, Kaouk JH (2010) Novel robotic da Vinci instruments for laparoendoscopic single-site surgery. Urology 76(6):1279–1282
Escobar PF, Haber GP, Kaouk J, Kroh M, Chalikonda S, Falcone T (2011) Single-port surgery: laboratory experience with the daVinci single-site platform. JSLS 15(2):136–141
Spinoglio G, Lenti LM, Maglione V, Lucido FS, Priora F, Bianchi PP, Grosso F, Quarati R (2012) Single-site robotic cholecystectomy (SSRC) versus single-incision laparoscopic cholecystectomy(SILC): comparison of learning curves. First European experience. Surg Endosc 26:1648–1655
Pietrabissa A, Sbrana F, Morelli L, Badessi F, Pugliese L, Vinci A, Klersy C, Spinoglio G (2012) Overcoming the challenges of single-incision cholecystectomy with robotic single-site technology. Arch Surg 147(8):709–714
Marks JM, Phillips MS, Tacchino R, Roberts K, Onders R, Denoto G, Gecelter G, Rubach E, Rivas H, Islam A, Soper N, Paraskeva P, Rosemurgy A, Ross S, Shah S (2013) Single-incision laparoscopic cholecystectomy is associated with improved cosmesis scoring at the cost of significantly higher hernia rates: 1-year results of a prospective randomized, multicenter, single-blinded trial of traditional multiport laparoscopic cholecystectomy vs single-incision laparoscopic cholecystectomy. J Am Coll Surg 216(6):1037–1047
Poon CM, Chan KW, Lee DW, Chan KC, Cho CW, Cheung HY, Lee KW (2003) Two-port vs four-port laparoscopic cholecystectomy. A prospective randomized controlled trial. Surg Endosc 17(10):1624–1627
Novitsky YW, Kercher KW, Czerniach DR, Kaban GK, Khera S, Gallagher-Dorva KA, Callery MP, Litwin DE, Kelly JJ (2005) Advantages of mini-laparoscopic vs conventional laparoscopic cholecystectomy. Results of a prospective randomized trial. Arch Surg 140(12):1178–1183
Bisgaard T, Klarskov B, Rosenberg J, Kehlet H (2001) Characteristics of early pain after laparoscopic cholecystectomy. Pain 90(3):261–269
Cuschieri A (2011) Single-incision laparoscopic surgery. J Minim Access Surg. 7(1):3–5
Greaves N, Nicholson J (2011) Single incision laparoscopic surgery in general surgery: a review. Ann R Coll Surg Engl 93(6):437–440
Pollard JS, Fung AK, Ahmed I (2012) Are natural orifice transluminal endoscopic surgery and single-incision surgery viable techniques for cholecystectomy? J Laparoendosc Adv Surg Tech A 22(1):1–14
Ma J, Cassera MA, Spaun GO, Hammil CW, Hansen PD, Aliabadi-Wahle S (2011) Randomized controlled trial comparing single-port laparoscopic cholecystectomy and four-port laparoscopic cholecystectomy. Ann Surg 254(1):22–27
Marks J, Tacchino R, Roberts K, Onders R, Denoto G, Paraskeva P, Rivas H, Soper N, Rosemurgy A, Shah S (2011) Prospective randomized controlled trial of traditional laparoscopic cholecystectomy versus single-incision laparoscopic cholecystectomy: report of preliminary data. Am J Surg 201(3):369–372
Brown KM, Moore BT, Sorensen GB, Boettger CH, Tang F, Jones PG, Margolin DJ (2013) Patient-reported outcomes after single-incision versus traditional laparoscopic cholecystectomy: a randomized prospective trial. Surg Endosc 27(9):3108–3115
Luna RA, Nogueira DB, Varela PS, Rodrigues Neto Ede O, Norton MJ, Ribeiro Ldo C, Peixoto AM, de Mendonca YL, Bendet I, Fiorelli RA, Dolan JP (2013) A prospective, randomized comparison of pain, inflammatory response, and short-termoutcomes between single port and laparoscopic cholecystectomy. Surg Endosc 27(4):1254–1259
Gonzalez AM, Rabaza JR, Donkor C, Romero RJ, Kosanovic R, Verdeja JC (2013) Single-incision cholecystectomy: a comparative study of standard laparoscopic, robotic, and SPIDER platforms. Surg Endosc 27(12):4524–4531
Hahn RA (1997) The nocebo phenomenon: concept, evidence, and implications for public health. Prev Med 26(5 Pt 1):607–611
Spiegel H (1997) Nocebo: the power of suggestibility. Prev Med 26(5 Pt 1):616–621
Turchetti G, Palla I, Pierotti F, Cuschieri A (2012) Economic evaluation of da Vinci-assisted robotic surgery: a systematic review. Surg Endosc 26(3):598–606
Buzad FA, Corne LM, Brown TC, Fagin RS, Hebert AE, Kaczmarek CA, Pack AN, Payne TN (2013) Single-site robotic cholecystectomy: efficiency and cost analysis. Int J Med Robot. 9(3):365–370
Funding
No funding was received for this work by any of the following organizations: National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI); or others.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Andrea Pietrabissa, Luigi Pugliese, Alessio Vinci, Andrea Peri, Francesco Paolo Tinozzi, Emma Cavazzi, Eugenia Pellegrino and Catherine Klersy have no conflict of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pietrabissa, A., Pugliese, L., Vinci, A. et al. Short-term outcomes of single-site robotic cholecystectomy versus four-port laparoscopic cholecystectomy: a prospective, randomized, double-blind trial. Surg Endosc 30, 3089–3097 (2016). https://doi.org/10.1007/s00464-015-4601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4601-3