Abstract
This study aimed to improve the yield of cyclodextrins (CDs) production in repetitive batches. An innovative ultrafiltration system was used to remove the inhibitory products that accumulated in the medium and to recover the enzyme. The assays were performed with the CGTase from Bacillus firmus strain 37 in purified, semi-purified, and crude extract forms. Maltodextrin (10 % w/v) and corn starch (5 % w/v) were used as substrates. After eight repetitive 24-h batches, the yield of β-CD obtained with the purified enzyme and the corn starch substrate was 0.54 mmol/L/h, which was 36 % greater than that observed with the 10 % maltodextrin substrate. The crude CGTase extract with the corn starch substrate showed a productivity of 0.38 mmol/L/h, which was 29 % lower than using the purified enzyme and the corn starch substrate but 7 % higher than using the purified enzyme and the maltodextrin substrate. The crude extract, assayed with the corn starch substrate in the presence of 10 % ethanol reached 0.43 mmol/L/h productivity, which was 12 % higher compared to the assay without ethanol. The semi-purified enzyme was assayed with the corn starch substrate in the presence of 10 % ethanol for eight batches lasting 12 h and an excellent selectivity for the β-CD was obtained, reaching a mean percentage of 96.0 %. Therefore, this ultrafiltration system enabled several batches of CD production, with efficient removal of products inhibitory to the CGTase and recovery of the enzyme. The possibility of industrial application of this system is promising.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs), also called Schardinger dextrins, cycloamylose, or cyclomaltose, are a family of non-reducing cyclic oligosaccharides, in which the glucose units are joined by α-1, 4-glycosidic bonds. The most common structures are formed by 6, 7, or 8 glucopyranose units, and named α-CD, β-CD or γ-CD, respectively [1–4]. They are produced from the cyclization reaction of starch, catalyzed by the enzyme cyclodextrin glycosyltransferase (CGTase) [5].
CGTase is a monomeric enzyme with a molecular mass of approximately 74.5 kDa. CGTase is an enzyme found in bacteria, generally extracellular produced, and converts starch and related substrates into CDs [6, 7]. Its amino acid sequence shows a structural similarity with the α-amylase enzyme. The CDs are formed through reversible intramolecular transglycosylation reactions, or cyclization. However, CGTases also have activity in reactions of intermolecular transglycosylation (coupling and disproportionation) and, to a lesser extent, in the hydrolysis of starch [8].
The hydrophobic nature of the cavity of CDs confers a strong ability to encapsulate a large number of organic and inorganic molecules, forming stable complexes by molecular inclusion [1, 9]. The inclusion complexes modify the physicochemical properties of the guest molecule, greatly increasing their stability and solubility [10, 11]. These features allow CDs or their derivatives to be suitable for applications in the pharmaceutical, food, cosmetic, textile, chemical, and other industries [12–14].
Thus, many researchers have been searching for innovative technology for the production of CDs, including Gawande and Patkar [15], who assayed the effect of enzyme concentration on the production of CDs from wheat starch (125 g/L) in the presence of 2 % (v/v) butan-1-ol. As expected, higher concentrations of enzyme led to higher initial production rates of CDs; however, the final yield of α-CD after 6 h was nearly the same at concentrations between 20 and 40 U/g of enzyme. Although the reason for the insensitivity of the CD yield to higher enzyme concentrations is unknown, two main hypotheses were proposed. The first considers that the cyclizing CGTase activity can be inhibited by high concentrations of product, which causes a decrease in the production of CDs as product concentration in the medium increases. The second possibility is that the reaction mixture is close to equilibrium at the end of the experiments, therefore only higher concentrations of enzyme would cause the equilibrium to be reached more quickly.
Many studies have reported an increase in the production of CDs with the presence of ethanol in the reaction medium [15]. Calsavara et al. [16], working with the Toruzyme® in the presence of 10 % ethanol, obtained an 18 % increase in production of β-CD, while the yield of α-CD almost tripled.
A strong inhibition of CGTase by CDs produced in the medium has been reported. Gaston et al. [17] showed that the CDs competitively inhibited the CGTase from Bacillus circulans DF 9R, with an increase in the K m values, while the maximum speed of the reaction remained constant. The results showed that the strongest inhibitor is γ-CD, followed by β-CD and then α-CD. Maltodextrins also had inhibitory effects on the activity of CGTase, and maltose was the most potent inhibitor. Thus, the yield of CDs can be improved by removing its inhibitory products that accumulate in the reaction medium, which can be obtained by the use of an ultrafiltration system coupled to the reactor [15, 17, 18].
Considering the importance of CDs and their numerous industrial applications, this research aimed to optimize their production by the CGTase from Bacillus firmus strain 37, in purified, semi-purified, and crude extract forms, using an ultrafiltration system for the separation of CDs and enzyme recovery steps, removing their inhibitory products and allowing the reuse of CGTase in repetitive batches. Furthermore, the effect of different substrates and the presence of ethanol were evaluated for improving the production of these molecules.
Materials and methods
Reagents, microorganism, and culture conditions
The α-, β-, and γ-CDs were purchased from Sigma (St. Louis, MO, USA). The corn starch substrate used in the assays was food grade (Maizena, Unilever Company), and it was purchased in a local market. The maltodextrin (dextrin 10 from maize starch, article 31410) was obtained from Fluka (Buchs, Switzerland). The other chemicals used were of analytical grade.
The CGTase enzyme was obtained from B. firmus strain 37 isolated from cassava plantation soil by Matioli et al. [19] and maintained on a sterile soil sample. The microorganism was seeded in Petri plates containing solid medium with the following composition (%w/v): soluble starch 1.0; polypeptone 0.5; yeast extract 0.5; K2HPO4 0.1; MgSO4·7H2O 0.02; Na2CO3 1.0 and agar 1.5 [20]. After incubation at 37 °C for 48 h, the colonies formed in the culture medium were freeze-dried. For freeze-drying, the colonies were removed from the plates, suspended in a small amount of sterile water and 2 mL of this suspension were transferred to glass vials. The samples were frozen in a freezer and subsequently placed inside the freeze dryer (LIOTOP–L101). The samples were then stored in a freezer until use.
Production and obtainment of CGTase in purified, semi-purified, and crude extract forms
To produce the enzyme, a pre-inoculum was prepared by adding 350 mg of freeze-dried cells of B. firmus strain 37 to a 500 mL Erlenmeyer flask containing 250 mL of liquid culture medium (with the same composition of the solid medium, but without agar), for turbidity corresponding to absorbance 1.0 at a wavelength of 660 nm [21]. The pre-inoculum was incubated at 37 °C and shaken at 80 rpm for 48 h. Aliquots of 50 mL of the pre-inoculum were transferred to 2 L Erlenmeyer flasks containing 1 L of liquid medium. After inoculation of each medium, a 1.0 mL aliquot was collected (t0) for the determination of enzyme activity. The media were incubated at 37 °C and shaken at 80 rpm for 5 days.
During the incubation period, 1.0 mL aliquots were collected at 24, 48, 72, 96 and 120 h for monitoring enzyme activity. After collecting the last aliquot, the contents of all 4 Erlenmeyer flasks were pooled and centrifuged for 10 min at 8,000 rpm at 4 °C. The pellet, composed of cells and insoluble components in the medium, was discarded. A sample of the supernatant containing the enzyme was set aside to be used in CD production assays as crude extract of CGTase and for the determination of enzymatic activity and protein concentration.
To the remainder of the supernatant, ammonium sulfate was added at a ratio of 561 g per 1 L of supernatant to obtain 80 % saturation for protein precipitation. The solution was kept in a refrigerator (4 °C) for 48 h. The precipitate was separated by centrifugation at 4 °C and 8,000 rpm for 20 min. The pellet was suspended in a small volume of 50 mM Tris–HCl buffer pH 8.0/5 mM CaCl2 solution in a 60:40 ratio. Then, a sample of the pellet was set aside to be subjected to semi-purification procedure. The sample was suspended in 50.0 mL of 50 mM Tris–HCl pH 8.0/5 mM CaCl2 solution in a 60:40 ratio, and then subjected to ultrafiltration through a membrane of 30 kDa NMWL (nominal molecular weight limit) and 44.5 mm diameter, in an Amicon® device. For washing, 50.0 mL of the solution 50 mM Tris–HCl buffer/5 mM CaCl2 (60:40) was added and a new ultrafiltration was performed. Next, the enzymatic activity and the protein concentration of the semi-purified CGTase were determined.
The enzymatic activity of the remaining pellet was determined and was kept refrigerated until the purification procedure. Purification of the CGTase from B. firmus strain 37 was performed using biospecific affinity chromatography (BAC) according to the methodology described by Moriwaki et al. [22].
Determination of enzymatic activity and protein concentration of purified, semi-purified, and crude extract CGTase
The protein concentration was determined by the Bradford method (Bradford [23]) using bovine serum albumin as the standard. The enzymatic activity of the samples was determined according to the production of β-CD, measured by the spectrophotometric method. One unit of activity corresponds to the amount of CGTase that produces 1 μmol of β-CD/min under the defined reaction conditions [22, 24].
Preliminary assay of CD production by the purified CGTase during 24 h
For the CD production assays using the CGTase from B. firmus strain 37, three compositions of the reaction medium were used, which differed with regard to the substrate used, its concentration, or whether in the presence of ethanol. The general composition of the medium was the substrate (at the specified concentration); 50 mM Tris–HCl (pH 8.0) 20 % (v/v); 5 mM CaCl2 solution 10 % (v/v); and purified water (q.s. 100 %).
To determine the duration of each batch for the CD production assays, a previous CD production assay lasting 24 h was performed for each substrate using the CGTase from B. firmus strain 37.
Maltodextrin substrate 10 % (w/v)
The assay was conducted in a jacketed reactor containing 1.0 mL of the purified CGTase and 99.0 mL of production medium containing the maltodextrin substrate 10 % (w/v). The reaction medium remained under constant stirring and the temperature was maintained at 50 °C with the aid of a magnetic stirrer and a thermostat bath connected to the jacketed reactor. During the reaction, aliquots were collected at 0, 30, 60, 90, 120, 180, 240, 360, 540, 720, 960, 1,200, and 1,400 min, and stored for later quantification of the CDs produced.
Corn starch substrate 5 % (w/v)
The same procedure described in the preceding section was performed on the reaction medium containing the corn starch substrate 5 % (w/v) at pH 8.0. The lower concentration used, compared to maltodextrin substrate, is due to the high viscosity conferred by starch substrate.
Repetitive batches of CD production with recovery of CGTase by ultrafiltration system
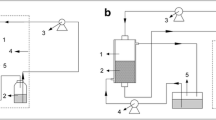
After obtaining the CGTase from B. firmus, the CD production proceeded in repetitive batches using the purified, semi-purified, and crude extract CGTase preparations, following the reaction parameters optimized by Matioli et al. [25]. At the end of each batch, one ultrafiltration system (Fig. 1) was used to remove the CDs and other inhibitory products formed during the reaction (glucose, maltooligosaccharides, and etc) [24]. The CGTase was recovered and then immediately reused in the next production batch.
Purified CGTase and maltodextrin substrate 10 % (w/v)
The assay was performed in a jacketed glass reactor with a capacity for 50 mL of medium. The dilution of the CGTase in the medium was 1:100, which corresponded to a concentration of 8.70 × 10−4 mg protein/mL of medium. First, the maltodextrin substrate in a concentration of 10 % (w/v), pH 8.0, was used. The reaction medium remained at constant stirring and the temperature was maintained at 50 °C with the aid of a magnetic stirrer and a thermostat bath connected to the jacketed reactor. The duration of each batch was set at 24 h.
After the first 24-h batch, a small aliquot was collected for the measurement of CDs produced and next the entire volume of medium was transferred by a peristaltic pump to an Amicon® ultrafiltration device (equipped with a membrane of 10 kDa NMWL and 44.5 mm diameter). The ultrafiltration was performed under nitrogen gas pressure and agitation (Fig. 1). The CGTase from B. firmus strain 37 has a molecular mass of approximately 78,000 Da [25] and thus is retained by the membrane. CDs have a size of 972, 1,135, and 1,297 Da for α, β, and γ-CD, respectively, and were subsequently filtered [26].
The filtrate was collected and stored for determining the CD concentration by high-performance liquid chromatography (HPLC). All of the retained volume containing the enzyme was suspended in a new reaction medium until the volume reached 50.0 mL and was transferred back to the reactor with the aid of the peristaltic pump. A second 24-h batch was then performed under the same conditions as the first batch, applying again the ultrafiltration procedure at the end. Repetitive batches were performed until the enzyme lost considerable activity which was determined by monitoring the β-CD yield using the spectrophotometric method at the end of each batch.
Purified CGTase and corn starch substrate 5 % (w/v)
The same assay conditions described in the preceding section were performed for the CD production with the reaction medium containing the corn starch substrate 5 % (w/v) at pH 8.0.
Crude CGTase extract and corn starch substrate 5 % (w/v)
Considering that the crude enzyme extract is easily obtainable and inexpensive compared to the enzyme purification, this extract was used in the CD production. Its enzymatic activity was correlated with the purified CGTase activity, allowing us to determine the volume of crude extract required to achieve the activity equivalent to that observed in the assays with the purified enzyme. Thus, the amount of crude extract used was 72-fold the volume of the purified enzyme.
Due to the good results obtained with regard to the β-CD yield with the 5 % (w/v) corn starch substrate, this substrate was selected for the assay with the crude extract. The assay followed the methodology cited for the purified enzyme in “Purified CGTase and maltodextrin substrate 10% (w/v).”
Crude CGTase extract and corn starch substrate 5 % (w/v) in the presence of ethanol 10 % (v/v)
A new assay was performed to increase the yield of CDs obtained with the CGTase crude extract and corn starch substrate, considering that both offer economic advantages for production.
Because of the reported increase in the CD yield in the presence of ethanol, and considering the satisfactory results obtained by Calsavara et al. [23] with a concentration of 10 % (v/v), the same assay conditions described in “Crude CGTase extract and corn starch substrate 5% (w/v)” were repeated to include the addition of ethanol to the reaction medium at a concentration of 10 % (v/v) at the beginning of each batch.
Semi-purified CGTase and corn starch substrate 5 % (w/v) in the presence of ethanol 10 % (v/v)
The methodology used to obtain the semi-purified enzyme is simplified compared to the full purification of the enzyme, comprising only the initial stages. Furthermore, semi-purification enables the CGTase to be obtained in greater concentration and purity than that observed in the crude extract. The CD production using semi-purified CGTase was performed in repetitive batches lasting 12 h because the most significant production of CDs occurred in this time interval.
For this assay, the substrate used was 5 % (w/v) corn starch in the presence of 10 % (v/v) ethanol. The same procedures and conditions of pH and temperature described in “Purified CGTase and maltodextrin substrate 10% (w/v)” were used. At the end of each 12-h batch, the medium was subjected to ultrafiltration in an Amicon® device, as described in “Purified CGTase and maltodextrin substrate 10% (w/v).”
Determination of the starch conversion rate in CGTase products
To define the amount of starch remaining in the medium retained by ultrafiltration, as well as to calculate the rate of conversion of starch into CDs and linear oligosaccharides, some samples of the assay with the semi-purified enzyme were selected for the determination of total sugars by the phenol sulfuric method (Dubois et al [27]).
Spectrophotometric and chromatographic determination of CDs
During the CD production assays, yield measurements were performed by spectrophotometric determination of the concentration of β-CD by the method described by Tardioli et al. [24] and Moriwaki et al. [27]. This method is based on the discoloration of a phenolphthalein solution at 550 nm, which occurs after complexation with β-CD present in the sample. Because the relationship between absorbance and β-CD concentration remains stable only at concentrations up to 0.4 mmol/L, the Complexing Theory was used, as previously mentioned in the literature [24, 28], which provides the non-linear relationship between absorbance and β-CD concentration.
The concentration of α-CD, β-CD, and γ-CD produced in different assays with CGTase from B. firmus strain 37 were determined by HPLC. A CG-480C liquid chromatograph (Varian) was used, which was equipped with the refractive index detector IR-CG 410, and an aminopropylsilane column (SGE) (particle size 5 mm, length 25 cm, and internal diameter 4.6 mm). The development was made by isocratic conditions using a solution of acetonitrile and water (70:30) as the mobile phase and a flow rate of 0.7 mL/min at room temperature (21 °C). Standards and samples were filtered through a 0.45-µm membrane. Analytical curves were constructed for α-CD, β-CD, and γ-CD in different concentration ranges. For α-CD, the values ranged from 1.0 to 7.0 mg/mL; for β-CD, the range was from 1.0 to 11.0 mg/mL; and for γ-CD, the range was from 1.0 to 3.0 mg/mL. The results of peaks area of each CD as a function of the concentration on the pattern were adjusted by linear regression.
Statistical analysis
The CD yield results were evaluated by analysis of variance (ANOVA), and means were compared by a Tukey test at a 5 % significance level.
Results and discussion
Enzymatic activity and protein concentration of purified, semi-purified, and crude extract CGTase
For purified CGTase, the protein concentration determined by the Bradford method corresponded to 0.09 mg/mL, while the enzyme activity related to β-CD reached 9.36 µmol β-CD/min/mL. Thus, the specific enzyme activity, expressed as activity per milligram of protein, corresponded to 108.92 µmol β-CD/min/mg. These parameters were satisfactory, justifying the use of the sample in CD production assays.
For crude extract CGTase, the protein concentration was determined to be 0.03 mg/mL, with an enzymatic activity of 0.13 µmol β-CD/min/mL with specific enzyme activity of 4.21 µmol β-CD/min/mg. These parameters were considered the concentration of enzyme typically obtained from B. firmus strain 37 after 5 days in culture. Despite showing approximately 72-fold lower activity than purified CGTase, the possibility of using the crude extract to produce CDs is very advantageous due to the ease of obtaining it.
For the semi-purified CGTase, a protein concentration of 0.10 mg/mL and an enzymatic activity of 2.32 µmol β-CD/min/mL were observed. Thus, the specific enzyme activity of semi-purified CGTase corresponded to 23.10 µmol β-CD/min/mg. The enzyme semi-purification process allowed it to be concentrated, significantly reducing the volume of enzyme solution added to the reactor. It was necessary to use 36.0 mL of the crude extract to obtain the same enzymatic activity of the purified enzyme when diluted 1:100, while the semi-purified enzyme required only 2.0 mL. Thus, the enzyme semi-purification provided operating order advantages. Furthermore, the semi-purification eliminated much of the contaminants (cyclodextrins, oligosaccharides, low molecular weight proteins, and etc.) that could exert inhibitory activity against the CGTase.
Preliminary assay of CD production by the purified CGTase during 24 h
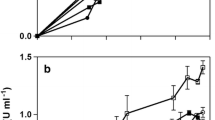
In agreement with previous studies on CGTase from B. firmus strain 37, the β-CD production was significantly higher compared with other CDs (Fig. 2), therefore characterizing it as a β-CGTase.
The β-CD concentration in the medium increased rapidly in the first 5 h of the reaction, and thereafter the concentration continued to increase more gradually. The production curves suggest that after 24 h the reaction is close to equilibrium (Fig. 2), subsequently showing limited change in CD concentration.
With a 10 % (w/v) maltodextrin substrate (Fig. 2a), the production was 11.9 mmol/L of β-CD, 1.9 mmol/L of α-CD, and 1.1 mmol/L of γ-CD. The 5 % (w/v) corn starch substrate gave a higher production of β-CD at the end of 24 h, reaching 13.5 mmol/L (Fig. 2b). Simultaneously, the α-CD production was affected dramatically, approaching zero. The γ-CD yield barely changed (1.0 mmol/L).
Repetitive batches of CD production with recovery of CGTase by ultrafiltration system
Considering the results obtained from the preliminary CD production assay, the duration of each batch was initially set at 24 h. The purified enzyme was assayed with the 10 % (w/v) maltodextrin substrate (Fig. 3a) and the 5 % (w/v) corn starch substrate (Fig. 3b) during eight repetitive batches of CD production. The effectiveness of the ultrafiltration system for batch CD production was shown in the first few batches of production, proving to be simple and eliminating the need for sophisticated reactors. The retention and recovery of the total CGTase enzyme present in the reaction medium and the removal of most inhibitory enzyme products using an Amicon® ultrafiltration device enabled an almost constant CD production for the initial batches.
The β-CD yield obtained was 0.54 mmol/L/h when the assay was performed with purified enzyme and the corn starch substrate and 0.35 mmol/L/h when using purified enzyme and the maltodextrin substrate. Specifically, the use of the corn starch substrate gave a 36 % increase in productivity, proving the superiority of the corn starch substrate over maltodextrin for producing this CD. Notably, 5 % (w/v) corn starch was used compared to 10 % (w/v) maltodextrin for the substrate; therefore, better results were obtained using corn starch even at half the concentration of maltodextrin (Table 1).
The CGTase activity may be related to the size of the substrate molecules. Maltodextrin, being smaller, would discourage the production of CDs and accelerate other reactions such as the coupling and degradation [29]. Furthermore, the interaction of the crystalline structure of starch with amino acids in the enzyme active site influences the specificity of the CGTase [30]. Therefore, it is likely that the corn starch best fit to the active site of the CGTase from B. firmus strain 37. Additionally, the proportion of amylose and amylopectin should be considered. The helical structure of amylose, with spins of six to seven glucose units, may favor the action of CGTase. Finally, increasing the substrate concentration and the degree of branching of polysaccharides can increase the β-CD yield [31].
The almost zero α-CD yields for some batches with the corn starch substrate can be understood as a consequence of the shift in production toward a higher β-CD yield, due to the higher specificity of the CGTase. Moriwaki et al. [32] obtained similar results when they immobilized B. firmus strain 37 and Bacillus sphaericus strain 41 in a loofa sponge, in which there was practically no α-CD production when the corn starch substrate was used.
The crude extract was assayed with the corn starch substrate 5 % (w/v) (Fig. 4a) and also with the corn starch substrate 5 % (w/v) in the presence of 10 % (v/v) ethanol (Fig. 4b), for eight repetitive batches of CD production.
When the CGTase crude extract was assayed using only the corn starch substrate, the productivity was 0.38 mmol/L/h, showing a 29 % decrease compared to the assay that used purified enzyme and the same substrate, with productivity of 0.54 mmol/L/h (Table 1). Although the volumes of extract used were equivalent to the same amount of enzyme in the assays with purified CGTase, this decrease can be due to numerous contaminant compounds in the supernatant used as the extract. These substances can act as interfering factors, disrupting the CD production by the CGTase. Even so, the assay using the crude extract and corn starch substrate had a 7 % greater productivity compared to the assay in which the purified enzyme and maltodextrin substrate were used.
Ethanol has been cited as an alternative for increasing CD production. It has the property of reducing the water activity in the enzyme active site, preventing hydrolytic reactions and reverse reactions, and thus decreases the decomposition of newly formed CDs [23]. The crude extract assayed with corn starch substrate in the presence of 10 % (v/v) ethanol showed a productivity of 0.43 mmol/L/h, or approximately 12 % higher compared to the assay without ethanol (Table 1).
The assay with crude extract, corn starch substrate, and ethanol showed a 19 % higher productivity when compared to the assay that used purified enzyme and the maltodextrin substrate. The test with crude extract, corn starch substrate, and ethanol only had 20 % lower productivity than the assay with the corn starch substrate and purified enzyme, in which the best β-CD yield was obtained. However, the purification process is laborious and expensive, which could offset the benefit of a better yield of CDs [33].
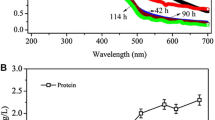
In an attempt to optimize CD production even more, a new assay was defined with the use of semi-purified enzyme and shorter-duration batches (Fig. 5). Thus, the duration of each CD production batch was set at 12 h because it was observed in preliminary assays with purified enzyme and corn starch substrate 5 % (w/v) (Fig. 2) that the most significant CD production occurred in this range of time, in which 88 % of the β-CD yield had already been achieved. For this new assay, 5 % (w/v) corn starch substrate in the presence of 10 % (v/v) ethanol was used, considering the good results observed with this combination in the assay with the CGTase crude extract.
The β-CD productivity observed for eight repetitive batches lasting 12 h was 0.72 mmol/L/h, and the thermal inactivation of the enzyme was very low throughout the repetitive batches. For comparison purposes, in the assay which used the CGTase crude extract and 5 % (w/v) corn starch substrate in the presence of 10 % (v/v) ethanol, the productivity for the first four batches lasting 24-h corresponded to 0.50 mmol/L/h, demonstrating that in the same range of time, the productivity was 30 % higher for the assay with the semi-purified enzyme and 12-h batches (Table 1).
An important observation in the assay with semi-purified enzyme refers to the percentage of β-CD relative to the total amount of CDs produced. In this assay with batches of 12 h production, an excellent selectivity for the β-CD was obtained, reaching a mean percentage of 96.0 % (Table 2). The lower selectivity observed in the 24-h assays can be understood as an effect of approaching the β-CD production equilibrium, making the CGTase adjust its production to α- and γ-CDs.
Determination of the starch conversion rate in CGTase products
This assay was performed with the semi-purified CGTase, a 12-h production batch and 5 % (w/v) corn starch substrate. The amount of starch remaining in the reaction medium at the end of the first batch after ultrafiltration was obtained by determining the total sugars using the phenol sulfuric method, which corresponded to 1.85 g. Whereas the total corn starch amount present in 50.0 mL of production medium at the beginning of the batch was 2.50 g, it was observed that 0.65 g starch was converted to CDs and linear oligosaccharides, which were separated by ultrafiltration using a 10 kDa membrane. Therefore, the starch conversion rate in the first batch reached 26 %.
The remaining starch at the end of the eighth CD production batch after the last ultrafiltration was 13.5 g, and the total amount of starch added during the assay was 18.0 g. Therefore, the starch amount converted in CGTase products, particularly CDs, was 4.5 g. Thus, the starch conversion rate for the whole assay (eight repetitive 12-h batches) was 25 %.
The satisfactory β-CD yield during the eight repetitive 12-h batches demonstrates that the higher starch concentrations used in the final batches did not affect the activity of the CGTase. However, for all assays in which the corn starch substrate was used, the gradual increase of the medium viscosity during the batches made the ultrafiltration process become progressively slower. This suggests that considering the conversion rate, it may be advantageous to add a progressively lower starch amount to each subsequent batch. These conditions need to be studied in the future and may save on time and raw materials, resulting in a reduction of CD production costs.
Conclusions
The ultrafiltration system proposed in this research to produce CDs in repetitive batches allowed for the performance of eight high-productivity batches. Provided removal of inhibitory products accumulated in the medium, and at the same time, the CGTase recovery. The utilization of a corn starch substrate 5 % (w/v) was beneficial for obtaining β-CD, both by increasing the yield and by serving as a low-cost raw material with wide availability in Brazil. Even using half the concentration of the maltodextrin substrate, the corn starch substrate resulted in better CD production. The crude extract and semi-purified CGTase preparations provided advantages for CD production, as they are easily obtained and produced satisfactory yields in the tested model. The CD production in the presence of 10 % (v/v) ethanol was shown to be an effective option to increase the yield, and may be associated with other economic advantages, making the use of the crude extract and semi-purified CGTase preparations and the corn starch substrate even more attractive for β-CD production. The 12-h batches showed more advantageous results compared to the 24-h batches, in addition they greatly increased the CGTase selectivity for β-CD production. The suggested ultrafiltration system proved to be efficient for CD production in repetitive batches. The possibility of industrial application for this innovative system, together with the assay conditions studied in this research, is promising.
Abbreviations
- CDs:
-
Cyclodextrins
- α-CD:
-
Alpha-cyclodextrin
- β-CD:
-
Beta-cyclodextrin
- γ-CD:
-
Gamma-cyclodextrin
- CGTase:
-
Cyclodextrin glycosyltransferase
- NMWL:
-
Nominal molecular weight limit
References
Del Valle EMM (2004) Cyclodextrins and their uses: a review. Process Biochem 39:1033–1046
Pinto FST, Flôres SH, Ayub MAS, Hertz PF (2007) Production of cyclodextrin glycosyltransferase by alkaliphilic Bacillus circulans in submerged and solid-state cultivation. Bioprocess Biosyst Eng 30:377–382
Singh M, Sharma R, Banerjee UC (2002) Biotechnological applications of cyclodextrins. Biotechnol Adv 20:341–359
Szejtli J (1997) Utilization of cyclodextrins in industrial products and processes. Mater Chem 7:575–587
Van der Veen BA, Uitdehaag JCM, Dijkstra BW, Dijkhuizen L (2000) Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim Biophys Acta 1543:336–360
Leemhuis H, Kelly RM, Dijkhuizen L (2010) Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl Microbiol Biotechnol 85:823–835
Mathew S, Adlercreutz P (2013) Regioselective glycosylation of hydroquinone to α-arbutin by cyclodextrin glucanotransferase from Thermoanaerobacter sp. Biochem Eng J 79:187–193
Vetter D, Thorn W (1992) Directed enzymatic synthesis of linear and branched glucooligosaccharides, using cyclodextrin-glucanosyltransferase. Carbohyd Res 223:61–69
Li Z, Wang M, Wang F, Gu Z, Du G, Wu J, Chen J (2007) γ -Cyclodextrin: a review on enzymatic production and applications. Appl Microbiol Biotechnol 77:245–255
Biwer A, Antranikian G, Heinzle E (2002) Enzymatic production of cyclodextrins. Appl Microbiol Biotechnol 59:609–617
Van der Veen BA, Uitdehaag JCM, Dijkstra BW, Dijkhuizen L (2000) Engineering of cyclodextrin glycosyltransferase reaction and product specificity. Biochim Biophys Acta 1543:336–360
Brewster ME, Loftsson T (2007) Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliver Rev 59:645–666
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications. Int J Pharm 329:1–11
Szente L, Szejtli J (2004) Cyclodextrins as food ingredients. Trends Food Sci Tech 15:137–142
Gawande B, Patkar A (2001) Alpha-Cyclodextrin Production using Cyclodextrin Glycosyltransferase from Klebsiella pneumonia AS-22. Starch-Starke 53:75–83
Calsavara LPV, Cunha ARD, Balbino TA, Zanin GM, Moraes FF (2011) Production of Cyclodextrins from Cornstarch Granules in a Sequential Batch Mode and in the Presence of Ethanol. Appl Biochem Biotech 165:1485–1493
Gastón JAR, Szerman N, Costa H, Krymkiewicz N, Ferrarotti SA (2009) Cyclodextrin glycosyltransferase from Bacillus circulans DF 9R: Activity and kinetic studies. Enzyme Microb Tech 45:36–41
Avci FG, Huccetogullari D, Azbar N (2013) The effects of cell recycling on the production of 1,3 propanediol by Klebsiella pneumoniae. Bioprocess Biosyst Eng 37:513–519
Matioli G, Zanin GM, Guimarães MF, Moraes FF (1998) Production and purification of CGTase of alkalophylic Bacillus isolated from Brazilian soil. Appl Biochem Biotech 70(2):267–275
Nakamura N, Horikoshi K (1976) Characterization and some cultural conditions of a cyclodextrin glucanotransferase from alkalophilic Bacillus sp. Agr Biol Chem Tokyo 40:753–757
Pazzeto R, Delani TCO, Fenelon VC, Matioli G (2011) Cyclodextrin production by Bacillus firmus strain 37 cells immobilized on loofa sponge. Process Biochem 46:46–51
Moriwaki C, Mazzer C, Pazzetto R, Matioli G (2009) Produção, purificação e aumento da performance de ciclodextrina glicosiltransferases para produção de ciclodextrinas. Quím Nova 32:2360–2366
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Tardioli PW, Zanin GM, Moraes FF (2006) Characterization of Thermoanaerobacter cyclomaltodextrin glucanotransferase immobilized on glyoxilagarose. Enzyme Microb Tech 39:1270–1278
Matioli G, Zanin GM, Moraes FF (2001) Characterization of cyclodextrin glycosyltransferase from Bacillus firmus strain nº 37. Appl Biochem Biotech 91(3):643–654
Szejtli J (1987) Cyclodextrins and the molecular encapsulation. Chim Oggi 3:17–21
Dubois N, Gilles KA, Hamilton JK, Rebers BA, Smith F (1956) Colorimetric Method Determination of Sugar and Substances. Anal Chem 23:350–356
Hamon V, Moraes FF (1990) Etude Preliminaire a L’immobilisation de L’enzyme CGTase WACKER. In: Research report, laboratoire de tecnologie enzymatique. Université de Tecnologie de Compiègne, Compiègne
Sakinah AMM, Ismail AF, Hassan O, Zularisam AW, Illias RM (2009) Influence of starch pretreatment on yield of cyclodextrins and performance of ultrafiltration membranes. Desalination 239:317–333
Charoenlap N, Dharmsthiti S, Sirisansaneeyakul S, Lertsiri S (2004) Optimization of cyclodextrin production from sago starch. Bioresource Technol 92:49–54
Alves-Prado HF, Carneiro AAJ, Pavezzi FC, Gomes E, Boscolo M, Franco CML et al (2008) Production of cyclodextrins by CGTase from Bacillus clausii using different starches as substrates. Appl Biochem Biotech 146:3–13
Moriwaki C, Mangolim CS, Ruiz GB, Morais GR, Baesso ML, Matioli G (2014) Biosynthesis of CGTase by immobilised alkalophilic bacilli and crystallisation of betacyclodextrin: Effective techniques to investigate cell immobilisation and the production of cyclodextrins. Biochem Eng J 83:22–32
Szerman N, Schroh I, Rossi AL, Rosso AM, Krymkiewicz N, Ferrarotti SA (2007) Cyclodextrin production by clyclodextrin glycosyltransferase from Bacillus circulans DF 9R. Bioresource Technol 98:2886–2891
Acknowledgments
The authors are thankful to the Brazilian Agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação Araucária for their financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fenelon, V.C., Aguiar, M.F.A., Miyoshi, J.H. et al. Ultrafiltration system for cyclodextrin production in repetitive batches by CGTase from Bacillus firmus strain 37. Bioprocess Biosyst Eng 38, 1291–1301 (2015). https://doi.org/10.1007/s00449-015-1369-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1369-8