Abstract
The effects of both biomass age and cell recycling on the 1,3-propanediol (1,3-PDO) production by Klebsiella pneumoniae were investigated in a membrane-supported bioreactor using hollow-fiber ultrafiltration membrane module in two separate experiments. It was determined that older cells have a negative effect on 1,3-PDO production. The concentrations of by-products, such as acetic acid and ethanol, increased in cultures with older cells, whereas the concentrations of succinic acid, lactic acid and 2,3-butanediol decreased. The effect of cell recycling was comparatively studied at a cell recycling ratio of 100 %. The results showed that cell recycling had also negative effects on 1,3-PDO fermentation. It was hypothesized that both cell recycling and biomass age caused metabolic shifts to undesired by-products which then inhibited the 1,3-PDO production. On the other hand, the use of hollow-fiber ultrafiltration membrane module was found to be very effective in terms of removal of cells from the fermentation broth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, biodiesel production has increased substantially, due to the interest in alternative energy sources [1]. During the production of biodiesel, massive amounts of glycerol are produced as a by-product in the range of 10 % (w w−1) of the biodiesel production [2]. One of the attractive management solutions for this problem is the conversion of this waste into value-added chemicals [3, 4].
Glycerol is a common carbon and energy source for several microorganisms for the biotechnological production of biochemicals such as citric acid, hydrogen, ethanol and 1,3-propanediol (1,3-PDO), which is one of the promising and environmentally friendly approaches [1, 5]. 1,3-PDO has been known and used for more than 100 years widely in many industrial applications, such as composites, adhesives, laminates, aliphatic polyester, cosmetics, foods, medicines and antifreeze [6, 7]. It is especially well known as a monomer for the synthesis of polytrimethylene terephthalate (PTT) by the polymerization of terephthalic acid. PTT is a polyester with excellent properties for fibers, textiles, carpets and coatings [8].
1,3-propanediol (1,3-PDO) can be produced by chemical synthesis or microbial fermentation. There are two different processes for chemical production: the conversion of acrolein to 1,3-PDO (Degussa/DuPont process) and the conversion of ethylene oxide to 1,3-PDO (Shell process). These processes are highly energy and pressure intensive, use expensive catalysts and release toxic intermediates [9, 10].
In recent years, the microbial production of 1,3-PDO has become increasingly attractive due to the utilization of inexpensive and renewable substrates, mainly glycerol [11]. A wide variety of wild-type bacteria are known to have the ability of converting glycerol to 1,3-PDO, including Klebsiella pneumoniae [7, 12], Klebsiella oxytoca [13, 14], Klebsiella planticola [14], Enterobacter agglomerans [15], Citrobacter freundii [16, 17], Ilyobacter polytropus [18], Clostridium butyricum [19, 20], Clostridium pasteurianum [21, 22], Lactobacillus brevis, Lactobacillus buchneri [23] and Lactobacillus reuteri [24]. Among the wild-type strains, K. pneumoniae and C. butyricum are currently considered as the most promising microorganisms for industrial applications due to high product yields and productivities [10]. The microbial conversion of glycerol to 1,3-PDO was mainly achieved under anaerobic or micro-aerobic conditions [25].
For the microbial production of 1,3-PDO, batch, fed-batch, continuous fermentations with suspended systems and whole cell immobilization (entrapment and attachment) were reported [26]. But the potential of cell recycling is still unknown for this fermentation. Bioreactors with cell recycling have been investigated for production of various types of compounds produced by anaerobic bacteria such as lactic acid [27], ethanol [28], propionic acid [29] and acetone–butanol–ethanol [30, 31]. However, there is only one report on this method for 1,3-PDO production [32].
A membrane-supported bioreactor allows integrating the fermentation and separation steps [33]. The microbial culture passes through a membrane system which separates the cell mass from the fermentation broth and then recirculates the concentrated cell suspension back into the bioreactor. The system is very effective in achieving a higher cell inventory than conventional methods and a cell-free culture liquid for following downstream processes [32, 34]. It is well known that the inoculum age and density can markedly influence the productivity and economics of bioprocesses. For example, some literature reports demonstrated the use of cell recycling for the production of acetone and butanol by Clostridium acetobutylicum in continuous culture [30]. However, no systematic studies to elucidate the effects of the biomass inoculum age and cell recycling on 1,3-PDO production have been reported before. Thus, this study was carried out to shed light on to this issue. The purpose of this study is to investigate the effects of biomass age and cell recycling on the 1,3-PDO production. There are very limited literature reports and we believe that further work remains to be done.
Materials and methods
Strain
Locally isolated K. pneumoniae (GenBank accession no. 27F HM063413) was kindly provided by the Faculty of Pharmacy, Ege University, Izmir, Turkey. The microorganisms were grown in Nutrient Broth (Merck KGaA, Darmstadt, Germany) with an initial inoculum ratio of 1 % (v v−1). This ratio was then increased to 10 % (v v−1) for the fermentation step.
Fermentation media
1 L of the fermentation media used throughout the study contained 40 g technical-grade (99 %) glycerol, 5.72 g K2HPO4, 1.5 g KH2PO4, 2.0 g (NH4)2SO4, 0.24 g MgSO4·7H2O, 1.0 g yeast extract, 0.5 mL FeSO4·7H2O (0.05 g L−1) and 1.0 mL trace element solution. The trace element solution consisted of ZnCl2 (70 mg L−1), MnCl2·4H2O (0.1 mg L−1), H3BO3 (60 mg L−1), CoCl2·2H2O (0.2 g L−1), CuCl2·2H2O (20 mg L−1), NiCl2·6H2O (25 mg L−1), Na2MoO4·2H2O (35 mg L−1) and 0.9 mL L−1 HCl (37 % v v−1) [35]. All the chemicals were provided from Merck KGaA, Darmstadt, Germany. The fermentation media were sterilized at 115 °C for 15 min.
Analytical methods
Cell concentration was estimated by measuring optical density (OD) at 620 nm using a spectrophotometer (Thermo, USA) [36]. Total suspended solid (TSS) measurements were carried out according to the standard methods [37]. The culture samples collected from the fermenter were centrifuged at 7,379×g using a microcentrifuge (Sigma 1-14 (10014), USA) with 24 × 0.22 L angle rotor, followed by filtration using 0.22 μm pore size cellulose acetate membrane filters (Sartorius, Germany) before high-performance liquid chromatography (HPLC) analysis. The desired product 1,3-PDO, substrate glycerol, residual glycerol and the by-products such as 2,3-butanediol (2,3-BD), lactic acid, acetic acid, succinic acid and ethanol concentrations were determined in cell-free supernatant using a HPLC system (Thermo, USA) with a Phenomenex Rezex RHM Monosaccharide (H+) 300 × 7.8 mm ion exchange column and a Thermo Refractive Index Detector (Thermo, USA). The column and detector temperatures were 65 and 45 °C, and the injection volume was 15 μL. A solution of H2SO4 (5 mM) was used as the mobile phase at a flow rate of 0.8 mL min−1 [35]. Analytical standards, solvents and reagents were provided by Ehrenstorfer, Germany.
Experimental setup for cell recycling and determination of biomass age
Continuous cultivation was carried out in a 2 L glass fermenter (Sartorius A+, Germany) with a working volume of 1 L. The temperature was maintained at 37 °C and the agitation rate was controlled at 150 rpm. The pH was kept at 7.0 by addition of 2 M NaOH automatically. K. pneumoniae is a facultative anaerobe microorganism; therefore no strict anaerobic conditions were provided.
In a previous study (data not shown), various hydraulic retention time (HRT) values (0.5–24 h) were applied during the continuous culture of K. pneumoniae for the maximum production of 1,3-PDO. An optimum HRT of 16 h was found to result in maximum concentrations of 1,3-PDO. As a result, the experiments were carried out at HRT of 16 h.
For cell recycling, a hollow-fiber ultrafiltration membrane module (Daicen Membrane Systems Ltd., Japan) made from polyethersulfone (inner diameter 0.8 mm, membrane area 0.26 m2) was connected to the fermenter (Fig. 1). The medium (feed) was added to the fermenter at a rate of 0.0625 L h−1 (HRT 16 h). At the same time, the culture from the fermenter was pumped through the hollow-fiber ultrafiltration membrane module at the same rate simultaneously. The concentrated cells were recycled to the fermenter. Permeate was removed at the same rate as the addition of feed to keep a constant volume in the fermenter, following which it was used for downstream processes (data not shown). The pressure was measured by manometers to prevent plugging. Maximum feed inlet and transmembrane pressures were 0.4 and 0.3 MPa, respectively.
To investigate the effect of cell recycling, a comparative experiment was done with and without cell recycling at HRT 16 h. To determine the optimum biomass age, TSS concentrations in the fermenter and recycling line were measured every day. The amount of biomass that should be removed from the system was calculated according to the equation below [38]. Four different biomass ages (0.67, 5, 15, and 30 days) were studied for the determination of optimum biomass age. A biomass age value of 0.67 day corresponding to an HRT value of 16 h was studied without cell recycling. On the other hand, other experiments using biomass age between 5, 15, and 30 days were operated with cell recycling using a hollow-fiber ultrafiltration membrane unit.
where θ c is the biomass age (day), V the working volume (L), X TSS the concentration in the fermenter (mg L−1), X r TSS the concentration of recycled biomass to the fermenter (mg L−1) and θ w the volume of biomass that should be removed from the recycling line per day (L day−1).
Hydraulic retention time (h) was defined as the working fermenter volume (L) divided by the feeding medium flow rate (L h−1). 1,3-PDO productivity (g L−1 h−1) was calculated as 1,3-PDO concentration (g L−1) divided by the HRT (h).
Error bars represent the samples taken during the pseudo-steady state conditions which were determined by coefficient of variation. The error bars were calculated for these samples using the average of last 5 days’ data in this period. All statistical calculations were performed using Systat Software Inc. (San Jose, California) Sigma Plot 11.0 Trial Version.
Results and discussion
Determination of the optimum biomass age
To control the biomass age, the determined amount of the biomass, calculated according to the equation given in “Experimental set-up for cell recycling and determination of biomass age”, was removed from the system. As a result, TSS and OD values were increased with the increase in biomass ages (Table 1).
As seen in Table 1, 1,3-PDO concentrations were decreased with the increase in biomass ages. The highest 1,3-PDO concentration was obtained at a biomass age of 0.67 day. Also, molar yields (given as 1,3-PDO production to glycerol consumed) and volumetric productivities (given as g 1,3-PDO L−1 h−1) are shown in Table 1. ANOVA tests (analysis of variance test) for 1,3-PDO concentrations, 1,3-PDO yields and 1,3-PDO volumetric productivities indicated statistically difference in concentrations, yields and volumetric productivities (p value <0.05). Therefore, pairwise t tests were further performed to determine the differences. At the end of the statistical analyses, 1,3-PDO concentration, 1,3-PDO yield and 1,3-PDO volumetric productivity for biomass age of 0.67 day were statistically different than the other biomass ages (p < 0.05). There are no statistically significant difference at 1,3-PDO concentrations, 1,3-PDO yields and 1,3-PDO volumetric productivities for biomass ages of 5, 15 and 30 days.
Figure 2 presents the data for the concentrations of residual glycerol and by-products. Lower concentrations were detected for succinic acid and lactic acid at higher biomass ages in general. As seen in Fig. 2, ~95 % of glycerol was consumed at every biomass age. Acetic acid concentration was decreased to 0.8–0.9 g L−1 at 5 and 15 days, while it was 2.35 g L−1 at 0.67 day. The concentration was increased to 2.62 g L−1 at 30 days again. 2,3-BD was not produced at 0.67 and 30 days. 2,3-BD concentration was 0.63 g L−1 and 0.22 g L−1 at 5 and 15 days, respectively. Among all other by-products, ethanol concentration was the highest, reaching up to 7.24 g L−1 at 30 days of biomass age. This value was really close to 1,3-PDO concentration (7.46 g L−1) at this biomass age. The growth curve for K. pneumoniae is given in Fig. 3. According to OD (620 nm) values in Table 1, the cells seem to be in stationary phase. This might be the explanation for high ethanol production. Succinic acid, lactic acid and 2,3-BD concentrations were decreased with the increase in biomass age, in general. This is an advantage for downstream processes. During the experiments, an increase was observed in the concentrations of acetic acid and ethanol. Cheng et al. [39] showed that acetic acid was the most inhibitory by-product during the 1,3-PDO fermentation under anaerobic conditions, with lactic acid and ethanol the next most inhibitory for K. pneumoniae. The critical concentrations of acetic acid, lactic acid and ethanol were assessed to be 15, 19, 26 g L−1, respectively. The effects of by-products on cell growth and 1,3-PDO production were investigated in batch cultures. At the beginning fermentations, 5 g L−1 acetic acid, 5 g L−1 lactic acid and 9 g L−1 ethanol were added to the fermentation media. Cell growth and 1,3-PDO production were decreased compared to the control group without addition. According to Zhang et al. [40], ethanol is the key competitor to the formation of 1,3-PDO in K. pneumoniae. Ethanol, contrary to organic acids, was shown to be more inhibitory to K. pneumoniae growth under aerobic than anaerobic conditions, with a critical concentration of 17 and 26 g L−1, respectively. The low 1,3-PDO concentrations can be explained by the potential inhibition due to the by-product formation at higher biomass ages. Furthermore, as the biomass age gets longer, microorganisms shift from their optimum 1,3-PDO production condition, which is exponential growth phase. Biomass age (θ c ) controls the microbial growth rate. Long-term operations (higher biomass ages) might be a problem because of the accumulation of older cells. This situation may reduce the system’s performance with time.
Effect of cell recycling
To determine the effect of cell recycling on 1,3-PDO production, all the fermentation broth was passed through the hollow-fiber ultrafiltration membrane module and the separated biomass was recycled back into the fermenter (100 % recycle). Cell recycling was stopped at the end of 192nd hour and then fermentation was continued without cell recycling at HRT 16 h. This experiment was ended after 387 h. Cell-free liquid (permeate) after ultrafiltration was used for the downstream processes for purification purpose.
Figure 4 shows the values of TSS and OD measurements throughout the experiments. It was observed that both TSS and OD values dropped down when cell recycling was stopped after the 192nd hour of the study as expected.
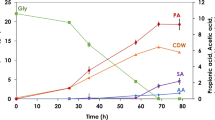
Throughout the experiment, almost 90 % of glycerol was consumed. As shown in Fig. 5, while the highest 1,3-PDO concentration was observed at the 24th hour (8.71 g L−1), it then kept decreasing up to a concentration of 3.98 g L−1. Microorganisms showed adaptation following the end of recycling. The 1,3-PDO concentration increased after the 339th hour of the experiment. 2,3-BD was not observed. Succinic acid and lactic acid concentrations showed the same trends with or without cell recycling. Their average concentrations were 0.33 and 0.54 g L−1, respectively. As seen in Fig. 5, acetic acid and ethanol were the main by-products. Their concentrations were increased when cell recycling was used. Ethanol was produced more than 1,3-PDO.
Increase in biomass concentration in the medium did not show any significant rise in 1,3-PDO probably due to the inhibition of by-products such as ethanol and acetic acid. The conversion capacities of the cells not only depend on the concentration, but also on the accumulation of the by-products. It is suggested that the high production of by-products was a result of metabolic shift from reduction to oxidation of glycerol. The yield of 1,3-PDO depends on the availability of NADH, which is generated in the oxidative branch pathway of glycerol. The formation of pyruvate-derived by-products competes with the biosynthesis of 1,3-PDO for NADH, resulting in a decrease of 1,3-PDO yield [41].
In biotechnological productions, higher biomass concentrations and smaller fermenter volumes are desirable for higher volumetric productivities. There are two generally accepted methods for high productivity: cell immobilization and cell recycle. Reimann et al. [32] reported the first attempt of continuous cultivation with cell recycling using hollow-fiber membrane modules for production of 1,3-PDO by C. butyricum. It was reported that the concentration could not be increased by cell recycling when it was compared with the un-recycled cultures (28.4 g L−1) for Clostridial 1,3-PDO fermentation. However, the system failed at higher glycerol concentration of 92 g L−1 due to the inability of cells to grow at such high substrate concentration. The productivity was at its highest value at a dilution rate of 0.7 h−1 for two substrate concentrations (32 and 56 g L−1). On the other hand, it decreased with the increasing dilution rates, which was particularly due to inhibition by the fermentation products. Previous studies showed that inhibition imposed by the products was the major limitation of this method, as in this study. Also, there were other biotechnological productions using cell recycling which were affected by by-product inhibitions such as 2,3-BD [42], lactic acid [27, 43], propionic acid [44], acetic acid [45], ethanol [46] and acetone/butanol [30]. Cells might also be affected by the mechanical stress caused by pumping the viscous culture through the hollow-fiber membrane module and through tubings in peristaltic pumps.
The use of hollow-fiber ultrafiltration membrane module helped to eliminate time-consuming and challenging methods for downstream processing such as flocculation, coagulation and centrifugation. It seems that inhibition of the by-products is one of the major limitations of this method. Because of the high cost of product recovery, a high final product concentration is desirable. To achieve this, the formation of by-products should be minimized, which was achieved up to a certain extent in this study. Also, metabolic engineering can be used to manipulate the pathways so that by-product pathways can be controlled or even eliminated.
References
Mu Y, Xiu ZL, Zhang DJ (2008) A combined bioprocess of biodiesel production by lipase with microbial production of 1,3-propanediol by Klebsiella pneumoniae. Biochem Eng J 40(3):537–541
Willke T, Vorlop K (2008) Biotransformation of glycerol into 1,3-propanediol. Eur J Lipid Sci Technol 110:831–840
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Nemeth A, Sevella B (2008) Development of a new bioprocess for production of 1,3-propanediol I.: modeling of glycerol bioconversion to 1,3-propanediol with Klebsiella pneumoniae enzymes. Appl Biochem Biotechnol 144(1):47–58
Wendisch VF, Lindner SN, Meiswinkel TM (2011) Use of glycerol in biotechnological applications. In: Montero G, Stoytcheva M (eds) Biodiesel—quality, emissions and by-products. InTech - Open Access Publisher, Rijeka
Liu H, Ou X, Zhou S, Liu D (2010) Microbial 1,3-propanediol, its copolymerization with terephthalate, and applications. In: Chen GQ (ed) Plastics from bacteria: natural functions and applications. Springer, New York
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng/Biotechnol 74:239–259
Zeng AP, Sabra W (2011) Microbial production of diols as platform chemicals: recent progresses. Curr Opin Biotechnol 22:749–757
Kraus GA (2008) Synthetic methods for the preparation of 1,3-propanediol. Clean 36(8):648–651
Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27(6):859–913
Hao J, Lin R, Zheng Z, Liu H, Liu D (2008) Isolation and characterization of microorganisms able to produce 1,3-propanediol under aerobic conditions. World J Microbiol Biotechnol 24:1731–1740
Huang H, Gong CS, Tsao GT (2002) Production of 1,3-propanediol by Klebsiella pneumoniae. Appl Biochem Biotechnol 98–100:687–698
Yang G, Tian J, Li J (2007) Fermentation of 1,3-propanediol by a lactate deficient mutant of Klebsiella oxytoca under microaerobic conditions. Appl Microbiol Biotechnol 73:1017–1024
Homann T, Tag C, Biebl H, Deckwer WD, Schink B (1990) Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl Microbiol Biotechnol 33:121–126
Barbirato F, Claret CC, Grivet JP, Bories A (1995) Glycerol fermentation by a new 1,3-propanediol-producing microorganism: Enterobacter agglomerans. Appl Microbiol Biotechnol 43:786–793
Boenigk R, Bowien S, Gottschalk G (1993) Fermentation of glycerol to 1,3-propanediol in continuous cultures of Citrobacter freundii. Appl Microbiol Biotechnol 38:453–457
Pflugmacher U, Gottschalk G (1994) Development of an immobilized cell reactor for the production of 1,3-propanediol by Citrobacter freundii. Appl Microbiol Biotechnol 41:313–316
Stieb M, Bernhard S (1984) A new 3-hydroxybutyrate fermenting anaerobe, Ilyobacter polytropus, gen. nov. sp. nov., possessing various fermentation pathways. Arch Microbiol 140:139–146
Abbad-Andaloussi S, Manginot-Durr C, Amine J, Petitdemange E, Petitdemange H (1995) Isolation and characterisation of Clostridium butyricum DSM 5431 mutants with increased resistance to 1,3-propanediol and altered production of acids. Appl Environ Microbiol 61:4413–4417
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Biebl H (2001) Fermentation of glycerol by Clostridium pasteurianum-batch and continuous culture studies. J Ind Microbiol Biotechnol 27:18–26
Nakas JP, Schaedle M, Parkinson CM, Coonley CE, Anenbaum SWT (1983) System development for linked-fermentation products of solvents from algal biomass. Appl Environ Microbiol 46:1017–1023
Veiga-da-Cunha MV, Foster MA (1992) 1,3-Propanediol: NAD+ oxidoreductases of Lactobacillus brevis and Lactobacillus buchneri. Appl Environ Microbiol 58:2005–2010
Lüthi-Peng Q, Dileme FB, Puhan Z (2002) Effect of glucose on glycerol bioconversion by Lactobacillus reuteri. Appl Microbiol Biotechnol 59:289–296
Liu HJ, Zhang DJ, Xu YH, Mu Y, Sun YQ, Xiu ZL (2007) Microbial production of 1,3-propanediol from glycerol by Klebsiella pneumoniae under micro-aerobic conditions up to a pilot scale. Biotechnol Lett 29:1281–1285
Hao J, Lin R, Zheng Z, Sun Y, Liu D (2008) 3-Hydroxypropionaldehyde guided glycerol feeding strategy in aerobic 1,3-propanediol production by Klebsiella pneumoniae. J Ind Microbiol Biotechnol 35:1615–1624
Kwon S, Yoo IK, Lee WG, Chang HN, Chang YK (2001) High-rate continuous production of lactic acid by Lactobacillus rhamnosus in a two-stage membrane cell-recycle bioreactor. Biotechnol Bioeng 73:25–34
Chang HN, Yang JW, Park YS, Kim DJ, Han KC (1992) Extractive ethanol production in a membrane cell recycle bioreactor. J Biotechnol 24:329–343
Colomban A, Roger L, Boyaval P (1993) Production of propionic acid from whey permeate by sequential fermentation, ultrafiltration, and cell recycling. Biotechnol Bioeng 42:1091–1098
Afschar AS, Biebl H, Schaller K, Schügerl K (1985) Production of acetone and butanol by Clostridium acetobutylicum in continuous culture with cell recycling. Appl Microbiol Biotechnol 22:394–398
Schlote D, Gottschalk G (1986) Effect of cell recycle on continuous butanol–acetone fermentation with Clostridium acetobutylicum under phosphate limitation. Appl Microbiol 24:1–5
Reimann A, Biebl H, Deckwer WD (1998) Production of 1,3-propanediol by Clostridium butyricum in continuous culture with cell recycling. Appl Microbiol Biotechnol 49:359–363
Tejayadi S, Cheryan M (1995) Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl Microbiol Biotechnol 43:242–248
Park YS, Othake H, Toda K, Fukaya M, Okumura H, Kawamura Y (1988) Acetic acid production using a fermentor equipped with a hollow fiber filter module. Biotechnol Bioeng 33:918–923
Casali S, Gungormusler M, Bertin L, Fava F, Azbar N (2012) Development of a biofilm technology for the production of 1,3-propanediol (1,3-PDO) from crude glycerol. Biochem Eng J 64:84–90
Barbirato F, Himmi EH, Conte T, Bories A (1998) 1,3-propanediol production by fermentation: an interesting way to valorize glycerin from the ester and ethanol industries. Ind Crop Prod 7:281–289
APHA, AWWA, WPCF (1995) Standard methods for the examination of water and wastewater, vol 19. American Public Health Association, Washington
Von Sperling M (2007) Biological wastewater treatment activated sludge and aerobic biofilm reactors, vol 5. IWA publishing, London
Cheng KK, Liu HJ, Liu DH (2005) Multiple growth inhibition of Klebsiella pneumoniae in 1,3-propanediol fermentation. Biotechnol Lett 27:19–22
Zhang Y, Li Y, Du C, Liu M, Cao Z (2006) Inactivation of aldehyde dehydrogenase: a key factor for engineering 1,3-propanediol production by Klebsiella pneumoniae. Metab Eng 8:578–586
Maervoet VET, De Mey M, Beauprez J, De Maeseneire S, Soetaert WK (2011) Enhancing the microbial conversion of glycerol to 1,3-propanediol using metabolic engineering. Org Process Res Dev 15:189–202
Zeng AP, Biebl H, Deckwer WD (1991) Production of 2,3-butanediol in a membrane bioreactor with cell recycle. Appl Microbiol Biotechnol 34:463–468
Ohleyer E, Blanch HW, Wilke CR (1985) Continuous production of lactic acid in a cell recycle reactor. Appl Biochem Biotech 11:317–332
Blanc P, Goma G (1987) Kinetics of inhibition in propionic acid fermentation. Bioprocess Eng 2:175–179
Parekh SR, Cheryan M (1994) Continuous production of acetate by Clostridium thermoaceticum in a cell-recycle membrane bioreactor. Enzyme Microb Technol 16:104–109
Ben Chaabane F, Aldiguier AS, Alfenore S, Cameleyre X, Blanc P, Bideaux C, Guillouet SE, Roux G, Molina-Jouve C (2006) Very high ethanol productivity in an innovative continuous two-stage bioreactor with cell recycle. Bioprocess Biosyst Eng 29:49–57
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avci, F.G., Huccetogullari, D. & Azbar, N. The effects of cell recycling on the production of 1,3-propanediol by Klebsiella pneumoniae . Bioprocess Biosyst Eng 37, 513–519 (2014). https://doi.org/10.1007/s00449-013-1018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1018-z