Abstract
Cyclodextrin glycosyltransferase (CGTase) catalyzes starch conversion into cyclic or linear oligosaccharides, important industrial products for the complexation of non-polar substances. In this work, conditions to increase CGTase production from Bacillus circulans strain DF 9R were optimized by two systems. On one hand, free cells were grown in batch fermentation experiments to optimize aeration and pH. The highest activity (1.47 ± 0.21 U ml−1) was achieved after 48 h of growth, aeration of 1.5 vvm and pH regulated to 7.6. On the other hand, bacterial cells were immobilized on loofa and synthetic sponge, and used for CGTase production in a semi-continuous process. An initial biomass of 30 mg of lyophilized cells and an immobilization time of 24 h with loofa or synthetic sponge were enough to achieve increased production of CGTase: 0.91 ± 0.10 and 0.95 ± 0.11 U ml−1, respectively. Sponges with immobilized bacteria were reused in 12 successive cycles. Besides, in our conditions, CGTase was not adsorbed onto the supports used for immobilization, which ensured the total recovery of the enzyme from the culture medium. The two CGTase production processes studied showed similar productivity and could be potentially scaled up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclodextrins (CDs) are non-reducing cyclic oligosaccharides. The most common CDs, i.e. cyclomaltohexaose (α-CD), cyclomaltoheptaose (β-CD) and cyclomaltooctaose (γ-CD), are composed of six, seven and eight glucose units, respectively, linked by α-1,4 glycosidic bonds. Due to their non-polar cavity, they are able to form complexes with other molecules, especially hydrophobic residues, thus changing physicochemical properties such as solubility and stability of the guest compounds. Because of that, CDs are suitable to be used in the food, pharmaceutical, cosmetic, agricultural, textile and chemical industries, as well as, in environmental protection [1–3]. The extensive industrial application of CDs is limited by their cost; as a consequence, many alternatives, such as the use of inexpensive raw substrates and inexpensive biocatalysts, have been tested to improve the production of these cyclic oligosaccharides [4, 5].
CDs are obtained by starch bioconversion using cyclodextrin glycosyltransferase (EC 2.4.1.19; CGTase). This enzyme is produced by a variety of bacteria, mainly Bacilli class, and also by Klebsiella, Anaerobranca, Thermoanaerobacter, Thermoanaerobacterium and Thermococcus [6]. Many wild-type or genetically modified bacterial strains have been used in the industry to produce CGTases by means of different fermentation systems, such as batch and continuous culture [7]. On the other hand, microorganism immobilization is being explored in the design of new processes using supports such as chitosan, alginate, agar and polyacrylamide [8–13]. In these processes, immobilized bacteria are physically localized on a defined matrix, can be used repeatedly and continuously, and are also less subject to the effects of inhibitory compounds, nutrient depletion and microbial contamination [14]. Traditional cell immobilization methods have been associated with problems such as alterations of the cell physiology, and decreased efficiency of transport of nutrients and products.
Recently, fibrous matrices have been used as alternative supports for cell immobilization [15]. Loofa sponge is derived from the fruit of the cucumber family. Since loofa sponge is composed of a network of dried cellulose fibers, it is an inexpensive and renewable matrix for microorganism immobilization. Besides, it is non-reactive, non-toxic, easy to handle, biodegradable and highly stable during repeated use. It has been used in production processes of several compounds such as gibberellic acid from milk permeate by Fusarium moniliforme cells [16], clavulanic acid by Streptomyces clavuligerus [17] and lactic acid by Lactobacillus salivarius [18]. Recently, cyclodextrin production [19] and CGTase synthesis [20] have been successfully carried out by Bacillus strain cells immobilized on loofa sponge.
In a previous work carried out at our lab, the conditions to obtain CGTase from B. circulans DF 9R were optimized in shake flasks [21]. Herein, fermentation experiments were performed to optimize the aeration and regulate the pH and, thus, increase CGTase production. On the other hand, bacterial cells were immobilized on loofa and synthetic sponge and used for CGTase production in a semi-continuous process, by transferring the immobilized cells, in successive cycles, to appropriate culture medium. To achieve the best system performance, it was necessary to optimize the initial biomass of bacterial cells and the immobilization time.
Materials and methods
Materials
Loofa sponge, from Luffa cylindrica dried fruit, and cassava starch were obtained from Brazilian suppliers. Synthetic polyurethane sponge was obtained from Scotch Brite, 3 M Company, Brazil. β-CD and antifoam 204 were obtained from Sigma Chemical Co., Mo, USA. All other chemicals were of analytical grade (Merck, Darmstadt, Germany).
Microorganism and growth conditions
B. circulans DF 9R was isolated from rotten potatoes by Ferrarotti et al. [22] and deposited in the Microbial Culture Collection, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires (Buenos Aires, Argentina), catalog number CCM-A-29: 1290 from the WFCC. The strain was cultured in a minimum saline medium with starch (MAS) consisting of 1.5 % cassava starch, 0.4 % (NH4)2SO4, 100 mM phosphate buffer pH 7.6, 0.002 % MgSO4 and 0.002 % FeSO4 [21]. After incubation at 37 °C and 120 rpm for 48 h, the bacterial suspension was centrifuged at 4 °C and 2,940×g for 10 min. After washing the pellet with sterile saline solution, 2-mL aliquots of these bacterial suspensions were lyophilized in glass vials using a Christ Beta 1–16 freeze dryer. The lyophilized cells were stored at −20 °C for at least six months. The lyophilized cells were then reactivated in 250-mL Erlenmeyer flasks containing 100 mL of MAS culture medium and incubated in an orbital shaker at 37 °C and 120 rpm for 24 h for later use as inocula or cell immobilization. At the end of the reactivation time, cells were grown in MAS agar medium and incubated for 48 h at 37 °C to rule out possible contaminations. Cell growth was determined by spectrophotometric measurement of absorbance at 620 nm. Optical density values were converted to cell dry weight using a standard curve. One unit of optical density at 620 nm equals to 0.19 g L−1 cell dry weight.
Batch fermentation process
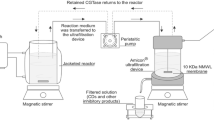
Fermentation processes were performed in a 3-L BioFlo 110 fermenter (New Brunswick Scientific, USA). An aliquot of 20 mL the inocula grown for 24 h (OD620nm = 0.3) was added to 1-L of MAS culture medium with 0.05 % antifoam. The temperature was maintained at 37 °C and the stirring speed at 100 rpm. The aeration rate was tested from 0 to 3.0 vvm and the pH from 7.2 to 8.0.
Influence of aeration and pH on CGTase production in batch process
Using the medium previously optimized in Rosso et al. [21], aeration and pH were tested in a stirred-tank bioreactor to increase enzyme production. The effect of aeration was studied by fixing agitation at 100 rpm and pH at 7.6. To study the effect of pH, fermentations were carried out adjusting the pH to 7.2, 7.6 or 8.0 by KOH addition as the process progressed.
CGTase cyclizing activity
The cyclizing activity of CGTase was determined according to the phenolphthalein method [23], measuring β-CD production spectrophotometrically at 550 nm on the basis of its ability to form a colorless inclusion complex with this dye. One unit of CGTase was defined as the amount of enzyme that catalyzes the production of 1 μmol of β-CD per min under the reaction conditions.
Bacterial cell immobilization
Loofa and synthetic polyurethane sponges were used as immobilization supports. Both sponges were cut into discs of 24 mm in diameter and 2–4 mm thick. Discs were soaked in boiling water for 10 or 30 min for synthetic and loofa sponges, respectively, washed thoroughly and left for 24 h in distilled water, changing the water three times. They were then dried in an oven at 70 °C and sterilized in an autoclave at 121 °C for 20 min. These discs were added to flasks (three discs each) containing different amounts of lyophilized cells and reactivated as described in section microorganism and growth conditions.
Influence of bacterial biomass and immobilization time on CGTase production in semi-continuous process
To optimize bacterial biomass, different amounts of lyophilized cells (30, 50 and 70 mg each) were reactivated as described in section microorganism and growth conditions. After 24 h incubation, three discs of loofa or synthetic sponge were placed in each flask containing fresh MAS culture medium and incubated for 24, 48, 72 and 96 h at 37 °C and 120 rpm. To optimize the immobilization time, the biomass selected in previous experiments was used and incubated under the above conditions for 24, 48, 72 and 96 h. After each immobilization period, washed sponge discs were transferred to fresh MAS medium for CGTase production, and enzyme activity was measured at 0, 4, 10, 24, 48, 72, 96 and 120 h. Controls, i.e. free cells without sponges, performed in the same conditions. All the experiments were carried out in triplicate.
CGTase production using immobilized bacteria
Sponge discs with immobilized biomass were washed with sterile saline and transferred to 250-mL Erlenmeyer flasks with 100 mL of MAS medium and incubated in a shaker at 120 rpm and 37 °C. Samples were taken at different times, as described in section influence of bacterial biomass and immobilization time on CGTase production in semi-continuous process, to determine CGTase activity. In addition, free bacteria used as controls were centrifuged for 5 min at 2,940×g and 25 °C; the pellet was washed with sterile saline and transferred to MAS medium just like sponge discs. Further, successive operational cycles of CGTase production were carried out for which sponge discs with immobilized bacteria were repeatedly transferred to fresh culture medium for reuse. From one cycle to the next, discs were washed with sterile saline. At the end of each cycle, CGTase activity was determined in the culture medium by the method of Goel and Nene [23].
Scanning electron microscopy
Supports containing bacterial cells were placed into 2.5 % glutaraldehyde in 100 mM potassium phosphate buffer pH 7.6 for 24 h. After that, discs were washed successively with solutions of 30, 50, 70, 90 and 100 % ethanol and finally subjected to dehydration in a supercritical fluid-extraction system using CO2 under high pressure. Photomicrographs were taken using a scanning electron microscope (Shimadzu Model SS 550) with an acceleration voltage of 10 kV. The samples were placed on the surface of a double-faced conductive tape and coated with a gold layer. This procedure was described by Moriwaki et al. [12].
Statistical analysis
The results were subjected to analysis of variance (ANOVA) and Tukey means tests, at 5 % significance level using Statistica 8.0/2008 (Stat Soft, Inc. Tulsa, OK, USA).
Results and discussion
Production of CGTase by B. circulans DF 9R in batch process
Both the productivity and product yield of processes that involve microorganisms depend on the specific growth rate, the biomass concentration and the specific rate of product formation. These variables are a function of the medium composition and culture conditions. As previously demonstrated in our experiments in shake flasks, this B. circulans strain requires the absence of glucose and the presence of starch as carbon source to grow and express the CGTase gene. On the other hand, the most nutrient-rich media lead to increased growth of B. circulans DF 9R, but not to increased synthesis of CGTase [21]. Although we tested starches from different sources, we used cassava starch as substrate because it allowed the best CGTase yield [21]. Besides, the minimal medium selected contained ammonium, Mg2+ and Fe2+ as essential nutrients and did not require complex nitrogen sources of unknown composition [21]. After optimizing the composition of the culture medium, we selected an initial pH of 7.6 and observed that the pH decreased to 5.9 during the process of fermentation in shake flasks because we could not keep it regulated. Similarly, we could not control the aeration in this system [21]. To increase the enzyme production, in the present study, we tested the effects of aeration and pH in a stirred-tank bioreactor, maintaining the medium and the previously optimized conditions. We obtained the highest growth with an aeration of 2 vvm (Fig. 1a), but achieved most enzyme production with an aeration of 1.5 vvm (Fig. 1b). Therefore, results suggest that moderate aeration and agitation are keys for optimal oxygen transfer, an essential factor for CGTase production. The results are in agreement with those reported by Pinto et al. [24], who reached the highest activity with aeration of 1.7 vvm using an alkalophilic strain of B. circulans. Regarding the effect of pH, the best enzyme production was achieved after 48 h incubation at a pH regulated to 7.6, but the greatest growth was observed when the pH was not regulated (Fig. 2a, b). Acidification of the medium to pH values below 6.0 leads to the inactivation of the enzyme [25]. This is why in the culture medium with an initial pH of 7.6 but unregulated, a marked decrease in enzyme activity was observed. The pH behavior of the different Bacillus that produces CGTase is very diverse. In the case of certain alkalophilic Bacillus, the maximum cell growth is achieved at pH 9.2 and higher production of enzyme at pH 9.8, whereas other Bacillus species are able to grow and produce the enzyme at pH 10.1 [26]. To compare the enzymatic activities obtained with different bacteria, it is necessary to consider the way in which the activity is defined. In this study, one unit of CGTase was defined as the amount of enzyme that catalyzes the production of 1 μmol of β-CD per min under the reaction conditions, whereas in other studies it has been defined as the amount of enzyme that catalyzes the production of 1 μg of β-CD per min. Differences in the reaction conditions and substrate used are also observed. Other authors have reported the production of CGTase in stirred tank with activities below those obtained in this work [27, 28].
Effect of aeration rate on Bacillus circulans DF 9R growth and CGTase production in batch process. a Bacterial growth measured as absorbance at 620 nm. b CGTase production. Samples were analyzed along 50 h of bacillus cultivation in a 1-L fermenter containing MAS medium at 37 °C, initial pH 7.6 and different aeration rates: 0 vvm (circle), 1 vvm (triangle), 1.5 vvm (open square), 2 vvm (open circle) and 3 vvm (square)
Influence of pH on Bacillus circulans DF 9R growth and CGTase production in batch process. a Bacterial growth measured as absorbance at 620 nm. b CGTase production. Samples were analyzed along 50 h of bacillus cultivation in a 1-L fermenter containing MAS medium at 37 °C, aeration rate 1.5 vvm and different pH values: 7.2 (square), 7.6 (circle), 8.0 (triangle) and 7.6 not regulated (open circle)
As seen in Figs. 1, 2, the highest enzyme yield was not linked to greater development of biomass, as the culture medium was optimized to increase the production of enzyme. Thus, a minimal culture medium with the presence of starch and salts without complex nitrogen sources was defined. On the other hand, the release of the enzyme to extracellular medium is related to the need of the microorganism to use available starch as a carbon source for growth [21]. In the present study, the highest enzyme activity (1.4 U mL−1) was reached after 48 h of growth, with cell dry weight of 0.39 g L−1 culture medium. These results are similar to those obtained by Pinto et al. [7] with CGTase from alkaliphilic B. circulans, but different from those reported by Ibrahim et al. [29] and Abdel-Naby et al. [9], who worked with Bacillus sp. and Bacillus cereus NRC7 and reached maximum activity at 12 and 24 h of culture, respectively.
Immobilization conditions
B. circulans DF 9R cells were successfully immobilized on loofa and synthetic polyurethane sponges using the culture medium and incubation conditions previously optimized for free cells. Good adsorption of bacteria both on the synthetic and loofa sponges was possible to observe using scanning electron microscopy techniques (Fig. 3). This technique has been used to confirm the efficient immobilization of cells to various supports [15, 19]. Regarding the matrix-cell interaction, recent FTIR studies suggest that adsorption of the bacteria to the sponge would be through van der Waals forces, without participation of covalent bonds [20]. Besides, in our conditions, CGTase was not adsorbed onto the supports used for immobilization, which ensured the total recovery of the enzyme from the culture medium.
To evaluate the minimal initial biomass and the optimal time for bacterial immobilization on the support that allow the best CGTase yield, experiments were performed as described in section influence of bacterial biomass and immobilization time on CGTase production in semi-continuous process. Increasing the initial biomass from 30 to 70 mg resulted in the same amount of CGTase produced (data not shown). Regarding the immobilization times, the results obtained showed that higher immobilization times (more than 24 h) did not improve CGTase yield (Fig. 4). The difference in the immobilization time may be related to the affinity of the bacteria to the sponge, as also suggested by Moriwaki et al. [20], who needed an immobilization time of 8–10 days to immobilize cells of Bacillus firmus and Bacillus sphaericus on loofa. By contrast, the production of enzyme decreased significantly after 96 h of immobilization and the yield obtained using synthetic sponge was similar to that obtained with free cells (Fig. 4).The data for the different immobilization times were subjected to analysis of variance (ANOVA) and Tukey test, and the significance was set at 5 %.
Effect of immobilization time of the cells onto the support on CGTase production. Lyophilized cells (30 mg) were reactivated in MAS medium for 24 h. Three discs of each support, loofa (triangle) or synthetic sponge (square), were placed in each flask containing fresh MAS medium and incubated at 37 °C and 120 rpm for a 24 h, b 48 h, c 72 h, d 96 h. Free cells (circle) were assayed as controls. Enzyme production was measured as β-CD cyclizing activity
Production of CGTase by B. circulans DF 9R in semi-continuous process
The bacterial cells immobilized on both sponge types were reused to produce CGTase in 12 successive cycles (Fig. 5). Each culture medium replacement was considered a new production cycle, which lasted for 24 h. In another set of experiments, the same amount of free cells was inoculated into the culture medium. In cycles 7–12, only 40 % of the activity produced in the first cycle was obtained. The activity obtained with the immobilized cells on loofa was 0.91 ± 0.10 U ml−1, similar to that obtained in the synthetic support (0.95 ± 0.11 U ml−1).The activity of the free cells was slightly lower than that of the immobilized cells at all cycles. To calculate productivity, only the first six cycles were considered. Moriwaki et al. [20] conducted a maximum of three consecutive cycles of reuse of 3 days each to produce CGTase by immobilized cells of B. firmus and B. sphaericus on loofa, after an immobilization period of 8–10 days. However, the purpose of their work was to obtain β-CD and enzyme production was not detailed. Catalyst systems in heterogeneous phase, as immobilized cells on a porous support, are affected by problems in the diffusion of nutrients, especially when working with high-molecular weight substrates such as starch. However, the pore size of the matrix used in this study (around 500 µm in synthetic sponge and larger in loofa, Fig. 3) is large enough to not significantly affect the substrate diffusion [15, 30].
Production of CGTase by Bacillus circulans DF 9R in semi-continuous process. Bacterial cells immobilized on loofa (black) and synthetic sponges (gray) were reused to produce CGTase in 12 successive cycles of 24 h each in fresh MAS medium and incubated at 37 °C and 120 rpm. Free cells (mottled) were assayed as controls
A decrease in the final CGTase production level was observed. However, when the cells were counted in plates of growth culture media for each of the 12 operating cycles, the results showed very low counts (data not shown). According to Behera et al. [31], the decrease in the production of enzyme might be due to marginal leakage of cells from the matrix during each cycle of CGTase production. Moriwaki et al. [20] showed by FTIR studies that matrix-cell interactions are mediated by van der Waals forces, which are weak bonds and would thus allow the separation of cells from the support. According to Iqbal and Zafar [32], the release of cells into the culture medium could be due either to desorption of the originally immobilized cells or to the release of daughter cells which could not be prevented. Saudagar et al. [17] argued that the decreased production of enzyme along the cycles could be due to cell death.
The values of activity and productivity achieved in batch fermentation and by immobilized cells on loofa or synthetic sponge are shown in Table 1. Regarding enzyme activity, the values obtained were higher in the batch process than in the semi-continuous process, whereas regarding specific productivity, similar results were obtained in the two types of processes. Abdel-Naby et al. [8] reported the specific productivity of several CGTase-producing microorganisms, such as: Bacillus amyloliquefaciens strain 312, 89.9 U g−1 dry cells h−1; Bacillus macerans strain 3,185, 94.8 U g−1 dry cells h−1; B. macerans strain 3,168, 66.4 U g−1 dry cells h−1 and Bacillus megaterium strain NR C4, 91 U g−1 dry cells h−1. These results agree with that obtained here using CGTase from B. circulans DF 9R. Recently, Abdel-Naby et al. [9] achieved a specific productivity of 315.2 U g−1 dry cells h−1 using Bacillus cereus.
Conclusions
In the optimization of the batch process with free cells, most enzyme production was achieved with aeration of 1.5 vvm, after 48 h of growth and at pH regulated to 7.6. The highest enzyme yield was not linked to greater development of biomass. Thus, we were able to improve the results obtained in Rosso et al. [21] in shake flasks. The optimization of the semi-continuous process with immobilized cells showed that an initial biomass above 30 mg did not result in an increase in CGTase production and that an immobilization time of 24 h with loofa sponge was enough to achieve the highest enzyme production, demonstrating that more prolonged incubation times were not required. The batch process using free cells provided higher enzyme activity than the semi-continuous process using immobilized cells in loofa or synthetic sponge. However, the specific productivity was similar in both processes. It is worth noting that the semi-continuous process with immobilized cells is inexpensive; matrices are renewable, non-toxic and biodegradable. Cells can be used repeatedly and separation from the fermentation medium is easy. Besides, scaling up of this process is very simple since fixed beds in large-scale reactors can be easily constructed with the loofa sponges [15, 31]. In conclusion, both processes of CGTase production, by free cells in batch fermentation or immobilized cells in a semi-continuous process, have similar productivity and can be potentially scaled up.
References
Loftsson T, Duchêne D (2007) Cyclodextrins and their pharmaceutical applications. Int J Pharm 329:1–11
Venturini CG, Nicolini J, Machado C, Machado VG (2008) Properties and recent applications of cyclodextrins. Quím Nova 31:360–368
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gándara J (2009) A review on the use of cyclodextrins in foods. Foods Hydrocoll 23:1631–1640
Biwer A, Antranikian G, Heinzle E (2002) Enzymatic production of cyclodextrins. Appl Microbiol Biotechnol 59:609–617
Szerman N, Schroh I, Rossi AL, Rosso AM, Krymkiewicz N, Ferrarotti SA (2007) Cyclodextrin production by cyclodextrin glycosyltransferase from Bacillus circulans DF 9R. Bioresour Technol 98:2886–2891
Costa H, Distefano AJ, Marino-Buslje C, Hidalgo A, Berenguer C, Biscoglio M, Ferrarotti S (2012) The residue 179 is involved in product specificity of the Bacillus circulans DF 9R cyclodextrin glycosyltransferase. Appl Microbiol Biotechnol 59:609–617
Pinto FS, Flôres SH, Ayub MAZ, Hertz PF (2007) Production of cyclodextrin glycosyltransferase by alkaliphilic Bacillus circulans in submerged and solid-state cultivation. Bioprocess Biosyst Eng 30:377–382
Abdel-Naby MA, Reyad RM, Abdel-Fattah AF (2000) Biosynthesis of cyclodextrin glucosyltransferase by immobilized Bacillus amyloliquefaciens in batch and continuous cultures. Biochem Eng J 5:1–9
Abdel-Naby MA, El-Refai EI, Abdel-Fattah AF (2011) Biosynthesis of cyclodextrin glucosyltransferase by the free and immobilized cells of Bacillus cereus in batch and continuous cultures. J Appl Microbiol 111:1129–1137
Kunamneni A, Prabhakar T, Jyothi B, Ellaiah P (2007) Investigation of continuous cyclodextrin glucanotransferase production by the alginate-immobilized cells of alkalophilic Bacillus sp. in an airlift reactor. Enzyme Microb Technol 40:1538–1542
Mazzer C, Rosas Ferreira L, Tedesco Rodella JR, Moriwaki C, Matioli G (2008) Cyclodextrin production by Bacillus firmus strain 37 immobilized on inorganic matrices and alginate gel. Bioch Eng J 41:79–86
Moriwaki C, Pelissari FM, Correia Gonçalves RA, Gonçalves JE, Matioli G (2007) Immobilization of Bacillus firmus strain 37 in inorganic matrix for cyclodextrin production. J Mol Catal Enzyme 49:1–7
Vassileva A, Beschkov V, Ivanova V, Tonkova A (2005) Continuous cyclodextrin glucanotransferase production by free and immobilized cells of Bacillus circulans ATCC 21783 in bioreactors. Process Biochem 40:3290–3295
Phillips CR, Poon YC (1988) Immobilization of cells, 5th edn., Biotechnology monographsSpringer, New York
Kilonzo P, Margaritis A, Bergougnou M (2009) Airlift-driven fibrous-bed bioreactor for continuous production of glucoamylase using immobilized recombinant yeast cells. J Biotechnol 143:60–68
Meleigy SA, Khalaf MA (2009) Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme cells immobilized on loofa sponge. Bioresour Technol 100:374–379
Saudagar PS, Shaligram NS, Singhal RS (2008) Immobilization of Streptomyces clavuligerus on loofah sponge for the production of clavulanic acid. Bioresour Technol 99:2250–2253
Chantawongvuti R, Veerajetbodithat J, Jaturapiree P, Muangnapoh C (2010) Immobilization of Lactobacillus salivarius ATCC 11741 on loofa sponge coated with chitosan for lactic acid fermentation. J Microbiol Biotechnol 20:110–116
Pazzetto R, de Oliveira Delani TC, Carvalho Fenelon V, Matioli G (2011) Cyclodextrin production by Bacillus firmus strain 37 cells immobilized on loofa sponge. Process Biochem 46:46–51
Moriwaki C, Mangolim CS, Ruiz GB, Rodrigues de Morais G, Baesso ML, Matioli G (2014) Biosynthesis of CGTase by immobilized alkalophilic bacilli and crystallization of beta-cyclodextrin: effective techniques to investigate cell immobilization and the production of cyclodextrins. Biochem Eng J 83:22–32
Rosso AM, Ferrarotti SA, Krymkiewicz N, Nudel BC (2002) Optimization of batch culture conditions for Cyclodextrin Glucanotransferase production from Bacillus circulans DF 9R. Microb Cell Fact 1:3
Ferrarotti SA, Rosso AM, Maréchal MA, Krymkiewicz N, Maréchal LR (1996) Isolation of two strains (S-R type) of Bacillus circulans and purification of a cyclomaltodextrin-glucanotransferase. Cell Mol Biol 42:653–657
Goel A, Nene S (1995) Modifications in the phenolphthalein method for spectrophotometric estimation of beta Cyclodextrin. Starch 47:399–400
Pinto FST, Flôres SH, Schneider CE, Ayub MAZ, Hertz PF (2011) The influence of oxygen volumetric mass transfer rates on cyclodextrin glycosyltransferase production by Alkaliphilic Bacillus circulans in batch and fed-batch cultivations. Food Bioprocess Technol 4:559–565
Marechal L, Rosso A, Marechal M, Krymkiewicz N, Ferrarotti S (1996) Some properties of a cyclomaltodextrin-glucanotransferase from Bacillus circulans DF 9R Type. Cell Mol Biol 42:659–664
Yap PW, Ariff AB, Woo KK, Hii SL (2010) Production of Cyclodextrin Glycosyltransferase (CGTase) by Bacillus lehensis S8 using Sago Starch as Carbon Source. J Biol Sci 10:676–681
Vassileva A, Burhan N, Beschkov V, Spasova D, Radoevska S, Ivanova V, Tonkova A (2003) Cyclodextrin glucanotransferase production by free and agar gel immobilized cells of Bacillus circulans ATCC 21783. Process Biochem 38:1585–1591
Alves-Prado HF, Gomes E, Da Silva R (2006) Evaluation of solid and submerged fermentations for the production of cyclodextrin glycosyltransferase by Paenibacillus campinasensis H69-3 and characterization of crude enzyme. Appl Biochem Biotechnol 129:234–246
Ibrahim HM, Yusoff WMW, Hamid AA, Omar O (2010) Enhancement of cyclodextrin glucanotransferase production by Bacillus G1 using different fermentation modes. Biotechnol 9:506–512
Hilal-Alnaqbi A, Hu AY, Zhang Z, Al-Rubeai M (2013) Growth, metabolic activity, and productivity of immobilized and freely suspended CHO cells in perfusion culture. Biotechnol Appl Biochem 60:436–445
Behera S, Mohanty RC, Ray RC (2011) Ethanol production from mahula (Madhuca latifolia L.) flowers with immobilized cells of Saccharomyces cerevisiae in Luffa cylindrica L. sponge discs. Appl Energy 88:212–215
Iqbal M, Zafar SI (1994) Vegetable sponge as a matrix to immobilize micro-organisms: a trial study for hyphal fungi, yeast and bacteria. Appl Microbiol 18:214–217
Acknowledgments
We thank the financial support of the following institutions: Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), PICT 2013-0880 préstamo BID, Universidad Nacional de Luján and Ministerio de Ciencia, Tecnología e Innovación Productiva (MINCyT) from Argentina, and Universidade Estadual de Maringá and Coordenação de Aperfeiçoamento de Pessoa de Nível Superior (CAPES) from Brazil. HC is a career member of the Argentine Council of Scientific and Technical Research (CONICET). We thank professor Victoria Eusevi for language supervision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costa, H., Gastón, J.R., Lara, J. et al. Cyclodextrin glycosyltransferase production by free cells of Bacillus circulans DF 9R in batch fermentation and by immobilized cells in a semi-continuous process. Bioprocess Biosyst Eng 38, 1055–1063 (2015). https://doi.org/10.1007/s00449-014-1347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-014-1347-6