Abstract
For thousands of years, crop production has almost entirely depended on conventional agriculture. However, the reality is changing. The ever-growing population, global climate change, soil degradation and biotic/abiotic stresses are a growing threat to food production and security. Thus, sustainable alternatives to increase crop production for a population projected to reach 9.8 billion by 2050 are a major priority. In addition to vertical and soilless farming, innovative products based on bioresources, including plant growth stimulants, have been a target for sustainable food production. Such solutions have led to the exploitation of microorganisms, including microalgae and cyanobacteria as potential bioresources for food and plant biostimulant products. Microalgae (eukaryotic) and cyanobacteria (prokaryotic) are photosynthetic microorganisms with the capacity to synthesize a vast array of bioactive metabolites from atmospheric CO2 and inorganic nutrients. The present review outlines the nutritional value of microalgae and cyanobacteria as alternative food resources. The potential aspects of microalgae and cyanobacteria as stabilizers of the net change in soil organic carbon (C) levels for reduced farmland degradation are also highlighted. The applications of microalgae and cyanobacteria as remedies for improved soil structure and fertility, and as enhancers of crop productivity and abiotic stress tolerance in agricultural settings are outlined. This review also discusses the co-cultivation of crops with microalgae or cyanobacteria in hydroponic systems to favor optimum root CO2/O2 levels for optimized crop production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural production is critically affected by plant diseases, soil quality, global climate change and the exponentially growing population. Global climate change leads to increased weather-related disasters, such as floods and droughts, causing food shortages and famine (Myers et al. 2017). Plant diseases and soil degradation also affect plant growth and development (Teng and Johnson 1988; Savary et al. 2012), leading to reduced crop yield and food production. This scenario threatens food security and is aggravated by the growing population projected to reach 9.8 billion by 2050.
Over the years, the development of chemical products such as synthetic fertilizers and pesticides significantly improved crop productivity. However, the extensive application of such chemical products cause negative impacts on the environment and the ecosystem (Bhandari 2014; Prashar and Shah 2016; Zhang et al. 2018). Thus, modern agriculture is challenged to search for sustainable food sources and ecofriendly methods of improving crop resilience and productivity. In this context, organic products such as plant biostimulants have been described as sustainable alternatives to improve plant growth, nutrient uptake, nutrient use efficiency, tolerance to abiotic stress and/or crop quality (Calvo et al. 2014; du Jardin 2015). The emerging development of innovative sustainable agricultural products has led to the exploitation of microorganisms including microalgae and cyanobacteria as potential bioresources for food and plant biostimulants (Gonçalves 2021; Alvarez et al. 2021). Microalgae (eukaryotic) and cyanobacteria (prokaryotic) are unicellular microscopic photosynthetic organisms that grow in diverse aquatic habitats and even humid soils (Khan et al. 2018). Microalgae and cyanobacteria have been described as high-nutrient food resources (García et al. 2017). Several species are presently exploited for the generation of protein food supplements and nutraceutical products such as Omega-3, astaxanthin and beta-carotene (Nethravathy et al. 2019; Rahman 2020), making them sustainable alternative food resources with health benefits. In agricultural settings, microalgal and cyanobacterial biomass application to farmlands can stabilize the net change in soil organic carbon (C) levels and reduce farmland degradation. Microalgae and cyanobacteria can also produce bioactive substances such as sulfated exopolysaccharides (EPS) and phytohormones that are beneficial for soil structure and plant growth (Gayathri et al. 2015; Abinandan et al. 2019). Microalgae and cyanobacteria extracts improve plant growth and nutrient uptake, contributing to crop growth and yield (Renuka et al. 2018; Alvarez et al. 2021). Microalgae and cyanobacteria can be grown in hydroponic system substrates, fixing carbon dioxide (CO2) through photosynthesis and releasing bioactive compounds such as sulfated EPS and phytohormones into the nutrient substrate (Zhang et al. 2017; Barone et al. 2019).
The present review outlines the nutritional value of microalgae and cyanobacteria as sustainable alternative food resources. This review also discusses how the utilization of microalgae and cyanobacteria can be exploited to help restore degrading or abandoned farmlands. The applications of microalgae and cyanobacteria as remedies for improved soil structure and fertility, and as enhancers of crop productivity and abiotic stress tolerance in agricultural settings are outlined. The review also highlights the use of microalgae and cyanobacteria for optimized crop production in hydroponic co-culture systems.
Microalgae and Cyanobacteria
Microalgae and cyanobacteria are microorganisms that exist in various aquatic and terrestrial ecosystems (Rajvanshi and Sharma 2012), and carry out oxygenic photosynthesis, a high-energy demanding process of water oxidation to molecular oxygen (O2) and reduction of CO2 to organic compounds (Tamagnini et al. 2002). Oxygenic photosynthesis first appeared in the ancestors of present-day cyanobacteria more than 3.7 billion years ago. Cyanobacteria are considered among the oldest life forms on Earth and are the original producers of the Earth's oxygenic atmosphere (Saad and Atia 2014). The close association of cyanobacteria with green algae, green plants and other organisms arose more than 1.2 billion years ago from an early endosymbiosis event where a cyanobacterium was taken up into a heterotrophic organism (Björn and Govindjee 2008). Cyanobacteria were originally classified as blue-green algae (Cyanophyta) under botanical codes (Oren 2014a; Demoulin et al. 2019), until their prokaryotic features were established in the 1960s and a proposal was made to include cyanobacteria within the bacteriological code (Stanier et al. 1978). Cyanobacteria in nature are all oxygenic photoautotrophs, with the possible exception of their capacity for facultative anoxygenic photosynthesis (Lau et al. 2015; Garcia-Pichel et al. 2020). Microalgae and cyanobacteria are capable of producing a broad variety of unique, potent substances (Chu and Phang 2019; Kini et al. 2020).
Microalgae and cyanobacteria are suitable bioresources for food and commodities production, and can address the underlying challenges related to food sources and sustainable agriculture (Stephens et al. 2013; Saifullah et al. 2014).
Microalgae and Cyanobacteria are Reliable Food Resources for Human Nutrition
Food security is a major priority worldwide, affecting both developing and developed countries (Rosegrant and Cline 2003; Schmidhuber and Tubiello 2007; Savary et al. 2012). The rapidly growing population will demand 60% increase in agricultural output by 2050, but climate change threatens the current and future agricultural production (Rosenzweig et al. 2014). The human population growth and agricultural intensification are major drivers of environmental degradation. Croplands and pastures are one of the largest terrestrial biomes on the planet, covering ~ 40% of the land surface and making agricultural production the planet’s single most extensive form of land use (Foley et al. 2005). In the tropics, new farmlands are cleared to the detriment of rainforests, savannas and diverse ecosystems (Gibbs et al. 2010; Costantini 2015). Microalgae and cyanobacteria are sustainable resources for food and agricultural product innovation; they are primary producers that exist in various aquatic and terrestrial ecosystems, and require no arable land for production (Rajvanshi and Sharma 2012; Hopes and Mock 2015).
Microalgae and cyanobacteria can produce a broad variety of nutritional value compounds, including proteins, lipids, carbohydrates, different pigments, vitamins and anti-oxidants. Microalgae are a pivotal food source since their first use by the Chinese 2000 years ago to survive during famine (Mobin et al. 2019). Microalgae species such as Dunaliella tertiolecta and Euglena gracilis are effective sources of healthy food in Japan (Nethravathy et al. 2019). Cyanobacterial species such as Arthrospira spp., also known as Spirulina, have also been used as sources of food by many civilizations. The Aztecs were among the first people who harvested this cyanobacterium in Lake Texcoco within the valley of Mexico (Barrios et al. 2017). Spirulina is rich in proteins (55% to 70% protein content per total dry weight), iron and essential unsaturated fatty acids such as omega 3 (Tokuşoglu and Ünal 2003), and are one of the richest natural green sources of vitamin B12 (Dochi et al. 2010). Spirulina also has various beneficial effects on human health, including antihypertensive effects, prevention of renal failure and the growth of beneficial intestinal Lactobacillus bacteria (Beheshtipour et al. 2013). Arthrospira ssp. are part of the diet of certain human populations where these cyanobacteria grow naturally, such as in the lakes of Chad in Africa (Spolaore et al. 2006; Mata et al. 2010; Hamed 2016), where spirulina (locally known as Dihé by the local people) is harvested for food (Fig. 1a and b) (Caterina et al. 2004).
Women of Chad preparing (a) and selling (b) spirulina dried on sand, locally known as Dihé by the local people of Chad (Caterina et al. 2004)
Microalgal species such as Dunaliella salina produce β-carotene under stress conditions, which provide numerous benefits for human health (Hosseini and Shariati 2009; Oren 2014b; Wu et al. 2020). β-Carotene is a major carotenoid present in the human diet and the main source of vitamin A in humans (Johnson 2002; Elvira-Torales et al. 2019), but research is still needed for dietary recommendations of algal sourced β-carotene, and caution should be taken for large doses (EFSA 2012). Microalgal species such as Haematococcus pluvialis produce astaxanthin, a carotenoid well known for its antioxidant activity as well as anticancer, photoprotection, and anti‐inflammatory properties (Yuan et al. 2011). Astaxanthin was approved by the U.S. FDA as an effective nutritional supplement with potent antioxidant properties (Nethravathy et al. 2019). Microalgae and cyanobacteria also produce long-chain polyunsaturated fatty acids (PUFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which are effective nutraceuticals with many health benefits (Sathasivam et al. 2019). Table 1 illustrates examples of dietary ingredients produced by microalgal and cyanobacterial species, beneficial for human consumption.
Microalgal and Cyanobacterial Products Market
Microalgal and cyanobacterial biomass production can be used to obtain different kinds of extracts economically important in commerce. Leading commercial players in microalgal and cyanobacterial products include Cyanotech Corporation (U.S.), DIC Lifetec Co. Ltd. (Japan), Cellana Inc. (U.S.), Alltech, Inc. (U.S.), Algaetech International Sdn Bhd (Malaysia), BlueBioTech GmbH (Germany) and Parry Nutraceuticals Limited (India) (Algae Products Market Information 2018). The Cyanotech Corporation sources Arthrospira sp. food supplements and astaxanthin from natural microalgae Haematococcus pluvialis. The current market value of astaxanthin is around $2,000/kg (Shah et al. 2016; Nethravathy et al. 2019). Although consumed by humans, microalgae-derived astaxanthin is principally consumed by the salmon feed industry (Nethravathy et al. 2019). H. pluvialis produced astaxanthin gives salmonids the typical salmon coloration, which is desired by the customers. Major microalgal astaxanthin producers in the market include: Cyanotech corporation (USA), Alga technologies (Israel), Jingzhou Natural Astaxanthin Inc (China), Fuji chemical industry Co. Ltd (Japan), and Parry Nutraceuticals (India) (Shah et al. 2016; Nethravathy et al. 2019).
Other beneficial ingredients such as lutein are commercially marketed in the form of powder, capsules, or oleoresins containing 3% to 80% of lutein (Nethravathy et al. 2019). microalgae-based lutein production provides a promising alternative to marigold petals, the current main commercial supply of lutein (Fernández-Sevilla et al. 2010; Lin et al. 2015; Xie et al. 2021). Several microalgae species including Chlorella spp., Scenedesmus spp., Muriellopsis spp., and Dunaliella spp. produce significant lutein content. However, production of microalgal lutein at commercial scale is still at the laboratory stage and not yet implemented in large-scale commercial production (Lin et al. 2015). Microalgae and cyanobacteria can be dried as food or consumed in the form of capsules and tablets as food supplements (Spolaore et al. 2006; Mata et al. 2010), or incorporated into other food and dairy products, such as pasta, bread, soft drinks, yogurt or snacks (Gross 2004; Mohamed et al. 2013). High value molecules produced by microalgae and cyanobacteria such as DHA and lutein are found in human breast milk and can be incorporated in formulas (Eggersdorfer and Wyss 2018).
Microalgae and cyanobacteria are suitable alternatives for consumers who are increasingly concerned about their health and diet control (Hamed 2016). Illnesses such as high cholesterol and heart disease linked to poor eating habits could be reduced by the substantial consumption of healthy microalgal or cyanobacterial food products due to their high nutrition value (Hamed 2016). For example, β‐1,3‐glucan polysaccharides, the main component in Chlorella spp. are active immunostimulators, free-radical scavenger and reducers of blood lipids (de Jesus Raposo et al. 2015; Sathasivam et al. 2019).
Microalgae and cyanobacteria production and commercialization is often limited by the high cost of cultivation. The optimization of culture medium is an important strategy for increasing the cost-effectiveness of the productions (Ronga et al. 2019). Low-cost resources such as nutrient-rich wastewaters, agricultural by-products and inexpensive synthetic fertilizers, are effective strategies for increasing the cost-effectiveness of the production (Mata et al. 2010; Gong and Jiang 2011). However, such strategies could not be suitable for algal biomass production destined for human consumption. Microalgae and cyanobacteria cultivation for food production can be grown in controlled optimized conditions to maximally promote the growth rate and productivity (Andrianantoandro et al. 2006; Narala et al. 2016; Tan et al. 2020). Culture parameters in photobioreactors (PBRs) can also be controlled to trigger enhanced production of specific value-added products under artificial growth conditions for the industrialization of microalgae and cyanobacteria (Kothari et al. 2017). Cultivation parameters including pH, temperature, light intensity, nutrient composition and concentration and CO2 supply are major growth factors of microalgae and cyanobacteria (Renhe et al. 2021; Figueroa-Torres et al. 2021).
Microalgae and Cyanobacteria vs. Crop Plants
Microalgae and cyanobacteria are good sources of proteins, carbohydrates, lipids and several useful metabolites. Microalgae and cyanobacteria do not require arable land (they are cultivated in controlled culture systems) as they can grow in brackish and even seawater, which is unusable for normal agriculture (Masojídek and Torzillo 2014). In addition, the microalgal yield of storable compounds can greatly exceed that of traditional crops. Table 2 illustrates the advantages of cultivation of microalgae and cyanobacteria over crop plants, including higher efficiency of CO2-fixation and energy conversion, biomass productivity, and lower resources requirements.
Microalgae can be produced at a large scale in artificial open systems (circular and raceway ponds where the culture is directly exposed to open air) or closed systems, where the culture is fully enclosed in a PBR (Narala et al. 2016). Open systems can be divided into natural ponds (eutrophic lakes or small natural basins) and artificial raceway ponds (Hamed 2016). Raceway ponds are the most widely used systems for the commercial production of microalgae (Jerney and Spilling 2018). They are generally the cheapest to construct, and their function is quite simple (Borowitzka 2013; Enzing et al. 2014). They are generally constructed as oval‐shaped recirculation channels in which the culture medium flow is guided around bends by baffles placed in the flow channel, and they are stirred with a paddlewheel to ensure culture homogenization, as shown in Fig. 2a. PBRs are reactors that offer a closed‐culture environment in which phototrophs are grown or used to carry out photobiological reactions (Tredici 2004) (Fig. 2b and c). PBRs allow better control of culture growth conditions, and most importantly, the microalgae are protected and relatively safe from invasion by competing microorganisms (Vishwanath Patil1 et al. 2005; Narala et al. 2016).
PBRs are the best culture systems for optimized culture production and biomass quality, despite their high setup, maintenance and energy input costs. In comparison to PBRs, circular and raceway ponds have higher contamination risks, culture evaporation and exposure to weather components, making these culture systems highly dependent on geographical conditions (Kumar et al. 2015; Muhammad et al. 2020). Open culture systems can be a great challenge for countries in the equatorial and monsoon climate regions of Africa but are highly suitable for the warm desert climate of Northern Africa. On the other hand, large-scale microalgal or cyanobacterial production using PBRs may not be practical in most parts of sub-Saharan Africa, considering that the larger population of these regions consists of smallholder farmers (Langyintuo 2020) with less financial capacity for sophisticated large-scale algal production.
Technological Developments in Microalgal and Cyanobacterial Cultivation to Improve the Algal Production and Industrialization
The latest technological developments in microalgal and cyanobacterial cultivation and harvesting, such as automation, phenotyping, and synthetic biology, were described by Fabris et al. (2020). A significant body of research has focused on optimizing conditions that maximally promote microalgae and cyanobacteria growth rates, or trigger enhanced production of specific value-added products under artificial growth conditions for the industrialization of microalgae and cyanobacteria (Andrianantoandro et al. 2006). Figure 3 shows cyanobacterial culture optimization by manipulating different growth parameters for oriented production of EPS.
The cultivation of microalgae and cyanobacteria has an advantage over heterotrophic systems due to their photosynthetic mechanisms and ability to fix C from inorganic sources (Fajardo et al. 2020). Despite the several advantages and inconveniences reported, there is no single "best practice" method for cultivating microalgae and cyanobacteria, especially at a large scale (Fajardo et al. 2020). The final design of the system is dependent on the final product, the geographical location, and local resources available (e.g., accessibility to water, CO2, and waste streams) (Fabris et al. 2020).
Advanced molecular techniques for research and development in microalgae are still underdeveloped compared to fungi, bacteria or even higher plants (Fajardo et al. 2020). Sophisticated studies including the overexpression or downregulation of several genes in a single organism, are only practically possible in Chlamydomonas reinhardtii (Wijffels and Barbosa 2010; Doron et al. 2016; Sanchez-Tarre and Kiparissides 2021). However, new advances in microalgae and cyanobacteria biotechnology are rapidly emerging, paving the way to the establishment of a sustainable, algae-based bioeconomy (Hamed 2016; Fu et al. 2019; Fabris et al. 2020; Fajardo et al. 2020). The available number of full or near‐full genome sequences of diverse microalgal species has increased over the last decade (Kumar et al. 2020). Such species include Chlamydomonas reinhardtii (Colina et al. 2018; Merchant et al. 2007; Nguyen et al. 2011; Schmollinger et al. 2014), Chlorella pyrenoidosa (Duan et al. 2019; Kumar et al. 2020), Chlorella sorokiniana (Tejano et al. 2019; Kumar et al. 2020), Chlorella vulgaris, Dunaliella salina (Yue et al. 2016; Wang et al. 2019; Kumar et al. 2020), Phaeodactylum tricornutum (Bowler et al. 2008; Yang et al. 2014; Longworth et al. 2016) and Thalassiosira pseudonana (Armbrust et al. 2004). Genome sequencing of microalgal species will provide a significant genetic resource for the study of their metabolic pathways, regulatory networks, and genetic potentials. Web-based resources such as pico-PLAZA (http://bioinformatics.psb.ugent.be/pico-plaza/), AlgaePath (http://algaepath.itps.ncku.edu.tw) and ALCOdb (http://alcodb.jp) are available databases for algal genomics (Kumar et al. 2020). These emerging technologies will provide tools for highly efficient algae-based solutions to a range of societal needs. In-depth understanding of algal biology, genetics and biochemical capacities, will improve both their production and processing for consumption or product innovation in agriculture.
Microalgae and Cyanobacteria for Sustainable Agriculture
Microalgal and Cyanobacterial Biomass Application to Degraded Soils: A New Approach to Restoring Organic Matter on Degraded Farmlands
The conversion of natural ecosystems to agriculture results in the depletion of soil organic C levels, releasing 50 to 100 GT of C from soil into the atmosphere (Lal 2009). This C loss is mainly caused by reductions in the amount of plant roots and residues returned to the soil, and higher soil erosion (Lemus et al. 2005). The soil C deficit created by the depletion of soil organic C stocks represents an opportunity to store C in soil through diverse land management approaches. Naturally, soil organic C levels are based on the interactions between many ecosystem processes, of which photosynthesis, respiration, and decomposition are key. Carbonic matter in degraded soils may take years to restore (Ovsepyan et al. 2019), considering that soil organic C input rates are primarily determined by plant roots and litter deposited from plant shoots (Lemus et al. 2005).
The microalgal and cyanobacterial biomass is rich in organic matter due to the organic C incorporated through photosynthesis, and it is therefore a suitable target for the development of innovative products for soil quality recovery. The total carbohydrate and starch contents of microalgae are approximately 20% and 10% dry weight (DW), respectively (Laurens et al. 2012; Cheng et al. 2017). Cyanobacterial species also have high carbohydrate contents, up to 60% DW. The carbohydrate contents vary with microalgal or cyanobacterial species but can be significantly increased according to cultivation conditions and time (Cheng et al. 2017). The restoration of C storage on degraded land parcels can begin from the decay of microalgae and cyanobacteria organic compounds, that are highly enriched in C (Ontl and Schulte 2012). Other organic compounds produced by microalgae and cyanobacteria, including proteins and lipids, also contribute to soil organic matter (Ontl and Schulte 2012).
Microalgal and cyanobacterial biomass can also provide soil microbes with energy in the form of C. Although decomposition of this biomass by soil microbes will result in C loss from the soil as CO2 from microbial respiration, a small proportion of the original C will be retained in the soil through the formation of humus, which is beneficial for soil water retention (Ontl and Schulte 2012; Bastida et al. 2012).
Microalgal and cyanobacterial biomass can boost the productivity of arable land parcels as soil amendments with the capacity to increase soil organic C and stabilize the balance between soil C inputs and outputs in farm soils (Alvarez et al. 2021). Various factors including climate change, historic land use patterns, land management strategies, and topographic heterogeneity influence soil C changes on farmlands, (Ontl and Schulte 2012). Other ecosystem processes, such as soil erosion and leaching of dissolved C into groundwater, can also lead to C loss in soils (Tiefenbacher et al. 2021; Thaler et al. 2021). C inputs from photosynthesis on agricultural farmlands are less than C losses from the soil; therefore, soil organic C levels decrease over time (Ontl and Schulte 2012). Thus, alimentation of the soil with C-rich products such as microalgal and cyanobacterial biomass could be an important method to stabilize the net change in soil organic C levels. When C inputs and outputs are in balance with each other, there is no net change in soil organic C levels.
Role of Microalgae and Cyanobacteria in Soil Fertility and Structure
Microbes play a vital role in determining soil structure and fertility (Vaishampayan et al. 2001; Singh Jay Shankar 2014; Singh et al. 2016). Although most microalgal species can utilize both organic and inorganic nitrogen (N), they can only assimilate nitrite (NO2·−), nitrate (NO3·−), and ammonium (NH). Cyanobacteria, on the other hand, can convert atmospheric N2 to ammonia by N2-fixation (Cai et al. 2013; Ahmed et al. 2014). Cyanobacteria can also tolerate various stresses, such as drought, high or low temperatures, pH and salinity, giving them an advantage over various competitors in different ecological niches (Gröniger et al. 2000; Ibañez et al. 2012; Sand-Jensen and Jespersen 2012). Cyanobacteria in particular have been a specific target in agriculture due to their ability to fix atmospheric N2 and CO2 and produce a diversity of biologically active metabolites (Vaishampayan et al. 2001; Singh Jay Shankar 2014; Singh et al. 2016). Cyanobacteria establish symbiotic associations (Subashchandrabose et al. 2011; Hamouda et al. 2016). Certain cyanobacteria species also adapt to local climatic conditions and survive in wet soils (Nisha et al. 2007). Up to 25% of the total biomass of the cyanobacteria surviving in wet soils is from EPSs (Nisha et al. 2007). EPS significantly contribute to the soil’s nutritional status, structural stability and crop productivity. They act as a gluing agent on soil particles, leading to soil aggregation, organic content accumulation, and an increase in the water holding capacity of the top layer of soil (Malam Issa et al. 2001; Nisha et al. 2007). An increase in water holding capacity and organic content favors the growth of plant-growth promoting rhizobacteria (PGPR) (Xiao and Zheng 2016). Therefore, microalgal or cyanobacterial growth in humid soils could subsequently ameliorate soil chemical, physical and biological properties that sustain soil fertility (Flaibani et al. 1989; Zulpa et al. 2003; Paul and Nair 2008).
The aerobic N-fixing capacity of some cyanobacteria makes them important players in the biogeochemical N cycle of both aquatic and terrestrial environments (Garcia-Pichel 2009). Cyanobacteria are a natural component of paddy fields and a beneficial contributor to rice production in tropical countries. Rice production in tropical countries is largely dependent on biological N2 fixation by cyanobacteria (Vaishampayan et al. 2001), adding approximately 20–30 kg fixed N ha−1 in addition to organic matter to paddy fields (Ahmed et al. 2014; Singh et al. 2016). Cyanobacteria also improve the availability of phosphorus (P) to crops through the solubilization and mobilization of insoluble organic phosphates (Singh et al. 2016; Jhala et al. 2017; Gonçalves 2021).
Microalgal and cyanobacterial biofertilization enhanced the photosynthetic performance and growth of willow (Salix viminalis L.) (Grzesik et al. 2017) and rice plants (oryza sativum) (Jochum et al. 2018). The application of microalgal biomass (Chlorella vulgaris and Scenedesmus dimorphus) to rice plants resulted in significant plant height increase under greenhouse conditions (Jochum et al. 2018), indicating that microalgal biomass can provide a biological option for rice fertility programs. This approach could be especially important for organic rice production where synthetic fertilizers cannot be used (Jochum et al. 2018).
Microalgal and cyanobacterial biomass recovered from wastewater treatments, CO2 sequestration or biodiesel generation could be valuable in agriculture as organic soil amendments (Ansari et al. 2019). The use of wastewater could provide inexpensive solutions to microalgae and cyanobacteria cultivation, which require significant nutrient input (Renuka et al. 2018). Conversely, agricultural commercialization of microalgae or cyanobacteria biomass generated from wastewater requires thorough quality evaluation and validation. Although wastewaters are rich in nutrients such as organic and inorganic forms of C, N and P, which can be effectively utilized by microalgae, they contain undesirable substances such as heavy metals, pesticides, pharmaceutical compounds, chemicals and pathogens (Rana et al. 2014; Arora et al. 2021). Microalgae and cyanobacteria accumulate heavy metals. Thus, direct application of wastewater Microalgal or cyanobacterial biomass to the soil may have potential risks on the environment, due to the presence of unwanted hazardous metals, toxins and pathogens (Renuka et al. 2018). Cyanotoxins produced by cyanobacteria can also be absorbed and accumulated by plants, including agricultural food crops, which can have direct negative effect on human and animals (Corbel et al. 2014).
Microalgal and Cyanobacterial Extracts as Biostimulants of Plant Growth and Productivity
Several experimental studies under open-field and greenhouse conditions have demonstrated the plant growth-enhancing capacity of microalgal and cyanobacterial extracts (Garcia-Gonzalez and Sommerfeld 2016; Puglisi et al. 2018; Chanda et al. 2019), indicating their potential use in agriculture as plant growth enhancers.
Crude bioextracts obtained from microalgae and cyanobacteria can ameliorate the growth and development of crops. Liquid extracts of microalgae and cyanobacteria enhanced tomato plant growth through increased chlorophyll content and uptake of N, P and potassium (K) (Chanda et al. 2020). Gas chromatography–mass spectrometry (GC–MS) analysis also showed that treatment with microalgal and cyanobacterial extracts enhanced the production of total lipids and pyridine-3-carboxamide, an amide active form of vitamin B3 (Surjana et al. 2010). The germination of lettuce in 2 and 3 g kg−1 soil with dry microalgal extracts derived from C. vulgaris promoted plant growth at the early stages of development (Faheed and Fattah 2008). In the same study, enhanced plant growth based on shoot and root dry weight and length was correlated with improved carotenoid and chlorophyll pigment biosynthesis. Sugar beet seedlings supplemented with extracts of the microalgae C. vulgaris or Scenedesmus quadricauda also exhibited higher total root length, surface area, and number of root tips (Barone et al. 2018). The different changes in root architecture/morphology induced by the microalgal extracts were attributed to the upregulation of several genes that may intervene in various metabolic pathways (Barone et al. 2018).
The biostimulant activity of microalgal and cyanobacterial extracts is associated with their complex biochemical composition. Biologically active molecules derived from microalgal and cyanobacterial extracts include key amino acids (arginine and tryptophan), sulfated polysaccharides, polyunsaturated fatty acids, vitamins, osmolytes and phytohormones (Ronga et al. 2019; Colla and Rouphael 2020). This complex and multicomponent nature of extracts complicates the study of the mode of action and the production, registration and use of many biostimulants (Yakhin et al. 2017). Studies carried out in evaluating the effect of microalgae and cyanobacteria or their extracts on plant growth and tolerance mechanisms have been summarized in Table 3. Further studies such as hormone profiling, transcriptomics, proteomics, and metabolomics analysis of treated and untreated plants could also reveal biostimulant activated signaling pathways involved in the stimulation of plant responses (Yakhin et al. 2017).
Cyanobacteria and Microalgae as Biostimulants of Crop Abiotic Stress Tolerance
Crop productivity is often affected by diverse abiotic stresses usually provoked by drought, soil salinization and low or high temperatures. Soil salinization is a major agricultural constraint (Shrivastava and Kumar 2015; Ronga et al. 2019) affecting ~ 800 million ha globally (Munns and Tester 2008) and is exacerbated by inappropriate chemical fertilization management practices that lead to osmotic imbalance in the soil, affecting crop growth and productivity (Goykovic Cortés and Saavedra del Real 2007). Many studies have highlighted the biostimulant properties of extracts from different microalgal and cyanobacterial species for improving abiotic stress tolerance in higher plants, including salt stress (Rodríguez et al. 2006; Shrivastava and Kumar 2015; El Arroussi et al. 2018).
Dunaliella sp. and Phaeodactylum sp. mitigated salt stress in bell pepper (Capsicum annuum L.) seedlings by reducing superoxide O2− production and lipid peroxidation (Guzmán-Murillo et al. 2013). Crude extracts of the cyanobacteria Arthrospira sp. and the microalga Chlorella sp. improved the salt stress tolerance of wheat (Triticum aestivum L.) and enhanced the antioxidant capacity and protein content of grains from treated plants (Abd El-Baky et al. 2010). Extracts from Nannochloris sp. also alleviated hydric stress in tomato (Solanum lycopersicum L.) as determined by improved root length and increased leaf area and plant height (Oancea et al. 2013). The application of EPS from halophilic microalgae (Dunaliella salina) also alleviated the effect of salt stress on the growth of tomato (Solanum lycopersicum L.) subjected to high salinity concentrations (3 and 6 g L−1 NaCl) by mitigating the decrease in length and dry weight of the plant’s shoot and root systems, and increasing K uptake and the K+/Na+ ratio (El Arroussi et al. 2018). In the same study, the accumulation of proline, phenolic compounds and Na+, as well as catalase (CAT), peroxidase (POD) and superoxide dismutase (SOD) activities triggered by salt stress, were attenuated after EPS treatment. Metabolic pathways involved in the plant’s tolerance to stress, such as jasmonic acid-dependent pathways, were also enhanced.
Microalgal or cyanobacterial biostimulants are usually applied as total crude extracts containing diverse organic compounds, including hormones. This mixture of diverse organic compounds could contain molecules mimicking compatible solutes, signaling molecules or elicitors; hence, they could have multiple direct or indirect effects on plant growth and stress tolerance (Renuka et al. 2018; Colla and Rouphael 2020). Figure 4 illustrates the possible biostimulant effects of Microalgae-cyanobacteria Extract Formulation (MEF) on salt-stressed tomato plants. Bioactive organic compounds in MEF can stimulate the antioxidative enzyme system, and consequently attenuate lipid peroxidation in plant cells. MEF can also enhance root growth, which favors nutrient uptake and consequent ion homeostasis in leaves and photosynthetic activity.
Possible biostimulant effects of Microalgae-cyanobacteria Extract Formulation (MEF) on salt-stressed tomato plants. MEF enhances root growth, which favors nutrient uptake and consequent ion homeostasis in leaves and photosynthetic activity. MEF stimulate antioxidative enzymes, which consequently attenuate lipid peroxidation in plant cells. Reactive Oxygen Species (ROS), Hydrogen Peroxidase (H2O2), Catalase (CAT), Peroxidases (POD), Superoxide Dismutase (SOD), and Salt Overly Sensitive pathway (SOS)
Co-cultivation of Microalgae/Cyanobacteria and Plants in Hydroponic Systems: An Emerging Innovative Method for Biologically Stimulated Crop Growth and Productivity
Soilless cultivation (hydroponics) is the future for agricultural production sectors. Research studies such as those on NO3− management or crop quality increases by managing the electrical conductivity of the solution are commonly exploited in hydroponics. Emerging new and innovative methods, such as the use of nanoparticles and beneficial microorganisms, including PGPRs, have also been described. Crop co-cultivation with microorganisms such as microalgae or cyanobacteria in hydroponics can ameliorate plant growth. Microalgae and cyanobacteria produce and release a wide range of bioactive compounds in the culture solution.
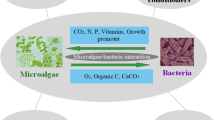
In hydroponics, microalgae and cyanobacteria can be grown in the nutrient solution. The release of bioactive compounds such as EPS and other metabolites in the substrate solution may trigger mechanisms related to plant tolerance, productivity and vigor (Fig. 5). Soil application of EPSs obtained from D. salina mitigated the effect of salinity stress by enhancing reactive oxygen species (ROS)-scavenging enzyme activities, phenolic compounds and key metabolites involved in antioxidative stress mechanisms (El Arroussi et al. 2018). Hydroponic co-cultivation of tomato plants with microalgae also stimulated crop performance and growth in terms of fresh and dry plant weight by ameliorating the substrate solution (Zhang et al. 2017; Barone et al. 2019). The plant growth stimulation was attributed to the constant use of CO2 and delivery of O2 to the hydroponic nutrient solution, through microalgal photosynthesis (Barone et al. 2019). The lower CO2 levels, resulting from CO2 fixation by microalgae or cyanobacteria, and the liberation of O2 in the root zone of hydroponically grown plants can positively regulate plant growth. CO2 accumulation in an O2-deficient root zone can be a detrimental factor to certain crop plants in flooded fields or aeroponic systems (Boru et al. 2003a, b).
Possible biostimulant effects of hydroponics co-cultivation with microalgae or cyanobacteria on crops. Bioactive metabolites such as sulfated exopolysaccharides and phytohormones released in the substrate solution enhance plant growth. CO2 fixation and O2 release from photosynthesis by microalgae or cyanobacteria in the substrate solution ameliorate root respiration
Crop co-culture with photosynthetic microorganisms in hydroponics releases more O2 in the nutrient solution to favor optimum root CO2/O2 levels for plant growth. The sole accumulation of CO2 in the root zone does not negatively affect plant growth unless O2 is deficient. Plant roots are naturally colonized by microorganisms and evolve more CO2 (CO2 concentrations in the rhizosphere is > 10-fold higher than in the atmosphere) (He et al. 2010). Exposure to no O2 combined with elevated CO2 levels attaining 30% (v/v) of total dissolved gases caused severe necrosis and stunted growth in soybean plants (Boru et al. 2003b). These results indicate that the co-culture technique could especially benefit crop plants with lower tolerance to higher dissolved CO2 in O2-deficient media.
The co-cultivation of crops with Microalgae or Cyanobacteria in hydroponic systems has been studied for several years (Hultberg et al. 2013; Ronga et al. 2019; Huo et al. 2020; Ergun et al. 2020; Supraja et al. 2020) as a new emerging innovative method for biologically stimulated crop growth and productivity. A plant-microalgae consortia relatively increased the pH and dissolved O2 from microalgae photosynthesis, and significantly improved the removal rate of total dissolved solids, total nitrogen (TN), and total phosphorus (TP) of the nutrient solution (Huo et al. 2020). Aqueous extracts prepared from microalgae collected from a greenhouse hydroponics solution significantly inhibited the growth of Fusarium oxysporum on slow nutrient agar (Schwarz and Gross 2015). Conversely, Lettuce fresh weight, shoot/root ratio, water and N uptake were significantly reduced in the presence of the microalgae Chlamydomonas spp. and Scenedesmus spp., compared to treatments which excluded microalgae (Schwarz and Gross 2015).
Commercial crop co-cultivation with photosynthetic microorganisms may have limiting factors. Co-cultivation will require optimized nutrient solution for the growth of both crops and microalgal or cyanobacterial species. The source and concentration of N can affect the growth and biochemical composition of microalgal and cyanobacterial species (Ronga et al. 2019). Several factors such as nutrient composition and concentration, light intensity, pH and electroconductivity (EC) affect the growth and the chemical composition of the microalgae or cyanobacteria (Danesi et al. 2002; Colla et al. 2007; Ogbonda et al. 2007). Crop co-cultivation with photosynthetic microorganisms is also limited to soft water microalgal or cyanobacterial species, as many crops poorly develop in highly saline sea water (Shrivastava and Kumar 2015). Hydroponic containers made of opaque material influences the growth of microalgae or cyanobacteria (Tocquin et al. 2003; Bawiec et al. 2019). In industrial setups, the hydroponic system could be mounted with a compartmentalized PBR, containing the photosynthetic microorganism and nutrient solution, and connected to the plant growth tray made of opaque material for optimized root growth, as illustrated in the drip system and nutrient film technique by Lee and Lee (2015). Challenges resulting from pH changes and growth of mold in the reservoir or tubing system can also be overcome by the use of non-recovery drip system. In this system, the hydroponic nutrient solution in the reservoir is delivered to each plant or pot using a pump, with the amount of nutrient solution for each plant adjusted by an electronic timer (Lee and Lee 2015). The nutrient solution is not collected and returned to the reservoir for recirculation through the system, thereby preventing fungal growth and pH changes (Lee and Lee 2015).
The biostimulant effects of microalgae or cyanobacteria in hydroponics may depend on a wide range of factors, including microalgae-excreted metabolites and phytohormones and O2/CO2 ratio changes in the root zone (nutrient solution). Analyzing all these factors will allow better exploitation of these microorganisms in developing microalgal and cyanobacterial biotechnology. Furthermore, hydroponic waste solutions contain high nutrients (Lee and Lee 2015). Thus, microalgae and cyanobacteria can be an effective method of removing nutrients from hydroponic waste solution, before they are discharged. The use of microalgae or cyanobacteria will prevent the generation of waste materials and hydroponic waste solution. The resulting biomass can be valorized in agriculture as soil conditioners, biostimulants or biofertilizers.
Conclusion
The rapidly growing population and climate change require further innovation to address the practical limitations and serious environmental concerns associated with current agricultural practices. Microalgae and cyanobacteria are targets for next-generation sustainable bioresources. They can be utilized to produce value-added bioproducts, and their rapid growth rates and higher biomass productivity offer several advantages over terrestrial crops. Microalgae and cyanobacteria can also be utilized in agricultural settings as soil conditioners and biostimulants of plant growth and abiotic stress tolerance.
Mass cultivation and commercial production of microalgae and cyanobacteria are highly dependent on the economics of their biomass production. The utilization of waste-substrates for the production of microalgal and cyanobacterial biomass is an economically viable strategy. Conversely, challenges associated with waste generated biomass, such as the presence of unwanted hazardous metals, toxins and pathogens still require thorough studies and field scale evaluation before their commercialization. In-depth studies of the biostimulatory effects of microalgal and cyanobacterial extracts on plant growth-related parameters will also facilitate their production and processing for product innovation in agriculture. Technological developments in microalgal and cyanobacterial cultivation and harvesting, such as automation, phenotyping, and synthetic biology will provide tools for highly efficient algae-based solutions to meet a range of societal needs. Genetic manipulation, such as the overexpression or downregulation of several genes in a single organism, is also possible in the microalga Chlamydomonas reinhardtii, and is a practical method to improve product development of microalgae and cyanobacteria for large scale production.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Abd El-Baky HH, El-Baz FK, El Baroty GS (2010) Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J Sci Food Agric 90:299–303. https://doi.org/10.1002/jsfa.3815
Abinandan S, Subashchandrabose SR, Venkateswarlu K, Megharaj M (2019) Soil microalgae and cyanobacteria: the biotechnological potential in the maintenance of soil fertility and health. Crit Rev Biotechnol 39:981–998. https://doi.org/10.1080/07388551.2019.1654972
Ahmed F, Schenk PM (2017) UV–C radiation increases sterol production in the microalga Pavlova lutheri. Phytochemistry 139:25–32. https://doi.org/10.1016/J.PHYTOCHEM.2017.04.002
Ahmed AI, Mohamed HA-A, Takuji O (2014) Nitrogen fixing cyanobacteria: future prospects. In: Ohyama Takuji (ed) Advances in biology and ecology of nitrogen Fixation. IntechOpen, London, pp 23–46
Algae Products Market Information (2018) Algae products market: cagr of 5.3% from 2018 to 2023 | industry trend, business revenue, professional survey and in-depth analysis research report. https://www.abnewswire.com/pressreleases/algae-products-market-cagr-of-53-from-2018-to-2023-industry-trend-business-revenue-professional-survey-and-indepth-analysis-research-report_221796.html. Accessed 5 Aug 2021
Alvarez AL, Weyers SL, Goemann HM et al (2021) Microalgae, soil and plants: a critical review of microalgae as renewable resources for agriculture. Algal Res 54:102200. https://doi.org/10.1016/j.algal.2021.102200
Amorim ML, Soares J, dos Coimbra JS, R, et al (2020) Microalgae proteins: production, separation, isolation, quantification, and application in food and feed. Crit Rev Food Sci Technol 61:1976–2002. https://doi.org/10.1080/10408398.2020.1768046
Andrianantoandro E, Basu S, Karig DK, Weiss R (2006) Synthetic biology: new engineering rules for an emerging discipline. Mol Syst Biol. https://doi.org/10.1038/msb4100073
Ansari F, Ravindran B, Gupta S et al (2019) Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J Environ Manage 240:293
Armbrust EV, Berges JA, Bowler C et al (2004) The genome of the diatom Thalassiosira Pseudonana: ecology, evolution, and metabolism. Science 306:79–86. https://doi.org/10.1126/science.1101156
Arora K, Kaur P, Kumar P et al (2021) Valorization of wastewater resources into biofuel and value-added products using microalgal system. Front Energy Res. https://doi.org/10.3389/FENRG.2021.646571
Barone V, Baglieri A, Stevanato P et al (2018) Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J Appl Phycol 30:1061–1071. https://doi.org/10.1007/s10811-017-1283-3
Barone V, Puglisi I, Fragalà F et al (2019) Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. J Appl Phycol 31:465–470. https://doi.org/10.1007/s10811-018-1518-y
Barrios CAZ, Nandini S, Sarma SSS (2017) Effect of crude extracts from cyanobacterial blooms in Lake Texcoco (Mexico) on the population growth of Brachionus calyciflorus (Rotifera). Toxicon 139:45–53. https://doi.org/10.1016/J.TOXICON.2017.09.013
Bastida F, Jindo K, Moreno JL et al (2012) Effects of organic amendments on soil carbon fractions, enzyme activity and humus–enzyme complexes under semi-arid conditions. Eur J Soil Biol 53:94–102. https://doi.org/10.1016/J.EJSOBI.2012.09.003
Bawiec A, Garbowski T, Pawęska K, Pulikowski K (2019) Analysis of the algae growth dynamics in the hydroponic system with LEDs nighttime lighting using the laser granulometry method. Water Air Soil Pollut. https://doi.org/10.1007/s11270-018-4075-8
Beheshtipour H, Mortazavian AM, Mohammadi R et al (2013) Supplementation of spirulina platensis and chlorella vulgaris algae into probiotic fermented milks. Compr Rev Food Sci Food Saf 12:144–154. https://doi.org/10.1111/1541-4337.12004
Benedetti M, Vecchi V, Barera S, Dall’Osto L (2018) Biomass from microalgae: the potential of domestication towards sustainable biofactories. Microb Cell Fact. https://doi.org/10.1186/s12934-018-1019-3
Bhandari G (2014) An overview of agrochemicals and their effects on environment in Nepal. Appl Ecol Environ Sci
Bhola V, Swalaha F, Ranjith Kumar R et al (2014) Overview of the potential of microalgae for CO2 sequestration. Springer. https://doi.org/10.1007/s13762-013-0487-6
BjörnGovindjee, LO (2008) The evolution of photosynthesis and its environmental impact. Photobiol Sci Life Light Second Ed. https://doi.org/10.1007/978-0-387-72655-7_12
Borowitzka MA (2013) Energy from microalgae: a short history. Algae for biofuels and energy. Springer, Dordrecht, pp 1–15
Boru G, Van Ginkel M, Trethowan RM et al (2003a) Oxygen use from solution by wheat genotypes differing in tolerance to waterlogging. Euphytica 132:151–158. https://doi.org/10.1023/A:1024622405505
Boru G, Vantoai T, Alves J et al (2003b) Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Acad Ann Bot. https://doi.org/10.1093/aob/mcg040
Bowler C, Allen A, Badger J et al (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456:239–244
Cai T, Park S, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19:360–369
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41
Caterina B, Marzio M, Adamou HT (2004) The future is an ancient lake. In: Tradit. knowledge, Biodivers. Genet. Resour. food Agric. Lake Chad Basin Ecosyst. http://www.fao.org/3/y5118e/y5118e.pdf. Accessed 11 Mar 2021
Chanda M-J, Merghoub N, El Arroussi H (2019) Microalgae polysaccharides: the new sustainable bioactive products for the development of plant bio-stimulants? World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-019-2745-3
Chanda M, Redouane B, Najib, et al (2020) Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci Rep. https://doi.org/10.1038/s41598-020-59840-4
Chen CY, Zhao XQ, Yen HW et al (2013) Microalgae-based carbohydrates for biofuel production. Biochem Eng J 78:1–10. https://doi.org/10.1016/J.BEJ.2013.03.006
Cheng D, Li D, Yuan Y, et al (2017) Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol Biofuels 10:. doi: https://doi.org/10.1186/s13068-017-0753-9
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chiu S-Y, Kao C-Y, Chen C-H et al (2007) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol. https://doi.org/10.1016/j.biortech.2007.08.013
Chu W-L, Phang S-M (2019) Bioactive compounds from microalgae and their potential applications as pharmaceuticals and nutraceuticals. Gd Challenges Biol Biotechnol. https://doi.org/10.1007/978-3-030-25233-5_12
Colina F, Amaral J, Carbó M et al (2018) Genome-wide identification and characterization of CKIN/SnRK gene family in Chlamydomonas reinhardtii. Sci Rep. https://doi.org/10.1038/s41598-018-35625-8
Colla G, Rouphael Y (2020) Microalgae: new source of plant biostimulants. Agronomy 10:1240
Colla LM, Oliveira Reinehr C, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493. https://doi.org/10.1016/J.BIORTECH.2005.09.030
Corbel S, Mougin C, Bouaïcha N (2014) Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 96:1–15. https://doi.org/10.1016/J.CHEMOSPHERE.2013.07.056
Costantini D (2015) Land-use changes and agriculture in the tropics: pesticides as an overlooked threat to wildlife. Biodivers Conserv 24:1837–1839. https://doi.org/10.1007/s10531-015-0878-8
Danesi EDG, Rangel-Yagui CDO, De Carvalho JCM, Sato S (2002) An investigation of effect of replacing nitrate by urea in the growth and production of chlorophyll by Spirulina platensis. Biomass Bioenerg 23:261–269. https://doi.org/10.1016/S0961-9534(02)00054-5
de Jesus Raposo MF, de Morais AMMB, de Morais RMSC (2015) Bioactivity and applications of polysaccharides from marine microalgae. Polysaccharides. Springer, Cham, pp 1683–1727
Demoulin CF, Lara YJ, Cornet L et al (2019) Cyanobacteria evolution: Insight from the fossil record. Free Radic Biol Med 140:206–223
Dineshkumar R, Subramanian J, Gopalsamy J et al (2017) The impact of using microalgae as biofertilizer in Maize (Zea mays L.). Waste Biomass Valorization 10:1101–1110. https://doi.org/10.1007/S12649-017-0123-7
Dochi H, Ray A, Kothari IL (2010) Spirulina Biotechnology. In: Geomicrobiol.—Google Books. https://books.google.co.ma/books?hl=en&lr=&id=s2_RBQAAQBAJ&oi=fnd&pg=PA209&dq=(Doshi+et+al.,+2010)+vitamin+B12+in+Spirulina&ots=ocxxaWXyN0&sig=7mWoq11hmfXzDdBorE1tT6uoOl8&redir_esc=y#v=onepage&q&f=false. Accessed 6 Oct 2020
Doron L, Segal N, Shapira M (2016) Transgene expression in microalgae—from tools to applications. Front Plant Sci. https://doi.org/10.3389/FPLS.2016.00505
Doughman S, Krupanidhi S, Sanjeevi C (2007) Omega-3 fatty acids for nutrition and medicine: considering microalgae oil as a vegetarian source of EPA and DHA. Curr Diabetes Rev 3:198–203. https://doi.org/10.2174/157339907781368968
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic (amsterdam) 196:3–14. https://doi.org/10.1016/J.SCIENTA.2015.09.021
Duan L, Chen Q, Duan S (2019) Transcriptional analysis of chlorella pyrenoidosa exposed to bisphenol A. Int J Environ Res Public Heal 16:1374. https://doi.org/10.3390/IJERPH16081374
EFSA (2012) Scientific opinion on the re-evaluation of mixed carotenes (E 160a (i)) and beta-carotene (E 160a (ii)) as a food additive. EFSA J 10:1–67. https://doi.org/10.2903/j.efsa.2012.2593
Eggersdorfer M, Wyss A (2018) Carotenoids in human nutrition and health. Arch Biochem Biophys 652:18–26. https://doi.org/10.1016/J.ABB.2018.06.001
El Arroussi H, Elbaouchi A, Benhima R, et al (2016) Halophilic microalgae Dunaliella salina extracts improve seed germination and seedling growth of Triticum aestivum L. under salt stress. In: Acta Horticulturae. International Society for Horticultural Science, pp 13–26
El Arroussi H, Benhima R, Elbaouchi A et al (2018) Dunaliella salina exopolysaccharides: a promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). J Appl Phycol 30:2929–2941. https://doi.org/10.1007/s10811-017-1382-1
Elvira-Torales LI, García-Alonso J, Periago-Castón MJ (2019) Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants. https://doi.org/10.3390/antiox8070229
Enzing C, Ploeg M, Barbosa M et al (2014) Microalgae-based products for the food and feed sector: an outlook for Europe. https://doi.org/10.2791/3339
Ergun O, Dasgan HY, Isik O (2020) Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic 1273:169–175. https://doi.org/10.17660/ACTAHORTIC.2020.1273.23
Fabris M, Abbriano R, Pernice M (2020) Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front Plant. https://doi.org/10.3389/fpls.2020.00279
Faheed F, Fattah Z (2008) Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J Agric Soc Sci 4:165–169
Fajardo C, De Donato M, Carrasco R et al (2020) Advances and challenges in genetic engineering of microalgae. Rev Aquac 12:365–381
Farid R, Mutale-joan C, Redouane B et al (2019) Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Appl Biochem Biotechnol 188:225–240. https://doi.org/10.1007/s12010-018-2916-y
Fernández-Sevilla JM, Acién Fernández FG, Molina Grima E (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 861(86):27–40. https://doi.org/10.1007/S00253-009-2420-Y
Figueroa-Torres GM, Pittman JK, Theodoropoulos C (2021) Optimisation of microalgal cultivation via nutrient-enhanced strategies: the biorefinery paradigm. Biotechnol Biofuels 141(14):1–16. https://doi.org/10.1186/S13068-021-01912-2
Flaibani A, Olsen Y, Painter T (1989) Polysaccharides in desert reclamation: compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr Res. https://doi.org/10.1016/0008-6215(89)84128-X
Foley JA, DeFries R, Asner GP et al (2005) Global consequences of land use. Science 309:570–574
Fu W, Nelson DR, Mystikou A et al (2019) Advances in microalgal research and engineering development. Curr Opin Biotechnol 59:157–164
García JL, de Vicente M, Galán B (2017) Microalgae, old sustainable food and fashion nutraceuticals. Microb Biotechnol 10:1017–1024. https://doi.org/10.1111/1751-7915.12800
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061. https://doi.org/10.1007/s10811-015-0625-2
Garcia-Pichel F, Zehr JP, Bhattacharya D, Pakrasi HB (2020) What’s in a name? The case of cyanobacteria. J Phycol 56:1–5. https://doi.org/10.1111/JPY.12934
Garcia-Pichel F (2009) Cyanobacteria . In: Encycl. Microbiol. - Google Books. https://books.google.co.ma/books?hl=en&lr=&id=rLhdW5YzuO4C&oi=fnd&pg=PP2&dq=Cyanobacteria+F.+Garcia-Pichel,+in+Encyclopedia+of+Microbiology+(Third+Edition),+2009&ots=p0c8oo2eQM&sig=er4XkvAWloiKuxh6P2GhFbtfPqM&redir_esc=y#v=onepage&q&f=false. Accessed 6 Oct 2020
Garrido-Cardenas JA, Manzano-Agugliaro F, Acien-Fernandez FG, Molina-Grima E (2018) Microalgae Research Worldwide. Algal Res 35:50–60. https://doi.org/10.1016/J.ALGAL.2018.08.005
Gayathri M, Kumar PS, Prabha AML, Muralitharan G (2015) In vitro regeneration of Arachis hypogaea L. and Moringa oleifera Lam. using extracellular phytohormones from Aphanothece sp. MBDU 515. Algal Res 7:100–105. https://doi.org/10.1016/J.ALGAL.2014.12.009
Gibbs HK, Ruesch AS, Achard F et al (2010) Tropical forests were the primary sources of new agricultural land in the 1980s and 1990s. Natl Acad Sci. https://doi.org/10.1073/pnas.0910275107
Gonçalves AL (2021) The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating and biopesticide roles. Appl Sci 11:871. https://doi.org/10.3390/app11020871
Gong Y, Jiang M (2011) Biodiesel production with microalgae as feedstock: from strains to biodiesel. Biotechnol Lett 337(33):1269–1284. https://doi.org/10.1007/S10529-011-0574-Z
Goykovic Cortés V, Saavedra del Real G (2007) Algunos efectos de la salinidad en el cultivo del tomate y prácticas agronómicas de su manejo. Idesia (arica) 25:47–58. https://doi.org/10.4067/s0718-34292007000300006
Gröniger A, Sinha R, Klisch M, Häder D (2000) Photoprotective compounds in cyanobacteria, phytoplankton and macroalgae—a database. J Photochem. https://doi.org/10.1016/S1011-1344(00)00112-3
Gross OPW (2004) Mini-review: valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648. https://doi.org/10.1007/s00253-004-1647-x
Grzesik M, Romanowska-Duda Z, Kalaji HM (2017) Effectiveness of cyanobacteria and green algae in enhancing the photosynthetic performance and growth of willow (Salix viminalis L) plants under limited synthetic fertilizers application. Photosynthetica. https://doi.org/10.1007/s11099-017-0716-1
Guzmán-Murillo MA, Ascencio F, Larrinaga-Mayoral JA (2013) Germination and ROS detoxification in bell pepper (Capsicum annuum L) under NaCl stress and treatment with microalgae extracts. Protoplasma. https://doi.org/10.1007/s00709-011-0369-z
Hamed I (2016) The evolution and versatility of microalgal biotechnology: a review. Compr Rev Food Sci Food Saf 15:1104–1123. https://doi.org/10.1111/1541-4337.12227
Hamouda R, Sorour N, El RA et al (2016) Biodegradation of crude oil by Anabaena oryzae, Chlorella kessleri and its consortium under mixotrophic conditions. Int Biodeterior Biodegrad. 4:6. https://doi.org/10.1016/j.ibiod.2016.05.001
He J, Austin PT, Lee SK (2010) Effects of elevated root zone CO2 and air temperature on photosynthetic gas exchange, nitrate uptake, and total reduced nitrogen content in aeroponically grown lettuce plants. J Exp Bot 61:3959–3969. https://doi.org/10.1093/JXB/ERQ207
Hopes A, Mock T (2015) Evolution of microalgae and their adaptations in different marine ecosystems. Wiley, New York. https://doi.org/10.1002/9780470015902.a0023744
Hosseini TA, Shariati M (2009) Dunaliella biotechnology: methods and applications. J Appl Microbiol 107:14–35
Hsieh-Lo M, Castillo G, Ochoa-Becerra MA, Mojica L (2019) Phycocyanin and phycoerythrin: strategies to improve production yield and chemical stability. Algal Res. https://doi.org/10.1016/J.ALGAL.2019.101600
Hüfner K (2010) UNESCO–United Nations Educational, Scientific and Cultural Organization. brill.com
Hultberg M, Carlsson AS, Gustafsson S (2013) Treatment of drainage solution from hydroponic greenhouse production with microalgae. Bioresour Technol 136:401–406. https://doi.org/10.1016/J.BIORTECH.2013.03.019
Huo S, Liu J, Addy M et al (2020) The influence of microalgae on vegetable production and nutrient removal in greenhouse hydroponics. J Clean Prod. https://doi.org/10.1016/J.JCLEPRO.2019.118563
Ibañez E, Herrero M, Mendiola JA, Castro-Puyana M (2012) Extraction and characterization of bioactive compounds with health benefits from marine resources: macro and micro algae, cyanobacteria, and invertebrates. In: Hayes M (ed) Marine bioactive compounds: sources, characterization and applications. Springer, New York, pp 55–98
Jacob-Lopes E, Maroneze MM, Deprá MC et al (2019) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci 25:1–7. https://doi.org/10.1016/J.COFS.2018.12.003
Jerney J, Spilling K (2018) Large scale cultivation of microalgae: open and closed systems. Methods Mol Biol 1980:1–8. https://doi.org/10.1007/7651_2018_130
Jhala YK, Panpatte DG, Vyas RV (2017) Cyanobacteria: source of organic fertilizers for plant growth. In: Panpatte DG, Jhala YK, Vyas RV, Shelat HN (eds) Microorganisms for green revolution. Springer, Singapore, pp 253–264. https://doi.org/10.1007/978-981-10-6241-4_13
Jochum M, Moncayo LP, Jo YK (2018) Microalgal cultivation for biofertilization in rice plants using a vertical semi-closed airlift photobioreactor. PLoS ONE. https://doi.org/10.1371/journal.pone.0203456
Johnson EJ (2002) The role of carotenoids in human health. Nutr Clin Care 5:56–65
Karthikeyan N, Prasanna R, Nain L, Kaushik BD (2007) Evaluating the potential of plant growth promoting cyanobacteria as inoculants for wheat. Eur J Soil Biol 43:23–30. https://doi.org/10.1016/J.EJSOBI.2006.11.001
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Fact 17:36. https://doi.org/10.1186/s12934-018-0879-x
Kini S, Divyashree M, Mani MK, Mamatha BS (2020) Algae and cyanobacteria as a source of novel bioactive compounds for biomedical applications. Adv Cyanobacterial Biol. https://doi.org/10.1016/B978-0-12-819311-2.00012-7
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63. https://doi.org/10.1016/J.ALGAL.2014.09.002
Kothari R, Pandey A, Ahmad S et al (2017) Microalgal cultivation for value-added products: a critical enviro-economical assessment. 3 Biotech. https://doi.org/10.1007/S13205-017-0812-8
Kumar K, Mishra SK, Shrivastav A et al (2015) Recent trends in the mass cultivation of algae in raceway ponds. Renew Sustain Energy Rev 51:875–885. https://doi.org/10.1016/J.RSER.2015.06.033
Kumar G, Shekh A, Jakhu S et al (2020) Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol. https://doi.org/10.3389/FBIOE.2020.00914
Lafarga T, Sánchez-Zurano A, Villaró S et al (2021) Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci Technol 116:176–185. https://doi.org/10.1016/J.TIFS.2021.07.018
Lal R (2009) Soils and food sufficiency: a review. Sustainable agriculture. Springer, Dordrecht, pp 25–49
Langley N, Harrison S, Van Hille R (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng J. 68:70–75
Langyintuo A (2020) smallholder farmers’ access to inputs and finance in Africa. Role Smallhold Farms Food Nutr Secur. https://doi.org/10.1007/978-3-030-42148-9_7
Lau NS, Matsui M, Abdullah AAA (2015) Cyanobacteria: photoautotrophic microbial factories for the sustainable synthesis of industrial products. Biomed Res Int. https://doi.org/10.1155/2015/754934
Laurens LML, Dempster TA, Jones HDT et al (2012) Algal biomass constituent analysis: method uncertainties and investigation of the underlying measuring chemistries. Anal Chem 84:12. https://doi.org/10.1021/ac202668c
Lee S, Lee J (2015) Beneficial bacteria and fungi in hydroponic systems: types and characteristics of hydroponic food production methods. Sci Hortic (Amsterdam) 195:206–215. https://doi.org/10.1016/J.SCIENTA.2015.09.011
Lemus R, Lal R, Charles Brummer E (2005) Bioenergy crops and carbon sequestration. CRC Crit Rev Plant Sci 24:1–21. https://doi.org/10.1080/07352680590910393
Li X, Wang X, Duan C et al (2020) Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol Adv 43:107602. https://doi.org/10.1016/J.BIOTECHADV.2020.107602
Lin JH, Lee DJ, Chang JS (2015) Lutein production from biomass: marigold flowers versus microalgae. Bioresour Technol 184:421–428. https://doi.org/10.1016/J.BIORTECH.2014.09.099
Longworth J, Wu D, Huete-Ortega M, Wright P (2016) Proteome response of Phaeodactylum tricornutum, during lipid accumulation induced by nitrogen depletion. Algal Res 18:213–224
Malam Issa O, Le Bissonais Y, Défarge C et al (2001) Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma. https://doi.org/10.1016/S0016-7061(00)00093-8ï
Maqubela MP, Mnkeni PNS, Issa OM et al (2008) (2008) Nostoc cyanobacterial inoculation in South African agricultural soils enhances soil structure, fertility, and maize growth. Plant Soil 3151(315):79–92. https://doi.org/10.1007/S11104-008-9734-X
Masojídek J, Torzillo G (2014) Mass cultivation of freshwater microalgae
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280
Merchant SS, Prochnik SE, Vallon O et al (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–251. https://doi.org/10.1126/science.1143609
Mobin SMA, Chowdhury H, Alam F (2019) Commercially important bioproducts from microalgae and their current applications—a review. Energy Procedia 160:752–760. https://doi.org/10.1016/J.EGYPRO.2019.02.183
Mohamed AG, Abo-El-Khair BE, Shalaby SM, Sciences WA (2013) Quality of novel healthy processed cheese analogue enhanced with marine microalgae chlorella vulgaris biomass. World Appl Sci J. https://doi.org/10.5829/idosi.wasj.2013.23.07.13122
Muhammad G, Alam MA, Xiong W et al (2020) Microalgae biomass production: an overview of dynamic operational methods. Microalgae Biotechnol Food, Heal High Value Prod. https://doi.org/10.1007/978-981-15-0169-2_13
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Myers SS, Smith MR, Guth S et al (2017) Climate change and global food systems: potential impacts on food security and undernutrition. Annu Rev Public Health 38:259–277. https://doi.org/10.1146/annurev-publhealth-031816-044356
Narala RR, Garg S, Sharma KK et al (2016) Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front Energy Res 4:1. https://doi.org/10.3389/fenrg.2016.00029
Nethravathy UM, Mehar JG, Mudliar SN, Shekh AY (2019) Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf 18:1882–1897. https://doi.org/10.1111/1541-4337.12500
Nguyen HM, Baudet M, Cuine S et al (2011) Proteomic profiling of oil bodies isolated from the unicellular green microalga Chlamydomonas reinhardtii: with focus on proteins involved in lipid metabolism. Wiley Online Libr 11:4266–4273. https://doi.org/10.1002/pmic.201100114
Nisha R, Kaushik A, Kaushik C (2007) Effect of indigenous cyanobacterial application on structural stability and productivity of an organically poor semi-arid soil. Geroderma 138:49–56
Oancea F, Velea S, Fatu V (2013) Micro-algae based plant biostimulant and its effect on water stressed tomato plants
Ogbonda KH, Aminigo RE, Abu GO (2007) Influence of temperature and pH on biomass production and protein biosynthesis in a putative Spirulina sp. Bioresour Technol 98:2207–2211. https://doi.org/10.1016/J.BIORTECH.2006.08.028
Ontl TA, Schulte LA (2012) Soil Carbon Storage. In: Nat. Educ. Knowl. . https://www.nature.com/scitable/knowledge/library/soil-carbon-storage-84223790/. Accessed 6 Oct 2020
Oren A (2014a) Cyanobacteria: biology, ecology and evolution. Wiley, Chichester
Oren A (2014b) The ecology of Dunaliella in high-salt environments. J Biol Res 21:23
Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF (2010) Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol Fertil Soils 468(46):861–875. https://doi.org/10.1007/S00374-010-0491-7
Ovsepyan L, Kurganova I, de Gerenyu VL, Kuzyakov Y (2019) Recovery of organic matter and microbial biomass after abandonment of degraded agricultural soils: the influence of climate. L Degrad Dev 30:1861–1874. https://doi.org/10.1002/LDR.3387
Parwani L, Bhatt M, Singh J (2021) Potential biotechnological applications of cyanobacterial exopolysaccharides. Brazilian Arch Biol Technol 64:1–13. https://doi.org/10.1590/1678-4324-2021200401
Patil V, Reitan KI, Knutsen G et al (2005) Microalgae as source of polyunsaturated fatty acids for aquaculture. In: Review. http://www.buggypower.eu/wp-content/uploads/2017/04/Microalgae-as-a-source-of-polyunsaturated-fatty-acids-for-aquaculture.pdf. Accessed 5 Oct 2020
Paul D, Nair S (2008) Stress adaptations in a Plant Growth Promoting Rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol 48:378–384. https://doi.org/10.1002/jobm.200700365
Pignolet O, Jubeau S, Vaca-Garcia C, Michaud P (2013) Highly valuable microalgae: biochemical and topological aspects Highly valuable microal-gae: biochemical and topological aspects. J Ind Microbiol Biotechnol. https://doi.org/10.1007/s10295-013-1281-7ï
Pimentel D, Berger B, Filiberto D, et al (2004) Water resources: agricultural and environmental issues
Prashar P, Shah S (2016) Impact of fertilizers and pesticides on soil microflora in agriculture. Sustain Agric Rev. https://doi.org/10.1007/978-3-319-26777-7_8
Puglisi I, Barone V, Sidella S et al (2018) Biostimulant activity of humic-like substances from agro-industrial waste on Chlorella vulgaris and Scenedesmus quadricauda. Eur J Phycol 53:433–442. https://doi.org/10.1080/09670262.2018.1458997
Rachidi F, Benhima R, Kasmi Y et al (2021) Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci Rep 11:1–16. https://doi.org/10.1038/s41598-020-78820-2
Rahman KM (2020) Food and high value products from microalgae: market opportunities and challenges. Microalgae Biotechnol Food Heal High Value Prod. https://doi.org/10.1007/978-981-15-0169-2_1
Rajvanshi S, Sharma MP (2012) Micro algae: a potential source of biodiesel. J Sustain Bioenergy Syst 02:49–59. https://doi.org/10.4236/jsbs.2012.23008
Ramanan R, Kannan K, Deshkar A et al (2010) Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour Technol 101:2616–2622. https://doi.org/10.1016/j.biortech.2009.10.061
Rana RS, Singh P, Kandari V et al (2014) (2014) A review on characterization and bioremediation of pharmaceutical industries’ wastewater: an Indian perspective. Appl Water Sci 71(7):1–12. https://doi.org/10.1007/S13201-014-0225-3
Randhir A, Laird DW, Maker G et al (2020) Microalgae: a potential sustainable commercial source of sterols. Algal Res 46:101772. https://doi.org/10.1016/J.ALGAL.2019.101772
Renhe Q, Song G, Paola A. L, Kimberly LO (2021) Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. https://www.osti.gov/servlets/purl/1580748. Accessed 9 Aug 2021
Renuka N, Guldhe A, Prasanna R et al (2018) Microalgae as multi-functional options in modern agriculture: current trends, prospects and challenges. Biotechnol Adv 36:1255–1273. https://doi.org/10.1016/J.BIOTECHADV.2018.04.004
Rodolfi L, Zittelli GC, Bassi N et al (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112. https://doi.org/10.1002/bit.22033
Rodríguez A, Stella A, Storni M et al (2006) Effects of cyanobacterial extracellular products and gibberellic acid on salinity tolerance in Oryza sativa L. Saline Syst 2:7. https://doi.org/10.1186/1746-1448-2-7
Ronga D, Biazzi E, Parati K et al (2019) Microalgal biostimulants and biofertilisers in crop productions. Agronomy. https://doi.org/10.3390/agronomy9040192
Rosegrant MW, Cline SA (2003) Global food security: challenges and policies. Science. 302:1917–1919
Rosenzweig C, Elliott J, Deryng D et al (2014) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci 111:3268–3273. https://doi.org/10.1073/PNAS.1222463110
Saad A, Atia A (2014) Review on freshwater blue-green algae (Cyanobacteria): occurrence, classification and toxicology. Biosci Biotechnol Res ASIA 11:1319–1325. https://doi.org/10.13005/bbra/1522
Saifullah AZ, Abdul Karim M, Ahmad-Yazid A (2014) Microalgae: an alternative source of renewable energy. Am J Eng Res 03:330–338
Samiee-Zafarghandi R, Karimi-Sabet J, Abdoli MA, Karbassi A (2018) Increasing microalgal carbohydrate content for hydrothermal gasification purposes. Renew Energy 116:710–719. https://doi.org/10.1016/J.RENENE.2017.10.020
Sanchez-Tarre V, Kiparissides A (2021) The effects of illumination and trophic strategy on gene expression in Chlamydomonas reinhardtii. Algal Res 54:102186. https://doi.org/10.1016/J.ALGAL.2021.102186
Sand-Jensen K, Jespersen TS (2012) Tolerance of the widespread cyanobacterium Nostoc commune to extreme temperature variations (-269 to 105°C), pH and salt stress. Oecologia 169:331–339. https://doi.org/10.1007/s00442-011-2200-0
Sathasivam R, Radhakrishnan R, Hashem A, Abd Allah EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci 26:709–722
Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security. Food Secur 4:519–537
Schmidhuber J, Tubiello FN (2007) Global food security under climate change. Proc Natl Acad Sci USA 104:19703–19709
Schmollinger S, Mühlhaus T, Boyle NR et al (2014) Nitrogen-sparing mechanisms in Chlamydomonas affect the transcriptome, the proteome, and photosynthetic metabolism. Plant Cell 26:1410–1435. https://doi.org/10.1105/tpc.113.122523
Schwarz D, Gross W (2015) Algae affecting lettuce growth in hydroponic systems. J Horticult Sci Biotechnol 79:554–559. https://doi.org/10.1080/14620316.2004.11511804
Senthil-Kumar M, Wang K, Mysore KS (2013) AtCYP710A1 gene-mediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal Behav. https://doi.org/10.4161/psb.23142
Shah MMR, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci. https://doi.org/10.3389/FPLS.2016.00531
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529
Shankar SS (2014) Cyanobacteria: a vital bio-agent in eco-restoration of degraded lands and sustainable agriculture-Indian journals. http://www.indianjournals.com/ijor.aspx?target=ijor:cces&volume=2&issue=2&article=006. Accessed 5 Oct 2020
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96. https://doi.org/10.1263/jbb.101.87
Stanier RY, Sistrom WR, Hansen TA (1978) Proposal to place the nomenclature of the cyanbacteria (blue-green algae) under the rules of the international code of nomenclature of bacteria. Int J Syst Bacteriol 28:335–336. https://doi.org/10.1099/00207713-28-2-335
Stephens E, Ross IL, Hankamer B (2013) Expanding the microalgal industry—continuing controversy or compelling case? Curr Opin Chem Biol 17:444–452
Subashchandrabose SR, Ramakrishnan B, Megharaj M et al (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29:896–907
Supraja KV, Behera B, Balasubramanian P (2020) Performance evaluation of hydroponic system for co-cultivation of microalgae and tomato plant. J Clean Prod 272:122823. https://doi.org/10.1016/J.JCLEPRO.2020.122823
Surjana D, Halliday GM, Damian DL (2010) Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J Nucleic Acids. https://doi.org/10.4061/2010/157591
Swarnalakshmi K, Prasanna R, Kumar A et al (2013) Evaluating the influence of novel cyanobacterial biofilmed biofertilizers on soil fertility and plant nutrition in wheat. Eur J Soil Biol 55:107–116. https://doi.org/10.1016/J.EJSOBI.2012.12.008
Tamagnini P, Axelsson R, Lindberg P et al (2002) Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev 66:1–20. https://doi.org/10.1128/mmbr.66.1.1-20.2002
Tan JS, Lee SY, Chew KW et al (2020) A review on microalgae cultivation and harvesting, and their biomass extraction processing using ionic liquids. Bioengineered 11:116–129. https://doi.org/10.1080/21655979.2020.1711626
Tarento TDC, McClure DD, Vasiljevski E et al (2018) Microalgae as a source of vitamin K1. Algal Res 36:77–87. https://doi.org/10.1016/J.ALGAL.2018.10.008
Tejano LA, Peralta JP, Yap EES et al (2019) Prediction of bioactive peptides from Chlorella sorokiniana proteins using proteomic techniques in combination with bioinformatics analyses. Int J Mol Sci 20:1786. https://doi.org/10.3390/IJMS20071786
Teng PS, Johnson KB (1988) Analysis of epidemiological components in yield loss assessment. Experimental techniques in plant disease epidemiology. Springer, Berlin, Heidelberg, pp 179–189
Thaler EA, Larsen IJ, Yu Q (2021) The extent of soil loss across the US Corn Belt. Proc Natl Acad Sci. https://doi.org/10.1073/PNAS.1922375118
Tiefenbacher A, Sandén T, Haslmayr HP et al (2021) Optimizing carbon sequestration in croplands: a synthesis. Agronomy 11:1–28. https://doi.org/10.3390/agronomy11050882
Tocquin P, Corbesier L, Havelange A et al (2003) (2003) A novel high efficiency, low maintenance, hydroponic system for synchronous growth and flowering of Arabidopsis thaliana. BMC Plant Biol 31(3):1–10. https://doi.org/10.1186/1471-2229-3-2
Tokuşoglu Ö, Ünal MK (2003) Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J Food Sci 68:1144–1148. https://doi.org/10.1111/j.1365-2621.2003.tb09615.x
Tredici MR (2004) Handbook of Microalgal Culture—Handbook of Microalgal Culture—Wiley Online Library. https://onlinelibrary.wiley.com/doi/pdf/10.1002/9780470995280#page=192. Accessed 5 Oct 2020
Vaishampayan A, Sinha RP, Häder DP et al (2001) Cyanobacterial biofertilizers in rice agriculture. Bot Rev 67:453–516
Van Krimpen MM, Bikker P, Van der Meer IM, et al (2013) Cultivation, processing and nutritional aspects for pigs and poultry of European protein sources as alternatives for imported soybean products. https://library.wur.nl/WebQuery/wurpubs/437524. Accessed 5 Oct 2020
Wang Y, Cong Y, Wang Y et al (2019) Identification of early salinity stress-responsive proteins in dunaliella salina by isobaric tags for relative and absolute quantitation (iTRAQ)-based quantitative proteomic analysis. Int J Mol Sci 20:599. https://doi.org/10.3390/IJMS20030599
Wijffels R, Barbosa M (2010) An outlook on microalgal biofuels. Science 10:67
Wu M, Zhu R, Lu J et al (2020) Effects of different abiotic stresses on carotenoid and fatty acid metabolism in the green microalga Dunaliella salina Y6. Ann Microbiol 70:48. https://doi.org/10.1186/s13213-020-01588-3
Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 34:1225–1244
Xie Y, Xiong X, Chen S (2021) Challenges and potential in increasing lutein content in microalgae. Microorganisms. https://doi.org/10.3390/MICROORGANISMS9051068
Yakhin OI, Lubyanov AA, Yakhin IA, Brown PH (2017) Biostimulants in plant science: a global perspective. Front Plant Sci 7:2049
Yang ZK, Zheng JW, Niu YF et al (2014) Systems-level analysis of the metabolic responses of the diatom Phaeodactylum tricornutum to phosphorus stress. Environ Microbiol 16:1793–1807. https://doi.org/10.1111/1462-2920.12411
Yuan JP, Peng J, Yin K, Wang JH (2011) Potential health-promoting effects of astaxanthin: a high-value carotenoid mostly from microalgae. Mol Nutr Food Res 55:150–165
Yue Z, Yuyong H, Zhiyong L, et al (2016) Identification of NaHCO3 Stress Responsive Proteins in Dunaliella salina HTBS using iTRAQ-based Analysis. In: Article. https://www.researchgate.net/profile/Yuyong-Hou/publication/303770280_Identification_of_NaHCO3_Stress_Responsive_Proteins_in_Dunaliella_salina_HTBS_using_iTRAQ-based_Analysis/links/575a29c608ae9a9c954f2c62/Identification-of-NaHCO3-Stress-Responsive-Proteins-in-Dunaliella-salina-HTBS-using-iTRAQ-based-Analysis.pdf. Accessed 13 Oct 2021
Zhang J, Wang X, Zhou Q (2017) Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenerg 97:132–138. https://doi.org/10.1016/j.biombioe.2016.12.024
Zhang L, Yan C, Guo Q et al (2018) The impact of agricultural chemical inputs on environment: global evidence from informetrics analysis and visualization. Int J Low-Carbon Technol 13:338–352. https://doi.org/10.1093/ijlct/cty039
Zhu X-G, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261. https://doi.org/10.1146/annurev-arplant-042809-112206
Zulpa G, Zaccaro MC, Boccazzi F et al (2003) Bioactivity of intra and extracellular substances from cyanobacteria and lactic acid bacteria on “wood blue stain” fungi. Biol Control 27:345–348. https://doi.org/10.1016/S1049-9644(03)00015-X
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
CMJ conceived the idea and wrote the first draft of the manuscript. CMJ designed the figures and drafted the table. EH contributed to manuscript revision, content modifications, scientific review and approval of the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Wendy Stirk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mutale-Joan, C., Sbabou, L. & Hicham, E.A. Microalgae and Cyanobacteria: How Exploiting These Microbial Resources Can Address the Underlying Challenges Related to Food Sources and Sustainable Agriculture: A Review. J Plant Growth Regul 42, 1–20 (2023). https://doi.org/10.1007/s00344-021-10534-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10534-9