Abstract
An economic and environmentally friendly approach of overcoming the problem of fossil CO2 emissions would be to reuse it through fixation into biomass. Carbon dioxide (CO2), which is the basis for the formation of complex sugars by green plants and microalgae through photosynthesis, has been shown to significantly increase the growth rates of certain microalgal species. Microalgae possess a greater capacity to fix CO2 compared to C4 plants. Selection of appropriate microalgal strains is based on the CO2 fixation and tolerance capability together with lipid potential, both of which are a function of biomass productivity. Microalgae can be propagated in open raceway ponds or closed photobioreactors. Biological CO2 fixation also depends on the tolerance of selected strains to high temperatures and the amount of CO2 present in flue gas, together with SOx and NOx. Potential uses of microalgal biomass after sequestration could include biodiesel production, fodder for livestock, production of colorants and vitamins. This review summarizes commonly employed microalgal species as well as the physiological pathway involved in the biochemistry of CO2 fixation. It also presents an outlook on microalgal propagation systems for CO2 sequestration as well as a summary on the life cycle analysis of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The impending threat of global climate change has amplified. This has been mainly attributed to greenhouse gas (GHG) emissions of which carbon dioxide (CO2) contributes up to 68 % of total emissions (Brennan and Owende 2010; Ho et al. 2011; Kumar et al. 2011). This is partly due to the high-energy usage and the dependence on coal for electricity generation (Kumar et al. 2011). According to a report by the Carbon Dioxide Information Analysis Center (CDIAC), CO2 emissions worldwide have increased from 3 metric tons in 1,751 to 8,230 metric tons in 2006. Further increases in emissions are projected to permanently alter future climates as well as cause significant dents to the economy. Therefore, it is imperative to develop an appropriate technology to reduce the emissions and accumulation of CO2. CO2 sequestration strategies implemented globally can be divided into physical and biological techniques (Khoo et al. 2011). However, due to numerous challenges associated with physical methods, there is a need to develop other suitable technologies. Biological CO2 fixation appears to be the only economical and environmentally viable technology of the future (Ho et al. 2011; Kumar et al. 2011). This presents an attractive development option as plants and other photosynthetic organisms naturally capture and use CO2 as part of their photosynthetic process. Terrestrial plants are able to sequester vast amounts of CO2 from the atmosphere. However, when compared to terrestrial plants, microalgae and cyanobacteria have faster growth rates, and their CO2-fixation efficiency is also between 10 and 50 times higher (Costa et al. 2000; Langley et al. 2012). The biological mitigation of CO2 using microalgae could therefore offer several advantages. No additional CO2 is created, while nutrient utilization is achieved in a continuous fashion leading to the production of biofuels and other secondary metabolites. Therefore, microalgal-mediated CO2 fixation coupled with biofuel production, and wastewater treatment could present a promising alternative to existing CO2 mitigation strategies (Wang et al. 2008; Lam et al. 2012). This paper focuses on commonly used microalgal species as well as the physiological pathway involved in the biochemistry of CO2 fixation. It also presents an outlook on microalgal propagation systems for CO2 sequestration as well as a summary on the life cycle analysis (LCA) of the process.
Carbon dioxide sequestration techniques
A number of efforts are currently under way to limit the amount of CO2 entering the atmosphere (Morais and Costa 2007). Selecting the most appropriate technology has sparked much debate globally. CO2 mitigation strategies applied worldwide can be divided into physical and biological techniques. Physical-based methodologies often entail three steps: capture, transportation and storage. CO2 is collected from a fixed source, for instance, from power plants or cement manufacturing facilities. Following capture, the gas mixture is converted to a supercritical fluid. This aids in transportation by pipeline or ship to a place of storage. Storage options often involve injection into deep oceanic or geological trenches and mineralization (Khoo et al. 2011; Pires et al. 2012). These currently employed disposal methods are considered unsustainable as they are expensive, require large amounts of space, are energy intensive and eventually lead to CO2 leakage over time (Stewart and Hessami 2005). However, even with the aforementioned drawbacks, this still remains a popular technology. It enables societies to maintain their existing carbon-based infrastructure while aiming to reduce the effects of CO2 on global warming (Pires et al. 2012).
Another option would be to utilize the collected CO2. For example, precipitated calcium carbonate (PCC), which is manufactured by controlling the reaction of CO2 with lime, can be used as a substitute for titanium dioxide or kaolin in the manufacture of paper products. CO2 could also be employed in the manufacture of paint, plastic, solvent and packaging. However, these industries would consume relatively minute quantities of CO2, as compared to the large amounts released annually into the atmosphere.
Biofixation of CO2 using microalgae could offer a sustainable alleviation technology without the aforementioned shortcomings (Stewart and Hessami 2005). Although terrestrial plants are responsible for fixing around 500 billion tones of CO2 per annum, they are expected to play a minor role (3–6 %) in the overall reduction in atmospheric CO2 (Skjanes et al. 2007). Microalgae and cyanobacteria have come to the forefront of research as they offer greater potential owing to their rapid growth rates, higher CO2-fixation ability and tolerance to extreme environments (Ho et al. 2011). Sydney (2010) stated that carbon uptake is often dependent on the metabolic activity of microalgae. Studies have suggested that microalgae exposed to increased levels of CO2 respond better (on a biomass basis), when compared to microalgae exposed to ambient air only. Microalgae are capable of generating roughly 280 tons of dry biomass per ha per year by utilizing 9 % of the freely available solar energy. During this process, approximately 513 tons of CO2 can be sequestered (Sydney 2010).
Research and development
Microalgae have been studied for several decades as a feedstock for renewable energy in an attempt to reduce global warming. These organisms are able to use concentrated amounts of CO2, present in power plant flue gases as well as from other sources. Therefore, they represent a powerful GHG mitigation strategy. During the mid-1970s, the US Department of Energy (DOE) began encouraging research pertaining to microalgal wastewater treatment (Benemann et al. 1977). The recovered microalgal biomass was subjected to anaerobic digestion which yielded methane gas. The “Aquatic Species Program” (US) funded by the office of fuels development started out as a project investigating the possibilities of using microalgae to sequester CO2 emissions from coal power plants (Sheehan et al. 1998). The project screened microalgae that could produce high amounts of oils as well as grow under adverse environmental conditions (extreme temperature, pH and salinity). This program was able to develop a culture collection system as well as support a pilot-scale project consisting of two raceway ponds in New Mexico. It was proposed that sufficient resources would be available in the southwest region of the USA for large-scale microalgae processes capable of capturing several hundred million tons of CO2 annually (Benemann and Oswald 1996). However, by the end of this program, it was concluded that in order for such a process to be economically feasible, favorable sites that yielded productivities near the theoretical maximum would be required.
During the 1990s in Japan, a major R&D program totaling over $250 million was carried out. The assignment focused on microalgae biofixation of CO2 as well as GHG abatement using closed photobioreactors (PBRs). However, owing to the high costs associated with PBRs (which still pose a problem today), the Japanese R&D initiative did not continue. The use of PBRs was then confined to inoculum production only (Lipinsky 1992; Nakajima and Ueda 2000).
The US DOE-NETL promoted microalgae R&D using closed PBRs. Other international participants of R&D pertaining to microalgae CO2 abatement included the following: Arizona Public Services, ENEL Produzione Ricerca, EniTecnologie, ExxonMobil and Rio Tinto. Due to the initiative of these governmental and private industries, the International Network on Microalgae Biofixation of CO2 and GHG Abatement was formed in 2000 in an effort to bring together the limited technical expertise in this field (Pedroni et al. 2001).
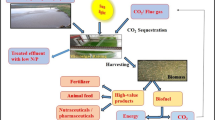
Past research initiatives suggest that practical CO2 utilization using microalgae still requires innovative scientific and technological breakthroughs to render this a feasible technology. Unless coupled with other technologies or co-processes, investments into microalgae R&D are unlikely to make a considerable contribution to solving the CO2 problem globally. The use of microalgae can be classified as a direct CO2 mitigation technology. Direct strategies usually encompass much higher economic projections, going into billions of dollars, as opposed to indirect approaches. Therefore, for this technology to be a success, future R&D should focus on achieving higher biomass productivities, culture stability over long periods of time, economical harvesting techniques and improved biomass-to-fuels conversion technologies. The economics of microalgal CO2 utilization may be improved by integrating this procedure with other co-processes. Potential co-processes include wastewater treatment, production of useful metabolites, as well as biofuels, animal feed and biofertilizer manufacturing. Wastewater treatment as a co-process has emerged as a viable approach as process requirements and objectives overlap significantly. Municipal wastewater treatment is more favorable when compared to agricultural wastewater treatment as it could yield an animal-feed co-product. This would greatly aid in the economics of the entire process as well as contribute to GHG abatement by not producing additional fossil fuel that is generally required for product formation (Ho et al. 2011).
Microalgae
Microalgae which are primitive, unicellular, microscopic (2–200 μm) organisms that can also be classified as thallophytes have an important ecological role (Khan et al. 2009; Brennan and Owende 2010; Greenwell et al. 2010; Mutanda et al. 2011). Besides the fact that they can serve as food and feed source for people and animals as they belong to the bottom of the food chain, they are the principal producers of O2 on earth (Khan et al. 2009). They can be autotrophic or heterotrophic, and in some cases even both. Autotrophic microalgae require inorganic compounds, salts and an appropriate light source for growth, whereas heterotrophic microalgae utilize external sources of organic compounds as well as nutrients, which are used as an energy source. Microalgae are mainly categorized based on their basic cellular structure, pigmentation and life cycle. Due to novel genetic and ultra-structural information constantly emerging, the evolutionary history and taxonomy of microalgae is complex (Brennan and Owende 2010; Mutanda et al. 2011). Microalgae can be classified into two prokaryotic divisions and nine eukaryotic divisions (Khan et al. 2009; Mutanda et al. 2011). These organisms show much promise for the production of value-added products and biofuels, as they are rich in minerals, vitamins, oils and fatty acid methyl esters (Spolaore et al. 2006; Del Campo et al. 2007; Khan et al. 2009; Mutanda et al. 2011). Microalgae are able to endure high concentrations of CO2, and this inherent ability makes them very advantageous in utilizing CO2 from flue gases of power plants. They are fast growers with biomass volumes that double within 24 h. At a flow rate of 0.3 L/min of air with 4 % CO2 concentration, most microalgal strains are able to achieve a carbon-fixation rate of roughly 14.6 gcm−2/day (Farrelly et al. 2013).
Sampling for highly CO2 tolerant microalgal strains
For effective CO2 sequestration using microalgae, the crucial step would be to search, collect and identify hyper-CO2-tolerant strains (Khan et al. 2009; Huang et al. 2010). Sampling for these microalgal strains is largely influenced by environmental factors as well as the aquatic system (Mutanda et al. 2011). Brackish aquatic environments appear to be ideal areas to sample for superior carbon sequesters as they are rich in dissolved CO2, O2 and dissolved salts. Also, most CO2 sources, such as power plants, are located along coastal areas. Proper sampling techniques need to be in place in order to ensure success, as damaged or dead cells often lead to failure. Temporal and spatial collection is often employed to offset any mishaps that may occur at the sampling site. The success of any microalgal mass culture ultimately depends on fast-growing, productive strains that are adapted to the local climatic conditions (Mutanda et al. 2011).
It is imperative that certain factors are measured on-site so that these conditions can be simulated when culturing the specimens under laboratory conditions. Some such parameters would be the light quality and quantity, water temperature, nutrient concentration, dissolved CO2 and O2, as well as pH and salinity. Over the years, microalgal collection and selection processes have been well established. Sampling equipment should include a knife, mesh net, scooping jar, vessels for sample collection, scalpels, dissolved CO2 and O2 analyzer, light meter, GPS, salinity meter and a multi-probe system (measuring pH, temperature, turbidity, conductivity and light intensity) (Mutanda et al. 2011). No definite sampling procedure has been recognized in literature. Researchers are encouraged to follow simple and cheap techniques when collecting microalgal samples (Mutanda et al. 2011).
Once samples are brought back to laboratories, microalgae are usually identified by means of microscope-based techniques. For species-level identification, conventional light microscopy has been extended to include fluorescence microscopy, phase-contrast microscopy, transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Conventional microscope techniques, however, could often prove misleading in precise microalgal identification as many cell types lack morphological markers. Microalgae are also known to alter cell size and shape during their life cycle (Godhe et al. 2001; Mutanda et al. 2011). A proper identification is impossible as most microalgae fail to survive fixation or in some cases shrink, lose pigmentation and flagella. Microalgal identification from field samples using microscopy is also time-consuming and requires significant experience in technical and taxonomic skills. Molecular-based techniques developed in recent years have led to rapid and precise monitoring, identification and quantification of microalgal species. Commonly analyzed DNA regions for phylogenetic purposes include mitochondria genes, ribosomal RNA genes (rRNA), internal transcribed sequences (ITS), plastid genes (rbcL) and microsatellite DNA sequences. Therefore, molecular-based techniques using species-specific molecular probes offer a powerful technology for rapid screening and identification of microalgal species when compared to conventional identification techniques.
Propagation systems
After selecting a strain of interest, the step to follow is appropriate cultivation of the organism. Artificial cultivation of microalgae ought to reproduce and enhance the optimum natural growth conditions (Brennan and Owende 2010; Vasumathi et al. 2012). Two systems that have been extensively proposed are based on open pond and closed PBR technologies (Molina et al. 2001; Suh and Lee 2003; Chisti 2008; Brennan and Owende 2010). However, there is ongoing debate pertaining to which of the open pond or closed PBR would be a better system for CO2 sequestration. Raceway ponds are the most commonly used artificial growth systems because they are cost-effective, but significant CO2 losses to the atmosphere occur. Open systems are also known to utilize CO2 much less efficiently than PBRs (Borowitzka 1999; Chisti 2007; Brennan and Owende 2010).
Open ponds are typically constructed of a closed-loop, oval-shaped recirculation channels, generally between 0.2 and 0.5 m deep (Chisti 2007; Brennan and Owende 2010). Mixing and circulation, which is required to stabilize microalgal growth and productivity, is maintained within the pond by a paddlewheel (Borowitzka 1999; Chisti 2007; Brennan and Owende 2010). The paddlewheel is a continuous operation that prevents sedimentation. Flow can be guided around bends by baffles placed in the flow channel (Chisti 2007). Open systems are built in concrete or compacted earth, and are usually lined with white plastic (Borowitzka 1999; Chisti 2007; Ugwu et al. 2008; Brennan and Owende 2010). For a continuous production cycle, microalgal broth and nutrients are introduced in front of the paddlewheel and circulated through the loop to the harvest extraction point. Broth is harvested behind the paddlewheel, on the completion of the circulation loop (Chisti 2007). In raceways, cooling is solely achieved by evaporation. Temperature fluctuates seasonally. Considerable loss of water due to evaporation can occur. Due to significant losses to the atmosphere, raceway ponds are known to use CO2 much less efficiently than PBRs (Chisti 2007). Microalgae present in open ponds usually obtain their CO2 requirement from the surface air, but submerged aerators have been known to be installed to enhance CO2 absorption (Brennan and Owende 2010).
Microalgal production using closed PBR technology has been implemented to overcome some of the key problems associated with the above-described open pond production systems. A major advantage of PBRs when compared to open raceway systems is that they permit culture of single species of microalgae for prolonged durations with lower risk of contamination (Brennan and Owende 2010). Harvesting costs may also be significantly reduced owing to the higher cell mass productivities attained, and CO2 is also utilized more effectively (Chisti 2007; Brennan and Owende 2010; Vasumathi et al. 2012). Despite the fact that a great deal of work has already been done to develop PBRs for microalgal cultures and effective CO2 utilization, more efforts are still required to improve PBR technologies and know-how of microalgal cultures. Photobioreactor design and development is perhaps one of the first major steps that should be undertaken for efficient mass cultivation of microalgae for carbon mitigation (Ugwu et al. 2008). Light distribution and higher biomass productivities are more efficient in PBRs that have a larger optical cross-sectional area. PBRs having special designed light systems have been investigated for the effective CO2 sequestration and biomass production (Lee 2001). In 2003, Suh and Lee designed and operated an internally illuminated airlift PBR. This reactor was employed to study the light distribution in an attempt to maximize the photosynthetic efficiency and hence carbon uptake of a Synechococcus sp. A flat-plate PBR was constructed by Zijffers et al. (2010). In this design, sunlight was focused on the top of the reactor by dual-axis positioning of lenses. Solar radiation was captured by vertical plastic light guides and then distributed into the PBR. This design allowed for a more uniform distribution of light throughout the reactor and hence, more effective light utilization (Kumar et al. 2011).
Volumetric gas transfer coefficient is another important characteristic that should be taken seriously when designing reactors. Gas transfer and cell growth rate vary at different regions of liquid flow. Liquid flow within reactors is often divided into three regions: bubble flow, transitional flow and a heterogeneous zone. These zones are often dependent on gas velocity. The bubble flow region is an area in which gas hold-up, interfacial area as well as the volumetric gas transfer coefficient is proportional to the gas superficial velocity. Increases in the gas transfer coefficient promote cell growth rate. However, toward the end of the transition zone, a reduction in growth rate can be observed. The drop in specific growth rate could be attributed to shear stress (Kumar et al. 2011). In 2002, Zhang et al. conducted a series of experiments to comparatively analyze gas transfer in different PBRs at various CO2 percentages. They were able to conclude that the gas transfer coefficient increases with a decrease in the CO2 concentration from the inlet gas stream.
Ascertaining the CO2 solubility within the cultivation media is essential as this will determine the amount of carbon available for growth of microalgal cells. The solubility and speciation of CO2 in the medium is dependent on pH, temperature and nutrient concentration. Henry’s law states that CO2 dissolves in water to an extent determined by its partial pressure (PCO2), temperature, as well as the interaction of dissolved CO2 with other solutes in the water (Carroll and Mather 1992). CO2 solubility is known to increase with increasing pressures and decrease with increasing temperatures. Due to the thermodynamics of the reaction, CO2 becomes more soluble at lower temperatures. Solubility of CO2 in freshwater is also significantly higher as opposed to solubility in salt water (Carroll and Mather 1992).
A study by Borkenstein et al. (2011) investigated the cultivation of C. emersonii for 30 days using both flue gas and pure CO2 in 5.5 L airlift PBRs. The experimental setup is shown in Fig. 1. Results showed that the Chlorella sp. supplied with CO2 containing flue gas resulted in a biomass yield of 2.00 g L−1; when cultivated with pure CO2, it yielded 2.06 g L−1 biomass. There was no significant difference in biomass yields. When supplied with flue gas, C. emersonii was able to grow as successfully as when supplied with pure CO2. It can be concluded that the concentrations of the components within the flue gas together with the size and proximity of the culture vessel and characteristics of the microalgal species are imperative in developing an effective CO2 remediation technology.
Schematic representation of an airlift photobioreactor (Borkenstein et al. 2011)
Microalgal species employed for carbon bio-mitigation
Ambient CO2 levels are generally low (approximately 0.036 %) when compared to industrial areas, which release large amounts of flue gases into the atmosphere. CO2 concentrations present in flue gases vary from industry to industry, but are normally within the range of 3–30 %. Microalgae are able to fix CO2 from a range of sources, such as the atmosphere, industrial exhaust gases (flue and flaring gas) as well as in the form of soluble carbonates (NaHCO3 and Na2CO3) (Wang et al. 2008).
Carbon dioxide (CO2) is an essential nutrient for all photosynthetic life forms. Terrestrial plants naturally capture and utilize CO2 from the atmosphere. Microalgae, however, possess a greater capacity to fix CO2 (Kumar et al. 2011). This can be attributed to their photosynthetic apparatus and chlorophyll being present within a single microalgal cell permitting rapid biomass generation. Listed below in Table 1 are some advantages of using microalgae over higher plants for CO2 sequestration.
In the context of propagation, atmospheric CO2 (0.036 %) is most often not sufficient to support microalgal growth as microalgae requires an enriched point source of carbon. Due to this, biofixation of CO2 from power plants and other point sources using microalgae has emerged as a likely alternative to combat the growing concerns of global warming due to CO2 emissions (Ho et al. 2011; Kumar et al. 2011).
An increase in the maximum growth rate of microalgal species due to higher CO2 concentrations has been investigated by many researchers (Cheng et al. 2006; Ono and Cuello 2006; López et al. 2010; Ho et al. 2011; Kumar et al. 2011). Microalgal-CO2 fixation occurs via photoautotrophic growth. Therefore, the CO2-fixation potential of microalgal species should positively correlate with their light utilization efficiency and cell growth rate. Increases in temperature (>20 °C) can cause significant reduction in CO2 solubility, which eventually leads to a decline in the photosynthetic efficiency.
Strains often favored are those that can directly utilize CO2 from industrial flue gas, those that grow well under natural day-night cycles, strains with high productivities that are easy to harvest and most importantly those that produce biomass that can be used in the production of desirable co-products. Unfortunately, it is rare for a single microalgal strain to possess all these attributes. Most often, a highly productive strain functions poorly in dense mass culture, while a robust strain could have low growth rates (Farrelly et al. 2013). In recent years, there has been talk of genetically manipulating microalgal strains to enhance their properties pertaining to carbon mitigation and mass culture (Farrelly et al. 2013). Over the years, research has largely focused on single effective carbon sequesters and trying to upscale these strains for mass culture. However, it would appear that a more effective approach would be to isolate strains from nature that thrive in mass culture, and subsequently manipulate these strains to grow in dense culture while altering their physiology to increase their productivity and ease of harvesting (Farrelly et al. 2013).
Microalgae and cyanobacterial species routinely used for CO2 mitigation include Anabaena sp., Botryococcus braunii, Chlamydomonas reinhardtii, Chlorella sp., Chlorocuccum littorale, Scenedesmus sp., and Spirulina sp. (de Morais and Costa 2007; Ota et al. 2009; Packer 2009; Chen et al. 2010; Chiang et al. 2011; Ho et al. 2011). Table 2 represents commonly employed carbon sequesters cultivated in different bioreactors.
Green microalgae that are effective carbon sequesters generally belong to the genera Chlorococcum, Chlorella, Scenedesmus and Euglena. In 1970, Seckbach and Libby isolated species from the aforementioned genera that were able to survive after exposure to pure (100 %) CO2. Experiments on the Scenedesmus sp. revealed that this strain thrived under 100 % CO2 concentration and cell concentration increased for up to 30 days, reaching 3.65 g L−1. This was a significant increase in cell concentration, when compared to the 1.19 g L−1 obtained under conditions of atmospheric CO2 (0.036 %). Chloroccum littorale represents a fast-growing species that is able to tolerate high CO2 concentrations. In another experiment, Seckbach and Libby (1970) demonstrated that C. caldarium could tolerate 10 atm of CO2 but not 50 atm of CO2. They were able to conclude that growth was inhibited due to the effect of high CO2 concentrations and not due to the high pressure. These species, however, are known to have low growth rates, and hence, productivity and CO2 recovery were found to be low (Satoh et al. 2001).
A Chlorella Tx 71105 strain was supplied with pure CO2 at a rate of 3.3 mL min−1 over a 28-day period. During the first 6 days, the effluent gas contained more than 96 % O2. On the 12th day of cultivation, a spike in temperature from 37 to 39 °C was observed. On the 13th day, a 32 % CO2 concentration was noted in the effluent gas. The gas flow was then turned off for 3.5 h. Upon re-addition of CO2, the effluent gas then contained 97.8 % O2. During the last 6 days, no alterations were made to the experiment, and over this period, it was noted that the effluent gas contained 18 % CO2. When this strain was cultured under 41, 71 and 100 % CO2, the mean biomass concentration recorded was 3.15, 2.71 and 2.49 g L day−1, respectively. These cell concentrations are reasonably similar to those obtained with other Chlorella species (Table 2) (Geckler et al. 1962).
In another study, Zhao et al. (2011) did a comparative study of the growth and CO2 biofixation of a Chlorella sp. under two different cultivation modes. Findings showed that closed cultivation substantially enhanced microalgal performance with regard to growth and carbon biofixation. Specific growth rate and CO2-fixation rate during closed cultivation were observed to be 1.78 and 5.39 times higher that of open cultivation, respectively. Closed systems also allow for effective gas bubble motion, which plays a vital role in reducing dissolved O2 build-up. Under the proper cultivation mode, Chlorella sp. exhibit much potential as effective carbon sequesters.
A study by Kurano et al. (1995) showed that C. littorale was able to reach a maximum cell concentration of 4.9 g L−1 at a 20 % CO2 concentration. When exposed to CO2 concentrations of more than 20 %, a short lag phase was observed prior to active photosynthesis. It must be noted that the performance of microalgal strains does not solely depend on CO2 concentrations, but also on culture and experimental conditions, such as culture medium, temperature, light intensity as well as reactor design. Variation in any of these conditions could have an effect on the CO2-fixation efficiency of the strains (Ho et al. 2011).
Physiological pathway involved in the biochemistry of CO2 fixation
Photosynthesis (Fig. 2) within microalgae cells occurs in two stages. The first stage involves light-dependent or light reactions that only take place when cells are illuminated. This step exploits light energy to form the energy-storage molecules adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH). Carbon-fixation or dark reactions that form the second stage of photosynthesis transpire both in the presence and in the absence of light. Energy-storage products generated during light reactions are used at this step to capture and reduce CO2 (Calvin 1989).
Microalgal photosynthesis: modified from Zeng et al. (2011)
Photosystem I (PSI) and photosystem II (PSII) are major photoactive complexes responsible for transferring sunlight into the electron transport chain via the excited chlorophyll dimer (Calvin 1989; Iverson 2006; Ho et al. 2011). Photosynthesis begins in the PSII complex. Once a chlorophyll molecule at the core of the PSII complex attains ample excitation energy, an electron is transferred to the primary electron acceptor molecule. This process is often termed photo-induced charge separation. Electrons are then transported across the membrane through an electron transport chain. PSI accepts the electrons transferred from PSII and transports them via the P700 dimer of chlorophyll, which is oxidized from light-excited antenna chlorophyll to strongly reducing ferredoxin and NADPH (Cerveny et al. 2009; Ho et al. 2011). Energy harvested via the light reaction can be stored by the formation of ATP during photophosphorylation (Yang et al. 2000). Research has concluded that 1.3 ATP molecules are formed per pair of electrons moving through the photosynthetic electron transport chain (Yang et al. 2000). NADPH that is the main reducing agent in chloroplasts is responsible for supplying electrons to fuel other reactions. Due to its formation, a deficit of electrons exist in the chlorophyll of PSI. These electrons are replaced from the electron transport chain by plastocyanin.
Carbon-fixation reactions (dark reactions) involve the Calvin cycle (Calvin 1989; Iverson 2006; Yang et al. 2000). During the Calvin cycle, CO2 is converted into sugar with the aid of ATP by the carboxylase activity of the enzyme RuBisCO (Ribulose 1,5-bisphosphate carboxylase/oxygenase). Ribulose 1,5-bisphosphate carboxylase/oxygenase that has low affinity for CO2 also carries out oxygenase activity and produces glycolate 2-phosphate as an end product. This end product is of no use to the cell and its synthesis consumes considerable amounts of cellular energy. It is also responsible for releasing previously fixed CO2 by the carboxylase activity of RuBisCO. The oxygenase activity of RuBisCO can hinder around 50 % of biomass formation (Giordano et al. 2005; Kumar et al. 2011).
As mentioned previously, light supply (both light intensity and quality) is a key variable that greatly impacts the photosynthetic activity and hence growth kinetics of microalgae. Culture systems are illuminated by sunlight, artificial light or a combination of both. Light/dark cycle durations also play a significant role in microalgal growth (Pires et al. 2012). Grobbelaar et al. (1996) used a Scenedesmus obliquus strain to demonstrate that photosynthetic rates increase exponentially with increasing light/dark frequencies. Research suggests that microalgae cells associate a low light/dark cycle with low light conditions and vice versa. It was also observed during this study that the microalgal cells became increasingly more proficient in the overall exploitation of light energy during a longer dark phase relative to light phase. However, it must be noted that a relatively longer dark phase did not necessarily achieve higher photosynthetic rates nor did the microalgal cells acclimatize to a precise light/dark cycle. High photon flux densities over a long duration of time eventually led to damage to protein D1 in PSII. Damage to protein D1 led to poor trapping of photons. This then causes an overall reduction in the photosynthetic activity. During the dark phase, the photo-induced damage can be repaired by the algae. Airlift reactors are extremely beneficial in this regard, allowing cells to experience adequate amounts of shading in which time they are able to repair damage to PSII. Within airlift reactors, light flux decreases exponentially with the distance from the irradiated surface. As a result of this, cells near the irradiation source are exposed to a high photon density when compared to cells at the center. These cells at the center receive less light owing to shading. Photosynthesis can be measured as rates of carbon accumulation or O2 evolution. Either measurement can be converted into the other using the photosynthetic quotient (Pires et al. 2012).
The influence of photoperiods on the rates of CO2 sequestration was also studied by Jacob-Lopes et al. (2010) using a cyanobacterial strain in both BGN medium and refinery wastewater. A linear decrease in biomass productivity was observed during a longer dark period in BGN medium. A photosynthetic quotient of 0.74 was achieved using refinery wastewater as the growth medium. This essentially means that 1 g of CO2 consumed corresponds to the release of 0.74 g of O2. From this study, it can be concluded that the gas-exchange pattern within a system is greatly influenced by the intermittent light cycle. During dark periods, microalgal cells consume organic carbon through heterotrophic metabolism and release CO2 in the process. Microalgal growth can, therefore, be enhanced through sequential changing in the light intensity. Furthermore, it is imperative that irradiance be regulated according to the culture density. Low culture densities exposed to high light intensities would lead to photoinhibition of cells; and at high culture densities, light penetration becomes the limiting factor. Studies have also suggested that growth under red light (600–700 nm) enhanced PSII relative to PSI, whereas blue light (400–500 nm) could induce PSI. These findings suggest that blue and red lights are more suitable than others for both microalgal cell growth and CO2 mitigation (You and Barnett 2004; Ravelonandro et al. 2008).
Microalgae possess a unique, inherent ability of accumulating large quantities of inorganic carbon in their cytoplasm. These concentrations are usually several orders of magnitude higher when compared to that on the outside. This system is called a CO2-concentrating mechanism (CCM). CO2 concentration is an important factor for photosynthesis. Too high a concentration would increase the CO2 mass transfer mechanism from the gas mixture to the medium, resulting in a pH drop. This sudden reduction in pH hinders the growth of most microalgal species. Flue gases typically contain very high concentrations of CO2 (sometimes even greater than 30 %). It should also be noted that another key inhibitor of microalgal growth is the O2 produced during photosynthesis. It is imperative that this gas be removed routinely and not be allowed to accumulate within the system (Pires et al. 2012).
Numerous studies have been documented on improving the CO2-fixation rate via the Calvin cycle, PEP carboxylase and/or through synthetic pathways (Rosgaard et al. 2012; Gimpel et al. 2013). These efforts have met with varying degrees of success. They can broadly be classified into these categories: engineering of RuBisCO for increased catalysis rates of carboxylation and reduction of the oxygenation reaction, enhancing the activation state of RuBisCO, enhancing the regeneration phase of the Calvin cycle and CO2 enrichment around RuBisCO in an effort to inhibit the oxygenase reaction. Findings from most of these studies indicate that the challenge lies in the activity of RuBisCO for carbon flux through the Calvin cycle when CO2 is not supplemented in the media, or under conditions of high temperature/light. Chlamydomonas reinhardtii strains are RuBisCO deficient and are able to complete their life cycle heterotrophically. This makes them ideal candidates for engineering of RuBisCO. Chlamydomonas sp. containing varying amounts of RuBisCO have been engineered for the effective utilization of energy, carbon and nitrogen. This has been achieved using the nuclear genome of an MRL1-deficient strain and expressing the rbcl mRNA maturation factor MRL1 at different levels. When compared to the wild type, results for the deficient strain showed that RuBisCo could maintain phototrophic growth even when it was lowered up to 15 %. These findings suggest that based on the culture conditions (light intensity or CO2 concentration), an inducible promoter for MRL1 could effectively be applied to modify RuBisCo accumulation (Rosgaard et al. 2012; Gimpel et al. 2013).
Influence of flue gas composition on microalgal cultivation
The ability of microalgae to utilize CO2 from flue gas and thereby mitigate the amount of carbon discharged into the atmosphere is an attractive idea. However, there are several major challenges that need to be addressed before this idea can be implemented:
When compared to atmospheric air, CO2 is an effective supplement to stimulate microalgal growth. However, at high concentrations of CO2 (>5 %), microalgal growth can be suppressed. This is often attributed to acidification of the cellular content which eventually hinders growth (Lee and Lee 2003). A study by Watanabe et al. (2000) showed that strains that grew well at CO2 concentrations between 5 and 10 % had drastic decreases in their growth rate above CO2 concentrations of 20 %. At elevated CO2 concentrations, pH can drop down to 5 or even lower due to the formation of high amounts of bicarbonate buffer. This environmental stress causes a biological reduction in the capacity of microalgal cells to sequester CO2. Slight decreases in pH due to increased CO2 concentrations usually have a minor impact on microalgal growth. Strong pH changes, however, could inhibit all growth (Kumar et al. 2011). Considering that flue gas usually contains between 3 and 30 % CO2, it is imperative to identify strains capable of growing under very high CO2 concentrations. Screening studies have yielded strains that grow well in CO2 concentrations between 30 and 70 % (Hanagata et al. 1992; Iwasaki et al. 1996; Sung et al. 1999). Findings by Olaizola (2003) indicate that microalgal growth may even be sustained at a 100 % CO2 concentration by controlling changes in pH and only releasing CO2 to the microalgae on demand.
Flue gases are often made up of CO2, water vapor, NOx, SOx and heavy metals such as nickel, vanadium and mercury (Packer 2009). CO2 is a major component of industrial flue gases. Table 3 shows the approximate global CO2 flue gas emissions per industrial sector. The use of flue gas to culture microalgae is advantageous in that a carbon-free nutrient medium could be used as the carbon requirement will be provided for by CO2. Pre-treatment costs could be minimized by direct utilization of flue gas; however, this would depend on the presence and quantities of the aforementioned components (Benemann 1993; Ono and Cuello 2007). Some researchers argue that the presence of NOx in flue gases pose little or no problem to microalgal growth, while the difficulty arises in the presence of SOx, which decreases the pH due to the formation of sulfurous acid (Maeda et al. 1995; Packer 2009; Kumar et al. 2011). Others, however, further argue that some strains are not inhibited by CO2 with <50 ppm SOx, but can be inhibited by CO2 when NOx are also present (Negoro et al. 1993; Lee et al. 2002; Ho et al. 2011). Therefore, denitrification and desulphurization along with cooling and dedusting represent scrubbing options that may be necessary for pre-treatment of flue gases. It should also be noted that nickel concentrations above 1 and 0.1 ppm of vanadium decrease microalgal productivity while mercury can be remediated by certain microalgal species (Packer 2009; Hende et al. 2012). A few tolerant microalgae exist: Dunaliella tertiolecta (Nagase et al. 1998), Tetraselmis sp. (Matsumoto et al. 1995), Chlorella sp.T-1 and Chlorella sp. HA-1 (Maeda et al. 1995) are capable of growing at NOx concentrations of up to 300 ppm. In these cases, excess NO is absorbed by the cultivation medium and transformed to NO2, which is then further utilized as a nitrogen source by the microalgae. Therefore, it must be noted that the tolerance of microalgae to NOx and SOx varies depending on the species. By buffering the medium, pH drops could be prevented leading to little or no changes in growth rates (Kumar et al. 2011; Hende et al. 2012).
Thermal stability is another essential characteristic required by microalgal strains involved in CO2 sequestration. Flue gas temperatures are extremely high (around 120 °C) and could have an adverse effect on cells when introduced to the system (Ono and Cuello 2007; Kumar et al. 2011). Therefore, the feasibility of sequestering CO2 from flue gas would either depend on using thermophilic microalgal species or installing a heat-exchange system. Over the years, numerous species capable of tolerating temperatures up to 60 °C have been identified. Miyairi (1995) studied the effects of various CO2 concentrations at different temperatures on the growth of the cyanobacteria Synechococcus elongates. The study revealed that a drop in pH at 52 °C with 60 % CO2 was comparable to a drop in pH at 25 °C with 20 % CO2. These findings suggest that the temperature-dependent solubility of CO2 gives an advantage to the thermophilic microalgae, enabling them to endure a higher concentration of CO2. As temperature increases so too does the ratio of O2 to CO2 solubility. This leads to considerable O2 fixation by the oxygenase activity of RuBisCO. Increases in temperature also lead to a decrease in RuBisCOs affinity for CO2 (Kumar et al. 2011).
Light is one of the principal requirements for photosynthesis. The relationship between light and photosynthesis can be illustrated using the photosynthesis–irradiance response (P–I) curve, which has three distinctive regions: light-limited photosynthesis, light-saturated photosynthesis and photoinhibition (Ralph and Gademann 2003). For effective CO2 fixation leading to biomass production, optimum light intensity is necessary. Light becomes the limiting factor for microalgal cultivation when it is below the optimum, while exposure of cells to a high light intensity over long periods of time leads to photoinhibition. Photoinhibition is due to damage to the repair mechanism of PSII, which leads to the inactivation of other systems (electron carriers, oxygen evolving systems and the related D1/D2 proteins). Light intensity is dependent on wavelength, cell concentration and the penetrating distance of light as well as the geometry of the system (Kumar et al. 2011).
Challenges and economics associated with microalgal CO2 sequestration
There are numerous hurdles that need to be overcome before microalgae can be employed to significantly reduce CO2 emissions at a commercial level. Strain selection and design of the culturing system are key factors in maximizing CO2 mitigation rates. Even though open systems are much more cost-effective compared to closed PBRs, it is difficult to maintain culture purity in such systems. Closed systems are efficient vessels for sustaining axenic cultures as well as minimizing CO2 loss to the atmosphere. However, cleaning and sterilizing of large-scale PBRs is difficult, and this then poses a problem in the production of high value-added products. Land availability for set-up of propagation vessels also becomes a problem in developing countries. For example, Kadam (2001) demonstrated that 1,000 ha of land area will be required for the construction of open ponds to mitigate CO2 emissions from a 50-MW power plant. Carbon-fixation rates for microalgal cultures differ under varying operational conditions. Preliminary ideas of these values are required so as to estimate space requirements for reactor implementation for effective CO2 fixation. A Portuguese cement industry annually produces ±450 kt of CO2. If two types of reactors (open ponds and light-diffusing optical fiber reactors) are considered for the effective sequestration of CO2 emitted from this industry, the estimated space required would be very different. Studies showed that under natural day/night cycles, a 4,000 m3 pond could sequester up to 2.2 kt of CO2 per year. If ponds were to be scaled up to a height of 30 cm (to prevent dark zones), open ponds occupying an area of 2.72 × 106 m2 would be required to sequester almost all the CO2 from this cement company. CO2-fixation rates for light-diffusing optical fiber reactors were reported to be 4.44 g L−1 d−1. Therefore, a reactor height of 1 m and a culturing area of 2.78 × 105 m2 would be needed to effectively sequester CO2 from this cement plant (Stewart and Hessami 2005; Pires et al. 2012).
Geographical considerations must also be taken into account: fluctuations in temperature and solar irradiation over the seasons. Tropical areas are often considered most suitable for microalgal cultivation. To maximize the overall economic and environmental efficiency of microalgal CO2 sequestration, culturing systems should be located as close as possible to the point source. Furthermore, a comprehensive plan should be compiled for the large-scale production of microalgae. This scheme should encompass modeling and LCA of the overall process. Failure in doing so could render many algal production systems unsustainable. It should also be noted that potential leaks from large-scale algal systems could cause ecological damage by eutrophication (Pires et al. 2012; Farrelly et al. 2013).
Bio-mitigation of carbon at large scale may entail the cultivation of non-transgenic as well as transgenic microalgae. Even under the best of conditions, minor releases of microalgae into the surrounding environment are bound to occur. While natural disasters would lead to massive releases. Very little has been discussed in the literature pertaining to minor or massive spills, especially those involving large amounts of non-transgenic microalgae. Possible risks from non-transgenic strains should not be taken lightly as the introduction of many non-native species to terrestrial and aquatic environments could have far-reaching ecological effects. In recent years, public awareness on the possible risks of commercial production of algae (especially non-transgenic algae) has increased. There have been suggestions that detailed scientific risk analysis reports for each strain be compiled, prior to large-scale cultivation (Gressel et al. 2013).
Biomass recovery poses a challenge in microalgal biomass production processes. This phase generally requires one or more solid–liquid separation steps and usually accounts for 20–30 % of the total costs of production (Wang et al. 2008; Brennan and Owende 2010). Common harvesting practices include flocculation, filtration, flotation and centrifugal sedimentation. Some of these procedures can be highly energy intensive. Selecting an appropriate harvesting technology during microalgal cultivation is crucial to economic production of microalgal biomass (Brennan and Owende 2010).
The choice of harvesting technique is dependent on microalgae characteristics such as size, density and the value of the target products (Packer 2009; Brennan and Owende 2010). According to Brennan and Owende (2010), microalgae harvesting is a two-stage process, involving bulk harvesting and thickening. Bulk harvesting aims at separating biomass from the bulk suspension. Thickening is generally a more energy-intensive step as it involves concentrating the slurry through techniques such as centrifugation, filtration and ultrasonic aggregation (Packer 2009).
Flocculation is a popular technique as it is straightforward and cost-effective. This harvesting mode uses multivalent cations to overcome the overall negative charge present on the surface of microalgae. Multivalent metal salts (ferric chloride, aluminum sulfate and ferric sulfate) and polymers (polyelectrolyte and chitosan) are usually effective flocculants. An ideal flocculant is one that can be applied at low concentrations, inexpensive, non-toxic, and further downstream processing is not adversely affected by its use. For effective microalgal harvesting, flocculation is often combined with “floating.” This simple technique allows microalgae to float on the surface of the medium and can be easily removed as scum (Packer 2009). Dissolved air flotation (DAF) is a costly process that uses fine bubbles, which are injected under high pressure into the water column and then rise to the surface (Packer 2009). Most microalgae can be easily harvested from suspension by centrifugation. However, while centrifugal recovery is quick and easy, it is highly energy intensive (Ho et al. 2011). For low-value products (biofuels or animal feed), gravity sedimentation enhanced via flocculation is probably the most appropriate method (Ho et al. 2011).
Life cycle analysis (LCA) is a methodical environmental technique used for evaluating the input–output inventory of a product system throughout its entire life cycle. This process encompasses acquisition of raw material, production, use and ultimate disposal (from cradle to grave) (Tsoutsos et al. 2010). Such an assessment helps detect problem-shifting during life cycle stages (lower energy utilization during use, attained at a much higher manufacturing energy consumption cost), transfer from one medium to another (lower air emissions, but increased solid waste), identify technological innovation opportunities as well as project the environmental performance based on a selected functional unit of the product (Gnansounou et al. 2009; Tsoutsos et al. 2010). Energy balance, an essential factor within a microalgal system, is usually calculated by evaluating energy inputs required at each LCA stage, against the total required inputs of the embodied energy related to the specific product/idea of interest. Energy balance may be influenced at each or any life cycle phase. It is thus imperative that each stage be closely monitored so as to prevent any mishaps that could have far-reaching implications on the rest of the chain.
In theory, an efficient GHG capture mechanism would entail the capture of CO2 flue gas from power stations and its subsequent utilization in promoting the growth of microalgae for biofuel production (Khoo et al. 2011). However, according to Campbell et al. (2011), such a theory is only marginally correct. They propose that permissible carbon credits do not arise from captured flue gas due to the fact that the algae-derived fuel will ultimately be burnt and returned to the atmosphere. Carbon credits arise from the replacement of the fossil fuel that would have been used if the biofuel had not become accessible. Additional carbon credits may become available if the spent microalgal biomass went into the generation of electricity. Hence, the biomass would displace any coal, gas or other materials that would have originally been used for energy production (Campbell et al. 2011). It is necessary to therefore carry out comprehensive life cycle calculations of the processing energy required to create the biofuel. These calculations would allow one to quantify GHG emissions at each stage of the process, enabling the researcher to establish whether the process does in fact emit less CO2 than the use of fossil fuels and if this is the case to quantify the associated GHG savings (Gnansounou et al. 2009; Khoo et al. 2011). Different microalgal propagation systems can only be compared if they perform the same function. After selection of a shared function, a unit is chosen that will enable one to compare the systems on the same quantitative basis. All energy and mass flows within the operating system will then be standardized to this functional unit (Kadam 2001). When assessing the CO2 balance of a system, it is necessary to take into account the total discharge from fossil energy versus the CO2 uptake of the microalgae during cultivation (Khoo et al. 2011).
Life cycle analysis (LCA) essentially covers cultivation, harvesting, lipid extraction and finally product formation. Wastewater emissions as well as waste (solids or wastewater) treatment is generally not covered in a LCA study (Khoo et al. 2011).
Alabi et al. (2009) did a detailed cost analysis on different algal production systems. Findings from this study revealed that capital investment required per liter volume for each production facility was as follows: US$52L−1 for raceway ponds, US$2L −1 for fermenters and US$111L−1 for PBRs. Production costs (which included labor and running costs) for a kg of dried algal biomass showed that PBRs were the most expensive system to operate (US$7.32 kg−1), followed by raceway ponds (US$2.66 kg−1). Surprisingly, fermenters proved the least expensive, at a cost of US$1.54 kg−1. Another cost analysis study by Molina-Grima et al. (2003) illustrated that it would cost US$32.16 to produce a kg of microalgal biomass (dry) in a standard PBR. This study further serves to highlight the high costs associated with closed systems. Despite years of research on the commercial viability of closed PBRs, a breakthrough has yet to be developed.
Carbon dioxide (CO2) sequestration costs depend largely on microalgal productivity, carbon content of selected strains and overall process efficiency. Stewart and Hessami (2005) investigated the carbon uptake rate of Synechocystis aquatilis and found that this strain was able to uptake carbon at a rate of 1.5 g/L/day. It was then proposed that under natural light conditions, this strain could mitigate up to 2.2 kt CO2/4,000 m3 pond/yr. In a separate study, Kadam (2001) demonstrated that a 1,000-ha open raceway pond could mitigate 210,000 t/yrCO2 of the 414,000 t/yrCO2 generated by a 50-MW power plant. This suggests that a 50 % reduction in CO2 from the flue gas emissions could be achieved. Carbon sequestration strategies routinely implemented in the USA entail the injection of carbon in saline aquifers and amine scrubbing of CO2 from flue gases. Carbon burial in saline aquifers is far cheaper (US$40 t−1 CO2) as opposed to amine scrubbing (US$150 t−1 CO2). The combined costs of these 2 processes, however, are still significantly lower when compared to carbon mitigation using microalgal open ponds (US$793t−1 CO2). As can be seen, this figure is extremely cost intensive. However, mitigation costs using algae could be reduced if recovered biomass was utilized in the production of valuable by-products (Pires et al. 2012; Farrelly et al. 2013).
Conclusion
The use of microalgae for the purpose of CO2 sequestration is a unique environmental technology. Microalgae are promising candidates for CO2 mitigation, which aids in combating GHG-related environmental impacts and has the added benefit of producing renewable biomass. In comparison with terrestrial plants, microalgae are capable of fixing CO2 at a rate several times higher than plants owing to their high photosynthetic efficiencies. Additionally, selected microalgal strains can assimilate CO2 from industrial flue gas within various ranges of concentrations from ambient (0.036 % v/v) to extremely high (100 % v/v). CO2 fixation from industrial flue gas coupled with nutrient recycling from wastewater makes algae ideal organisms for the production of useful by-products. Several algal strains capable of growing in and consuming high CO2 concentrations from flue gas streams have been isolated and propagated; however, they are just a fraction of the vast majority of total algal species that have yet to be isolated and exploited for this purpose. An interesting approach would be to utilize a consortium of hyper-CO2-tolerant microalgal strains instead of a single strain. For effective CO2 sequestration, an in-depth knowledge of flue gas composition and biology of microalgal cells would be required. Temperature, pH, SOx, NOx, heavy metals, light, culture strain and density, as well as CO2 mass transfer and O2 accumulation are major factors that affect CO2 sequestration and biomass production. LCA is imperative to ascertain economic feasibility and environmental sustainability of algal CO2 sequestration systems. For example, harvesting and dewatering are processes that are highly energy intensive; thus, research efforts should focus on developing an optimal harvesting strategy. Furthermore, strategic engineering decisions should be taken into consideration to realize effective microalgal CO2 sequestration systems. Microalgal cultivation requires the development of suitable reactors with features such as high S/V ratio, mixing, mass transfer, scalability and ease of operation. Airlift bioreactors that distribute light through optical fibers could be a possible solution (this increases the ratio between the illumination surface and reactor volume).
Technical viability of algal CO2 sequestration has already been demonstrated in a few systems; however, the major challenges are the strategic and holistic development of technologies that will improve economic feasibility of algal CO2 sequestration and make this a viable industrial approach to GHG remediation.
References
Alabi AO, Tampier M, Bibeau E (2009) Microalgae technologies and processes for biofuels/bioenergy production in British Columbia. The British Columbia Innovation Council, Winnipeg
Aresta M, Dibenedetto A, Barberio G (2005) Utilization of macro-algae for enhanced CO2 fixation and biofuels production: development of a computing software for an LCA study. Fuel Process Technol 86:1679–1693
Benemann JR (1993) Utilization of carbon dioxide from fossil fuel—burning power plants with biological system. Energy Convers Manag 34:100–999
Benemann JR, Oswald WJ (1996) Systems and economic analysis of microalgae ponds for conversion of CO2 to biomass. Final report US DOE. http://www.osti.gov/bridge/servlets/purl/493389-FXQyZ2/webviewable/493389.pdf. Accessed 16 July 2012
Benemann JR, Koopman BL, Weissman JC, Eisenberg DM, Oswald WJ (1977) Species control in large scale microalgae biomass production. Report to University of California Berkeley SERL 77-5, SAN/740-77/1
Borkenstein CG, Knoblechner J, Frühwirth H, Schagerl M (2011) Cultivation of Chlorella emersonii with flue gas derived from a cement plant. J Appl Phycol 23:131–135
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, tubes and fermenters. J Biotechnol 70:313–321
Brennan L, Owende P (2010) Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Calvin M (1989) 40 years of photosynthesis and related activities. Photosynth Res 21:3–16
Campbell PK, Beer T, Batten D (2011) Life cycle assessment of biodiesel production from microalgae in ponds. Bioresour Technol 102:50–56
Carroll JJ, Mather AE (1992) The system carbon dioxide-water and the Krichevsky–Kasarnovsky equation. J Solut Chem 21:1201–1209
Cerveny J, Setlik I, Trtilek M, Nedbal L (2009) Photobioreactor for cultivation and real-time, in situ measurement of O2 and CO2 exchange rates, growth dynamics, and of chlorophyll fluorescence emission of photoautotrophic microorganisms. Eng Life Sci 9:247–253
Chen CY, Yeh KL, Su HM, Lo YC, Chen WM, Chang JS (2010) Strategies to enhance cell growth and achieve high-level oil production of a Chlorella vulgaris isolate. Biotechnol Prog 26:679–686
Cheng LH, Zhang L, Chen HL, Gao CJ (2006) Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol 50:324–329
Chiang CL, Lee CM, Chen PC (2011) Utilization of the cyanobacteria Anabaena sp. CH1 in biological carbon dioxide mitigation processes. Bioresour Technol 102:5400–5405
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chiu SY, Kao CY, Chen CH, Kuan TC, Ong SC, Lin CS (2008) Reduction of CO2 by a high density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819
Costa JAV, Linde GA, Atala DIP (2000) Modelling of growth conditions for cyanobacterium Spirulina platensis in microcosms. World J Microbiol Biotechnol 16:15–18
de Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Del Campo JA, Garcia-Gonzalez M, Guerrero MG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:117–1163
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manag 52:163–170
Farrelly DJ, Everard CD, Fagan CC, McDonnell KP (2013) Carbon sequestration and the role of biological carbon mitigation: a review. Renew Sustain Energy Rev 21:712–727
Geckler RP, Sane JO, Tew RW (1962) Highly concentrated carbon dioxide as a carbon source for continuous algae cultures [Online]. http://contrails.iit.edu/DigitalCollection/1962/AMRLTDR62-116article06.pdf [2013, 03/06]
Gimpel JA, Specht EA, Georgianna DR, Mayfield SP (2013) Advances in microalgae engineering and synthetic biology applications for biofuel production. Curr Opin Chem Biol 17:1–7
Giordano M, Beardall J, Raven JA (2005) Mechanisms in algae: mechanisms, environmental modulation, and evolution. Ann Rev Plant Biol 56:99–131
Gnansounou E, Dauriat A, Villegas J, Panichelli L (2009) Life cycle assessment of biofuels: energy and greenhouse gas balances. Bioresour Technol 100:4919–4930
Godhe A, Anderson DM, Rehnstam-Holm AS (2001) PCR amplification of microalgal DNA for sequencing and species identification: studies on fixatives and algal growth stages. Harmful Algae 1:375–382
Greenwell HC, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interf 7:703–726
Gressel J, van der Vlugt CJB, Bergmans HEN (2013) Environmental risks of large scale cultivation of microalgae: Mitigation of spills. Algal Research. http://dx.doi.org/10.1016/j.algal.2013.04.002
Grobbelaar JU, Nedbal L, Tichy V (1996) Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. J Appl Phycol 8:335–343
Hanagata N, Takeuchi T, Fukuju Y, Barnes DJ, Karube I (1992) Tolerance of microalgae to high CO2 and high-temperature. Phytochemistry 31:3345–3348
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkes L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs—a summary. Aquac Res 31:637–659
Hende SVD, Vervaeren H, Boon N (2012) Flue gas compounds and microalgae: (Bio-) chemical interactions leading to biotechnological opportunities. Biotechnol Adv. doi:10.1016/j.biotechadv.2012.02.015
Ho SH, Chen CY, Lee DJ, Chang JS (2011) Perspectives on microalgal CO2-emission mitigation systems-a review. Biotechnol Adv 29:189–198
Hsueh HT, Li WJ, Chen HH, Chu H (2009) Carbon bio-fixation by photosynthesis of Thermosynechococcus sp. CL-1 and Nannochloropsis oculta. J Photochem Photobiol, B 95:33–39
Hu Q, Kurano N, Kawachi M, Iwasaki I, Miyachi S (1998) Ultra high-cell-density culture of a marine green alga Chlorococcum littorale in a flat-plate photobioreactor. Appl Microbiol Biotechnol 49:655–662
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87:38–46
Huntley M, Redalje D (2007) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strateg Global Change 12:573–608
Iverson TM (2006) Evolution and unique bioenergetic mechanisms in oxygenic photosynthesis. Curr Opin Chem Biol 10:91–100
Iwasaki I, Kurano N, Miyachi S (1996) Effects of high-CO2 stress on photosystem II in a green alga, Chlorococcum littorale, which has a tolerance to high CO2. J Photochem Photobiol B Biol 36:327–332
Jacob-Lopes E, Lacerda LMCF, Franco TT (2008) Biomass production and carbon dioxide fixation by Aphanothece microscopica Nageli in a bubble column photobioreactor. Biochem Eng J 40:27–34
Jacob-Lopes E, Revah S, Hernandez S, Shirai K, Franco TT (2009) Development of operational strategies to remove carbon dioxide in photobioreactors. Chem Eng J 153:120–126
Jacob-Lopes E, Scoparo CHG, Queiroz MI, Franco TT (2010) Biotransformations of carbon dioxide in photobioreactors. Energy Convers Manag 51:894–900
Kadam KL (2001) Microalgae production from power plant flue gas: Environmental implications on a life cycle basis. Technical report, National Renewable Energy Laboratory Contract No. DE-AC36-99-GO10337
Khan SA, Rashmi, Hussain MZ, Prasad S, Banerjee UC (2009) Prospects of biodiesel production from microalgae in India. Renew Sust Energy Rev 13:2361–2372
Khoo HH, Sharratt PN, Das P, Balasubramanian RK, Naraharisetti PK, Shaik S (2011) Life cycle energy and CO2 analysis of microalgae-to-biodiesel: preliminary results and comparisons. Bioresour Technol 102:5800–5807
Kumar A, Ergas S, Yuan X, Sahu A, Zhang Q, Dewulf J, Malcata FX, Langenhove HV (2011) Enhanced CO2 fixation and biofuels production via microalgae: recent developments and future directions. Trends Biotechnol 28:371–380
Kuramochi T, Ramirez A, Turkenburg W, Faaij A (2012) Comparative assessment of CO2 capture technologies for carbon-intensive industrial processes. Prog Energy Combust 38:87–112
Kurano N, Ikemoto H, Miyashita H, Hasegawa T, Hata H, Miyachi S (1995) Fixation and utilization of carbon dioxide by microalgal photosynthesis. Energy Convers Manag 36:689–692
Lam MK, Lee KT, Mohamed AR (2012) Current status and challenges on microalgae-based carbon capture. Int J Greenh Gas Con 10:456–469
Langley NM, Harrison STL, Van Hille RP (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng J 68:70–75
Lee YK (2001) Microalgal mass culture systems and methods: their limitation and potential. J Appl Phycol 13:307–315
Lee JS, Lee JP (2003) Review of advances in biological CO2 mitigation technology. Biotechnol Bioproc E 8:354–359
Lee JS, Kim DK, Lee JP, Park SC, Koh JH, Cho HS, Kim SW (2002) Effects of SO2 and NO2 on growth of Chlorella sp. KR-1. Bioresour Technol 8:1–4
Lipinsky ES (1992) R&D status of technologies for utilization of carbon dioxide. Energy Convers Manag 33:505–512
López CVG, Fernández FGA, Sevilla JMF, Fernández JFS, García MCC, Grima EM (2010) Utilization of the cyanobacteria Anabaena sp. ATCC 33047 in CO2 removal processes. Bioresour Technol 100:5904–5910
Maeda K, Owada M, Kimura N, Omata K, Karube I (1995) CO2 fixation from flue gas on coal fired thermal power plant by microalgae. Energy Convers Manag 36:717–720
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
Matsumoto H, Shioji N, Hamasaki A, Ikuta Y, Fukuda Y, Sato M, Endo N, Tsukamoto T (1995) Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. Appl Biochem Biotech 51:681–692
Miyairi S (1995) CO2 assimilation in a thermophilic cyanobacterium. Energy Convers Manag 36:763–766
Molina GE, Belarbi EH, Fernandez FG, Medina AR, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Molina-Grima E, Belarbi EH, Ferna′ndez AFG, Robles MA, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102:57–70
Nagase H, Eguchi K, Yoshihara K, Hirata K, Miyamoto K (1998) Improvement of microalgal NOx removal in bubble column and airlift reactors. J Ferment Bioeng 86:421–423
Nakajima Y, Ueda R (2000) The effect of reducing light-harvesting pigment on marine microalgal productivity. J Appl Phycol 12:285–290
Negoro M, Hamasaki A, IKuta Y, Makita T, Hirayama K, Suzuki S (1993) Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. Appl Biochem Biotechnol 39(40):643–653
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20:459–466
Ono E, Cuello JL (2006) Feasibility assessment of microalgal carbon dioxide sequestration technology with photobioreactor and solar collector. Biosyst Eng 95:597–606
Ono E, Cuello JL (2007) Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst Eng 96:129–134
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL (2009) Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresour Technol 100:5237–5242
Packer M (2009) Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 37:3428–3437
Pedroni P, Davison J, Beckert H, Bergman P, Benemann J (2001) A proposal to establish an international network on biofixation of CO2 and greenhouse gas abatement with microalgae. J Energy Environ Technol 1:136–150
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sustain Energy Rev 16:3043–3053
Radakovits R, Jinkerson RE, Darzins AL, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501
Ralph PJ, Gademann R (2003) Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat Biol 82:222–237
Ramanan R, Kannan K, Deshkar A, Yadav R, Chakrabarti T (2010) Enhanced algal CO2 sequestration through calcite deposition by Chlorella sp. and Spirulina platensis in a mini-raceway pond. Bioresour Technol 101:2616–2622
Ravelonandro PH, Ratianarivo DH, Joannis-Cassan C, Isambert A, Raherimandimby M (2008) Influence of light quality and intensity in the cultivation of Spirulina platensis from Toliara (Madagascar) in a closed system. J Chem Technol Biotechnol 83:842–848
Rosgaard L, de Porcellinis AJ, Jacobsen JH, Frigaard NU, Sakuragi Y (2012) Bioengineering of carbon fixation, biofuels, and biochemicals in cyanobacteria and plants. J Biotechnol 162:134–147
Satoh A, Kurano N, Miyachi S (2001) Inhibition of photosynthesis by intracellular carbonic anhydrase in microalgae under excess concentrations of CO2. Photosynth Res 68:215–224
Scragg AH, Illman AM, Carden A, Shales SW (2002) Growth of microalgae with increased calorific values in a tubular bioreactor. Biomass Bioenergy 23:67–73
Seckbach J, Libby WF (1970) Vegetative life on Venus? Or investigations with algae which grow under pure CO2 in hot acid media at elevated pressures. Origins Life Evol B 2:121–143
Sharma YC, Singh B, Korstad J (2011) A critical review on recent methods used for economically viable and eco-friendly development of microalgae as a potential feedstock for synthesis of biodiesel (critical review). Green Chem 13:2993–3006
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the U. S. Department of Energy’s aquatic species program - biodiesel from algae. NREL/TP-580-24190. US Department of Energy’s Office of Fuels Development
Skjanes K, Lindblad P, Muller J (2007) BioCO2–a multidisciplinary, biological approach using solar energy to capture CO2 while producing H2 and high value products. Biomol Eng 24:405–413
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stewart C, Hessami MA (2005) A study of methods of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energy Convers Manag 46:403–420
Suh IS, Lee CG (2003) Photobioreactor engineering: design and performance. Biotechnol Bioproc E 8:313–321
Sung KD, Lee JS, Shin CS, Park SC, Choi MJ (1999) CO2 fixation by Chlorella sp.KR-1 and its cultural characteristics. Bioresour Technol 68:269–273
Sydney EB (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101:5892–5896
Tsoutsos T, Kouloumpis V, Zafiris T, Foteinis S (2010) Life cycle assessment for biodiesel production under Greek climate conditions. J Clean Prod 18:328–335
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Vasumathi KK, Premalatha M, Subramanian P (2012) Parameters influencing the design of photobioreactors for the growth of microalgae. Renew Sustain Energy Rev 16:5443–5450
Wang B, Li YQ, Wu N, Lan CQ (2008) CO2 bio-mitigation using microalgae. Appl Microbiol Biotechnol 79:707–718
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES-collection list of strains, microalgae and protozoa. Microbial culture collections (ed) National Institute for Environmental Studies, Tsukuba, Japan
Yang C, Hua Q, Shimizu K (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87–102
You T, Barnett SM (2004) Effect of light quality on production of extracellular polysaccharides and growth rate of Porphyridium cruentum. Biochem Eng J 19:251–258
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: from CO2 fixation to biofuel production. Renew Sustain Energy Rev 15:3252–3260
Zhang L, Happe T, Melis A (2002) Biochemical and morphological characterization of sulfur-deprived and H2-producing Chlamydomonas reinhardtii (green alga). Planta 214:552–561
Zhao B, Zhang Y, Xiong K, Zhang Z, Hao X, Liu T (2011) Effect of cultivation mode on microalgal growth and CO2 fixation. Chem Eng Res Des 9:1758–1762
Zijffers JWF, Schippers KJ, Zheng K, Janssen M, Tramper J, Wijffels RH (2010) Maximum photosynthetic yield of green microalgae in photobioreactors. Mar Biotechnol 12:708–718
Acknowledgments
The authors hereby acknowledge the National Research Foundation (South Africa) for the financial contribution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhola, V., Swalaha, F., Ranjith Kumar, R. et al. Overview of the potential of microalgae for CO2 sequestration. Int. J. Environ. Sci. Technol. 11, 2103–2118 (2014). https://doi.org/10.1007/s13762-013-0487-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0487-6