Abstract
Microalgae produce a wide array of bioactive compounds with potential applications such as pharmaceuticals and nutraceuticals that are yet to be fully explored. Metabolites from microalgae are known to have various bioactivities including antibacterial, antiviral, anticancer and immunomodulatory activities as well as beneficial effects against metabolic disorders such as obesity, diabetes and hyperlipidaemia. Production of pharmaceuticals and nutraceuticals derived from microalgae is attractive as the photosynthetic organisms can be grown on a large scale under controlled environment. Furthermore, the production platform can be integrated with other applications, especially biofuel production, to improve economic competitiveness based on a biorefinery concept. Microalgae that have been successfully cultured on a large scale for commercial production of pharmaceuticals and nutraceuticals include Spirulina (Arthrospira), Dunaliella, Chlorella and Haematococcus. While various bioactive compounds have been discovered from microalgae, most studies on those compounds are still at the preclinical stage, confined to laboratory experiments. Overall, only very few microalgal products have been successfully brought to the market. This chapter aims to give an overview of the wide range of potential pharmaceuticals and nutraceuticals from microalgae and current status of the production of such compounds as well as to highlight the future directions of research in this field.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microalgae comprise a diverse group of organisms with an estimated number of species of 72,500, but only about 44,000 have been described (de Clerck et al. 2013). This group of organisms consists of the eukaryotic (microalgae) and prokaryotic taxa (cyanobacteria). Apart from the rich biological diversity, microalgae are also known to produce a myriad of metabolites, many of which have potential biomedical applications that have yet to be fully explored (Coates et al. 2013). The breadth of chemical diversity of secondary metabolites from microalgae is related to the vast number of environments they inhabit. While microalgae are mainly found in aquatic environments (49.78% in freshwater and 44.48% in seawater), some species also grow in contaminated and extreme environments including thermal and glacial lakes (Cezare-Gomes et al. 2019). The production of secondary metabolites is related to their possible ecological roles including for defence of the microalgae against predators, protection against infection by various microorganisms and sexual communication (Coates et al. 2013). Interestingly, some of the bioactive compounds that were first isolated from invertebrates were later found to be produced by symbiotic microalgae in those animals. For instance, the anticancer compound dolastatin was originally isolated from the sea hare (Dolabella auricularia) but was later discovered to be synthesized by the cyanobacteria Lyngbya and Symploca (Luesch et al. 2002).
Microalgae are a potential and attractive source of bioactive compounds, as they can be easily cultured, and have short generation times, with doubling time of 3.9–8.1 h for some species (Table 12.1). The doubling time of microalgae can be further shortened by manipulating the culture conditions (de Swaaf et al. 2003; Chen et al. 2013; Table 12.1). Furthermore, microalgae can synthesize structurally complex molecules, which are difficult or impossible to produce by chemical synthesis (Dewi et al. 2018). The possibility of culturing those microalgae also allows an environmentally friendly way to drug discovery, overcoming problems such as overutilization of natural bioresources and use of destructive collection practices (Lauritano et al. 2016). In addition, a “milking” approach can be used where extraction of the high-value products (e.g. use of biocompatible organic solvents and pulsed electric field) does not kill the algae and thus, the process is more environmentally and economically friendly (Vinayak et al. 2015). Large-scale cultivation of microalgae in photobioreactors is possible to produce large volumes of cultures, ranging from 1000 to 6000 L of microalgae such as Chlorella and Spirulina, for the extraction of bioactive compounds (Borowitzka 1999). In addition, microalgae are potential candidates for exploration of new compounds due to their metabolic plasticity, which can be exploited for the production of several compounds with applications in various biotechnology sectors including food, energy, health, environment and biomaterials (Martinez Andrade et al. 2018).
Regulatory bodies such as the US Food and Drug Administration (FDA) set the standards that need to be met before any microalgal product is certified safe for human consumption (Gantar and Svircev 2008). Some of the microalgal products have attained GRAS (generally recognized as safe) status and are marketed as functional food (Gantar and Svircev 2008; Jha et al. 2017a). The most widely sold microalgal products as functional food include Spirulina (Arthrospira) and Chlorella (Andrade 2018). In addition, Haematococcus pluvialis has been approved for human consumption as dietary supplement in several European countries (Lorenz and Cysewski 2000). The food-grade microalgae are a rich source of nutraceuticals such as carotenoids (β-carotene and astaxanthin), C-phycocyanin, long-chain polyunsaturated fatty acids (LC-PUFA), vitamins and polysaccharides (Lorenz and Cysewski 2000; Andrade 2018).
Microalgae produce a wide array of metabolites with diverse bioactivities including anticancer, antibacterial, antiviral and immunomodulatory activities and ameliorating effects against metabolic disorders such as diabetes and hyperlipidaemia (Nagaoka et al. 2005; Renju et al. 2014a; Aboulthana et al. 2018; Dewi et al. 2018). Apart from the limited number of microalgal species that have reached commercial status, the rich microalgal resources with the mentioned bioactivities have not been fully exploited as sources of pharmaceuticals and nutraceuticals (Jha et al. 2017a). There are several reports on large-scale screening of microalgae for bioactive compounds. For instance, in an early study, screening of 1000 cyanobacterial strains from diverse habitats for novel antineoplastic agents identified the families Scytonemataceae and Stigonemataceae as prolific producers of novel cytotoxic compounds (Patterson et al. 1991). Recently, Caires et al. (2018) screened various strains of the benthic filamentous cyanobacteria Neolyngbya for antimicrobial and antioxidant activities. Solvent extracts of the cyanobacteria showed inhibitory activity against the tested bacteria and Candida albicans, and the methanolic extract showed the greatest antioxidant activity. In another study, Lauritano et al. (2016) screened 21 diatoms, seven dinoflagellates, and four flagellate species for antioxidant, anti-inflammatory, anticancer, antidiabetic, antibacterial, and antibiofilm activities.
The aim of this chapter is to give an overview of the wide range of potential pharmaceuticals and nutraceuticals derived from microalgae. The aspect on mass-culture systems, particularly photobioreactors, currently used to produce microalgal biomass for the extraction of pharmaceuticals and nutraceuticals is also discussed. This chapter also aims to highlight the challenges faced in the commercialization of microalgal products as pharmaceuticals and nutraceuticals, and to propose some directions for future research.
2 Bioactivities of Microalgal Compounds and Their Potential Applications as Pharmaceuticals and Nutraceuticals
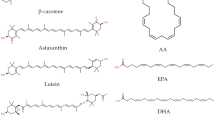
Microalgal compounds with potential for development into pharmaceuticals and nutraceuticals include astaxanthin, fucoxanthin, C-phycocyanin, sulphated polysaccharides and novel secondary metabolites (Figs. 12.1 and 12.2). In the subsequent sections, the following bioactivities of microalgal compounds will be covered in detail: antibacterial, antiviral, anticancer and immunomodulatory activities, and beneficial effects against metabolic disorders such as obesity, diabetes and hyperlipidaemia. A summary of the microalgal compounds with antibacterial, antiviral and immunomodulatory activities is given in Table 12.2 while those with anticancer activity are listed in Table 12.3.
Selected bioactive compounds derived from microalgae with potential for the development of pharmaceuticals and nutraceuticals. Example of microalgal producer for each compound is given in parenthesis (Burja et al. 2001; Chen et al. 2003; Mori et al. (2004); Mimouni et al. 2012; Coates et al. 2013; Rodrigues et al. 2015; Assunção et al. 2017; Bertin et al. 2017)
2.1 Antibacterial Activity
There has been a long history in attempts to isolate antibacterial compounds from microalgae since the discovery of chlorellin, which is a mixture of fatty acids, from Chlorella vulgaris and Chlorella pyrenoidosa for its activity against various Gram-positive and Gram-negative bacteria (Pratt et al. 1944). Since then, a variety of antibacterial compounds from microalgae have been characterized, including fatty acids (Desbois et al. 2009), phenolic compounds (Kuntzler et al. 2018), biphenyls (Volk and Furkert 2006), polysaccharides (de Jesus Raposo et al. 2015) and nanoparticles (Patel et al. 2015).
Amongst the fatty acids from microalgae which display antibacterial activity include eicosapentaenoic acid (EPA) and palmitic acid. For instance, Desbois et al. (2009) showed that EPA from Phaeodactylum tricornutum displayed potent antibacterial activity against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Recently, Wang et al. (2018a) obtained two active fractions from Phaeodactylum tricornutum, a pure fraction of EPA and another (fraction B) containing new antibacterial substances that displayed antibacterial activities against human pathogenic bacteria. In addition, fatty acid methyl esters (FAME), particularly palmitic acid methyl ester (C16:0), extracted from Scenedesmus intermedius have also been found to display antibacterial activities against both Gram-positive and Gram-negative bacteria (Davoodbasha et al. 2018).
Recently, Senhorinho et al. (2018) screened 40 freshwater green microalgae from water bodies near abandoned mine sites in northern Canada. Of the 40 methanol extracts from the microalgae tested, 37.5% displayed strong inhibitory activity against the human pathogen Staphylococcus aureus, with Minimum Inhibitory Concentrations (MIC) ranging from 16 to >1024 μg/mL. The metabolites identified in the active extracts include amino acids, glycerolipids, sphingolipids, acylcarnitines and biogenic amines. However, there was no significant correlation between the metabolites analysed and antibacterial activity observed.
Nanoparticles (NP) such as silver and gold NP are well known to possess antibacterial activity. There has been increasing interest in using microalgae as bio-factory for the synthesis of such NP (Shankar et al. 2016). The green synthesis approach that uses cell-free extracts from the microalgae is more attractive compared to conventional physical and chemical methods, which are costly and involve the use of hazardous chemicals (Narayanan and Sakthivel 2011). Synthesis of NP may involve extracellular process, where the metal ions are trapped on the cell surface and reduced to elemental metal atoms by specific enzymes. On the other hand, in intracellular synthesis of NP, the ions are transported into the algal cell to form NP in the presence of enzymes.
Da Silva Ferreira et al. (2017) synthesized silver chloride nanoparticles (AgCl-NP) by incubating conditioned medium of Chlorella vulgaris with AgNO3. The AgCl-NP formed was found to have potent antibacterial activity, reducing 98% the growth of Gram-positive Staphylococcus aureus and Gram-negative Klebsiella pneumoniae bacterial pathogens. In another study, Patel et al. (2015) evaluated the ability of 16 strains of cyanobacteria and microalgae to synthesize silver nanoparticles (Ag-NP). The Ag-NP were formed in the presence of the biomass as well as in the cell-free culture medium and might involve extracellular polysaccharide. In the same study, C-phycocyanin from Spirulina and Limnothrix were successfully used for the biosynthesis of Ag-NP. Further, the Ag-NP from all except one strain was found to display antibacterial activity against bacteria such as Staphylococcus aureus and Pseudomonas aeruginosa.
Some cyanotoxins have also been shown to be a promising source of novel antibiotics against multi-resistant bacteria. For instance, microcystin-LR displayed antimicrobial activity against four highly resistant species of Mycobacterium, with MIC ranging from 0.42 to 0.53 μM (Ramos et al. 2015). In addition, phenolic compounds from Spirulina LEB 18 were found to have antibacterial activity against Escherichia coli and Staphylococcus aureus when tested alone or incorporated into nanofibers (Kuntzler et al. 2018). The phenolic compounds in Spirulina contain organic acids such as caffeic, gallic, salicylic and trans-cinnamic acids (de Souza et al. 2015). In another study, simple phenols such as ferulic acid from the chloroform extracts of Dunaliella sp. accounted for the antibacterial activity against Bacillus subtilis ATCC 6633 and E. coli 0157:H7 ATCC 35150 (Kim et al. 2012a). In addition, both purified β-carotene and chlorophyll pigments from the organic solvent extracts of Chlorococcum humicola were shown to display antibacterial activity against Staphylococcus aureus, Vibrio cholerae and Klebsiella pneumoniae (Bhagavathy et al. 2011).
2.2 Antiviral Activity
As viral infection is one of the main threats to human health, there has been great interest in looking for safe, effective and specific antiviral agents. Screening of water, methanolic or ethanolic extracts from microalgae for antiviral activity has been reported by several authors. For instance, in an early study, Lau et al. (1993) reported that only 2% of approximately 900 strains of cyanobacteria were able to inhibit reverse transcriptase activity of the human immunodeficiency virus (HIV) and avian myeloblastosis virus. In another study, Fabregas et al. (1999) screened extracts from ten microalgae for antiviral activity against the viral haemorrhagic septicaemia virus (VHSV) of salmonid fish and the African swine fever virus (ASFV). The study showed that aqueous extracts from Porphyridium cruentum, Chlorella autotrophica and Ellipsoidon sp. displayed significant inhibition of the replication of both viruses, which could be attributed to sulphated polysaccharides. In addition, Abdo et al. (2012) tested methanol and water extracts of five freshwater microalgae against Adenovirus Type 40 and found that only extracts from Spirulina platensis showed significant activity at non-cytotoxic concentrations. In another study, Kok et al. (2011) assessed the inhibitory activities of methanol extracts from Ankistrodesmus convolutus, Synechococcus elongatus and Spirulina platensis against Epstein–Barr virus (EBV) in three Burkitt’s lymphoma (BL) cell lines. The methanol extracts were obtained by homogenizing the dried algal biomass in methanol and after centrifugation, the supernatant was evaporated and the dried extract was reconstituted in the solvent before use for the testing. The study found that the HPLC sub-fraction of the methanol extracts from Synechococcus elongatus (SEF1′) was most effective in reducing cell-free EBV DNA (EC50 = 1.38 μg/mL, therapeutic index >69). In addition, Komatsu et al. (2013) found that acidic polysaccharide fraction from Coccomyxa gleobotrydiformi displayed antiviral activity against influenza A virus through preventing the cell attachment and/or penetration of the virus.
Recently, Afify et al. (2018) found that protein hydrolysates obtained from Scenedesmus obliquus exhibited antiviral activity against Coxsackie B3 virus (CVB3). The inhibitory action of the hydrolysates was at the steps involving attachment, penetration, and adsorption of the viral particles. In addition, the antiviral activity of the hydrolysates was found to be positively correlated with the antioxidant activity of the extracts.
Polysaccharides are amongst the compounds that are known to possess antiviral activity and have great potential for pharmaceutical applications (Yu et al. 2018). For instance, a sulphated polysaccharide named calcium spirulan isolated from Spirulina platensis was shown to have potent antiviral activity against both HIV-1 and anti-herpes simplex virus type 1 (HSV-1) (Hayashi et al. 1996). In another study, Rechter et al. (2006) assessed the antiviral activity of intracellular and extracellular fractions containing spirulan-like molecules from Spirulina. The study showed that the spirulan-like fractions displayed strong inhibition against human cytomegalovirus, HSV-1, human herpesvirus type 6 and HIV-1, but only weak or no inhibition against EBV and influenza A virus. While for herpesviruses, the antiviral action of the fractions mainly targeted at viral entry, the action against HIV-1 occurred at a stage later than viral entry.
The sulphated polysaccharide p-KG03 purified from the dinoflagellate Gyrodinium impudium was found to exhibit antiviral activity against influenza type A virus (Kim et al. 2012b). The study further showed that the antiviral activity of the sulphated polysaccharide was associated with its interaction with the viral particles, particularly in the adsorption and internalization steps. In a subsequent study, Yim et al. (2004) showed that p-KG03 also displayed potent antiviral activity (EC50 = 26.9 μg/mL) against encephalomyocarditis virus (EMCV) without any cytotoxic effect. In addition, cell wall sulphated polysaccharide from the red microalga Porphyridium sp. was found to display strong antiviral activity against HSV-1 and 2 and Varicella zoster virus (VZV) (Huleihel et al. 2001). Another sulphated polysaccharide isolated from Navicula directa, named naviculan, was found to have antiviral activities against HSV-1 and 2, and influenza A virus (Lee et al. 2006). In addition, naviculan displayed inhibitory effect against cell–cell fusion between CD4-expressing and HIV gp160-expressing cells.
Amongst the cyanobacterial compounds, cyanovirin-N (CVN) is a potent antiviral agent against HIV that acts by inhibiting the virus cell entry in a highly specific manner (Lotfi et al. 2018). Cyanovirin-N (CVN) is a type of lectins, which are proteins of non-immune origin that are capable of recognizing and binding to glycoconjugate moieties non-covalently (Sharon and Lis 1989) There have been attempts to develop different expression systems for the recombinant production of CVN for use as an effective anti-HIV microbicide. Formulation of the microbicide can be in the form of vagina gels/rings, creams, lubricants or suppositories, delivering the active ingredients slowly during coitus or over extended periods of time.
Cyanovirin-N (CVN) was first isolated from the cultures of Nostoc ellipsosporum (Boyd et al. 1997). The protein was successfully produced recombinantly by expression of a corresponding DNA sequence in E. coli. The antiviral activity of CVN was partly due to its high-affinity interactions with the viral surface envelope glycoprotein gp120. Cyanovirin-N (CVN) has also been found to display antiviral activity against Ebola (Barrientos et al. 2003) and influenza virus (O’Keefe et al. 2003). The potency of CVN against viruses is attributed to the N-linked oligosaccharides with high mannose content that interact with the viral glycoproteins (Barrientos et al. 2006). This shows that the antiviral activity of CVN is not due to its induction of viral particle agglutination, but via inhibition of viral entry.
Other lectins from cyanobacteria which have been shown to exhibit anti-HIV activity include scytovirin (SVN) from Scytonema varium (Bokesch et al. 2003) and agglutinin from Oscillatoria agardhii (OAA) (Sato et al. 2007). O’keefe et al. (2011) have filed a patent on the use scytovirin for treating infections caused by high mannose-enveloped viruses including hepatitis C virus (HCV). Recently, Siqueira et al. (2018) performed a genomic screening of new putative antiviral lectins from the genomic sequences of Amazonian cyanobacteria using a bioinformatics approach. Amongst the seven strains of cyanobacteria screened, 75 unique coding sequences for one or more lectin domains were identified. Using homology modelling and molecular dynamics simulations, the study discovered that Nostoc sp. CACIAM 19 and Tolypothrix sp. CCAM 22 strains presented cyanovirin-N homologues.
2.3 Immunomodulatory Activity
Several bioactive compounds from microalgae have been shown to possess immunomodulatory activity, especially anti-inflammatory effect. For instance, lycopene (cis/all-trans 40:60) from Chlorella marina was found to show significant antioxidant and anti-inflammatory effect in high-cholesterol fed rats (Renju et al. 2014a). Inflammatory biomarkers such as cyclooxygenase, 15-lipoxygenase and myeloperoxidase activity, and C-reactive protein and ceruloplasmin levels in serum were found to decrease significantly in rats fed algal lycopene. In another study, lycopene from Chlorella marina was found to reduce significantly inflammatory marker enzymes such as cyclooxygenase, lipoxygenase and myeloperoxidase in an arthritis rat model, compared to tomato lycopene and the anti-inflammatory drug indomethacin (Renju et al. 2013). There was also a marked reduction in oedema of paw and joint tissues in the rats supplemented with the algal lycopene. The authors further suggest that algal lycopene could be a potential agent for the treatment of anti-inflammatory diseases such as arthritis. In addition, violaxanthin isolated from Chlorella ellipsoidea was found to significantly inhibit nuclear factor-κB (NF-κB) pathways in exerting its anti-inflammatory effect against lipopolysaccharide (LPS)-stimulated RAW 264.7 mouse macrophage cells (Soontornchaiboon et al. 2012).
Another pigment which has been shown to have immunomodulatory effect is C-phycocyanin, which is one of the major pigments in cyanobacteria, especially Spirulina. C-phycocyanin has been shown to display inhibitory activity against allergic responses, such as ear swelling, skin reactions and histamine release from mast cells in a rat model (Remirez et al. 2002). In addition, C-phycocyanin was found to reduce allergic inflammation by suppressing antigen-specific IgE response in a mouse model (Nemoto-Kawamura et al. 2004). In another study, water extract from Botryococcus braunii exerted anti-inflammatory effect by inhibiting the expression of nitric oxide synthase (iNOS) gene and the consequent production of nitric oxide (NO) under oxidative stress in RAW 264.7 murine macrophages (Buono et al. 2012). Recently, Wang et al. (2018b) showed that protein-rich materials after extraction of DHA from bioengineered Schizochytrium sp. ameliorated bowel inflammation in mice. The protein hydrolysate attenuated the induction of pro-inflammatory cytokines and increased the induction of anti-inflammatory cytokines. In addition, the protein hydrolysate promoted the proliferation of colonic crypt stem cells and progenitor cells required for crypt repair. In another study, feeding of Schizochytrium oil rich in DHA has been shown to increase plasma level of the anti-inflammatory cytokine TGF-β1 (Komprda et al. 2016). In another study, supplementation of Spirulina was found to enhance primary immune response in terms of antibody production following tetanus toxoid vaccination in a mouse model (Chu et al. 2013).
There has also been interest in screening of microalgae for compounds for skin health, including anti-inflammatory, wound-healing and antioxidative effects (Kim et al. 2018). For instance, Hidalgo-Lucas et al. (2014a) assessed the beneficial effect of a formulation based on Chlorella sorokiniana (ROQUETTE Chlorella sp., RC) on two dermatological disorder models in mice, namely skin inflammation and wound healing. Oral and topical administration of RC at high doses showed significant effects in reducing skin inflammation and had efficient effect on wound-healing process. Similarly, topical and oral administration of formulation based on Schizochytrium (ROQUETTE Schizochytrium sp.) had significant effects on macroscopic score of skin inflammation and efficient effect on healing process, especially at high doses (Hidalgo-Lucas et al. 2014b). In addition, supplementation of Chlorella vulgaris has been able to reduce atopic dermatitis-like symptoms, including dermatitis scores, epidermal thickness and skin hydration, in a mouse model (Kang et al. 2015). In the same study, treatment of Chlorella vulgaris was found to downregulate serum levels of thymus- and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC). Also, the treatment downregulated mRNA expression levels of IL-4 and IFN-γ. In another study, sulphated polysaccharide of Porphyridium was found to be an effective anti-inflammatory agent for topical use. Topical application of the polysaccharide on human subjects was found to inhibit the development of erythema induced by a powerful irritant (Matsui et al. 2003).
2.4 Anticancer Activity
Cancer is the second leading cause of death globally, accounting for an estimate of 9.6 million deaths in 2018 (WHO 2018). There has been much interest in exploring for new therapeutic agents for cancer, particularly from natural products, in view of the side effects posed by the current treatment strategies (Pádua et al. 2015) and the development of resistance of tumour cells against currently available drugs (Li et al. 2018). Microalgae are a potential source of anticancer compounds that have yet to be fully explored (Abd El-Hack et al. 2018). Potential anticancer compounds from microalgae include pigments (C-phycocyanin, astaxanthin and fucoxanthin), bioactive peptides, lipid compounds, alkaloids and dinoflagellate toxins (Table 12.3). The anticancer effects of the microalgal compounds are mediated through their action in inducing cytotoxicity, downregulating invasion of tumour cells, and enhancing cancer cell apoptosis.
A wide range of novel metabolites, especially bioactive peptides, from cyanobacteria have been shown to display anticancer activity (Gerwick and Moore 2012). For instance, symplopastin 1, a linear peptide isolated from Symploca hydroides, was found to be a potent microtubule inhibitor that was effective against drug-insensitive mammary tumour and drug-insensitive colon tumour (Luesch et al. 2001). In addition, TZT-1027 (auristatin PE), an analogue of dolastatin (linear peptide), was tested in phase 1 clinical trials due to its good preclinical activity, particularly its antitumour activity against breast carcinoma and lung carcinoma in mice (Kobayashi et al. 1997). In another development, a drug named brentuximab vedotin (Fig. 12.3) developed based on antibody conjugated to dolastatin 10 has been approved by the US FDA for treating Hodgkin’s lymphoma and anaplastic large-cell lymphoma (Gerwick and Moore 2012). Recently, a patent on novel derivatives of dolastatin 10 and auristatins, particularly on their methods of production and use as medicinal products in the treatment of cancer, was filed (Perez et al. 2018).
Brentuximab vedotin, conjugation of antibody with dolastatin 10 (cyanobacterial metabolite), is a drug that has been approved by the US FDA for the treatment of Hodgkin’s lymphoma (Alves et al. 2018)
Cryptophycin-52 (LY355703), an analogue of the cyanobacterial depsipeptide cryptophycin, is another compound that has been brought into phase 1 clinical trial (Sessa et al. 2002). The trial was on patients with advanced solid tumour; however, adverse effects related to neuropathy and myalgia were reported. This was followed by a multi-centre phase 2 clinical trial on patients with non-small-cell-lung cancer (NSCLC) (Edelman et al. 2003). However, the results showed that cryptophycin-52 only has limited activity in NSCLC cells.
Other metabolites from cyanobacteria that have been found to display anticancer activity include apratoxin A, tricophycin A, calothrixin A and hapalindole H (Table 12.3). For instance, apratoxin A, a cyclic depsipeptide isolated from Lyngbya spp. (=Moorea spp.), was found to display potent inhibitory activity against cancer cell growth by inducing apoptosis and cell cycle arrest at G1 phase (Luesch et al. 2006). In addition, apratoxin A was found to show potent antitumour activity against U2OS osteosarcoma cells and this was attributed to its inhibitory action on the cellular secretory pathways (Liu et al. 2009). Recently, Cai et al. (2018) synthesized an analogue of apratoxin, named apratoxin 10 (Apra S10), and demonstrated its potential as an antipancreatic cancer agent using an orthotopic pancreatic patient-derived xenograft mouse model. The compound inhibited growth of pancreatic cancer cells via downregulation of multiple receptor tyrosine kinase and inhibition of growth factor and cytokine secretion.
Trichophycin A, a polycavernoside (polyketide) isolated from Trichodesmium thiebautii, was found to exhibit cytotoxic activity against neuro-2a-neuroblastoma and human colon cancer cell lines (Bertin et al. 2017). In addition, calothrixin A, a phenanthridine alkaloid isolated from Calothrix sp., was found to induce apoptosis in human Jurkat cancer cells and caused G2/M cell cycle arrest (Chen et al. 2003). Recently, Acuna et al. (2018) found that hapalindole H, an alkaloid compound isolated from Fischerella muscicola, displayed selective cytotoxicity against PC-3 prostate cancer cells.
The cyanobacterial pigment C-phycocyanin is another potential compound for the development of anticancer agent (Beata and Katalin 2017). The cell targets of C-phycocyanin include MDR1 gene, cytoskeletal proteins and COX-2 enzymes, making it capable of killing drug-resistant cancer cells (Fernandes et al. 2018). C-phycocyanin has been shown to induce apoptosis and cell cycle arrest as well as suppress cell migration, proliferation and colony formation ability of NSCLC cells by regulating multiple key genes (Hao et al. 2018). In addition, C-phycocyanin was found to show potent anticancer activity against triple-negative MDA-MB-231 breast cancer cells (Ravi et al. 2015). In another study, C-phycocyanin from Spirulina platensis was found to display antiangiogenesis effect on B16-F10 melanoma tumours in a C57BL/6 mouse model (Dibaei et al. 2018). Recently, Liu et al. (2018b) demonstrated that selenium-enriched phycocyanin (Se-PC), when applied via photodynamic therapy approach, was effective in inhibiting tumour development in a mouse liver cancer model.
There have been many studies that evaluated the anticancer activity of algal carotenoids in extract or purified form (Gateau et al. 2017; Cha et al. 2008). For instance, Cha et al. (2008) demonstrated that extracts from Chlorella vulgaris and Chlorella ellipsoidea, containing mainly violaxanthin and lutein, inhibited the growth of HCT116 human colon cells and enhanced apoptosis. In another study, lycopene from Chlorella marina was found to display stronger inhibitory effect against the growth and colony formation of human prostate cancer cells than the carotene from tomatoes (Renju et al. 2014b).
Astaxanthin and fucoxanthin are amongst the major algal carotenoids that have been widely studied for their anticancer activity. Astaxanthin is a keto-carotenoid produced by Haematococcus pluvialis, especially when it is under unfavourable growth conditions. The carotenoid is accumulated outside the chloroplast and the content can reach up to 4.5% dry weight in Haematococcus pluvialis (Boussiba et al. 1999). The anticancer effect of astaxanthin is suggested to be due to its inflammation and oxidative stress-reducing properties (McCall et al. 2018). In a recent study, McCall et al. (2018) demonstrated that treatment of astaxanthin significantly reduced proliferation rates and migration of breast cancer cells. In another study, Rao et al. (2013) demonstrated that mono- and diester forms of astaxanthin were more potent than the unesterified form of the xanthophyll in reducing UV-DBMA-induced skin cancer in a rat model. In addition, astaxanthin was found to be able to resensitize gemcitabine-resistant human pancreatic cancer cells (GR-HPCC) to gemcitabine (Yan et al. 2017). In addition, Palozza et al. (2009) showed that an astaxanthin-rich lipid CO2 extract from Haematococcus pluvialis inhibited the growth of human colon cancer cell lines. The use of Haematococcus extract instead of purified astaxanthin is attractive, as it reduces the high costs involved in the production, isolation and purification of the carotenoid, which may limit its application in cancer therapy (Palozza et al. 2009).
Fucoxanthin is another algal carotenoid that has been shown to display anticancer activity (Nakazawa et al. 2009; Kim et al. 2010, 2013; Jin et al. 2018; Wang et al. 2018a). While brown seaweeds are the major producers of fucoxanthin, marine microalgae such as Phaeodactylum tricornutum and Isochrysis galbana have also been shown to have the potential for commercial production of the carotenoid (Kim et al. 2012c, d). Fucoxanthin was found to induce reactive oxygen species (ROS) production, promoting apoptosis in HL-60 human leukaemia cells (Kim et al. 2010). In addition, the carotenoid was shown to inhibit growth of other cancerous cells including colon cancer (Caco-2) and prostate cancer (PC-3 and LNCaP) cells by inducing apoptosis (Nakazawa et al. 2009). Recently, Jin et al. (2018) demonstrated that combination of fucoxanthin with tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) exerted a strong synergistic effect on apoptosis in human cervical cancer cells by targeting the P13K/Akt/NF-κβ signalling pathway. In another study, Wang et al. (2012b) demonstrated that fucoxanthin could significantly inhibit the growth of sarcoma in xenografted sarcoma 180 (S180) mice. Another in vivo model study showed that the administration of fucoxanthin could significantly inhibit the growth of tumour mass in mice implanted with melanoma B16F10 cells (Kim et al. 2013).
Several toxins produced by dinoflagellates including amphidinolides (APDN), amphidinols (APDL), karlotoxins (KTX), pectenotoxins (PTX) and yessotoxin (YTX) have been shown to display anticancer activity (Leira et al. 2002; Kobayashi and Tsuda 2004; Chae et al. 2005; Waters et al. 2010; Espiritu et al. 2017). For instance, APDN produced by dinoflagellates of the genus Amphidinium displayed strong cytotoxicity against murine lymphoma L1210 and human epidermoid carcinoma KB cell lines (Kobayashi and Tsuda 2004). A study on amphidinol-2 (AM-2) showed that the compound displayed cytotoxicity in HCT-116, HT-29 and MCF-7 cancer cell lines and upregulated the pre-apoptosis markers cfos and cjun in those cell lines (Espiritu et al. 2017). Karlotoxins (KTX), produced by Karlodinium venefictum, are potentially useful for lowering cholesterol or targeting cancer cells high in cholesterol (Waters et al. 2010). Pectenotoxins (PTX) are a group of toxins produced by dinoflagellates of the genus Dinophysis that cause diarrhetic shellfish poisoning (PSP) (Miles et al. 2006). The toxin was found to be highly effective in activating an intrinsic apoptosis pathway in p53-deficient tumour cells (Chae et al. 2005). Yessotoxin (YTX), a polyether toxin produced by Prorocentrum and Gonnyaulax, was found to induce apoptotic changes in BE(2)-M17 neuroblastoma cells (Leira et al. 2002).
Some of the microalgal polysaccharides have also been found to have anticancer activity. For instance, an extracellular polysaccharide (D-galactan sulphate) isolated from the toxic dinoflagellate Gymnodinium sp. A3 (GA3), exhibited cytotoxic effect against a panel of cancer cells (Umemura et al. 2003). The polysaccharide was found to exert its effect by inhibiting DNA topoisomerase I and II. In addition, cell wall sulphated polysaccharide from Porphyridium cruentum was found to exhibit strong antitumour activity against Graffi myeloid tumour in hamsters (Gardeva et al. 2014). The anticancer activity of the sulphated polysaccharide could be associated with its immunostimulating effect as well as with its direct cytotoxic properties.
Several lipid compounds from microalgae have been shown to display anticancer activity. For instance, nonyl 8-acetoxy-6-methyloctanate (NAMC), which is a novel fatty alcohol ester isolated from Phaeodactylum tricornutum, displayed strong suppression on the growth of HL-60 leukaemia cells by upregulating apoptotic pathways (Samarakoon et al. 2014). In addition, curacin A, a lipid compound isolated from Lyngbya masjucula, is another potent agent found to have selective antiproliferative activity against colon, renal and breast cancer cell lines (Gerwick et al. 1994). The compound acts by suppressing the binding of tubulin to colchicine. Another lipid compound, sigmasterol, isolated from Navicula incerta showed potent apoptotic effect against HepG2 liver cancer cells (Kim et al. 2014). Further, polyunsaturated aldehydyes (PUA), an allelopathic agent produced by diatoms, were reported to display cytotoxic effect against A549 lung cancer and COLO 205 colon cancer cell lines (Sansone et al. 2014). In a recent study, supplementation of algal oil rich in omega-3 PUFA was found to significantly suppress pulmonary metastases and outgrowth of melanoma cells in a mouse model (C57BL/6) (Tan et al. 2018).
2.5 Beneficial Effects Against Metabolic Disorders and Other Diseases
Several reports have highlighted the beneficial effects of consuming microalgae as a functional food in ameliorating metabolic diseases such as hyperlipidaemia, diabetes and obesity. For instance, feeding of rats with diets containing dietary fibre from pelleted red microalgal biomass (Porphyridium sp.) or their sulphated polysaccharides was found to significantly lower serum cholesterol levels (Dvir et al. 2000). In addition, the sulphated polysaccharides increased mucosa and muscularis cross-sectional area of the jejunum and caused hypertrophy in the muscularis layer. Similarly, feeding of Spirulina platensis concentrate was found to have hypocholesterolaemic effect in rats and the action was suggested to be due to the action of C-phycocyanin in inhibiting both jejunal cholesterol absorption and ileal bile acid reabsorption (Nagaoka et al. 2005).
There have also been animal model studies which showed that consumption of microalgae (whole biomass or extract) could have antidiabetic effect. For instance, algal extract prepared from Nannochloropsis oculata was found to lower glucose and C-reactive protein levels and restore insulin to normal level in streptozotocin-induced diabetic rats (Aboulthana et al. 2018). A study on diabetic rats showed that oral supplementation of Spirulina platensis could lead to an increase in trace minerals and antioxidant enzymes and lowering of plasma concentrations of glucose and inflammatory markers such as TNF-α and IL-6 (Nasirian et al. 2018). Another study showed that supplementation of omega-3 fatty acids extracted from microalgae could prevent the appearance of health complications caused by inflammatory states in diabetic rats (Gutierrez-Pliego et al. 2018). Recently, Zhang et al. (2018) demonstrated that administration of fucoxanthin significantly improved glucose/lipid metabolism and insulin resistance and prevented pancreatic histological changes in a diabetic mouse model. In addition, administration of astaxanthin has been shown to prevent the progression of diabetic nephropathy induced by oxidative stress in a mouse model (Naito et al. 2004).
Another metabolic disorder that is of great concern is obesity, as it has become a global threat to public health and imposes a huge expenditure on healthcare costs (Chu and Phang 2016). Amongst the algal compounds, fucoxanthin is one of the most well studied in terms of antiobesity effect (Gammone and D’Orazio 2015). Feeding of fucoxanthin was found to reduce the levels of inflammatory markers such as interleukin-18 (IL-18), TNF-α, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in an obese mouse model (Tan and Hou 2014). In addition, a combination of fucoxanthin and conjugated linoleic acid was found to reduce serum levels of triacylglycerols, glucose and leptin in diet-induced obese rats (Hu et al. 2012). Fucoxanthin also affects hepatic lipid contents by regulating metabolic enzyme activities and stimulating fatty acid oxidation activity (Woo et al. 2010). In addition, fucoxanthin exerts its antiobesity effect by inducing uncoupling protein 1 (UCP-1) expression in white adipose tissue (WAT), which enhances energy dissipation through fatty acid oxidation and heat production (Maeda 2015).
Long-chain omega-3 PUFA such as EPA and docosahexaenoic acid (DHA) has long been recognized to provide significant health benefits, particularly in reducing cardiac diseases such as arrythmia, stroke and hypertension (Romieu et al. 2005; von Schacky 2008; Ulmann et al. 2017). Dietary DHA has also been shown to be important for proper development of the brain and eye (Ward and Singh 2005). While fish oil is the major dietary source of EPA and DHA, it is not suitable for vegetarians and the odour makes it unattractive. Furthermore, there is concern over the decreasing global stocks of fish as sustainable sources of EPA and DHA for human nutrition (Ulmann et al. 2017). There are a variety of alternative sources of EPA and DHA including microalgae that are being explored for commercial production (Adarme-Vega et al. 2012). Amongst the microalgae, species of Thraustochytrium, Schizochytrium and Crypthecodinium are rich in DHA, while those of Phaeodactylum and Monodus are good sources of EPA (Ward and Singh 2005). In addition, the diatom Odontella aurita is another source of EPA, which has been commercialized as a dietary supplement (Mimouni et al. 2012). Algal DHA from Crypthecodinium cohnii produced by Martek Inc. has received approval for inclusion in infant formulae in the US (Ward and Singh 2005).
2.6 Other Bioactivities
Microalgae are known to produce a variety of compounds with antioxidative activity, particularly carotenoids such as astaxanthin, β-carotene and lutein (Chu 2011; Gateau et al. 2017). Such carotenoids are not only consumed as dietary supplement but also as natural food additives, which are better accepted by consumers than synthetic ones (Gateau et al. 2017). Amongst the carotenoids, astaxanthin is a very powerful antioxidant, as its activity is ten times more than other carotenoids such as zeaxanthin, lutein, canthaxanthin and β-carotene, and 100 times higher than α-tocopherol (Higuera-Ciapara et al. 2006). The protective effects of astaxanthin against diseases such as cancer, inflammatory diseases, diabetes and cardiovascular disease involve antioxidative mechanisms that prevent oxidative damage to cells (Ambati et al. 2014).
Among the carotenoids, consumption of lutein and zeaxanthin has been shown to be beneficial in reducing the development and progression of age-related macular degeneration (AMD) (Carpentier et al. 2009). Lutein and zeaxanthin are the two major macular pigments in the retina, which function as blue light filters and antioxidants. Increasing age has been proven to be a risk factor for the development and progression of AMD (Carpentier et al. 2009). Lutein is currently produced from marigold oleoresin, but several microalgae have been shown to contain higher amounts of the pigment than most marigold cultivars (Fernandez-Sevilla et al. 2010). The potential microalgal producers of lutein include Muriellopsis sp. (Del Campo et al. 2001), Scenedesmus almeriensis (Sánchez et al. 2008) and Chlorella protothecoides (Shi and Chen 2002).
In another development, there have been several reports on the beneficial effects of microalgae consumption in enhancing cognitive activity and ameliorating memory loss. For instance, in a recent study, Choi et al. (2018a) showed that extract from Spirulina maxima fermented with Lactobacillus planterium HY-08 could ameliorate scopolamide-induced memory impairment in mice. The efficacy of the fermented extract was suggested to be due to the synergistic effects of β-carotene and other bioactive substances, particularly in increasing extracellular signal-regulated kinases (ERK)-signalling and inducing expression of p-cAMP response element-binding protein (p-CREB) and brain-derived neurotrophic factor (BDNF). In another study, feeding of Chlorella-supplemented diet was found to reduce oxidative stress and prevent the decline in memory loss in a transgenic mouse model of age-dependent dementia (Nakashima et al. 2009). The studies by both Choi et al. (2018a) and Nakashima et al. (2009) showed the beneficial effects of Chlorella and Spirulina as functional food in enhancing cognitive activities. In both studies, algal extract or whole algal meal was tested rather than pure compounds.
3 Mass Culture of Microalgae for the Production of Pharmaceuticals and Nutraceuticals
Scaling up of microalgal cultures is an important aspect that needs to be considered in commercial production of pharmaceuticals and nutraceuticals from microalgae, as critically reviewed by Borowitzka and Vonshak (2017). It has been estimated that to produce 1 tonne/year of protein, about 18,300 L of culture are required, while production of β-carotene requires about 182,600 L of culture and fucoxanthin would require about 548,000 L. Most commercial production of microalgae is operated using outdoor open ponds due to its lower costs rather than closed systems, although the processing conditions are far from being optimal (Guedes et al. 2011). However, open pond systems have numerous issues including the difficulty in controlling temperature, light distribution and CO2 concentration, possibility of microbial contamination, and low biomass productivity (Guedes et al. 2011; Borowitzka and Vonshak 2017). Of great concern is microbial contamination, as it may compromise the quality of the final products. Thus, the production of high-value pharmaceuticals and nutraceuticals needs to be operated using photobioreactors (PBR). Closed systems using PBR allow the culture to be well controlled with the benefits of low contamination risk, high biomass productivity and high CO2 fixation efficiency (Wang et al. 2012a).
Many different types of PBR have been developed since the first flat panel PBR described by Burlew (1953). There are a variety of reactor designs including vertical column, tubular and flat panel PBR. Some examples of PBR used for the culturing of microalgae to produce pharmaceuticals and nutraceuticals are given in Table 12.4. Tubular PBR, which consist of an array of transparent tubes built in straight, bent or spiral patterns are amongst the most popular configuration (Wang et al. 2011). Using a Computational Fluid Dynamics (CFD) simulation approach, Guler et al. (2019) compared the performance of three types of PBR, namely flat plate, airlift and stirred tank for biomass and fucoxanthin production from Phaeodactylum tricornutum. The authors found that the highest biomass and fucoxanthin production was from the cultures grown in the flat-plate PBR.
There are also instances where both open and closed systems are integrated for commercial production of high-value chemicals from microalgae. For instance, an outdoor culture system consisting of both PBR and open pond was successfully developed to generate dense biomass of Haematococcus pluvialis for the extraction of astaxanthin (Olaizola 2000). In this system, the microalga was first grown in an outdoor PBR (25,000 L) before being transferred daily to an open pond system, where astaxanthin accumulation was induced. In a recent study, a hybrid system consisting of open pond and PBR, with culture medium circulating between both, was used to grow the oleaginous microalga Scenedesmus dimorphus under indoor and outdoor conditions (Liu et al. 2018a). The algal biomass productivity of the hybrid system increased 46.3–74.3% compared with the open pond and was 12.5% higher than that of PBR. Recently, Wang et al. (2018a) set up a pilot plant of 20 specially designed polyethylene phytobags (volume 150 L each) for the culturing of Phaeodactylum tricornutum to produce EPA. The EPA was shown to have potent antibacterial activity and have potential use as a feed supplement. In a recent development, Dunaliella salina was successfully grown on seawater desalination concentrate in floating PBR anchored on the ocean field of Lingshui Bay in China for the production of β-carotene (Zhu et al. 2018). The floating PBR system is an innovative design as the ocean provides a natural habitat with large cultivable area for microalgae and it facilitates temperature control due to the high specific heat capacity of seawater (Kim et al. 2016). In addition, the energy from waves can be used to mix the culture.
There have been reports on the successful cultivation of microalgae for products such as carotenoids and DHA under heterotrophic condition. For instance, Chlorella protothecoides grown heterotrophically on glucose and urea in fermenters scaled up to 30 L produced high biomass concentration (45.8 g/L) and attained high lutein productivity (49.18 mg/L/day) (Shi and Chen 2002). Similarly, Crypthecodinium cohnii grown on acetic acid (feeding rate of 50% w/w) on a fed-batch mode under heterotrophic condition afforded final concentrations of 109 g/L dry biomass, 61 g/L lipids and 19 g/L DHA (de Swaaf et al. 2003). The use of fermentation strategy under heterotrophic mode is an attractive strategy to reduce costs in producing high-density algal biomass without the need of light. However, not all microalgae or microalgal products can be produced this way (Borowitzka 1999).
Dinoflagellate toxins have great potential in pharmaceutical applications, as discussed in the previous sections. However, only few of those compounds have led to commercial products. The main challenge faced is to generate sufficient biomass for the extraction of the bioactive compounds (Camacho et al. 2007). While producing large quantities of other microalgae such as Chlorella, Spirulina, Dunaliella salina and Haematococcus pluvialis has proved to be successful (Borowitzka 1999; Ben-Amotz 2004; Belay 2013), culturing of dinoflagellates poses new challenges. The growth rates of dinoflagellates are lower than typical microalgae and the biomass attained are usually low. As the toxin concentrations are very low, extremely large volumes of cultures are required for the extraction process (Camacho et al. 2007).
The design of PBR for the culturing of dinoflagellates needs to consider the extreme sensitivity of such microalgae to shear stress and hydrodynamic forces (Cao et al. 2015; Gallardo-Rodriguez et al. 2016). The levels of shear stress that dinoflagellates can withstand are two orders of magnitude lower than most animal and plant cells (Chisti 1999; Juhl et al. 2000). In one study, García Camacho et al. (2007) showed that the tolerance of Protoceratium reticulatum, a producer of yessotoxin, to shear stress was very low compared to other dinoflagellates and animal cells. The dinoflagellate cells were damaged to varying degrees under low shear stress of >0.12 to 0.20 mN/m2. Turbulence due to agitation, shaking, aeration and stirring has been reported to inhibit the growth of dinoflagellate cultures (Pollingher and Zemel 1981; Berdalet 1992; Juhl et al. 2001).
Despite the challenges, there have been several successful ventures in mass culturing of dinoflagellates to produce bioactive compounds. For instance, a pilot-scale bioprocess was developed for the production of karlotoxin-enriched extracts of Karlodinium veneficum using a bubble column and a flat-panel PBR (80–281 L) under outdoor conditions (Lopez-Rosales et al. 2018). The bubble column PBR proved to be a better culture system than the flat panel PBR based on the maximum cell productivity attained (58 × 103 cells/mL/day). In another pilot-scale study, Molina-Miras et al. (2018) developed a LED-illuminated bubble column PBR (80 L) to culture Amphidinium carterae for the production of amphidinols (APDN). As APDN were excreted by cells into the medium, a dry APDN-enriched extract (49 mg/L) was recovered from the supernatant.
Integration of bioprocessing of the algal biomass for bioactive compound production with other applications such as biofuel production, CO2 fixation and bioremediation based on a biorefinery concept is an attractive approach to improve economic competitiveness (Giraldo-Calderón et al. 2018; Costa et al. 2019). Figure 12.4 illustrates how the various applications of microalgae can be integrated using a biorefinery approach. An example of how this concept is applied is the culturing of Haematococcus pluvialis in bubble column reactors to remove flue gas, attaining high biomass and astaxanthin productivity (Choi et al. 2018b). In another set-up, Spirulina platensis grown in a flat-plate PBR was used to produce C-phycocyanin and simultaneously mitigate CO2 emissions during its growth (Chen et al. 2013).
Another example of biorefinery process, focusing on the downstream processing aspect, was reported for the production of fucoxanthin by Isochrysis galbana (Gilbert-López et al. 2015). A green chemistry approach was used to extract a variety of products from the microalgal biomass. The process involved first the extraction of fucoxanthin using supercritical CO2, followed by recovering other fractions with high antioxidant activity and, eventually, carbohydrates and proteins from the residual biomass. In another biorefinery process, lipids and carotenoids were extracted from Nannochloropsis sp. using supercritical CO2 and the residual biomass was subjected to dark fermentation for production of biohydrogen (Nobre et al. 2013). Details of various aspects of biorefinery for Nannochloropsis in relation to induction, harvesting and extraction of EPA-oil and other products such as high-value proteins were recently reviewed by Chua and Schenk (2017).
The use of wastewater as a low-cost substrate for the culturing of microalgae can be an important component of the biorefinery approach. For instance, Olguin (2012) highlighted the potential of growing Spirulina in seawater supplemented with anaerobically digested piggery waste for waste treatment and production of biogas, biodiesel and high-value chemicals such as C-phycocyanin using the biorefinery approach. However, strict regulatory requirements need to be complied with in ensuring that products derived from microalgae in wastewater are free from potential impurities if they are to be marketed as pharmaceuticals or nutraceuticals (Borowitzka and Vonshak 2017). Efficient extraction technologies (e.g. supercritical fluid extraction and enzyme-assisted extraction) are crucial in order to attain high yields and to obtain products of high purity (Sosa-Hernandez et al. 2018).
4 Future Directions of Research
The pharmaceutical and nutraceutical industries based on microalgae are still confined to a few well-established products such as astaxanthin and C-phycocyanin. Also, commercial production of microalgae as functional food is based on only a few species, notably Spirulina platensis, Haematococcus pluvialis, Chlorella spp. and Dunaliella salina. As highlighted in this chapter, there is a diverse range of potential bioactive compounds that have been discovered from microalgae. However, there are many hurdles that need to be overcome before such compounds can be further developed for the pharmaceutical and nutraceutical industries.
Despite the many reported studies on bioactive compounds from microalgae, most have been exploratory, focusing mainly on mechanistic aspects confining to laboratory investigations. Most studies on the bioactivities of microalgal compounds are still at the pre-clinical stage based on cell lines or animal models. Very few clinical trials have been conducted to validate the efficacy of such compounds in human subjects. Dolastatin 10 (Vaishampayan et al. 2000) and cryptophycin 52 (Edelman et al. 2003) are amongst the microalgal compounds that have been brought to clinical trials to evaluate their anticancer activity. There have been more clinical trials on astaxanthin compared to other microalgal compounds. The clinical trials on astaxanthin have been conducted on human subjects to assess its hypolipidaemic, hypotensive and antioxidant effects (Ambati et al. 2014). Despite the potential anticancer activity of astaxanthin, as highlighted in this chapter, there have been not been any clinical trials of astaxanthin conducted on cancer patients. So far, there has been only one drug developed from microalgal compound, brentuximab vedotin (dolastatin conjugated with antibody), which has been approved as a cancer therapeutic agent (Gerwick and Moore 2012). More clinical trials on microalgal compounds are urgently required before new therapeutic agents can be brought to the market.
Scaling up of microalgal cultures to ensure sufficient supply of biomass for the extraction of bioactive compounds is a crucial part in the development of the pharmaceutical and nutraceutical industries based on microalgae (Borowitzka and Vonshak 2017). Although many microalgae have been identified as a source of bioactive compounds, very few have been evaluated beyond laboratory culture conditions. The potential species should be assessed for their performance when grown on a large scale under outdoor conditions. An example of such large-scale study using 10 pilot-scale trials involving high-rate algae ponds (HRAP) and PBR (flat-panel and tubular forms) was reported by Wolf et al. (2016). The aim was to assess the interaction between solar energy input, ambient temperature and system surface area-to-volume ratio in influencing yield and areal and volumetric productivities of Chlorella sorokiniana and Chlorella sp. cultured in those systems. Moving forward, new tools such as artificial intelligence (AI), particularly neural learning and statistical and evolutionary learning-based techniques, should be applied in optimizing productivity and costs in mass culture of microalgae (Jha et al. 2017b). The AI tools that can be applied involve statistical models such as Bayesian and naïve Bayes, clustering, hidden Markov and nearest-neighbour models.
The use of molecular approaches, particularly “omics’” tools, to enhance production of the desired microalgal metabolites is another area that has seen much advances in recent years (Guarnieri and Pienkos 2015). Microalgae are now regarded as highly attractive candidates for development as microbial factories. More studies are needed to unravel the biosynthetic pathways for the high-value metabolites in microalgae. Metabolomics approach will be useful for a comprehensive analysis of the secondary metabolites and elucidation of the pathways involved in the synthesis of the novel compounds in microalgae (Prakash et al. 2018). Metabolic and genetic engineering can then be applied to manipulate production of the microalgal metabolites. For instance, modification of the carotenoid biosynthesis pathway by genetic engineering has resulted in an increase of 67% astaxanthin production in the transformed strains of Haematococcus pluvialis compared to the wild-type (Galarza et al. 2018). Genome mining is another approach that is worth further exploring in attempts to discover novel metabolites in microalgae for the development of new drugs. Using this approach, instead of whole-genome sequencing, it allows rapid identification of gene clusters that code for specific compounds (Shih et al. 2013). The chemical structure of the compound can then be predicted based on the sequence information using in silico modelling.
Improving the delivery and bioavailability of bioactive compounds from microalgae is another area that warrants further investigations. This area is particularly relevant to microalgal products such as astaxanthin, which is already in the market, and potential new drugs intended for commercialization. For instance, the low bioavailability of astaxanthin may result in low assimilation into the body system, reducing its effectiveness as a supplement (Anarjan et al. 2013). There have been some recent studies that aimed to develop astaxanthin formulations in encapsulated forms to enhance its bioavailability (Khalid and Barrow 2018; Niizawa et al. 2018). In another line of development, nanoformulations using a wide variety of nanoparticles and their polymerized forms is an emerging approach in the development of new cancer drugs (Bajpai et al. 2018). For instance, a carbon-based nano-delivery system for anticancer drugs, in combination with genetically bioengineered biosilica from Thalassiosira pseudonana, was developed to kill targeted cancer cells without any adverse effect on normal and healthy cells (Delalat et al. 2015).
5 Concluding Remarks
There are indeed a diverse range of bioactive compounds from microalgae that have great potential to be developed for pharmaceuticals and nutraceutical applications. The bioactivities of microalgal metabolites that have attracted much interest include anticancer, antibacterial, antiviral and immunomodulatory activities, and their beneficial effects in ameliorating metabolic disorders such as diabetes, obesity and hyperlipidaemia. However, there are only a few pharmaceuticals and nutraceuticals derived from microalgae that have been successfully brought to commercialization. Amongst the well-known commercial microalgal products include astaxanthin, fucoxanthin, C-phycocyanin, β-carotene and PUFA. Clinical trials to evaluate the efficacy and safety of other bioactive compounds from microalgae are urgently needed before the compounds can be considered for the development of new drugs. Also, the feasibility of mass culturing of the selected microalgae under outdoor conditions needs to be critically evaluated. Production of bioactive compounds integrated with other applications such as biofuel production and CO2 biofixation based on a biorefinery concept is an attractive strategy to enhance economic feasibility.
References
Abd El-Hack ME, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, Mahgoub SA, Elnesr SS, Gebriel MG (2018) Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother 111:42–50
Abdo SM, Hetta MH, El-Senousy WM, El RAS, Ali GH (2012) Antiviral activity of freshwater algae. J Appl Pharm Sci 2:21
Aboulthana WM, El-Feky AM, Ibrahim NE, Sahu RK, El-Sayed AEB (2018) Evaluation of the pancreatoprotective effect of Nannochloropsis oculata extract against streptozotocin-induced diabetes in rats. J Appl Pharm Sci. 8:46–58
Acuna UM, Mo S, Zi J, Orjala J, De Blanco EJ (2018) Hapalindole H induces apoptosis as an inhibitor of NF-kB and affects the intrinsic mitochondrial pathway in PC-3 androgen-insensitive prostate cancer cells. Anticancer Res 38:3299–3307
Adarme-Vega TC, Lim DK, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Afify AE, El Baroty GS, El Baz FK, El Baky HH, Murad SA (2018) Scenedesmus obliquus: antioxidant and antiviral activity of proteins hydrolyzed by three enzymes. J Genet Eng Biotechnol 16:399–408
Alvarez-Diaz PD, Ruiz J, Arbib Z, Barragan J, Garrido-Perez C, Perales JA (2014) Lipid production of microalga Ankistrodesmus falcatus increased by nutrient and light starvation in a two-stage cultivation process. Appl Biochem Biotechnol 174:1471–1483
Alves C, Silva J, Pinteus S, Gaspar H, Alpoim MC, Botana LM, Pedrosa R (2018) From marine origin to therapeutics: the antitumor potential of marine algae-derived compounds. Front Pharmacol 9:777
Ambati RR, Phang SM, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs 12:128–152
Anarjan N, Nehdi IA, Tan CP (2013) Influence of astaxanthin, emulsifier and organic phase concentration on physicochemical properties of astaxanthin nanodispersions. Chem Central J 7:127
Andrade LM (2018) Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements: an overview. MOJ Food Process Technol 6:45–58
Arbib Z, de Godos I, Ruiz J, Perales JA (2017) Optimization of pilot high rate algal ponds for simultaneous nutrient removal and lipids production. Sci Total Environ 589:66–72
Assunção J, Guedes A, Malcata F (2017) Biotechnological and pharmacological applications of biotoxins and other bioactive molecules from Dinoflagellates. Mar Drugs 15(12):393
Bajpai VK, Shukla S, Kang SM, Hwang SK, Song X, Huh YS, Han YK (2018) Developments of cyanobacteria for nano-marine drugs: relevance of nanoformulations in cancer therapies. Mar Drugs 16:179
Barrientos LG, O’Keefe BR, Bray M, Sanchez A, Gronenborn AM, Boyd MR (2003) Cyanovirin-N binds to the viral surface glycoprotein, GP1,2 and inhibits infectivity of Ebola virus. Antivir Res 58:47–56
Barrientos LG, Matei E, Lasala F, Delgado R, Gronenborn AM (2006) Dissecting carbohydrate-cyanovirin-N binding by structure-guided mutagenesis: functional implications for viral entry inhibition. Protein Eng Des Sel 19:525–535
Beata MK, Katalin S (2017) Phycobilins and phycobiliproteins used in food industry and medicine. Mini Rev Med Chem 17:1173–1193
Belay A (2013) Biology and industrial production of Arthrospira (Spirulina). In: Richmond A, Qiang H (eds) Handbook of microalgal culture: applied phycology and biotechnology. Wiley, Hoboken, pp 339–358
Ben-Amotz A (2004) Industrial production of microalgal cell-mass and secondary products-major industrial species: Dunaliella. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Wiley, Hoboken, pp 273–288
Berdalet E (1992) Effects of turbulence on the marine dinoflagellate Gymnodinium nelsonii 1. J Phycol 28:267–272
Bertin M, Wahome P, Zimba P, He H, Moeller P (2017) Trichophycin A, a cytotoxic linear polyketide isolated from a Trichodesmium thiebautii bloom. Mar Drugs 15:10
Bhagavathy S, Sumathi P, Bell IJ (2011) Green algae Chlorococcum humicola – a new source of bioactive compounds with antimicrobial activity. Asian Pac J Trop Biomed 1:S1–S7
Blair MF, Kokabian B, Gude VG (2014) Light and growth medium effect on Chlorella vulgaris biomass production. J Environ Chem Eng 2:665–674
Bokesch HR, O’Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, Turpin J, Watson K, Buckheit RW (2003) A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 42:2578–2584
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, and fermenters. Prog Ind Microbiol 35:313–321
Borowitzka MA, Vonshak A (2017) Scaling up microalgal cultures to commercial scale. Eur J Phycol 52:407–418
Boussiba S, Bing W, Yuan JP, Zarka A, Chen F (1999) Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol Lett 21:601–604
Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM (1997) Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother 41:1521–1530
Buono S, Langellotti AL, Martello A, Bimonte M, Tito A, Carola A, Apone F, Colucci G, Fogliano V (2012) Biological activities of dermatological interest by the water extract of the microalga Botryococcus braunii. Arch Dermatol Res 304:755–764
Burja AM, Banaigs B, Abou-Mansour E, Grant Burgess J, Wright PC (2001) Marine cyanobacteria—a prolific source of natural products. Tetrahedron 57:9347–9377
Burlew J (1953) Algal culture from laboratory to pilot plant. Carnegie Institute of Washington, Washington, DC
Cai W, Ratnayake R, Gerber MH, Chen QY, Yu Y, Derendorf H, Trevino JG, Luesch H (2018) Development of apratoxin S10 (Apra S10) as an anti-pancreatic cancer agent and its preliminary evaluation in an orthotopic patient-derived xenograft (PDX) model. Investig New Drugs 37:364–374
Caires TA, da Silva AMS, Vasconcelos VM, Affe HMJ, de Souza Neta LC, Boness HVM, Sant’Anna CL, Nunes JMC (2018) Biotechnological potential of Neolyngbya (Cyanobacteria), a new marine benthic filamentous genus from Brazil. Algal Res 36:1–9
Camacho FG, Rodriguez JG, Miron AS, Garcia MC, Belarbi EH, Chisti Y, Grima EM (2007) Biotechnological significance of toxic marine dinoflagellates. Biotechnol Adv 25:176–194
Cao Z, Li X, Zou X, Greenwood M, Gerwick WH, Murray TF (2015) Involvement of JNK and caspase activation in hoiamide A-induced neurotoxicity in neocortical neurons. Mar Drugs 13:903–919
Carpentier S, Knaus M, Suh M (2009) Associations between lutein, zeaxanthin, and age-related macular degeneration: an overview. Crit Rev Food Sci Nutr 49:313–326
Cezare-Gomes EA, Mejia-da-Silva LDC, Perez-Mora LS, Matsudo MC, Ferreira-Camargo LS, Singh AK, de Carvalho JCM (2019) Potential of microalgae carotenoids for industrial application. Appl Biochem Biotechnol 188(3):602–634
Cha KH, Koo SY, Lee DU (2008) Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agri Food Chem 56:10521–10526
Chae HD, Choi TS, Kim BM, Jung JH, Bang YJ, Shin DY (2005) Oocyte-based screening of cytokinesis inhibitors and identification of pectenotoxin-2 that induces Bim/Bax-mediated apoptosis in p53-deficient tumors. Oncogene 24:4813
Chen X, Smith GD, Waring P (2003) Human cancer cell (Jurkat) killing by the cyanobacterial metabolite calothrixin A. J Appl Phycol 15:269–277
Chen CY, Kao PC, Tsai CJ, Lee DJ, Chang JS (2013) Engineering strategies for simultaneous enhancement of C-phycocyanin production and CO2 fixation with Spirulina platensis. Bioresour Technol 145:307–312
Chisti Y (1999) Shear sensitivity. In: Flickinger MC, Drew SW (eds) Encyclopedia of bioprocess technology: fermentation, biocatalysis, and bioseparation, vol 5. Wiley, New York, pp 2379–2406
Choi WY, Kang DH, Lee HY (2018a) Effect of fermented Spirulina maxima extract on cognitive-enhancing activities in mice with scopolamine-induced dementia. Evid Based Complement Alternat Med Vol 2018, Article FD7218504, 9 p
Choi YY, Hong ME, Jin ES, Woo HM, Sim SJ (2018b) Improvement in modular scalability of polymeric thin-film photobioreactor for autotrophic culturing of Haematococcus pluvialis using industrial flue gas. Bioresour Technol 249:519–526
Chu WL (2011) Potential applications of antioxidant compounds derived from algae. Curr Top Nutra Res 9:83
Chu WL, Phang SM (2016) Marine algae as a potential source for anti-obesity agents. Mar Drugs 14:222
Chu WL, Quynh lV, Radhakrishnan AK (2013) Effect of Spirulina (Arthrospira) supplementation on the immune response to tetanus toxoid vaccination in a mouse model. J Diet Suppl 10:229–240
Chua ET, Schenk PM (2017) A biorefinery for Nannochloropsis: induction, harvesting, and extraction of EPA-rich oil and high-value protein. Bioresour Technol 244:1416–1424
Coates RC, Trentacoste E, Gerwick WH (2013) Bioactive and novel chemicals from microalgae. In: Richmond A, Qiang H (eds) Handbook of microalgal culture: applied phycology and biotechnology. Wiley, Hoboken, pp 504–531
Costa JAV, Freitas BCBD, Lisboa CR, Santos TD, Brusch LRDF, de Morais MG (2019) Microalgal biorefinery from CO2 and the effects under the Blue Economy. Renew Sustain Energy Rev 99:58–65
da Silva Ferreira V, ConzFerreira ME, Lima LM, Frases S, de Souza W, Sant’Anna C (2017) Green production of microalgae-based silver chloride nanoparticles with antimicrobial activity against pathogenic bacteria. Enzym Microb Technol 97:114–121
Davoodbasha M, Edachery B, Nooruddin T, Lee SY, Kim JW (2018) An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb Pathog 115:233–238
de Clerck O, Guiry MD, Leliaert F, Samyn Y, Verbruggen H (2013) Algal taxonomy: a road to nowhere? J Phycol 49:215–225
de Jesus Raposo MF, de Morais AM, de Morais RM (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13:2967–3028
de Souza TD, Prietto L, de Souza MM, Furlong EB (2015) Profile, antioxidant potential, and applicability of phenolic compounds extracted from Spirulina platensis. Afr J Biotechnol 14:2903–2909
de Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Del Campo JA, Rodrıguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 85:289–295
Delalat B, Sheppard VC, Rasi Ghaemi S, Rao S, Prestidge CA, McPhee G, Rogers ML, Donoghue JF, Pillay V, Johns TG, Kroger N, Voelcker NH (2015) Targeted drug delivery using genetically engineered diatom biosilica. Nat Commun 6:8791
Desbois AP, Mearns-Spragg A, Smith VJ (2009) A fatty acid from the diatom Phaeodactylum tricornutum is antibacterial against diverse bacteria including multi-resistant Staphylococcus aureus (MRSA). Mar Biotechnol 11:45–52
Dewi IC, Falaise C, Hellio C, Bourgougnon N, Mouget JL (2018) Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In: Levine IA, Fleurence J (eds) Microalgae in health and disease prevention. Academic, Cambridge, pp 235–261
Dibaei F, Fazilati M, Moenzadeh F, Kafayat A, Jazayeri N, Talebi A (2018) Anti-angiogenesis effect of C-phycocyanin of Spirulina platensis on B16-F10 melanoma tumors in C57BL/6 mouse. Pathobiol Res 21:141–146
Dvir I, Chayoth R, Sod-Moriah U, Shany S, Nyska A, Stark AH, Madar Z, Arad SM (2000) Soluble polysaccharide and biomass of red microalga Porphyridium sp. alter intestinal morphology and reduce serum cholesterol in rats. Br J Nutr 84:469–476
Edelman MJ, Gandara DR, Hausner P, Israel V, Thornton D, DeSanto J, Doyle LA (2003) Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer. Lung Cancer 39:197–199
Espiritu RA, Tan MCS, Oyong GG (2017) Evaluation of the anti-cancer potential of amphidinol 2, a polyketide metabolite from the marine dinoflagellate Amphidinium klebsii. Jordan J Biol Sci 10:297–302
Fabregas J, Garcia D, Fernandez-Alonso M, Rocha AI, Gomez-Puertas P, Escribano JM, Otero A, Coll JM (1999) In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antivir Res 44:67–73
Fernandes ESE, Figueira FDS, Lettnin AP, Carrett-Dias M, Filgueira D, Kalil S, Trindade GS, Votto APS (2018) C-Phycocyanin: cellular targets, mechanisms of action and multi drug resistance in cancer. Pharmacol Rep 70:75–80
Fernandez-Sevilla JM, Acien Fernandez FG, Molina Grima E (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86:27–40
Galarza JI, Gimpel JA, Rojas V, Arredondo-Vega BO, Henríquez V (2018) Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res 31:291–297
Gallardo-Rodriguez JJ, Lopez-Rosales L, Sanchez-Miron A, Garcia-Camacho F, Molina-Grima E, Chalmers JJ (2016) New insights into shear-sensitivity in dinoflagellate microalgae. Bioresour Technol 200:699–705
Gammone MA, D’Orazio N (2015) Anti-obesity activity of the marine carotenoid fucoxanthin. Mar Drugs 13:2196–2214
Gantar M, Svircev Z (2008) Microalgae and cyanobacteria: food for thought. J Phycol 44:260–268
García Camacho F, Gallardo Rodríguez JJ, Sánchez Mirón A, Cerón García MC, Belarbi EH, Molina Grima E (2007) Determination of shear stress thresholds in toxic dinoflagellates cultured in shaken flasks. Process Biochem 42:1506–1515
Gardeva E, Toshkova R, Minkova K, Gigova L (2014) Cancer protective action of polysaccharide, derived from red microalga Porphyridium cruentum – biological background. Biotechnol Biotechnolog Equip 23:783–787
Gateau H, Solymosi K, Marchand J, Schoefs B (2017) Carotenoids of microalgae used in food industry and medicine. Mini Rev Med Chem 17:1140–1172
Gerwick WH, Moore BS (2012) Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol 19:85–98
Gerwick WH, Proteau PJ, Nagle DG, Hamel E, Blokhin A, Slate DL (1994) Structure of curacin A, a novel antimitotic, antiproliferative and brine shrimp toxic natural product from the marine cyanobacterium Lyngbya majuscula. J Org Chem 59:1243–1245
Gilbert-López B, Mendiola JA, Fontecha J, van den Broek LAM, Sijtsma L, Cifuentes A, Herrero M, Ibáñez E (2015) Downstream processing of Isochrysis galbana: a step towards microalgal biorefinery. Green Chem 17:4599–4609
Giraldo-Calderón ND, Romo-Buchelly RJ, Arbeláez-Pérez AA, Echeverri-Hincapié D, Atehortúa-Garcés L (2018) Microalgae biorefineries: applications and emerging technologies. Dyna 85:219–233
Guarnieri MT, Pienkos PT (2015) Algal omics: unlocking bioproduct diversity in algae cell factories. Photosynth Res 123:255–263
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of high added-value compounds—a brief review of recent work. Biotechnol Prog 27:597–613
Guler BA, Deniz I, Demirel Z, Oncel SS, Imamoglu E (2019) Comparison of different photobioreactor configurations and empirical computational fluid dynamics simulation for fucoxanthin production. Algal Res 37:195–204
Gutierrez-Pliego LE, Martinez-Carrillo BE, Resendiz-Albor AA, Arciniega-Martinez IM, Escoto-Herrera JA, Rosales-Gomez CA, Valdes-Ramos R (2018) Effect of supplementation with n-3 fatty acids extracted from microalgae on inflammation biomarkers from two different strains of mice. J Lipids Vol 2018, Article ID 4765358, 10 p, https://doi.org/10.1155/2018/4765358
Hao S, Yan Y, Li S, Zhao L, Zhang C, Liu L, Wang C (2018) The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar Drugs 16:178
Hayashi K, Hayashi T, Kojima I (1996) A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res Hum Retroviruses 12:1463–1471
Hidalgo-Lucas S, Bisson JF, Duffaud A, Nejdi A, Guerin-Deremaux L, Baert B, Saniez-Degrave MH, Rozan P (2014a) Benefits of oral and topical administration of ROQUETTE Chlorella sp. on skin inflammation and wound healing in mice. Anti-Inflamm Anti-Allergy Agents Med Chem 13:93–102
Hidalgo-Lucas S, Rozan P, Guerin-Deremaux L, Violle N, Baert B, Saniez-Degrave MH, Bisson JF (2014b) Oral and topical administration of ROQUETTE Schizochytrium sp. alleviate skin inflammation and improve wound healing in mice. Anti-Inflamm Anti-Allergy Agents Med Chem 13:154–164
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196
Hu X, Li Y, Li C, Fu Y, Cai F, Chen Q, Li D (2012) Combination of fucoxanthin and conjugated linoleic acid attenuates body weight gain and improves lipid metabolism in high-fat diet-induced obese rats. Arch Biochem Biophys 519:59–65
Huerlimann R, De Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257
Huleihel M, Ishanu V, Tal J, Arad SM (2001) Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J Appl Phycol 13:127–134
Jha D, Jain V, Sharma B, Kant A, Garlapati VK (2017a) Microalgae-based pharmaceuticals and nutraceuticals: an emerging field with immense market potential. ChemBioEng Rev 4:257–272
Jha SK, Bilalovic J, Jha A, Patel N, Zhang H (2017b) Renewable energy: present research and future scope of artificial intelligence. Renew Sust Energ Rev 77:297–317
Jin Y, Qiu S, Shao N, Zheng J (2018) Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt/NF-κB signaling pathway. Med Sci Monitor 24:11–18
Juhl AR, Velazquez V, Latz MI (2000) Effect of growth conditions on flow-induced inhibition of population growth of a red-tide dinoflagellate. Limnol Oceanogr 45:905–915
Juhl AR, Trainer VL, Latz MI (2001) Effect of fluid shear and irradiance on population growth and cellular toxin content of the dinoflagellate Alexandrium fundyense. Limnol Oceangr 46:758–764
Kang H, Lee CH, Kim JR, Kwon JY, Seo SG, Han JG, Kim BG, Kim JE, Lee KW (2015) Chlorella vulgaris attenuates Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int J Mol Sci 16:21021–21034
Khalid N, Barrow CJ (2018) Critical review of encapsulation methods for stabilization and delivery of astaxanthin. J Food Bioactives 1:104–115–104–115
Kim KN, Heo SJ, Kang SM, Ahn G, Jeon YJ (2010) Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol In Vitro 24:1648–1654
Kim H, Lantvit D, Hwang CH, Kroll DJ, Swanson SM, Franzblau SG, Orjala J (2012a) Indole alkaloids from two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorg Med Chem 20:5290–5295
Kim M, Yim JH, Kim SY, Kim HS, Lee WG, Kim SJ, Kang PS, Lee CK (2012b) In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir Res 93:253–259
Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH, Chung D, Pan CH (2012c) A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol 166:1843–1855
Kim SM, Kang SW, Kwon ON, Chung D, Pan CH (2012d) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: characterization of extraction for commercial application. J Korean Soc Appl Biol Chem 55:477–483
Kim KN, Ahn G, Heo SJ, Kang SM, Kang MC, Yang HM, Kim D, Roh SW, Kim SK, Jeon BT, Park PJ, Jung WK, Jeon YJ (2013) Inhibition of tumor growth in vitro and in vivo by fucoxanthin against melanoma B16F10 cells. Environ Toxicol Pharmacol 35:39–46
Kim YS, Li XF, Kang KH, Ryu B, Kim SK (2014) Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep 47:433–438
Kim ZH, Park H, Hong SJ, Lim SM, Lee CG (2016) Development of a floating photobioreactor with internal partitions for efficient utilization of ocean wave into improved mass transfer and algal culture mixing. Bioprocess Biosyst Eng 39:713–723
Kim JH, Lee JE, Kim KH, Kang NJ (2018) Beneficial effects of marine algae-derived carbohydrates for skin health. Mar Drugs 16(11):459
Kobayashi JI, Tsuda M (2004) Amphidinolides, bioactive macrolides from symbiotic marine dinoflagellates. Nat Prod Rep 21:77–93
Kobayashi M, Natsume T, Tamaoki S, Watanabe J, Asano H, Mikami T, Miyasaka K, Miyazaki K, Gondo M, Sakakibara K, Tsukagoshi S (1997) Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Jpn J Cancer Res 88:316–327
Kok YY, Chu WL, Phang SM, Mohamed SM, Naidu R, Lai PJ, Ling SN, Mak JW, Lim PK, Balraj P, Khoo AS (2011) Inhibitory activities of microalgal extracts against Epstein-Barr virus DNA release from lymphoblastoid cells. J Zhejiang Univ Sci B 12:335–345
Komatsu T, Kido N, Sugiyama T, Yokochi T (2013) Antiviral activity of acidic polysaccharides from Coccomyxa gloeobotrydiformi, a green alga, against an in vitro human influenza A virus infection. Immunopharmacol Immunotoxicol 35:1–7
Komprda T, Sladek Z, Skultety O, Krizkova S, Rozikova V, Nemcova B, Sustrova T, Valova M (2016) Effect of dietary Schizochytrium microalga oil on selected markers of low-grade inflammation in rats. J Anim Physiol Anim Nutr 100:1169–1178
Kuntzler SG, Costa JAV, Morais MG (2018) Development of electrospun nanofibers containing chitosan/PEO blend and phenolic compounds with antibacterial activity. Int J Biol Macromol 117:800–806
Lau AF, Siedlecki J, Anleitner J, Patterson GM, Caplan FR, Moore RE (1993) Inhibition of reverse transcriptase activity by extracts of cultured blue-green algae (Cyanophyta). Planta Med 59:148–151
Lauritano C, Andersen JH, Hansen E, Albrigtsen M, Escalera L, Esposito F, Helland K, Hanssen KØ, Romano G, Ianora A (2016) Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci 3:68
Lee JB, Hayashi K, Hirata M, Kuroda E, Suzuki E, Kubo Y, Hayashi T (2006) Antiviral sulfated polysaccharide from Navicula directa, a diatom collected from deep-sea water in Toyama Bay. Biol Pharm Bull 29:2135–2139
Leira F, Alvarez C, Vieites J, Vieytes M, Botana L (2002) Characterization of distinct apoptotic changes induced by okadaic acid and yessotoxin in the BE (2)-M17 neuroblastoma cell line. Toxicol In Vitro 16:23–31
Li Y, Gao X, Yu Z, Liu B, Pan W, Li N, Tang B (2018) Reversing multidrug resistance by multiplexed gene silencing for enhanced breast cancer chemotherapy. ACS Appl Mat Interfaces 10:15461–15466
Liu Y, Law BK, Luesch H (2009) Apratoxin a reversibly inhibits the secretory pathway by preventing cotranslational translocation. Mol Pharmacol 76:91–104
Liu W, Chen Y, Wang J, Liu T (2018a) Biomass productivity of Scenedesmus dimorphus (Chlorophyceae) was improved by using an open pond–photobioreactor hybrid system. Eur J Phycol 54:1–8. https://doi.org/10.1080/09670262.2018.1519601
Liu Z, Fu X, Huang W, Li C, Wang X, Huang B (2018b) Photodynamic effect and mechanism study of selenium-enriched phycocyanin from Spirulina platensis against liver tumours. J Photochem Photobiol B 180:89–97
Lopez-Rosales L, Sanchez-Miron A, Garcia-Camacho F, Place AR, Chisti Y, Molina-Grima E (2018) Pilot-scale outdoor photobioreactor culture of the marine dinoflagellate Karlodinium veneficum: production of a karlotoxins-rich extract. Bioresour Technol 253:94–104
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Lotfi H, Sheervalilou R, Zarghami N (2018) An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. Bioimpacts 8:139–151
Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH (2001) Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J Nat Prod 64:907–910
Luesch H, Harrigan GG, Goetz G, Horgen FD (2002) The cyanobacterial origin of potent anticancer agents originally isolated from sea hares. Curr Med Chem 9:1791–1806
Luesch H, Chanda SK, Raya RM, de Jesus PD, Orth AP, Walker JR, Izpisua Belmonte JC, Schultz PG (2006) A functional genomics approach to the mode of action of apratoxin A. Nat Chem Biol 2:158–167
Maeda H (2015) Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review. J Oleo Sci 64:125–132
Martinez Andrade KA, Lauritano C, Romano G, Ianora A (2018) Marine microalgae with anti-cancer properties. Mar Drugs 16:165
Matsui MS, Muizzuddin N, Arad S, Marenus K (2003) Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl Biochem Biotechnol 104:13–22
McCall B, McPartland CK, Moore R, Frank-Kamenetskii A, Booth BW (2018) Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants 7:135
Miles CO, Wilkins AL, Munday JS, Munday R, Hawkes AD, Jensen DJ, Cooney JM, Beuzenberg V (2006) Production of 7-epi-pectenotoxin-2 seco acid and assessment of its acute toxicity to mice. J Agric Food Chem 54:1530–1534
Mimouni V, Ulmann L, Pasquet V, Mathieu M, Picot L, Bougaran G, Cadoret JP, Morant-Manceau A, Schoefs B (2012) The potential of microalgae for the production of bioactive molecules of pharmaceutical interest. Curr Pharm Biotechnol 13:2733–2750
Molina-Miras A, Morales-Amador A, de Vera CR, López-Rosales L, Sánchez-Mirón A, Souto ML, Fernández JJ, Norte M, García-Camacho F, Molina-Grima E (2018) A pilot-scale bioprocess to produce amphidinols from the marine microalga Amphidinium carterae: isolation of a novel analogue. Algal Res 31:87–98
Mori K, Ooi T, Hiraoka M, Oka N, Hamada H, Tamura M, Kusumi T (2004) Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar Drugs 2:63–72
Nagaoka S, Shimizu K, Kaneko H, Shibayama F, Morikawa K, Kanamaru Y, Otsuka A, Hirahashi T, Kato T (2005) A novel protein C-phycocyanin plays a crucial role in the hypocholesterolemic action of Spirulina platensis concentrate in rats. J Nutr 135:2425–2430
Naito Y, Uchiyama K, Aoi W, Hasegawa G, Nakamura N, Yoshida N, Maoka T, Takahashi J, Yoshikawa T (2004) Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic db/db mice. Biofactors 20:49–59
Nakashima Y, Ohsawa I, Konishi F, Hasegawa T, Kumamoto S, Suzuki Y, Ohta S (2009) Preventive effects of Chlorella on cognitive decline in age-dependent dementia model mice. Neurosci Lett 464:193–198
Nakazawa Y, Sashima T, Hosokawa M, Miyashita K (2009) Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J Funct Foods 1:88–97
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interf Sci 169:59–79
Nasirian F, Dadkhah M, Moradi-Kor N, Obeidavi Z (2018) Effects of Spirulina platensis microalgae on antioxidant and anti-inflammatory factors in diabetic rats. Diabetes Metab Syndr Obes 11:375–380
Nemoto-Kawamura C, Hirahashi T, Nagai T, Yamada H, Katoh T, Hayashi O (2004) Phycocyanin enhances secretary IgA antibody response and suppresses allergic IgE antibody response in mice immunized with antigen-entrapped biodegradable microparticles. J Nutr Sci Vitaminol 50:129–136
Niizawa I, Espinaco BY, Zorrilla SE, Sihufe GA (2018) Natural astaxanthin encapsulation: use of response surface methodology for the design of alginate beads. Int J Biol Macromol 121:601–608
Nobre BP, Villalobos F, Barragan BE, Oliveira AC, Batista AP, Marques PA, Mendes RL, Sovova H, Palavra AF, Gouveia L (2013) A biorefinery from Nannochloropsis sp. microalga—extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour Technol 135:128–136
O’keefe B, McMahon JB, Gustafson K, Takebe Y (2011) Antiviral activity of the protein scytovirin and methods of use. Google Patents
O’Keefe BR, Smee DF, Turpin JA, Saucedo CJ, Gustafson KR, Mori T, Blakeslee D, Buckheit R, Boyd MR (2003) Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob Agents Chemother 47:2518–2525
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12:499–506
Olguin EJ (2012) Dual purpose microalgae-bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol Adv 30:1031–1046
Pádua D, Rocha E, Gargiulo D, Ramos AA (2015) Bioactive compounds from brown seaweeds: phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem Lett 14:91–98
Palozza P, Torelli C, Boninsegna A, Simone R, Catalano A, Mele MC, Picci N (2009) Growth-inhibitory effects of the astaxanthin-rich alga Haematococcus pluvialis in human colon cancer cells. Cancer Lett 283:108–117
Patel V, Berthold D, Puranik P, Gantar M (2015) Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Rep 5:112–119
Patterson GML, Baldwin CL, Bolis CM, Caplan FR, Karuso H, Larsen LK, Levine IA, Moore RE, Nelson CS, Tschappat KD, Tuang GD, Furusawa E, Furusawa S, Norton TR, Raybourne RB (1991) Antineoplastic activity of cultured blue-green algae (Cyanophyta). J Phycol 27:530–536
Perez M, Rilatt I, Lamothe M (2018) Derivatives of dolastatin 10 and auristatins. Google Patents
Pollingher U, Zemel E (1981) In situ and experimental evidence of the influence of turbulence on cell division processes of Peridinium cinctum forma westii (Lemm.) Lefevre. Br Phycol J 16:281–287
Prakash B, Kujur A, Yadav A (2018) Drug synthesis from natural products: a historical overview and future perspective. In: Tewari A, Tiwari S (eds) Synthesis of medicinal agents from plants. Elsevier, Amsterdam, pp 25–46
Pratt R, Daniels T, Eiler JJ, Gunnison J, Kumler W, Oneto JF, Strait LA, Spoehr H, Hardin G, Milner H (1944) Chlorellin, an antibacterial substance from Chlorella. Science 99:351–352
Ramos DF, Matthiensen A, Colvara W, de Votto AP, Trindade GS, da Silva PE, Yunes JS (2015) Antimycobacterial and cytotoxicity activity of microcystins. J Venom Anim Toxins Incl Trop Dis 21:9
Rao AR, Sindhuja H, Dharmesh SM, Sankar KU, Sarada R, Ravishankar GA (2013) Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J Agric Food Chem 61:3842–3851
Ravi M, Tentu S, Baskar G, Rohan Prasad S, Raghavan S, Jayaprakash P, Jeyakanthan J, Rayala SK, Venkatraman G (2015) Molecular mechanism of anti-cancer activity of phycocyanin in triple-negative breast cancer cells. BMC Cancer 15:768
Rechter S, Konig T, Auerochs S, Thulke S, Walter H, Dornenburg H, Walter C, Marschall M (2006) Antiviral activity of Arthrospira-derived spirulan-like substances. Antivir Res 72:197–206
Remirez D, Ledon N, Gonzalez R (2002) Role of histamine in the inhibitory effects of phycocyanin in experimental models of allergic inflammatory response. Mediat Inflamm 11:81–85
Renju GL, Muraleedhara Kurup G, Saritha Kumari CH (2013) Anti-inflammatory activity of lycopene isolated from Chlorella marina on type II collagen induced arthritis in Sprague Dawley rats. Immunopharmacol Immunotoxicol 35:282–291
Renju GL, Kurup GM, Saritha Kumari CH (2014a) Effect of lycopene from Chlorella marina on high cholesterol-induced oxidative damage and inflammation in rats. Inflammopharmacology 22:45–54
Renju GL, Muraleedhara Kurup G, Bandugula VR (2014b) Effect of lycopene isolated from Chlorella marina on proliferation and apoptosis in human prostate cancer cell line PC-3. Tumour Biol 35:10747–10758
Rodrigues DB, Menezes CR, Mercadante AZ, Jacob-Lopes E, Zepka LQ (2015) Bioactive pigments from microalgae Phormidium autumnale. Food Res Int 77:273–279
Romieu I, Téllez-Rojo MM, Lazo M, Manzano-Patiño A, Cortez-Lugo M, Julien P, Bélanger MC, Hernandez-Avila M, Holguin F (2005) Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Resp Crit Care Med 172:1534–1540
Samarakoon KW, Ko JY, Lee JH, Kwon ON, Kim SW, Jeon YJ (2014) Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J Funct Foods 6:231–240
Sánchez JF, Fernández-Sevilla JM, Acién FG, Cerón MC, Pérez-Parra J, Molina-Grima E (2008) Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79:719–729
Sansone C, Braca A, Ercolesi E, Romano G, Palumbo A, Casotti R, Francone M, Ianora A (2014) Diatom-derived polyunsaturated aldehydes activate cell death in human cancer cell lines but not normal cells. PLoS One 9:e101220
Sato Y, Okuyama S, Hori K (2007) Primary structure and carbohydrate-binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium, Oscillatoria agardhii. J Biol Chem 282(15):11021–11029
Senhorinho GNA, Laamanen CA, Scott JA (2018) Bioprospecting freshwater microalgae for antibacterial activity from water bodies associated with abandoned mine sites. Phycologia 57:432–439
Sessa C, Weigang-Köhler K, Pagani O, Greim G, Mora O, de Pas T, Burgess M, Weimer I, Johnson R (2002) Phase I and pharmacological studies of the cryptophycin analogue LY355703 administered on a single intermittent or weekly schedule. Eur J Cancer 38:2388–2396
Shankar PD, Shobana S, Karuppusamy I, Pugazhendhi A, Ramkumar VS, Arvindnarayan S, Kumar G (2016) A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: formation mechanism and applications. Enzym Microb Technol 95:28–44
Sharon N, Lis H (1989) Lectins as cell recognition molecules. Science 246:227–234
Shi XM, Chen F (2002) High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol Prog 18:723–727
Shih PM, Wu D, Latifi A, Axen SD, Fewer DP, Talla E, Calteau A, Cai F, Tandeau de Marsac N, Rippka R, Herdman M, Sivonen K, Coursin T, Laurent T, Goodwin L, Nolan M, Davenport KW, Han CS, Rubin EM, Eisen JA, Woyke T, Gugger M, Kerfeld CA (2013) Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc Natl Acad Sci USA 110:1053–1058
Siqueira AS, Lima ARJ, Aguiar DCF, Santos AS, Vianez Júnior JLDSG, Gonçalves EC (2018) Genomic screening of new putative antiviral lectins from Amazonian cyanobacteria based on a bioinformatics approach. Proteins 86:1047–1054
Soontornchaiboon W, Joo SS, Kim SM (2012) Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull 35:1137–1144
Sosa-Hernandez JE, Escobedo-Avellaneda Z, Iqbal HMN, Welti-Chanes J (2018) State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 23:2953
Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, Mattern M, Gerwick WH, Jacobs RS, Marshall LA (2002) The identification and characterization of the marine natural product Scytonemin as a novel Antiproliferative Pharmacophore. J Pharmacol Exp Ther 303(2):858–866
Tan CP, Hou YH (2014) First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 37:443–450
Tan RH, Wang F, Fan CL, Zhang XH, Zhao JS, Zhang JJ, Yang Y, Xi Y, Zou ZQ, Bu SZ (2018) Algal oil rich in n-3 polyunsaturated fatty acids suppresses B16F10 melanoma lung metastasis by autophagy induction. Food Funct 9:6179–6186
Ulmann L, Blanckaert V, Mimouni VX, Andersson M, Schoefs B, Chenais B (2017) Microalgal fatty acids and their implication in health and disease. Mini Rev Med Chem 17:1112–1123
Umemura K, Yanase K, Suzuki M, Okutani K, Yamori T, Andoh T (2003) Inhibition of DNA topoisomerases I and II, and growth inhibition of human cancer cell lines by a marine microalgal polysaccharide. Biochem Pharmacol 66:481–487
Vaishampayan U, Glode M, Du W, Kraft A, Hudes G, Wright J, Hussain M (2000) Phase II study of dolastatin-10 in patients with hormone-refractory metastatic prostate adenocarcinoma. Clin Cancer Res 6:4205–4208
Vinayak V, Manoylov KM, Gateau H, Blanckaert V, Herault J, Pencreac’h G, Marchand J, Gordon R, Schoefs B (2015) Diatom milking: a review and new approaches. Mar Drugs 13:2629–2665
Volk RB, Furkert FH (2006) Antialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growth. Microbiol Res 161:180–186
von Schacky C (2008) Omega-3 fatty acids: antiarrhythmic, proarrhythmic or both? Curr Opin Clin Nutr Metab Care 11:94–99
Wang H, Fewer DP, Sivonen K (2011) Genome mining demonstrates the widespread occurrence of gene clusters encoding bacteriocins in cyanobacteria. PLoS One 6:e22384
Wang B, Lan CQ, Horsman M (2012a) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30:904–912
Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, Cao X (2012b) In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs 10:2055–2068
Wang S, Said IH, Thorstenson C, Thomsen C, Ullrich MS, Kuhnert N, Thomsen L (2018a) Pilot-scale production of antibacterial substances by the marine diatom Phaeodactylum tricornutum Bohlin. Algal Res 32:113–120
Wang X, Wang H, Pierre JF, Wang S, Huang H, Zhang J, Liang S, Zeng Q, Zhang C, Huang M, Ruan C, Lin J, Li H (2018b) Marine microalgae bioengineered Schizochytrium sp. meal hydrolysates inhibits acute inflammation. Sci Rep 8:9848
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem 40:3627–3652
Waters AL, Hill RT, Place AR, Hamann MT (2010) The expanding role of marine microbes in pharmaceutical development. Curr Opin Biotechnol 21:780–786
WHO (World Health Organization) Cancer (2018) http://www.who.int/en/news-room/fact-sheets/detail/cancer. Accessed 19 Jan 2019
Wolf J, Stephens E, Steinbusch S, Yarnold J, Ross IL, Steinweg C, Doebbe A, Krolovitsch C, Müller S, Jakob G, Kruse O, Posten C, Hankamer B (2016) Multifactorial comparison of photobioreactor geometries in parallel microalgae cultivations. Algal Res 15:187–201
Woo MN, Jeon SM, Kim HJ, Lee MK, Shin SK, Shin YC, Park YB, Choi MS (2010) Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem Biol Interact 186:316–322
Yan T, Li HY, Wu JS, Niu Q, Duan WH, Han QZ, Ji WM, Zhang T, Lv W (2017) Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol Lett 14:5400–5408
Yim JH, Kim SJ, Ahn SH, Lee CK, Rhie KT, Lee HK (2004) Antiviral effects of sulfated exopolysaccharide from the marine microalga Gyrodinium impudicum strain KG03. Mar Biotechnol 6:17–25
Yu Y, Shen M, Song Q, Xie J (2018) Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym 183:91–101
Zhang Y, Xu W, Huang X, Zhao Y, Ren Q, Hong Z, Huang M, Xing X (2018) Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J Funct Foods 48:515–524
Zhu C, Zhai X, Jia J, Wang J, Han D, Li Y, Tang Y, Chi Z (2018) Seawater desalination concentrate for cultivation of Dunaliella salina with floating photobioreactor to produce β-carotene. Algal Res 35:319–324
Acknowledgements
The first author would like to acknowledge the funding and support from the International Medical University for algal biotechnology research. Phang S.M. would like to acknowledge the following grants: UM Algae (GA003-2012); MOHE-HiCoE Grant Phase 2 (2019-2021); UM Grand Challenge-SBS No.GC002B-15SBS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chu, WL., Phang, SM. (2019). Bioactive Compounds from Microalgae and Their Potential Applications as Pharmaceuticals and Nutraceuticals. In: Hallmann, A., Rampelotto, P. (eds) Grand Challenges in Algae Biotechnology. Grand Challenges in Biology and Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-030-25233-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-25233-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-25232-8
Online ISBN: 978-3-030-25233-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)