Abstract
The characterization of nutrient and biostimulant effects in crops is complex and needs rigorous evaluations. In this study, we evaluated morphological and molecular responses induced by microalgae (Chlorella vulgaris and Scenedesmus quadricauda) extracts in Beta vulgaris L. The two microalgae extracts were firstly characterized by CNS, Fourier transform infrared spectroscopic analysis (FT-IR), and carbon-13 nuclear magnetic resonance (13C NMR). Seedlings were grown in Hoagland’s solution under controlled conditions. After 5 days of growth, 2 mL L−1 (1 mg Corg L−1) and 4 mL L−1 (2 mg Corg L−1) of the two microalgae extracts were added to the Hoagland solution. Roots were sampled 36 h after treatments. Inductively coupled plasma spectrometry (ICP-OES) and nanofluidic real-time PCR (OpenArray system) were used for sample profiling. Fifty-three sugar beet genes putatively involved in sulfate starvation were tested in treated and untreated samples. Root morphological traits were measured by means of a scanner-based image analysis system. Multivariate statistical analysis revealed no significant changes in the ionomic profile of Hoagland’s solutions treated with the two microalgae extracts with respect to that of the untreated solution. At the molecular level, microalgae extract supplies upregulated many of the evaluated genes. Functional categorization revealed these genes to be related to various biological pathways and processes including primary and secondary metabolism and intracellular transport. At the morphological level, the treated seedlings showed significantly higher values for root traits related to soil exploration and nutrient uptake, such as total root length, fine root length (diameter < 0.5 mm), and number of root tips, than the untreated plants. These data indicate that microalgae extracts have biostimulant effects on the expression of root traits and genes related to nutrient acquisition in sugar beet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the overuse of chemical fertilizers has contributed to increasing environmental pollution (Köhler and Triebskorn 2013). Production of greenhouse gases, acidification, and increase in soil and water pollution are some of the negative effects produced by an excessive use of chemical inputs. In order to promote the efficiency of plant nutrient uptake and the reduction of environmental pollution, a wide spectrum of compounds has been considered capable of enhancing plant growth and development when applied in small amounts to the soil or directly on the foliar surface. These are referred to as positive plant-growth regulators or metabolic enhancers, as well as biostimulants (Miller 1990; Chen et al. 2002). Biostimulants belong to four major groups: humic substances, amino acid-containing products, microbial inoculants (mycorrhizal fungi and rhizobacteria), and seaweed extracts (du Jardin 2015).
Biologists divide algae into four types according to their size and cell morphological characteristics (Nabti et al. 2016). These types are unicellular, colonial, filamentous, and multicellular. According to these features, algae are divided into two main groups: macro- and microalgae. Macroalgae are macroscopic organisms, multicellular, and generally associated with algae, with a maximum length of 65 m. Microalgae are microscopic unicellular or filamentous organisms with a much smaller size, from 1 to 50 μm.
In the last years, interest in the use of microalgae has increased due to their versatility and potential application in many different sectors. According to the literature, they can be utilized as animal feed, food, fuel, and fertilizers (Metting et al. 1990; Borowitzka 2013). Some microalgae species have successfully been applied to remove organic and inorganic pollutants, such as heavy metals and other toxic organic contaminants, from water and wastewater (Fierro et al. 2008; Baglieri et al. 2016). Since microalgal biodiversity is very high, they represent a resource worth exploring (Norton et al. 1996). Many microalgae-based products are available for use in agriculture as a new and promising source of crop nutrients (Vigani et al. 2015). Microalgae extracts have been shown to contain micro- and macronutrients, especially N, P, and K, and are considered as an organic slow-release fertilizer (Coppens et al. 2016). Many studies have also indicated that these microorganisms contain plant growth-promoting substances such as auxins, cytokinins, betaines, amino acids, vitamins, and antifungal compounds (Spolaore et al. 2006). The application of microalgae extract enhances plant development, increases crop production, and preserves plants from abiotic and biotic stresses. This aspect is particularly crucial given that environmental stresses can limit crop production up to 70% (Boyer 1982). Garcia-Gonzalez and Sommerfeld (2016) recently observed that aqueous extracts of Acutodesmus dimorphus induce a positive effect on tomato plant growth. Higher biomass accumulation and enhanced absorption of nutrients have been observed in lettuce (Faheed and Abd-El Fattah 2008).
About 40,000 species of microalgae have been classified. Among them, Chlorella vulgaris attracts the attention of scientists for its high protein content, reaching > 55% of biomass dry weight (Safi et al. 2014). Clorella vulgaris belongs to the green eukaryotic microalgae and was discovered as the first microalga with a well-defined nucleus (Beijerinck 1890). Many studies have already been done on morphology, composition, and production of C. vulgaris. Due to the high protein concentration, carbohydrates (15–55% dry weight), and lipids (5–40% dry weight), it is utilized in animal feed, human nutrition, and cosmetics and as a biofertilizer. Given this wide variety of uses, its annual production has reached 2000 t (dry weight). Shaaban (2001) evaluated the effect of different concentrations of water extract of C. vulgaris (25, 50, 75, and 100%) on the yield of wheat (Triticum aestivum L. cv. Giza 69) in a greenhouse. It was found that dry mass of the shoot treated previously with 50% algal extract had an 81.4% weight increase when compared with the control. The increase can be a reflection of the increased nutrient uptake. This concentration of algal extract also led to a more than 40% increase in weight of 100 grains when compared with the control.
Fewer studies have been conducted on Scenedesmus quadricauda, a colonial green microalgae. Like C. vulgaris, Scenedesmus cultures are easy to cultivate and have a rapid growth rate (Huang et al. 1994). In optimal growth conditions, S. quadricauda can reach 9–16% of lipids (dry weight), mainly composed of large amounts of long-chain hydrocarbons (Rocha et al. 2015). Scenedesmus quadricauda has been applied in wastewater management such as biosorption of heavy metals and the production of biofuels due to its triacylglycerol accumulation under stress (Devi et al. 2012), but it has never been tested as a biofertilizer.
Sugar beet (Beta vulgaris), the main crop for sugar production in Europe, is often affected by many abiotic stresses. Low temperatures, salinity, heat, and water deficiency are some of the major constraints (Biancardi et al. 2010). Water and nutrient stresses are particularly severe in the Mediterranean area, where sugar yield losses of 1 t ha−1 are estimated (Jones et al. 2003). Biofertilizer use could help farmers to overcome these abiotic stresses.
In this study, we evaluated the root morphophysiological changes and molecular response induced by two microalgae (C. vulgaris and S. quadricauda) extracts in B. vulgaris, aimed at studying their biostimulant effect on the roots.
Materials and methods
Preparation of microalgae extracts
Tests were conducted using two species of microalgae, Chlorella vulgaris and Scenedesmus quadricauda, both supplied by Swansea University (Wales, UK) and cultivated, for 30 days in a growth chamber using standard BG-11 algae culture medium (Stanier et al. 1971), bubbled with air and average photon flux (PPF) of 100 μmol photons m−2 s−1 (PHILIPS SON-T AGRO 400) with a 12-h photoperiod. The biomass of each species (referred to as CV and SQ for C. vulgaris and S. quadricauda, respectively) was harvested by centrifugation and freeze-drying. After lyophilization, the biomass was washed with distilled water (up conductivity < 200 μS cm−1). The final pellets obtained were added to methanol to lyse the cell wall and obtain the intracellular extracts. After centrifugation and evaporation of organic solvent, the extract was collected with distilled water (microalgae extracts stock solution, referred to as CVextr and SQextr from C. vulgaris and S. quadricauda, respectively). The water extracts were also lyophilized for subsequent chemical analyses.
Chemical, spectroscopic, and biochemical characterizations
The chemical and spectroscopic characterizations involved both lyophilized microalgae biomass and the obtained extracts (CVextr and SQextr).
Total carbon and nitrogen content was determined on samples using combustion analysis (Elementar vario MACRO CNS, Elementar Analysensysteme GmbH, Germany).
The FT-IR spectra were performed with a Perkin-Elmer FT-IR 2000 spectrometer (Perkin Elmer Italia, Italy), equipped with an IR source, KBr beam splitter, and DTGS KBr detector. For each sample, 64 scans were recorded with a 4 cm−1 resolution over a 4000 to 400 cm−1 range using pellets obtained by pressing a mixture of 1 mg organic freeze-dried sample and 400-mg dried KBr.
Solid-state 13C MAS NMR spectra were recorded, fully proton-decoupled, on a BrukerAvance II 400 MHz instrument (9.4 T) (Bruker Corp., USA) operating at 100.63 MHz. Rotors with a 7 mm diameter were filled with about 50 mg of the freeze-dried sample; spinning rate was 8000 Hz s−1. The experimental parameters adopted were as follows: spectral width 20,000 Hz, data points 2 K, 100,000 scans, 5 μs, 90° of excitation pulse, and 4 s of relaxation delay. The HPDEC pulse sequence was used with a decoupling power of 300 W (9H). The FID was zero-filled and processed with 5 Hz line broadening.
Lastly, in order to exclude any residual enzymatic activity in the microalgae extract, 11 hydrolytic enzymes involved in the principal nutrient cycles were determined in CVextr and SQextr. These were as follows: (i) C-cycle: α (α-gluc) and β-glucosidase (β-gluc), glucoronidase (glu), xylosidase (xyl), nonanoate esterase (nona); (ii) N-cycle: leucine-aminopeptidase (leu); (iii) P-cycle: acid (acP) and alkaline phosphomonoesterases (alkP), phosphodiesterase (diP), pyrophosphate-phosphodiesterase (piroP); (iv) S-cycle: arylsulphatase (aryS).
All potential enzymatic activities were measured in duplicate from all the samples. An aliquot of each algal extract was dispensed into 384-well white microplates with the appropriate buffer to fluorometrically quantify the enzymatic activities using fluorogenic, 4-methyl-umbelliferyl (MUF), and 4-amido-7-methyl-coumarine (AMC) substrates. All measurements were done in duplicate and the activities were expressed as nanomoles of MUF (or AMC) h−1 mL−1.
Plant material and growing conditions
The Department of Agronomy, Food, Natural resources, Animals and Environment (DAFNAE) of University of Padova (Italy) provided the sugar beet hybrid “Shannon” used in this study. Seeds were sterilized by soaking in 76% ethanol for 5 min and rinsed with sterilized water. They were then placed on distilled water-moistened filter paper and germination took place in a growth chamber in the dark at 25 °C for 48 h. After germination, seedlings were grown in a hydroponic Hoagland solution (Arnon and Hoagland 1940) and kept in a climatic chamber at 25 °C. After 5 days of growth, 2 mL L−1 (1 mg Corg L−1) and 4 mL L−1 (2 mg Corg L−1) of the two microalgae extract stock solutions were added to the Hoagland solution. Roots were sampled 36 h after the treatment.
Ionomic analysis
Samples of the Hoagland solution were collected before and after adding microalgae extracts and analyzed using inductively-coupled plasma (ICP-OES, Spectro, Germany). Treatments were arranged in a complete randomized block design and replicated three times. The experiment was repeated twice. At the end of the experiment, fresh roots were harvested and analyzed immediately, while part of the plant material was stored at − 80 °C for further molecular analyses.
Root morphological analysis
Root morphological parameters (total root length, root surface area, and total number of root tips) were determined by computerized scanning (STD 1600, Regent Instruments, Canada) and analyzed using WinRHIZO software (Regent Instruments).
Molecular analysis
One hundred milligram of root tissues was used to extract total RNA using a EuroGold TriFast TM Kit (Euro Clone, Italy) following the manufacturer’s recommendations. RNA quantification was done with a Qubit RNA HS Assay Kit on a Qubit Fluorometer (Thermo, USA).
FastGene 55-Scriptase (Nippon Genetics, Japan) was used to reverse transcribe 1 μg of total RNA. The expression level of 53 sugar beet genes, coming from a previous experiment of RNA-seq and present in multiple biological pathways, such as lipid metabolism, stress response, protein folding, and signal transduction (Moliterni et al., personal communication), were tested by means of a QuantStudio 12K Flex Real-Time PCR System (Life Technologies, USA). The reaction mix, prepared for gene expression analysis, was composed of 2.5 μL of 2× TaqMan Open Array master mix (Life Technologies, USA) and 2.5 μL of cDNA. The comparative cycle-threshold method was used for quantification of gene expression with normalization to reference genes (Tubulin; GAPDH; Histone H3) using the 2-ΔCCT formulas. The sequences of primers and TaqMan probes designed for the real-time PCR experiments are reported as supplementary material (Table S1). Data were analyzed by ANOVA using Statistica 8.0 (StatSoft Inc., USA). Differences between groups were considered significant at P < 0.01.
Results and discussion
Distribution of the diverse forms of carbon of microalgae biomass and extracts designated according to the areas of the different NMR spectra regions, as indicated by Baglieri et al. (2014) (aliphatic, 0 to 45 ppm; N and O alkyl, 45 to 95 ppm; aromatic, 95 to 160 ppm; carboxyl, 160 to 195 ppm), is reported in Table 1. The biomass spectra for CV and SQ are very similar in shape (Fig. 1) and distribution of the various carbon forms. Conversely, some differences are recognized between the two extracts. Indeed, the CVextr spectrum has three visible peaks 31, 128, and 182 ppm, whereas only two main peaks 31 and 128 ppm are detected in SQextr (Fig. 1). The latter peak occurs within the aromatic carbon region; however, it could also be produced by the characteristic double bond resonance from triacylglycerides (−C = C−), from 125 to 135 ppm, in some species of microalgae (Akhter et al. 2016). These compounds may be due to the storage reserve material extracted from whole cells. In fact, in microalgae, triacylglycerides are stored in the vacuoles, while the phospholipids and glycolipids are components of the cell membrane (Olofsson et al. 2012). The N and O alkyl carbon calculated from the integration of the signal in the 45 to 95 ppm region is lower in CVextr than that in SQextr, vice versa for the carboxyl carbon (from 160 to 195 ppm; 9.37 and 4.42% for CVextr and SQextr, respectively). Overall, distribution of various forms of carbon showed that the extracts from both species have a greater amount of alkyl and aromatic carbon than algal biomass. In contrast, extracts (CVextr and SQextr) showed a lower distribution of carbohydrates and protein carbon (N and O alkyl carbon) compared to biomass sources (Table 1). Similarly, in agreement with Akhter et al. (2016), what was observed for the carboxyl carbon may be attributable to protein molecules and/or lipids. Consequently, both extracts showed a higher degree of hydrophobicity (HB/HI) than that observed for the respective biomass from which they were extracted. The degree of hydrophobicity was determined according to Baglieri et al. (2014) for humic substances as follows: HB/HI = [(0 – 45) + (95 – 160)/(45 – 95) + (160 – 195)]. The degree of hydrophobicity for CVextr resulted as much higher than that calculated for SQextr; CVextr thus being more apolar than SQextr (Table 1).
The FT-IR spectra (Fig. 2) showed big differences between the biomass of microalgae (CV and SQ spectra in solid line) and their extracts (CVextr and SQextr spectra—dotted line). Assignment of the bands in the FT-IR spectra was performed according to Baglieri et al. (2012) and Duygu et al. (2012). Each peak was assigned to a functional group. Protein spectra were characterized by three strong features at 1665, 1556, and 1406 cm−1 in CV and SQ biomass. These bands were due, primarily, to C = O stretching vibration in amide I (signal at 1665 cm−1), N-H bending and C-N stretching vibration in amide II (signal at 1556 cm−1), and CH2 and CH3 bending of methyl for the latter signal. However, this band may also be attributed to C-O stretching of the carboxylate groups of the carboxylic acids or to (N(CH3)) bending of methyl of lipids (Sigee et al. 2002). Lipid spectra were characterized by two sets of vibrations, C-H stretching of methane at 2965 cm−1 and CH2 stretching of methylene. In CV and SQ microalgae biomass, carbohydrate absorption bands, due to C-O stretching of polysaccharides and complex sugar ring modes at 1162 cm−1 and at 1050 cm−1, are clearly visible. Finally, in the spectra of biomass sources, two other peaks are detectable. The first broad at about 3300 cm−1 (precisely at 3296 cm−1 in CV and 3303 cm−1 in SQ) attributed to O-H stretching of water or to N-H stretching of the amide A, and the second at 1260 cm−1 assigned to the >P = O stretching of phosphodiesters of nucleic acids (Fig. 2).
In microalgae extracts, only two strong signals attributable to the proteins at 1556 cm−1—more intense in CVextr than SQextr—and 1406 cm−1 were observed, whereas the signal at 1665 cm−1 was very weak (Fig. 2). These results are in agreement with data of 13C NMR which showed a lower protein content in the microalgae extracts compared to the respective biomass.
The bands attributed to lipids in the CVextr and SQextr spectra have similar intensities to those observed in the CV and SQ biomass, respectively. CVextr and SQextr also showed two additional bands, at 3019 and 1742 cm−1, also attributable to lipids. The first is assigned to C-H stretching of methane and the second one to C=O stretching of esters of fatty acids. As observed for proteins, a higher lipid content in microalgae extracts than that recorded in CV and SQ biomass was observed in 13C NMR spectra (Fig. 1).
In addition, the signal of carbohydrates at 1050 cm−1 (Fig. 2) in the extracts was evident, while the signal at 1162 cm−1 disappeared. Also, the signal (at 1260 cm−1) assigned to nucleic acids was very weak, whereas the one attributed to stretching of the O-H water was moved approximately to 3200 cm−1 (precisely 3221 cm−1 in CVextr and 3194 cm−1 in SQextr).
Table 2 shows the element composition of CV and SQ biomass and their respective extracts. The C and N contents were very similar in CV and SQ biomass, as well as their extracts. Our results showed that C and N content was higher in SQ (52.9 and 7.94%, respectively) than that found in other species of the same genus by other authors. In fact, Di Caprio et al. (2015) found that in a strain of Scenedesmus sp., grown for 31 days in BG11 medium, the content of C and N was 40.5 and 5.1%, respectively. Similar results (46.54% C and 4.52% N) were also obtained by Makarevičienė et al. (2012) for Scenedesmus sp. cultivated for 30 days in the same medium. However, Lopez et al. (2010) found a value of total N corresponding to 11% in S. almeriensis, after 10 days of growth in Mann and Myers medium (Mann and Myers 1968). This confirms that quite a variability in C and N content occurs in Scenedesmus sp.
The C and N content found in CV biomass was in accordance with the results reported by Bumbak et al. (2011), who determined values of C ranging between 51 and 72% and values of N between 6 and 8% in Chlorella spp. biomass, depending on the availability of N in the culture medium used (M-8). As regard the N content in this species, comparable values (between 6.2 and 7.7%) were found by Crofcheck et al. (2012). For single microalgal species, carbon content was higher in extracts than that in biomass (CVextr carbon > CV carbon; SQextr carbon > SQ carbon). By contrast, nitrogen content was much lower in CV and SQ biomass than CVextr and SQextr (Table 2). These data are in agreement with the results obtained by the 13C–NMR analysis. In extracts from the starting biomass, an increase in alkyl and aromatic carbon as well as a reduction of N and O alkyl carbon was detected in both species (Table 1). In fact, the percentage of carbon in unsubstituted aliphatic and aromatic compounds was higher than that registered in substituted compounds. Percentage contents of P, S, and Mg were similar between extracts and biomass, for each single species (Table 2); however, their contents in SQ species were higher than those measured in CV. In CV, S and Mg contents were in agreement with Bumbak et al. (2011) and Crofcheck et al. (2012), while P content in this species was much lower than that reported in the literature (0.2 vs 1.2%) (Bumbak et al. 2011).
The Ca and Fe contents were higher in biomass than those in the extracts for both species (Ca: 0.50% in CV and 0.05% in CV extract, 0.65% in SQ and 0.15% in SQ extract; Fe: 0.13% in CV and 0.01% in CV extract, 0.24% in SQ and 0.01% in SQ extract). Interestingly, in both extracts, the content of these elements was similar, whereas in biomass it was always higher in SQ than CV (Ca: 0.50% in CV and 0.65% in SQ; Fe: 0.13% in CV and 0.24% in SQ), suggesting that in extracts these elements may be influenced by the extraction procedure (Table 2).
Furthermore, Fe content in CV (0.13%) was in agreement with data reported in the literature (0.04%/0.55%) (Crofcheck et al. 2012), while Ca content was higher than that found in the same species by Crofcheck et al. (2012) (between 0.005 and 0.08%).
The K and Na contents in extracts of both species were always higher than those measured in the starting biomass. In particular, Na content increased by about nine times in CVextr and SQextr if compared to the corresponding starting biomass (Table 2).
Interestingly, the elements involved in osmosis and ionic balance (such as K) and pH regulation (Na) (Ferreira et al. 2004; Sanudo-Wilhelmy et al. 2014) recorded the highest increase in concentration in both extracts, suggesting that the extraction procedure may in some way affect their contents. Lastly, no enzyme activity was detected in CVextr and SQextr meaning that only free amino acids could be present in both solutions. In summary, NMR, FT-IR, and ICP-OES results give insight in the complexity and heterogeneity of various bioactive components of microalgae extracts. Moreover, the modes of action as biostimulants of these compounds remained largely unexplored, especially if provided in low concentration and taking into account their synergistic activity.
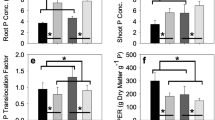
Agricultural biostimulants include different types of bio-active compounds such as microorganisms, plant growth regulators, enzymes, macro- and microalgae extracts. Biostimulants are known to act on plant physiology improving resistance to abiotic and biotic stresses, increasing yield and crop vigor. These products are able to modify root growth and architecture (Lucini et al. 2015). In our study, root apparatus was positively influenced by microalgae application as revealed by WinRhizo analysis. Total root length, root surface area, and number of root tips significantly (p < 0.01) increased in plants treated with the two doses of microalgae (Fig. 3). In particular, plants treated with SQ showed a higher number of root tips compared to those treated with CV. No difference between the two doses has been revealed, meaning that even a minimal concentration can produce a significant difference in root morphology, as previously observed by Bulgari et al. (2015). ICP-OES profiles have not revealed significant changes in the ionomic profile of Hoagland’s solutions treated with the two microalgae extracts with respect to the untreated solution. In sugar beet, greater root development especially at the seedling stage appears beneficial not only to increase water-nutrient capturing and competitive ability against weeds but also to improve final sugar yield (Stevanato et al. 2010, 2011). Therefore, the future development of this research will be focused on the root yield improvements achievable with field treatments based on evaluated microalgae extracts.
In order to study the role of CVextr and SQextr on plants’ genetic expression, we analyzed the transcript level of 53 genes related to nutrient acquisition on treated and untreated plants. Figure 4 shows the heat map of the expression pattern of the 53 genes in plants not treated (NT) and treated with two different doses (T1 and T2). The expression levels of all genes increased in response to the treatment. Red and green colors mean high and low levels of expression, respectively.
Heat map exhibiting the expression pattern of 53 sugar beet genes evaluated in the roots of untreated (T0) plants and treated with 2 mL L−1 (1 mg Corg L−1; T1) and 4 mL L−1 (2 mg Corg L−1; T2) of the two microalgae extracts. Colors range from green to red: low expression, green; high expression, red
The relative gene expression is generally higher in plants treated with SQ (Fig. 5) than CV (Fig. 6). The second dose (T2) of CV showed significantly higher levels of expression with respect to the first dose (T1) and control with the exception of genes Bv3_049600_wxxy (AILJKS3), Bv_24910_jato (AIVI56U), and Bv2_025660_etua (AI90PH) coding for “high affinity inorganic phosphate transporter,” “Aspartic proteinase-like protein 1,” and “Laccase,” respectively. The higher expression levels of evaluated genes observed in treated versus untreated plants may contribute to their superior metabolic activity sustaining nutrient acquisition and root development processes. A previous work highlighted positive and strong correlations between the expression levels of sulfate starvation genes and root morphogenetic responses as increased root elongation in sugar beet (Stevanato et al. 2015). Few experiments have been done on gene expression analysis of plants treated with seaweed as biostimulant. In Brassica napus L., Ascophillum nodosum extracts have been tested revealing a biostimulant activity on plant growth, nutrient uptake, and translocation, without changing the mineral composition of the nutrient solution (Billard et al. 2014). To our knowledge, this is the first report showing a correlation between the promoting effect of root traits and the overexpression of genes in plants treated with microalgae. The listed genes could therefore be considered as markers not only of nutritional status but also associated to root development.
Conclusion
Environmental stresses are strongly limiting the sugar beet production. The development of sustainable methods to attenuate the effect of these environmental stresses is the first priority in sugar beet research. Our study indicated that microalgae extracts perform biostimulant effects on the expression of root traits and genes related to nutrient acquisition in sugar beet improving plant growth and vigor. Therefore, the examined microalgae extracts could have a protection effect against a number of biotic and abiotic stresses and offer potential for field application.
References
Akhter M, Majumdar RD, Fortier-McGill B, Soong R, Liaghati-Mobarhan Y, Simpson M, Arhonditsis G, Schmidt S, Heumann H, Simpson AJ (2016) Identification of aquatically available carbon from algae through solution-state NMR of whole 13C-labelled cells. Anal Bioanal Chem 408:4357–4370
Arnon DI, Hoagland DR (1940) Crop production in artificial culture solution and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci 50:463–483
Baglieri A, Gennari M, Ioppolo A, Leinweber P, Nègre M (2012) Comparison between the humic acids characteristics of two andisols of different age by: FT-IR and 1 H-NMR spectroscopy and py-FIMS. Geochem Int 50:148–158
Baglieri A, Cadili V, Monterumici CM, Gennari M, Tabasso S, Montoneri E, Nardi S, Negre M (2014) Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci Hortic 176:194–199
Baglieri A, Sidella S, Barone V, Fragalà F, Silkina A, Nègre M, Gennari M (2016) Cultivating Chlorella vulgaris and Scenedesmus quadricauda microalgae to degrade inorganic compounds and pesticides in water. Environ Sci Pollut Res 23:18165–18174
Beijerinck MW (1890) Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Bot Ztg 48:1–23
Biancardi E, McGrath JM, Panella LW, Lewellen RT, Stevanato P (2010) Sugar beet. In: Bradshaw J (ed) Handbook of plant breeding, Tuber and Root Crops, vol 4. Springer, New York, pp 173–219
Billard V, Etienne P, Jannin L, Garnica M, Cruz F, Garcia-Mina JM, Yvin JC, Ourry A (2014) Two biostimulants derived from algae or humic acid induce similar responses in the mineral content and gene expression of winter oilseed rape (Brassica napus L.) J Plant Growth Regul 33:305–316
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bulgari R, Cocetta G, Trivellini A, Vernieri P, Ferrante A (2015) Biostimulants and crop responses: a review. Biol Agric Hortic 31:1–17
Bumbak F, Cook S, Zachleder V, Hauser S, Kovar K (2011) Best practices in heterotrophic high-cell-density microalgal processes: achievements, potential and possible limitations. Appl Microbiol Biotechnol 91:31
Chen SK, Subler S, Edwards CA (2002) Effects of agricultural biostimulants on soil microbial activity and nitrogen dynamics. Appl Soil Ecol 19:249–260
Coppens J, Lindeboom R, Muys M, Coessens W, Alloul A, Meerbergen K, Lievens B, Clauwaert P, Boon N, Vlaeminck SE (2016) Nitrification and microalgae cultivation for two-stage biological nutrient valorization from source separated urine. Bioresour Technol 211:41–50
Crofcheck C, Xinyi E, Shea A, Montross M, Crocker M, Andrews R (2012) Influence of media composition on the growth rate of Chlorella vulgaris and Scenedesmus acutus utilized for CO2 mitigation. J Biochem Technol 4:589–594
Devi MP, Subhash GV, Mohan SV (2012) Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: effect of nutrient supplementation. Renew Energy 43:276–283
Di Caprio F, Altimari P, Pagnanelli F (2015) Integrated biomass production and biodegradation of olive mill wastewater by cultivation of Scenedesmus sp. Algal Res 9:306–311
du Jardin P (2015) Plant biostimulants: definition, concept, main categories and regulation. Sci Hortic 196:3–14
Duygu DY, Udoh AU, Ozer TB, Akbulut A, Erkaya IA, Yildiz K, Guler D (2012) Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr J Biotechnol 11:3817–3824
Faheed FA, Abd-El Fattah Z (2008) Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J Agric Soc Sci 4:165–169
Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838
Fierro S, del Pilar S-SM, Copalcua C (2008) Nitrate and phosphate removal by chitosan immobilized Scenedesmus. Bioresour Technol 99:1274–1279
Garcia-Gonzalez J, Sommerfeld M (2016) Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J Appl Phycol 28:1051–1061
Huang CZ, Wang SL, Chen L, Lemieux C, Otis C, Turmel M, Liu XQ (1994) The Chlamydomonas chloroplast clpP gene contains translated large insertion sequences and is essential for cell growth. Mol Gen Genet 244:151–159
Jones PD, Lister DH, Jaggard KW, Pidgeon JD (2003) Future climate impact on the productivity of sugar beet (Beta vulgaris L.) in Europe. Clim Chang 58:93–108
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science 341:759–765
Lopez CVG, Garcia MDCC, Fernandez FGA, Bustos CS, Chisti Y, Sevilla JMF (2010) Protein measurements of microalgal and cynobacterial biomass. Biores Technol 101:7587–7591
Lucini L, Rouphael Y, Cardarelli M, Canaguier R, Kumar P, Colla G (2015) The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci Hortic 182:124–133
Makarevičienė V, Skorupskaitė V, Andrulevičiūtė V (2012) Biomass and oil production of green microalgae Scenedesmus sp. using different nutrients and growth. Environ Res Eng Manag 62:5–13
Mann JE, Myers J (1968) On pigments, growth and photosynthesis of Phaedactylum tricornutum. J Phycol 4:349–355
Metting B, Zimmerman WJ, Crouch IJ, van Staden J (1990) Agronomic uses of seaweed and microalgae. In: Akatsuka I (ed) Introduction to applied phycology. SPB Academic Publishing, The Hague, pp 269–307
Miller RH (1990) Soil microbiological inputs for sustainable agricultural systems. In: Edwards CA, Lal R, Madden P, Miller RH, House G (eds) Sustainable agricultural systems. Soil and Water Conservation Society, Ankeny, pp 614–623
Nabti E, Jha B, Hartmann A (2016) Impact of seaweeds on agricultural crop production as biofertilizer. Int J Environ Sci Technol 1–16
Norton TA, Melkonian M, Andersen RA (1996) Algal biodiversity. Phycologia 35:308–326
Olofsson M, Lamela T, Nilsson E, Bergé JP, Del Pino V, Uronen P, Legrand C (2012) Seasonal variation of lipids and fatty acids of the microalgae Nannochloropsis oculata grown in outdoor large-scale photobioreactors. Energies 5:1577–1592
Rocha GS, Pinto FHV, Melão MGG, Lombardi AT (2015) Growing Scenedesmus quadricauda in used culture media: is it viable? J Appl Phycol 27:171–178
Safi C, Ursu AV, Laroche C, Zebib B, Merah O, Pontalier PY, Vaca-Garcia C (2014) Aqueous extraction of proteins from microalgae: effect of different cell disruption methods. Algal Res 3:61–65
Sanudo-Wilhelmy SA, Gómez-Consarnau L, Suffridge C, Webb EA (2014) The role of B vitamins in marine biogeochemistry. Annu Rev Mar Sci 6:339–367
Shaaban MM (2001) Green microalgae water extract as foliar feeding to wheat plants. Pak J Biol Sci 4:628–632
Sigee DC, Dean A, Levado E, Tobin MJ (2002) Fourier-transform infrared spectroscopy of Pediastrum duplex: characterization of a micro-population isolated from a eutrophic lake. Eur J Phycol 37:19–26
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bact Rev 35:171
Stevanato P, Trebbi D, Saccomani M (2010) Root traits and yield in sugar beet: identification of AFLP markers associated with root elongation rate. Euphytica 173:289–298
Stevanato P, Trebbi D, Bertaggia M, Colombo M, Broccanello C, Concheri G, Saccomani M (2011) Root traits and competitiveness against weeds in sugar beet. Int Sugar J 113:497–501
Stevanato P, Fedito P, Trebbi D, Cagnin M, Saccomani M, Cacco G (2015) Effect of sulfate availability on root traits and microRNA395 expression in sugar beet. Biol Plantarum 59:491–496
Vigani M, Parisi C, Rodríguez-Cerezo E, Barbosa MJ, Sijtsma L, Ploeg M, Enzing C (2015) Food and feed products from micro-algae: market opportunities and challenges for the EU. Trends Food Sci Technol 42:81–92
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(XLSX 53 kb)
Rights and permissions
About this article
Cite this article
Barone, V., Baglieri, A., Stevanato, P. et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). J Appl Phycol 30, 1061–1071 (2018). https://doi.org/10.1007/s10811-017-1283-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1283-3