Abstract
Arsenic (As) being a toxic metalloid adversely affects plant growth and yield, as well as poses severe risks to human health. Hydrogen sulfide (H2S) has emerged a vital signaling molecule regulating key plant growth processes under stress conditions. However, till date little information is available regarding the role of H2S in mitigating As toxicity in pea plants. In the present study, the effect of externally applied H2S and its scavenger hypotaurine (HT) on various morphological, physiological and biochemical parameters of pea plants was evaluated. Our results showed significant decline in root length (RL), shoot length (SL), dry biomass, photosynthetic parameters such as pigment content and gas exchange characteristics in pea plants subjected to As stress. However, H2S supplementation significantly decreased As accumulation in the roots and shoot, as well as considerably enhanced growth and photosynthetic parameters. Hydrogen peroxide (H2O2), malondialdehyde (MDA) and electrolyte leakage (EL) increased significantly in the As-treated plants, while H2S supplementation considerably reduced the levels of H2O2 and MDA as well as EL. Arsenic stress accelerated the activities of antioxidant and AsA-GSH cycle enzymes except that of CAT; however, the activities of these enzymes were found to be further increased by H2S supply including that of CAT. Furthermore, ascorbate (AsA), glutathione (GSH) and methylglyoxal (MG) levels were significantly enhanced by As stress, and were further intensified in the H2S-supplemented plants. Our results demonstrated significant role of H2S in reducing As accumulation and inducing upregulation of the AsA-GSH cycle to overcome ROS-mediated oxidative damage to the cellular components of pea plants. Hence, H2S reduced oxidative damage and promoted growth of pea plants under As stress, suggesting an important role of H2S in plant priming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal stress is recognized as an important constraint for attaining optimum crop production world over. Of the various heavy metals known so far, arsenic (As) is a toxic heavy metal(loid), and is known as “King of Poisons” (Abbas et al. 2018). Arsenic is present in many forms with different mechanisms of toxicity, and its inorganic form is more toxic (Lizama et al. 2011). Arsenic is easily transported from root to upper parts, as it is highly mobile and can ultimately find its way to humans wherein it can cause various health problems (Abbas et al. 2018; Heikens et al. 2007; Zhao et al. 2011).
An increase in As concentration in soil might result due to anthropogenic activity, use of extremely high contents of pesticides and herbicides, irrigation with ground water polluted with heavy metals, etc. (Eliana Andrea et al. 2019; Tripathi et al. 2007; Zhao et al. 2010)) Arsenic is very toxic in nature even in small concentrations because it can cause a marked impairment in physio-biochemical processes of plants. Arsenic in soil is present in two inorganic forms viz, arsenite (AsIII) and arsenate (AsV). According to Ji et al. (2017), the AsIII is highly toxic compared with AsV, because AsIII is more soluble and mobile. Plants exposed to As are reported to show reduced growth and biomass yield as well as reduced crop production (Abbas et al. 2018; Rahman et al. 2008, 2007). According to Garg and Singla (2011), As toxicity hampers photosynthesis, respiration and other physiological activities as it disrupts the water transport (Verbruggen et al. 2009). Arsenic stress leads to ionic, osmotic as well as oxidative stress; however, plants are able to synthesize osmolytes like proline, glycine betaine, etc., which can effectively protect the cell organelles from the stress-induced toxicity. Prolonged metal stress also leads to generation of reactive oxygen species (ROS) like hydrogen peroxide (H2O2), superoxide (O2−), singlet oxygen (.O−) etc. which react with biomolecules and affect their normal functioning (Ahmed et al. 2010). Arsenic stress also induces higher accumulation of methylglyoxal (MG), a key byproduct of glycolysis, which is believed to be very harmful for plant organelles (Jan et al. 2018; Kumar and Yadav 2009; Yadav et al. 2005). MG and ROS work together and hamper the normal functioning of plant organelles, and if they are not properly removed they may cause cell death (Ahmad et al. 2019a). However, plants have their own defense mechanisms to offset the ionic, osmotic and oxidative stresses. Plants can accumulate osmolytes like proline and glycine betaine that protect the biomolecules from dehydration stress without interfering with the key functions of the cell. Proline has also been reported to have an antioxidant property that helps to quench the ROS. Plants under a stress also induce the activities of different antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione-S-transferase and other enzymes of the ascorbate glutathione cycle (ascorbate peroxidase, APX; glutathione reductase, dehydroascorbate reductase, monodehydroascorbate reductase, ascorbic acid, and glutathione). These antioxidants are known to scavenge the extra ROS from the cell and make it less prone to oxidative stress. Another system is glyoxalase system [glyoxalase I, (Gly I); glyoxalase II, (Gly II)] is thought to be responsible for the detoxification of MG (Jan et al. 2018).
Different strategies have been adopted to reclaim As polluted soils for achieving maximal crop production from the limited soil resources. One of the sustainable approaches is the use of external supplementation of nutrients, inorganic elements and phytohormones, etc. (Shivaraj et al. 2019).
Hydrogen sulfide (H2S), a gaseous molecule with a lot of health benefits, has been reported in animal system (du Toit 2015; Mancardi et al. 2009). However, within the last two decades, this gaseous molecule has gained a considerable ground because of its effective role in mitigating the adverse effects of environmental cues on plants (Ali et al. 2014; Christou et al. 2013; Mostofa et al. 2015; Shi et al. 2014; Shivaraj et al. 2019). Mitigation of an abiotic stress by H2S is attributed to its role in different defense mechanisms like antioxidant activities and ROS detoxification system (Chen et al. 2013; Mostofa et al. 2015). Hence, the key objective of the present study was to determine whether H2S has any role in the mitigation of As stress in pea plants. For this various morphological, physiological and biochemical parameter related to As tolerance were used for the evaluation of pea plants.
Materials and Methods
Plant Material, Treatment and Growth Conditions
Healthy and viable pea (Pisum sativum L.) seeds were selected for surface sterilization for 10 min using 5% sodium hypochlorite (NaOCl) solution, and were washed thoroughly with distilled water. The seeds were got germinated in pots containing a mixture of sand, perlite and peat (1:1:1); however, full strength Hoagland’s nutrient solution was used to grow the pea seedlings for two weeks by following detailed procedure of Singh et al. (2015). Thereafter, the seedlings were subjected to AsIII stress by mixing 20 µM sodium arsenite (NaAsO2) in Hoagland’s solution, whereas normal Hoagland solution was used to grow the control plants (Ahmad et al. 2020). The H2S donor, i.e., sodium hydrosulfide (NaHS; 200 µM) was mixed with Hoagland solution and then supplied to both control and As-treated plants. Moreover, a H2S scavenger i.e., hypotaurine (HT; 200 µM) was also applied with Hoagland solution in absence and presence of H2S to check if H2S really had a positive role under As stress by following (Kaya et al. 2020). Based on the following treatments, the plants were grouped as: (i) control (Hoagland solution only), (II) control + H2S, (III) As stress (20 mM), (IV) As + H2S, and (V) As + H2S + HT. The pots were placed in a growth chamber under controlled environmental conditions viz., photoperiod of 18 h, relative humidity (RH) of 70–75%, and day/night temperature 25 ± 2 °C/15 ± 2 °C. Forty-day old plants were harvested for estimating different morphological, physiological and biochemical parameters.
Growth and Photosynthetic Pigment Parameters
Morphological parameters viz., shoot length (SL), root length (RL), shoot fresh weight (SFW) and shoot dry weight (SDW) were estimated from 40-day-old plants following Singh et al. (2015). For the estimation of SDW, the samples were oven-dried at 70 °C for 48 h and final weights of the dry samples were recorded. Pigment related to photosynthesis viz., chlorophyll and carotenoids were extracted and estimated using the acetone extract method (Arnon 1949). Fresh leaf tissue was used for the extraction of pigments using 80% acetone, and a spectrophotometer (Beckman 640D USA) was used to record absorbance at 480 nm, 645 nm and 663 nm.
Horizontal fully expanded leaves were used for the estimation of leaf gas exchange parameters at full noon using an IRGA (LCA-4 model Analytical Development Company, Hoddesdon, England). These traits included transpiration rate (E), net photosynthetic rate (Pn) and stomatal conductance (gs).
Physiological and Biochemical Parameters
The Yamasaki and Dillenburg (1999) standard protocol were followed for leaf relative water content (LRWC) estimation. The upper most leaves were used to collect leaf disks, and the fresh weights of these leaf samples were recorded. The below mentioned formula was used to estimate LRWC:
The standard procedure of Bates et al. (1973), i.e., acid ninhydrin method, was followed for proline content estimation. A spectrophotometer (Beckman 640D, USA) was used to measure absorbance at 520 nm. Determination of proline content was done using a standard curve and worked out as μmol proline g−1 FW (Bates et al. 1973).
For the estimation of glycine betaine (GB) content, the method as described previously in detail by Grieve and Grattan (1983) was followed.
Oxidative Stress Biomarkers
Estimation of hydrogen peroxide (H2O2) was carried out following the detailed procedure of Velikova et al. (2000). A spectrophotometer (Beckman 640D, USA) was used to measure absorbance at 390 nm.
The protocol of Madhava Rao and Sresty (2000) was followed to measure malondialdehyde (MDA) content and lipid peroxidation. The MDA and lipid peroxidation were measured using a spectrophotometer (Beckman 640D, USA).
Electrolyte leakage (EL) was determined by following a procedure as described in detail by Dionisio-Sese and Tobita (1998).
Estimation of Enzymatic and Non-enzymatic Activity
Enzyme extract and assay were prepared by collecting fresh leaf tissue. Potassium phosphate buffer (100 mM, pH 7.0) containing polyvinyl pyrrolidone (1%) were used for homogenizing the leaf samples using a pestle and mortar. Centrifugation of the slurry was carried out for 30 min at 16,128 rcf/g-force at 4 °C. Enzyme activities were determined from the resulting supernatant (Ahmad et al. 2018).
The Dhindsa and Matowe (1981) protocol was followed to measure SOD (EU mg−1 protein) activity, which is based on nitroblue tetrazolium (NBT) reduction method. The procedure as described in detail by Aebi (1984) was used to measure CAT (EU mg−1 protein) activity. The activity of GST (EU mg−1 protein) was determined by following the detailed procedure of Hasanuzzaman and Fujita (2013). For the estimation of APX (EU mg−1 protein) activity, we followed the procedure as previously described by Nakano and Asada (1981). The method of Foster and Hess (1980) was used to estimate GR (EU mg−1 protein) activity.
The detailed procedure as previously described by Miyake and Asada (1992) was used to determine MDHAR (EU mg−1 protein) activity. A standard protocol of Nakano and Asada (1981) was used to estimate DHAR (EU mg−1 protein) activity. For estimating AsA and GSH contents, we followed the previously described methods of Huang et al. (2005) and Yu et al. (2003), respectively.
Statistical Analysis
In the present study, average values each estimated from five replicates were used for the data analysis. One-way analysis of variance (ANOVA) was carried out using the SPSS software (Version 17), and the significant differences among the means were worked out at P ≤ 0.05. Tukey’s HSD (Honestly Significant Difference) test was used for comparison between control and treatment’s means at P ≤ 0.05 significance level using SPSS 10 software.
Results
Phenotypic Evaluation
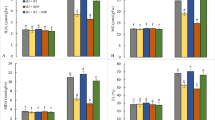
Arsenic stress significantly decreased SL and RL by 55.32% and 64.83%, respectively, in As-treated pea plants with respect to controls; however, H2S application slightly increased SL and RL in the control plants, whereas it enhanced SL and RL by 24.77% and 35.10%, respectively, in the As-treated plants (Fig. 1a). The SFW and SDW also decreased by 65.88% and 42.85%, respectively, in the As-treated plants relative to those in the untreated plants; however, exogenous supply of H2S to the As-treated plants increased the SFW and SDW by 41.17% and 23.80%, respectively, with reference to those in the plants solely treated with As (Fig. 1b). Our study observed that H2S scavenger i.e., hypotaurine (HT) reversed the positive effects of H2S. The As + HT-treated plants revealed almost similar phenotypic effects as those of plants treated with As only for the traits viz., SL, RL, SFW and SDW (Fig. 1a, b).
Arsenic Concentration, Tolerance Index and Translocation Factor
Accumulation of As in the shoot tissue decreased from 525 to 225 µg g−1 DW, and in roots it reduced from 715 to 492 µg g−1 DW with the supplementation of H2S. However, the effect of H2S on As concentration in the shoot and root tissues was nullified by the HT treatment; for example, As + HT + H2S and As-treated plants showed non-significant difference in shoot and root As accumulation, but showed significantly higher As concentration in both tissues relative to those in the As + H2S-treated plants (Table 1).
Shoot tolerance index (STI) and root tolerance index (RTI) also increased from 44.67 to 75.22% and 35.16 to 64.89%, respectively, by the exogenous supply of H2S in the As-treated plants. However, translocation factor decreased from 0.734 to 0.457 in the present of H2S in the As-treated plants (Table 1). The beneficial effect of H2S on STI, RTI and translocation factor in the As-treated plants was nullified by the application of HT, and these parameters showed non-significant difference between the As-treated and As + HT + H2S plants (Table 1).
Pigments and Gas Exchange Parameters
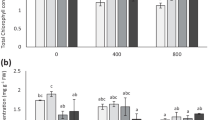
Arsenic stress reduced total chlorophyll and carotenoid contents by 37.41% and 57.57% in the pea plants compared to that in the control plants. However, As + H2S-treated plants showed only 24.45% and 36.36% decrease in total chlorophyll and carotenoid contents relative to the controls. Furthermore, exogenous supply of As + HT + H2S to the pea plants showed similar levels of total chlorophyll and carotenoid contents to those in the As-treated plants (Fig. 2a, b).
The Pn, gs, and E decreased by 51.60%, 82.20% and 75.46% under As stress in the pea plants compared to the controls. However, H2S application to the As-treated plants showed a marked reduction in Pn, gs and E by 35.47%, 57.37% and 42.33% with reference to the controls, which are significantly lower than those in the pea plants treated with only As (Fig. 2c–e). Moreover, addition of HT eliminated the positive effects of H2S in the As-stressed plants.
Physiological and Biochemical Analysis
Arsenic stress reduced RWC by 46.56% in the pea plants with respect to the control. However, supplementation of H2S to the As-treated plants exhibited enhanced RWC (70.62%) compared to that in the pea plants fed with As only (Fig. 3a). Proline and GB contents increased under As stress by 82.81% and 71.83%, respectively, compared to the controls. However, in the As + H2S-treated plants an increase of only 52.82% in proline and 57.26% in GB content, respectively, was observed relative to the controls, which is considerably low compared to those in the pea plants treated with As only (Fig. 3b, c). The As + H2S + HT and As-treated plants showed non-significant differences for RWC, proline and GB contents.
Oxidants, Electrolyte Leakage and Antioxidant Activity
Hydrogen peroxide (H2O2) and MDA contents increased by 74.22% and 63.09%, respectively, in the As-treated plants compared to the controls. However, application of H2S suppressed the H2O2 and MDA contents in the As + H2S-treated plants by 63.23% and 57.26%, respectively, compared to those in the As-treated plants (Fig. 4a, b). In addition, electrolyte leakage (EL) was recorded to be increased from 10.77% to 69.05% under As stress; however, H2S application reduced the EL to 44.15% (Fig. 4c). The HT supplementation to As + H2S-treated plants showed a similar behavior in H2O2, MDA and EL as that in the As-treated plants.
The activities of enzymatic antioxidants viz., SOD, CAT, APX, GR and GST, enhanced by 179.88%, 39.21%, 58.95%, 89.65% and 63.45%, respectively, in the As-treated plants with reference to the controls. However, H2S application to the As-stressed plants further showed enhanced activities of SOD, CAT, APX, GR and GST by 32.10%, 70.59%, 14.54%, 19.33% and 26.93% with reference to those in the As-stressed plants (Figs. 5a–c, 6a, b). Arsenic stress decreased the ascorbate recycling enzymes MDHAR and DHAR by 49.16% and 49.06%, respectively, relative to the controls. Supplementation of H2S increased the activities of MDHAR and DHAR by 43.38% and 70.79%, respectively, with respect to those in the As-stressed plants (Fig. 6c, d). However, the As + H2S + HT and As-treated plants showed non-significant differences for CAT, APX, GR, GST, MDHAR and DHAR.
As stress decreased AsA and GSSG by 55.00% and 42.76%, respectively, but increased GSH by 75.60% in the As-treated plants relative to the controls. The H2S supply enhanced the levels of AsA, GSH and GSSG by 66.66%, 25.38% and 61.34%, respectively, with respect to those in the plants treated with As only (Fig. 7a–c). However, HT supplemented to the As + H2S-treated plants showed non-significant effect on this trait.
Methyl Glyoxal and Glyoxalase Cycle
Arsenic stress applied to the pea plants resulted in increased amount of MG by 92.66% compared to the control; however, application of H2S decreased the MG content by 35.48% relative to that in the As-stressed plants (Fig. 8a). Application of HT to the As + H2S-treated plants nullified the effect of H2S, and As + H2S + HT and As-treated plants showed non-significant differences for MT.
Arsenic stress enhanced the Gly I by 63.23%, but decreased Gly II by 41.55% relative to the controls. Application of H2S to the As-treated plants further enhanced Gly I by 18.01%, and Gly II by 44.44% compared to those in the As-treated plants (Fig. 8b, c). The addition of HT to the As + H2S-treated plants showed non-significant difference between As + H2S + HT and As-alone-treated plants.
Discussion
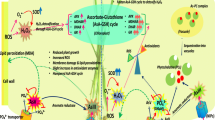
Hydrogen sulfide (H2S) has recently emerged as an important gaseous signaling molecule regulating numerous physiological processes in plants such as germination, regulation of stomatal aperture, photosynthesis and formation of lateral and adventitious root (Shivaraj et al. 2019). Besides, many studies have demonstrated important role of H2S in plant defense response against multiple abiotic stresses such as including drought stress (Jin et al. 2017), salt stress (Lai et al. 2014), chilling stress (Fu et al. 2013), osmotic stress (Khan et al. 2017) and heavy metal stress (Guo et al. 2016). Hence, the present study demonstrated a potential role of H2S in mitigating As stress in the pea plants, which was ascribed to varying regulation of various morphological, physiological and biochemical parameters. Our results revealed that As toxicity diminished growth (SL & RL) and biomass (SFW & SDW) of the pea plants. Similar to our results, many previous studies have reported As-induced reduction in plant growth and biomass in different plant species viz., Luffa acutangula (Singh et al. 2013), rice (Singh et al. 2017), and soybean (Chandrakar and Keshavkant 2019). Such As-induced reduction in plants growth may occur due to disturbance in cellular processes at molecular, biochemical and physiological levels in plants (Abbas et al. 2018; Gunes et al. 2010; Khalid et al. 2017; Rafiq et al. 2017, 2018). The supplementation of H2S significantly increased growth and biomass of As-treated pea plants, similar as previously demonstrated by Singh et al. (2015). Exogenous supply of H2S has been widely reported to increase plant growth parameters in various plants under toxic metal concentrations (Chen et al. 2013; Cui et al. 2014; Shi et al. 2014; Singh et al. 2015). Application of the H2S scavenger, i.e., HT, has been well documented to reverse the beneficial effects of H2S on plant growth under heavy metal stress (Kaya and Ashraf 2019; Kaya et al. 2020; Mostofa et al. 2015; Scuffi et al. 2014). It has been suggested that improvement in growth and biomass yield of As-treated plants by H2S might result from the reduced accumulation of As in the tissues (root and shoot), as well as its translocation from root to shoot of pea plants (Singh et al. 2015). For example, our results showed that H2S application significantly reduced the As concentration in the root and shoot tissues, and its translocation from root to shoot, suggesting some specific mechanisms utilized by the H2S to reduce As uptake, accumulation and transport to aboveground shoot tissues. Moreover, many authors have documented the key role of H2S in regulating uptake, accumulation and transport of metal(loid)s in plants (Li et al. 2013; Singh et al. 2015; Sun et al. 2013).
A negative effect of As stress has been demonstrated on photosynthesis and chlorophyll metabolism, leading to impaired biosynthesis and accelerated degradation of the pigments (Abbas et al. 2018; Singh et al. 2016). However, our study exhibited a considerable reduction in chlorophyll content of the pea plants by As stress which is analogous to some earlier studies on Zea mays (Emamverdian et al. 2015) and Trifolium pratense (Hasanuzzaman et al. 2017). Anjum et al. (2011) reported that a remarkable reduction in chlorophyll pigment synthesis under As stress results from the shortage of adaptive adjustments of photosystems-I and -II. In agreement with the present study, previous studies reported As-induced reduction in carotenoid pigment in different plant species such as mungbean (Srivastava et al. 2017) and chickpea (Dwivedi et al. 2012). However, supplementation of H2S to the As-treated plants considerably reduced the degradation of chlorophyll and carotenoid pigments in the pea plants, as previously demonstrated by Singh et al. (2015). This effect on photosynthetic pigment biosynthesis may have been due to the role of H2S in reducing the translocation and accumulation of As in photosynthetic organs (Abbas et al. 2018; Luo et al. 2020; Singh et al. 2015).
Arsenic induced reduction in leaf gas exchange characteristics such as Pn, E and gs is evident in the present investigation, which is in agreement with the findings earlier reported in maize (Wang et al. 2015) and cowpea (Dutta and Mondal 2014). Arsenic stress and ABA accumulation are directly correlated; higher concentration of As in plants leads to accumulation of ABA in plant cells including guard cells (Huang et al. 2012), thereby causing stomatal closure and reduced transpiration (Stoeva et al. 2004). For example, Armendariz et al. (2016) reported that As stress induces ABA accumulation and stomatal closure in soybean, and reduces transpiration. However, closure of stomata decreases CO2 fixation, that in turn reduces photosynthetic rate (Ahmad et al. 2018). The reduction in Pn can be attributed to the damage caused by As in both photochemical and biochemical steps of the photosynthesis process (Abbas et al. 2018). Application of H2S significantly enhanced gas exchange parameters in the As-treated pea plants. Many studies have reported that H2S can enhance gas exchange parameters under metal toxicity stress in different plants such as in nickel-stressed rice (Rizwan et al. 2019), chromium-stressed cauliflower (Ahmad et al. 2019b), and arsenic-stressed pea (Singh et al. 2015). However, HT application reversed the positive effects of H2S on photosynthetic and gas exchange parameters, which in line with some earlier studies (Baudouin et al. 2016; Khan et al. 2018; Wei et al. 2017).
The present study showed as significant reduction in RWC of pea plants under As stress, similar as earlier found in rice (Rahman et al. 2016) and soybean (Vezza et al. 2018). This effect most possibly results due to As-induced constraints to water uptake and damage to the root system architecture. Vezza et al. (2018) reported water absorption reduction of 25–39% in soybean under As stress. They demonstrated that As treatment applied could not decrease the osmotic potential, and suggested reduction in water absorption might be controlled instead of physiological or phenotypic changes. Armendariz et al. (2016) reported that As treatment to soybean plants resulted in thicker and lignified cell walls of root cells as well as deposition of huge quantity of dark deposits in the xylem vessels, and also observed a decline in root length and biomass. Many previous studies have also observed a similar kind of effect on the roots of several other plants under As and other heavy metals (Rai et al. 2011; Rui et al. 2016; Yoon et al. 2015). Heavy metal toxicity including As generally stimulates imbalance in plant water as well as induces osmolyte accumulation like Pro and GB. In the present study, we observed an inverse relationship of leaf RWC, Pro and GB levels under As stress, but the application of H2S recovered RWC by avoiding the accumulation of high amount of Pro. These findings show the direct role of H2S in other metabolic adjustment(s) by preventing accumulation of high Pro level. Importantly, the observed increment in the RWC might result in opening of stomata as well as increased transpiration in plants treated with H2S + As, which in turn may contribute to the improvement of photosynthesis (Bharwana et al. 2014; Duan et al. 2014) thereby improving the growth of the pea plants.
Hydrogen peroxide (H2O2), MDA and EL are prospective biomarkers of oxidative stress (Ahmad et al. 2017; Hasanuzzaman et al. 2014). Like many other metals, As can induce the production and accumulation of MDA and H2O2, that in turn may increase EL; these results are similar to those reported in by different authors (Choudhury et al. 2011; Ghosh et al. 2013; Mylona et al. 1998; Rafiq et al. 2018). In the present investigation, supplementation of H2S to the As-stressed pea plants resulted in reduction of H2O2, MDA and EL, suggesting an important function of H2S in mitigating oxidative damage resulting due to the effect of As stress. Three different mechanisms were suggested to mediate alleviation of As-induced oxidative damage by H2S: (1) H2S reduces accumulation of As, hence reducing the metal-induced e damage, (2) H2S induces higher levels of NO, and NO acts as a scavenger of ROS such as SOR and peroxy radicals (lipid peroxidation products) (Singh et al. 2013), and (3) both H2S and NO are important molecules involved in defense signaling molecules; these molecules regulate oxidative stress tolerance by inducing antioxidant defense system (Fang et al. 2016; Hancock and Whiteman 2014). Plants possess enzymatic and non-enzymatic antioxidants defense mechanisms to scavenge excess ROS and maintain normal redox balance within the cells, as well as prevent oxidative cellular damages. Under abiotic-induced oxidative stress, SOD scavenges O⋅− into oxygen (O2) and H2O2, and it acts as a first line of defense in managing oxidative stress. However, the APX, CAT and GPX act on the stress-generated H2O2, that is finally reduced to H2O. In our study, the activities of SOD and APX increased significantly under As stress, which is a similar response as earlier observed in other species like rice (Shri et al. 2009), and maize (Requejo and Tena 2005). In contrast, the activity of CAT decreased in the pea plants under As stress, that is in agreement with the findings reported in Taxithelium nepalense by Singh et al. (2007). Hence, an increase or a decrease in the activities of SOD and CAT, suggests accumulation of H2O2, which is also supported by H2O2 data presented here. However, the CAT plays an important function in the removal of excess H2O2 produced under oxidative stress. Application of H2S further increased the activities of SOD, APX, and CAT, which is in consistent with the findings reported in wheat (Zhang et al. 2008), pepper (Kaya et al. 2018), and Arabidopsis (Shan et al. 2018).
Four enzymes, i.e., MDHAR DHAR, APX and GR, and two non-enzymatic antioxidants i.e., AsA and GSH are important components of the AsA-GSH cycle (Foyer and Noctor 2011). These AsA-GSH cycle components interact with the ROS and induce specific changes in the ROS and antioxidant levels as well as the redox ratios of AsA and GSH (Kuźniak and Skłodowska 2005). Our results showed a significant (P < 0.05) inhibition in the MDHAR and DHAR activities under As stress, but those of APX and GR increased significantly. The decreased activities of MDHAR and DHAR resulted in reduced (P < 0.05) AsA and GSH pools. Hence, inhibition in the MDHAR and DHAR activities might have increased lipid and protein damage due to oxidative stress resulting from As stress as reported by Aravind and Prasad (2005). However, H2S supply ameliorated the inhibition of MDHAR and DHAR activities in the As-treated plants, as well as further increased the activities of APX and GR. These findings suggest the putative role of H2S in inducing enzyme activities of the AsA-GSH cycle, thereby regulating the redox status of AsA and GSH. This in turn might have resulted in improved growth of the pea plants relative to the plants treated with As only. However, Gao and Zhang (2008) demonstrated the protective role of AsA in the Arabidopsis ascorbate-deficient (vtc1) mutant against oxidative stress. Furthermore, the role of GSH in the synthesis of phytochelatins and ROS elimination has been well documented (Kalinowska and Pawlik-Skowrońska 2010), and GSH is known to regulate the detoxification of lipids and protein peroxidation products by acting as a substrate via the activity of glutathione-S-transferase (Gajewska and SkŁodowska 2010). Our results clearly showed As-mediated redox status alteration in the pea plants through its interference with the AsA and GSH pools. However, H2S application recovered the redox status as shown by increased accumulation of AsA and GSH, and thus regulates the redox buffering. The increased content of AsA and GSH is directly associated with the enzyme activities of the AsA-GSH cycle, suggesting the function of H2S in stimulating the antioxidant defense system. In agreement with our findings, the application H2S was shown to reestablish the redox status in Medicago sativa plants, thereby increasing plant salinity tolerance (Lai et al. 2014).
Over-accumulation of methylglyoxal (MG) in plant cells leads to adverse effects by inducing the generation of ROS as well as inhibiting the antioxidant defense system (Li 2016; Yadav et al. 2005). For avoiding MG-mediated cellular injury, plants have developed a well-developed detoxification mechanism for MG which is referred to as the glyoxalase (Gly) system. This system includes Gly I and Gly II (Kaur et al. 2016). By utilizing the GSH, the glyoxalase I converts MG to S-d-lactoylglutathione, while as S-d-lactoylglutathione is further converted to D-lactic acid by glyoxalase II, and during this reaction GSH is regenerated (Hossain et al. 2012). Higher accumulation of MG observed under As toxicity indicates clear signs of oxidative stress. In the present study, As stress mediated increase and decrease in the activity of Gly I and Gly II, respectively, suggests the inefficiency of the Gly system in the As-stressed plants. Enhanced Gly I activity under heavy metal toxicity has been reported in various studies (Hossain and Fujita 2010; Hossain et al. 2009; Kalapos et al. 1992; Lin et al. 2010). Mostofa et al. (2015) which suggest that decline in Gly II in response to heavy metal stress might result from proteolytic damage to enzymes. However, supplementation of H2S to the As-treated pea plants increased the activities of both Gly I and Gly II enzymes with a corresponding decrease in the MG levels. Previously, it was demonstrated that supplementation of rice seedlings with H2S induces Gly I and Gly II accumulation; hence, resulting in increased Cd tolerance by increasing MG accumulation (Mostofa et al. 2015). These findings also suggest that induction of Gly II activity assists in recycling GSH efficiently into the system, which in turn maintains GSH homeostasis and higher enzyme activity of the AsA and GSH cycle to effectively prevent oxidative stress. Barrameda-Medina et al. (2014) have demonstrated increased activities of Gly enzymes and antioxidant enzymes (APX, GST and GPX) which were found to alleviate Zn toxicity in Brassica oleracea (Barrameda-Medina et al. 2014). Thus, our results suggest an important role of H2S in the regulation of the antioxidant defense as well as the Gly systems to overcome ROS and MG toxicity induced by As stress in the pea plants. Hence, H2S alleviated the oxidative damage in the As-treated pea plants through regulating the activities of Gly I and Gly II enzymes, which suggests that H2S can effectively restore GSH and glutathione redox potential via the glyoxalase system.
However, the positive effects of H2S in regulating ROS levels, and antioxidant and glyoxalase systems under As stress in the pea plants were all reversed by the supplementation of HT (a H2S scavenger), which, suggest that H2S plays an important role in alleviating As-induced damage to pea plants.
Conclusion
Arsenic toxicity is an important constraint to growth and yield of crop plants. In higher plants, As has been analyzed under diverse perspectives such as its assimilation, transport, accumulation, and cellular metabolism etc. Recently, some gaseous signaling molecules such as H2S have been suggested to play an important role in the mitigation of As toxicity in crop plants. However, least efforts have been made to elucidate the role and mechanism involved in H2S mediated alleviation of As stress in pea crop. Hence, in the present study various morphological, physiological and biochemical parameters were appraised to determine the role of H2S in regulating As tolerance in pea plants. Our results showed significant adverse effects of As stress on plant growth, biomass, photosynthesis, ROS production and antioxidant system. However, supplementation of H2S to As-treated plants reversed all the negative effects of As on the pea plants leading to improved plant growth, biomass as well as reduced As-induced oxidative damage. Lastly, the present study provides a conclusive evidence for the role of H2S as a priming agent in mitigating As stress in pea and other such crops.
References
Abbas G et al (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Env Res Public Health 15:59. https://doi.org/10.3390/ijerph15010059
Aebi H (1984) Catalase in vitro. Methods in enzymology. Elsevier, Amsterdam, pp 121–126
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Egamberdieva D, Bhardwaj R, Ashraf M (2017) Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. J Plant Interact 12:429–437. https://doi.org/10.1080/17429145.2017.1385867
Ahmad P, Abd Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KHM (2018) Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate-glutathione cycle and secondary metabolites. Sci Rep 8:13515–13515. https://doi.org/10.1038/s41598-018-31917-1
Ahmad A, Kumari P, Ahmad M (2019) Apigenin attenuates edifenphos-induced toxicity by modulating ROS-mediated oxidative stress, mitochondrial dysfunction and caspase signal pathway in rat liver and kidney. Pestic Biochem Physiol 159:163–172. https://doi.org/10.1016/j.pestbp.2019.06.010
Ahmad R et al (2019) Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. https://doi.org/10.1111/ppl.13001
Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M (2020) Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 244:125480
Ahmed S et al (2010) Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect 119:258–264. https://doi.org/10.1289/ehp.1102086
Ali B, Gill RA, Yang S, Gill MB, Ali S, Rafiq MT, Zhou W (2014) Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol Environ Saf 110:197–207. https://doi.org/10.1016/j.ecoenv.2014.08.027
Anjum SA, Xie X-y, Wang L-c, Saleem MF, Man C, Lei W (2011) Morphological, physiological and biochemical responses of plants to drought stress. Afr J Agric Res 6:2026–2032
Aravind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate–glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116. https://doi.org/10.1016/j.plaphy.2005.01.002
Armendariz AL, Talano MA, Travaglia C, Reinoso H, Wevar Oller AL, Agostini E (2016) Arsenic toxicity in soybean seedlings and their attenuation mechanisms. Plant Physiol Biochem 98:119–127. https://doi.org/10.1016/j.plaphy.2015.11.021
Arnon DI (1949) Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Barrameda-Medina Y, Montesinos-Pereira D, Romero L, Blasco B, Ruiz JM (2014) Role of GSH homeostasis under Zn toxicity in plants with different Zn tolerance. Plant Sci 227:110–121. https://doi.org/10.1016/j.plantsci.2014.07.010
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/bf00018060
Baudouin E, Poilevey A, Hewage NI, Cochet F, Puyaubert J, Bailly C (2016) The significance of hydrogen sulfide for Arabidopsis seed germination. Front Plant Sci 7:930–930. https://doi.org/10.3389/fpls.2016.00930
Bharwana SA, Ali S, Farooq MA, Ali B, Iqbal N, Abbas F, Ahmad MSA (2014) Hydrogen sulfide ameliorates lead-induced morphological, photosynthetic, oxidative damages and biochemical changes in cotton. Environ Sci Pollut Res 21:717–731. https://doi.org/10.1007/s11356-013-1920-6
Chandrakar V, Keshavkant S (2019) Nitric oxide and dimethylthiourea up-regulates pyrroline-5-carboxylate synthetase expression to improve arsenic tolerance in Glycine max L. Environ Prog Sustain Energy 38:402–409. https://doi.org/10.1002/ep.12978
Chen J et al (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318. https://doi.org/10.1007/s11104-012-1275-7
Choudhury B, Chowdhury S, Biswas AK (2011) Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J Plant Interact 6:15–24. https://doi.org/10.1080/17429140903487552
Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64:1953–1966. https://doi.org/10.1093/jxb/ert055
Cui W et al (2014) Cadmium-induced hydrogen sulfide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo)glutathione and reactive oxygen species homeostases. PLoS ONE 9:e109669–e109669. https://doi.org/10.1371/journal.pone.0109669
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32:79–91. https://doi.org/10.1093/jxb/32.1.79
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9. https://doi.org/10.1016/s0168-9452(98)00025-9
du Toit A (2015) The health benefits of hydrogen sulphide. Nat Rev Mol Cell Biol 16:68–68. https://doi.org/10.1038/nrm3946
Duan B, Ma Y, Jiang M, Yang F, Ni L, Lu W (2014) Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul 75:33–44. https://doi.org/10.1007/s10725-014-9929-5
Dutta P, Mondal S (2014) Changes in pigments and photosynthetic parameters of cowpea under two inorganic arsenicals. IOSR J Agric Vet Sci 7:99–103. https://doi.org/10.9790/2380-074199103
Dwivedi S et al (2012) Arsenic affects essential and non-essential amino acids differentially in rice grains: inadequacy of amino acids in rice based diet. Environ Int 46:16–22. https://doi.org/10.1016/j.envint.2012.04.012
Eliana Andrea M-M, Ana Carolina T-E, Tito José C-B, José Luis M-N, Luis Carlos G-M (2019) Evaluation of contaminants in agricultural soils in an Irrigation District in Colombia. Heliyon 5:e02217–e02217. https://doi.org/10.1016/j.heliyon.2019.e02217
Emamverdian A, Ding Y, Mokhberdoran F, Xie Y (2015) Heavy metal stress and some mechanisms of plant defense response. Sci World J 2015:756120–756120. https://doi.org/10.1155/2015/756120
Fang H, Liu Z, Jin Z, Zhang L, Liu D, Pei Y (2016) An emphasis of hydrogen sulfide-cysteine cycle on enhancing the tolerance to chromium stress in Arabidopsis. Environ Pollut 213:870–877. https://doi.org/10.1016/j.envpol.2016.03.035
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487. https://doi.org/10.1104/pp.66.3.482
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
Gajewska E, SkŁodowska M (2010) Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol Environ Saf 73:996–1003. https://doi.org/10.1016/j.ecoenv.2010.02.013
Gao Q, Zhang L (2008) Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol 165:138–148. https://doi.org/10.1016/j.jplph.2007.04.002
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321. https://doi.org/10.1007/s10311-011-0313-7
Ghosh S et al (2013) Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 8:e82529–e82529. https://doi.org/10.1371/journal.pone.0082529
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/bf02374789
Gunes A, Inal A, Bagci EG, Kadioglu YK (2010) Combined effect of arsenic and phosphorus on mineral element concentrations of sunflower. Commun Soil Sci Plant Anal 41:361–372. https://doi.org/10.1080/00103620903462357
Hancock JT, Whiteman M (2014) Hydrogen sulfide and cell signaling: team player or referee? Plant Physiol Biochem 78:37–42. https://doi.org/10.1016/j.plaphy.2014.02.012
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596. https://doi.org/10.1007/s10646-013-1050-4
Hasanuzzaman M, Nahar K, Alam MM, Fujita M (2014) Modulation of antioxidant machinery and the methylglyoxal detoxification system in selenium-supplemented brassica napus seedlings confers tolerance to high temperature stress. Biol Trace Elem Res 161:297–307. https://doi.org/10.1007/s12011-014-0120-7
Hasanuzzaman M et al (2017) Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci 18:200. https://doi.org/10.3390/ijms18010200
Heikens A, Panaullah GM, Meharg AA (2007) Arsenic behaviour from groundwater and soil to crops: impacts on agriculture and food safety. Reviews of environmental contamination and toxicology. Springer, New York, pp 43–87
Hossain MA, Fujita M (2010) Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol Mol Biol Plants 16:19–29. https://doi.org/10.1007/s12298-010-0003-0
Hossain MA, Hossain MZ, Fujita M (2009) Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37. https://doi.org/10.1155/2012/872875
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049. https://doi.org/10.1093/jxb/eri301
Huang H, Jia Y, Sun G-X, Zhu Y-G (2012) Arsenic speciation and volatilization from flooded paddy soils amended with different organic matters. Environ Sci Technol 46:2163–2168. https://doi.org/10.1021/es203635s
Jan S, Alyemeni MN, Wijaya L, Alam P, Siddique KH, Ahmad P (2018) Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol 18:146–146. https://doi.org/10.1186/s12870-018-1359-5
Ji R et al (2017) Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1;1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS ONE 12:e0173681–e0173681. https://doi.org/10.1371/journal.pone.0173681
Kalapos MP, Garzó T, Antoni F, Mandl J (1992) Accumulation of S-d-lactoylglutathione and transient decrease of glutathione level caused by methylglyoxal load in isolated hepatocytes. Biochim Biophys Acta 1135:159–164. https://doi.org/10.1016/0167-4889(92)90132-u
Kalinowska R, Pawlik-Skowrońska B (2010) Response of two terrestrial green microalgae (Chlorophyta, Trebouxiophyceae) isolated from Cu-rich and unpolluted soils to copper stress. Environ Pollut 158:2778–2785. https://doi.org/10.1016/j.envpol.2010.03.003
Kaur C, Sharma S, Singla-Pareek SL, Sopory SK (2016) Methylglyoxal detoxification in plants: role of glyoxalase pathway. Indian J Plant Physiol 21:377–390. https://doi.org/10.1007/s40502-016-0260-1
Kaya C, Ashraf M (2019) The mechanism of hydrogen sulfide mitigation of iron deficiency-induced chlorosis in strawberry (Fragaria × ananassa) plants. Protoplasma 256:371–382. https://doi.org/10.1007/s00709-018-1298-x
Kaya C, Ashraf M, Akram NA (2018) Hydrogen sulfide regulates the levels of key metabolites and antioxidant defense system to counteract oxidative stress in pepper (Capsicum annuum L.) plants exposed to high zinc regime. Environ Sci Pollut Res 25:12612–12618. https://doi.org/10.1007/s11356-018-1510-8
Kaya C, Ashraf M, Alyemeni MN, Ahmad P (2020) Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol Plant. https://doi.org/10.1111/ppl.13012
Khalid S et al (2017) Arsenic behaviour in soil-plant system: biogeochemical reactions and chemical speciation influences. Enhancing cleanup of environmental pollutants. Springer, New York, pp 97–140
Khan MN, AlZuaibr FM, Al-Huqail AA, Siddiqui MH, Ali HM, Al-Muwayhi MA, Al-Haque HN (2018) Hydrogen sulfide-mediated activation of O-acetylserine (Thiol) lyase and l/d-cysteine desulfhydrase enhance dehydration tolerance in Eruca sativa mill. Int J Mol Sci 19:3981. https://doi.org/10.3390/ijms19123981
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157. https://doi.org/10.1002/jctb.2023
Kuźniak E, Skłodowska M (2005) Compartment-specific role of the ascorbate–glutathione cycle in the response of tomato leaf cells to Botrytis cinerea infection. J Exp Bot 56:921–933
Lai W-A et al (2014) Paenibacillus medicaginis sp. nov. a chitinolytic endophyte isolated from a root nodule of alfalfa (Medicago sativa L.). Int J Syst Evol Microbiol 65:3853–3860. https://doi.org/10.1099/ijsem.0.000505
Li Z-G (2016) Methylglyoxal and glyoxalase system in plants: old players, new concepts. Bot Rev 82:183–203. https://doi.org/10.1007/s12229-016-9167-9
Li Z-G, Ding X-J, Du P-F (2013) Hydrogen sulfide donor sodium hydrosulfide-improved heat tolerance in maize and involvement of proline. J Plant Physiol 170:741–747. https://doi.org/10.1016/j.jplph.2012.12.018
Lin F, Xu J, Shi J, Li H, Li B (2010) Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol Biol Rep 37:729–735. https://doi.org/10.1007/s11033-009-9578-3
Lizama AK, Fletcher TD, Sun G (2011) Removal processes for arsenic in constructed wetlands. Chemosphere 84:1032–1043. https://doi.org/10.1016/j.chemosphere.2011.04.022
Luo M, Lin H, He Y, Zhang Y (2020) The influence of corncob-based biochar on remediation of arsenic and cadmium in yellow soil and cinnamon soil. Sci Total Environ 717:137014. https://doi.org/10.1016/j.scitotenv.2020.137014
Madhava Rao KV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128. https://doi.org/10.1016/s0168-9452(00)00273-9
Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P (2009) Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta 1787:864–872. https://doi.org/10.1016/j.bbabio.2009.03.005
Miyake C, Asada K (1992) Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33:541–553. https://doi.org/10.1093/oxfordjournals.pcp.a078288
Mostofa MG, Saegusa D, Fujita M, Tran L-SP (2015) Hydrogen sulfide regulates salt tolerance in rice by maintaining Na(+)/K(+) balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front Plant Sci 6:1055–1055. https://doi.org/10.3389/fpls.2015.01055
Mylona PV, Polidoros AN, Scandalios JG (1998) Modulation of antioxidant responses by arsenic in maize. Free Radic Biol Med 25:576–585. https://doi.org/10.1016/s0891-5849(98)00090-2
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Rafiq M, Shahid M, Abbas G, Shamshad S, Khalid S, Niazi NK, Dumat C (2017) Comparative effect of calcium and EDTA on arsenic uptake and physiological attributes of Pisum sativum. Int J Phytoremediation 19:662–669. https://doi.org/10.1080/15226514.2016.1278426
Rafiq M et al (2018) A comparative study to evaluate efficiency of EDTA and calcium in alleviating arsenic toxicity to germinating and young Vicia faba L. seedlings. J Soils Sediments 18:2271–2281. https://doi.org/10.1007/s11368-017-1693-5
Rahman MA, Hasegawa H, Rahman MM, Rahman MA, Miah MAM (2007) Accumulation of arsenic in tissues of rice plant (Oryza sativa L.) and its distribution in fractions of rice grain. Chemosphere 69:942–948. https://doi.org/10.1016/j.chemosphere.2007.05.044
Rahman MA, Hasegawa H, Rahman MM, Miah MAM, Tasmin A (2008) Straighthead disease of rice (Oryza sativa L.) induced by arsenic toxicity. Environ Exp Bot 62:54–59. https://doi.org/10.1016/j.envexpbot.2007.07.016
Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Muthurajan R (2016) Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol 16(Suppl 1):35–35. https://doi.org/10.1186/s12896-016-0261-1
Rai A et al (2011) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:986–995. https://doi.org/10.1016/j.chemosphere.2010.10.070
Requejo R, Tena M (2005) Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry 66:1519–1528. https://doi.org/10.1016/j.phytochem.2005.05.003
Rizwan M et al (2019) Hydrogen sulfide enhances rice tolerance to nickel through the prevention of chloroplast damage and the improvement of nitrogen metabolism under excessive nickel. Plant Physiol Biochem 138:100–111. https://doi.org/10.1016/j.plaphy.2019.02.023
Rui M et al (2016) Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci 7:815–815. https://doi.org/10.3389/fpls.2016.00815
Scuffi D, Álvarez C, Laspina N, Gotor C, Lamattina L, García-Mata C (2014) Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166:2065–2076. https://doi.org/10.1104/pp.114.245373
Shan C, Wang T, Zhou Y, Wang W (2018) Hydrogen sulfide is involved in the regulation of ascorbate and glutathione metabolism by jasmonic acid in Arabidopsis thaliana. Biol Plant 62:188–193. https://doi.org/10.1007/s10535-017-0740-9
Shi H, Ye T, Chan Z (2014) Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol Biochem 74:99–107. https://doi.org/10.1016/j.plaphy.2013.11.001
Shivaraj SM et al (2019) Nitric oxide and hydrogen sulfide crosstalk during heavy metal stress in plants. Physiol Plant. https://doi.org/10.1111/ppl.13028
Shri M et al (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110. https://doi.org/10.1016/j.ecoenv.2008.09.022
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73. https://doi.org/10.1007/s10725-007-9205-z
Singh VP, Srivastava PK, Prasad SM (2013) Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol Biochem 71:155–163. https://doi.org/10.1016/j.plaphy.2013.07.003
Singh VP, Singh S, Kumar J, Prasad SM (2015) Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate–glutathione cycle: possible involvement of nitric oxide. J Plant Physiol 181:20–29. https://doi.org/10.1016/j.jplph.2015.03.015
Singh AP et al (2016) Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci 6:1272–1272. https://doi.org/10.3389/fpls.2015.01272
Singh AP et al (2017) A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice ( Oryza sativa L.). Plant Physiol Biochem 115:163–173. https://doi.org/10.1016/j.plaphy.2017.02.019
Srivastava S, Sinha P, Sharma YK (2017) Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J Plant Nutr 40:298–306. https://doi.org/10.1080/01904167.2016.1240189
Stoeva N, Berova M, Zlatev Z (2004) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296. https://doi.org/10.1007/s10535-005-3296-z
Sun J, Wang R, Zhang X, Yu Y, Zhao R, Li Z, Chen S (2013) Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol Biochem 65:67–74. https://doi.org/10.1016/j.plaphy.2013.01.003
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165. https://doi.org/10.1016/j.tibtech.2007.02.003
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66. https://doi.org/10.1016/s0168-9452(99)00197-1
Verbruggen N, Hermans C, Schat H (2009) Mechanisms to cope with arsenic or cadmium excess in plants. Curr Opin Plant Biol 12:364–372. https://doi.org/10.1016/j.pbi.2009.05.001
Vezza ME, Llanes A, Travaglia C, Agostini E, Talano MA (2018) Arsenic stress effects on root water absorption in soybean plants: physiological and morphological aspects. Plant Physiol Biochem 123:8–17. https://doi.org/10.1016/j.plaphy.2017.11.020
Wang H, Dai B, Shu X, Wang H, Ning P (2015) Effect of kinetin on physiological and biochemical properties of maize seedlings under arsenic stress. Adv Mater Sci Eng 2015:1–7. https://doi.org/10.1155/2015/714646
Wei Y et al (2017) Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour Technol 247:190–199. https://doi.org/10.1016/j.biortech.2017.09.092
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett 579:6265–6271. https://doi.org/10.1016/j.febslet.2005.10.006
Yamasaki S, Dillenburg LR (1999) Measurements of leaf relative water content in Araucaria angustifolia. Revista Brasilleira de fisiologia vegetal 11:69–75
Yoon Y, Lee W-M, An Y-J (2015) Phytotoxicity of arsenic compounds on crop plant seedlings. Environ Sci Pollut Res 22:11047–11056. https://doi.org/10.1007/s11356-015-4317-x
Yu C-W, Murphy TM, Lin C-H (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955. https://doi.org/10.1071/fp03091
Zhang H, Hu L-Y, Hu K-D, He Y-D, Wang S-H, Luo J-P (2008) Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50:1518–1529. https://doi.org/10.1111/j.1744-7909.2008.00769.x
Zhao F-J, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559. https://doi.org/10.1146/annurev-arplant-042809-112152
Zhao F-J, Stroud JL, Khan MA, McGrath SP (2011) Arsenic translocation in rice investigated using radioactive 73As tracer. Plant Soil 350:413–420. https://doi.org/10.1007/s11104-011-0926-4
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSURG-1438-039.
Author information
Authors and Affiliations
Contributions
AAA and PA designed and performed the experiments. JAB, MNA analyzed the data and helped in writing the first draft of this manuscript. MA and PA revised the manuscript to the present form.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exits.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alsahli, A.A., Bhat, J.A., Alyemeni, M.N. et al. Hydrogen Sulfide (H2S) Mitigates Arsenic (As)-Induced Toxicity in Pea (Pisum sativum L.) Plants by Regulating Osmoregulation, Antioxidant Defense System, Ascorbate Glutathione Cycle and Glyoxalase System. J Plant Growth Regul 40, 2515–2531 (2021). https://doi.org/10.1007/s00344-020-10254-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10254-6