Abstract
The study was conducted to consider the role of nitrate reductase (NR)-synthesized nitric oxide (NO) in the methyl jasmonate (MJ)-induced tolerance of arsenic (As) stress in rice plants. Before starting As treatment, rice plants were sprayed with 0.5 mM MJ for 3 days. Thereafter, rice plants were hydroponically treated with 50 μM As for 2 weeks. Arsenic treatment diminished growth and photosynthetic pigments and increased hydrogen peroxide (H2O2), methylglyoxal (MG) and malondialdehyde (MDA), electrolyte leakage (EL), nitrate reductase (NR), nitric oxide (NO) level, antioxidant enzymes, the glyoxalase cycle, and the leaf and root contents of glutathione (GSH) and phytochelatins (PCs) in rice. MJ lessened the root and leaf concentrations of As and the levels of H2O2, MG, MDA, and EL, enhanced plant growth and photosynthetic pigments, and led to further improvements in the activity of antioxidant enzymes, the glyoxalase cycle, NR activity, and the endogenous level of NO in rice plants under As stress. MJ enhanced the levels of GSH and PCs in the roots and leaves of As-stressed rice by regulating the expression of GSH1, PCS, and ABCC1 genes. However, the application of sodium nitroprusside as a NO donor reversed the inhibitory effects of sodium tungstate on MJ-induced As tolerance, suggesting that NR-synthesized NO is required for MJ-mediated As tolerance of rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a toxic metalloid, arsenic (As) is a potential hazard to ecological systems due to anthropogenic impacts and environmental activities such as dumping of industrial and municipal wastes, the use of different fertilizers, pesticides, mining, and fossil fuel consumption (Mehmood et al. 2017; Ramezani et al. 2021). Humans may be exposed to As toxicity in several ways, such as the plant-soil–water system and by drinking As-contaminated water (Khalid et al. 2017). Arsenic in irrigation water and soil enters the food chain through absorption by the plant. High levels of As in soil and irrigation water not only limit the growth and productivity of crops but can also be a dangerous threat to human health by entering the food chain (Abbas et al. 2018; Ghorbani et al. 2020). Toxic levels of As induce dysfunction and oxidation of bio-molecules in plant cells through the excessive accumulation of reactive oxygen species, including singlet oxygen, hydroxyl radical, and hydrogen peroxide (H2O2) (Kohli et al. 2019; Ghorbani et al. 2021). To counteract the oxidative stress induced by As toxicity, the antioxidant defense system is naturally activated in plants, which includes the accumulation of non-enzymatic compounds such as glutathione (GSH), as well as improving the activity of antioxidant enzymes and the glyoxalase system (Afzal et al. 2018; Ghorbani et al. 2020). The glyoxalase system plays a critical role in diminishing abiotic stress-induced oxidative stress by detoxifying methylglyoxal (MG) (Hossain et al. 2012). Chelation of toxic metals in the cytoplasm by phytochelatins (PCs) and their sequestration in vacuoles is recognized an important defense mechanism for plant tolerance to heavy metal (HM) toxicity (Park et al. 2013). Therefore, stimulation of plant defense systems can effectively alleviate the negative effects induced by As stress in plants. In this regard, methyl jasmonate (MJ), as an effective bio-stimulant involved in the plant defense mechanism under biotic and abiotic stresses (Bari and Jones 2009; del Amor and Cuadra-Crespo 2011), could play an outstanding role in improving plant tolerance under As toxicity.
Jasmonates are important phytohormones in higher plants that are derived from lipids and mainly include methyl jasmonate (MJ) and jasmonic acid. Jasmonates are known to act as signal agents or elicitors in many biochemical, physiological, and molecular processes, including leaf senescence, phenolic production, fruit ripening, root growth, germination, and defence responses (Reyes-Díaz et al. 2016; Asghari 2019; Mousavi et al. 2020). Furthermore, the exogenous application of MJ plays an important role in diminishing the phytotoxic effects of HM, e.g., As (Mousavi et al. 2020), cadmium (Per et al. 2016), copper (Poonam et al. 2013), and lead (Salavati et al. 2021). However, the defense mechanisms induced by MJ in improving As toxicity tolerance need to be examined more deeply with regard to the role of nitrate reductase (NR)-mediated nitric oxide (NO).
NR enzymes are one of the main enzymes that synthesize NO in plants exposed to stressful conditions (Fu et al. 2018). NO as a signaling molecule has been reported to play a positive role in modulating the defense mechanisms of plants under various stresses such as cadmium stress (Ahmad et al. 2018; Gerami et al. 2018), As stress (Singh et al. 2017), drought (Montilla-Bascón et al. 2017), cold stress (Fan et al. 2015), and salinity (Ahmad et al., 2016). Apart from the well-known protective properties of MJ and NO in plants' tolerance to various stresses, the role of MJ and NO interaction in the response of rice plants under As toxicity has not been well studied. Therefore, the present experiment was designed to investigate the possible functions of NR-triggered NO in MJ-mediated As tolerance with respect to the content of photosynthetic pigments, the activity of some antioxidant enzymes and the glyoxalase cycle, as well as the expression level of genes responsible for As sequestration in vacuoles using sodium tungstate (ST) as an NR inhibitor. The application of sodium tungstate (ST) as an NR inhibitor abolished the positive impacts of MJ on the induction of As tolerance in rice by diminishing NR-mediated endogenous NO.
Materials and Methods
Plant Materials and Treatments
The experiments were done on rice (Oryza sativa L. cv. IR64) in greenhouse conditions with 70–75% humidity. After treating the seeds with a NaOCl (1%) solution for surface sterilization, seed germination was performed in peat moss (autoclaved twice). A MJ treatment (0.5 mM) was applied to 12-day-old seedlings by foliar spray for 3 days (once a day). A MJ solution was prepared using MJ (C13H20O3) with 0.1% ethanol and 0.01% tween-20 in distilled water. Control plants were sprayed with an equal volume of distilled water containing 0.1% ethanol and 0.01% tween-20. Before transplanting the seedlings, the roots were immersed in a 0.1 mM sodium tungstate (ST) solution for 3 h as ST treatments (Kaya 2021). Fifteen-day-old seedlings were transferred to plastic containers filled with 1/2-strength Hoagland solution containing 50 µM As (NaAsO2) or without As, which were replaced with fresh solution every 3 days. After transplanting rice seedlings, a foliar spray of 0.1 mM sodium nitroprusside (SNP) was applied twice (once every week). Temperature, light period and light intensity of the greenhouse were maintained at 25 ± 3 °C, 16 h light and 300–350 μmol m−2 s−1, respectively (Ghorbani et al. 2011). Samples were collected 2 weeks after beginning As treatment. After recording the plant height, the total dry weight was estimated by incubating the samples at 75 °C for 48 h (Ghorbani et al. 2009). After harvesting, fresh samples were rapidly frozen in liquid nitrogen and kept at -80 °C for biochemical and molecular attributes.
Chlorophyll Florescence and Photosynthetic Pigments
The PAM fluorometer (PAM 2500, Walz) was employed to appraise the chlorophyll fluorescence value of rice leaves after 30 min dark adaptation (Ghorbani et al. 2018b). Fresh leaves were applied to assess the content of photosynthetic pigments using 80% acetone. After centrifugation and reading of the supernatants at 460, 645, and 663 nm, the contents of chlorophyll a, b, and carotenoids were calculated by the method outlined by Lichtenthaler (1987).
Nitric Oxide (NO) Content
By determining nitrite (NO2−) content using the Griess reagent, NO content was obtained according to Zhou et al. (2005). Extraction buffer (cold acetic acid (50 mM, pH 3.6) including 4% zinc diacetate) was used for the homogenization of fresh roots and leaves. After centrifugation at 12,000 g for 15 min at 4 °C, the supernatants were mixed with charcoal and incubated at room temperature for 30 min. Then, the Griess reagent was added to the samples and read at 540 nm.
Arsenic Content
By digesting the dried root and shoot tissues in HNO3:H2O2 (1:4 ratio), the root and shoot concentrations of As were obtained using an ICP-MS (Agilent 7500 cx).
Glutathione (GSH) and Phytochelatins (PCs)
Glutathione extraction was achieved using meta-phosphoric acid (6%, pH 2.8) containing EDTA (1 mM). After centrifugation, polyvinylpolypyrrolidone was added to the supernatants and centrifuged at 12,000 × g for 20 min (Yu et al. 2003). Supernatants were combined with yeast GSH reductase (GR, 20 IU mL–1), 5,5′-dithiobis(2-nitrobenzoic acid (2.4 mg mL–1), NADPH (1.9 mg mL–1), and potassium phosphate buffer (0.1 M, pH 7.0) containing EDTA (5 mM). After reading at 412 nm, the total GSH content was expressed as µmol g–1 FW following the method of Adams and Liyanage (1991). After determining non-protein thiols (Howe and Merchant, 1992), the content of PCs was obtained by subtracting the GSH content from the content of non-protein thiols (De Vos et al. 1992).
Nitrate Reductase (NR) Activity
An extraction buffer containing HEPES–KOH (0.1 M, pH 7.5), 1,4-dithiothreitol (5 mM), polyvinylpyrrolidone (1%), Triton X-100 (0.1%), glycerol (10%), flavin adenine dinucleotide (20 μM), EDTA (1 mM) and phenylmethylsulfonyl fluoride (0.5 mM) was applied to quantify the leaf activity of NR. After centrifugation, the supernatants were utilized to quantify NR activity by the method of Sun et al. (2014). Assay buffer containing HEPES–KOH (50 mM, pH 7.5), MgCl2 (10 mM), 1,4-dithiothreitol (1 mM), KNO3 (2 mM) and NADH (0.2 mM) was mixed with the supernatants and incubated at 30 °C for 30 min. After adding Zn-acetate (0.5 M), N-(1-naphthyl) ethylenediamine (0.02%) in HCl (0.2 M) and sulfanilamide (1%) in HCl (3 M), the produced nitrate was determined by reading at 520 nm.
Electrolyte Leakage (EL)
After washing the leaves with distilled water, the leaf discs were incubated in distilled water at room temperature on a shaker, and after 24 h, electrical conductivity was recorded (EC1). Then, to determine EC2, the samples were autoclaved at 120 °C for 20 min (Dionisio-Sese and Tobita (1998). After recording EC2, EL was calculated as: EL (%) = (EC1/EC2) × 100.
Hydrogen Peroxide (H2O2), Methylglyoxal (MG) and Malondialdehyde (MDA) Contents
The method of Loreto and Velikova (2001) was used to quantify the leaf H2O2 content. Trichloroacetic acid (TCA, 1%) was employed to extract fresh leaves. After centrifugation of the extracts, K buffer (10 mM) and potassium iodide (1 M) were added to the supernatants and read at 390 nm.
Homogenization of fresh leaves with perchloric acid (5%) was performed to quantify MG using the procedure of Wild et al. (2012). After centrifugation, charcoal and saturated potassium carbonate were utilized to decolorize and neutralize the supernatants. After adding N-acetyl-L-cysteine and NaH2PO4, the absorbance of the mixtures was recorded at 288 nm.
The method of Weisany et al. (2012) was applied to measure leaf MDA levels. Trichloroacetic acid (1%, w:v) was used to homogenize fresh leaves, and after centrifugation, thiobarbituric acid (0.5%) and TCA (20%) were mixed with the supernatants. The mixtures were placed in a water bath (90 °C) for 30 min, and after cooling, the absorbance of the samples was read at 532 nm.
Extraction and Assay of Enzymes
Extraction buffers including potassium phosphate buffer (50 mM, pH 7.0), glycerol (10%), KCl (100 mM), ascorbic acid (1 mM) and β-mercaptoethanol (5 mM) were employed to homogenize fresh leaves. After centrifugation of the extracts, supernatants were used to estimate enzyme activity and protein content (Bradford 1976).
The reaction mixtures containing enzyme extract, K-P buffer (50 mM, pH 7.0) and H2O2 (5.9 mM) were employed to quantify the activity of catalase (CAT) (Chance and Maehly 1955). CAT activity was quantified by recording a decline in the OD of 240 nm following the decomposition of H2O2 for 1 min. One unit of the CAT enzyme represents the amount needed to decompose H2O2 per min.
The reaction solution containing enzyme extract, phosphate buffer (50 mM, pH 7.8), EDTA (0.1 mM), nitroblue tetrazolium (NBT, 0.75 µM), riboflavin (2 mM), Na2CO3 (50 mM) and methionine (13.33 mM) was utilized to assess the activity of superoxide dismutase (SOD) based on the inhibition of photochemical reduction of NBT (Dhindsa et al. 1981).
The protocol of Nakano and Asada (1981) was utilized to assess the activity of the ascorbate peroxidase (APX) enzyme by recording a change in the absorbance of the reaction solution containing K–P buffer (50 mM, pH 7.0), enzyme extracts, EDTA (0.1 mM), ascorbic acid (0.5 mM) and H2O2 (0.1 mM) at 290 nm. The amount needed to oxidize ascorbate per min was defined as one unit of the APX enzyme.
By regarding the alteration in the absorbance of the mixture including enzymatic extracts, K-P buffer (0.1 mM, pH 7.0), NADPH (0.2 mM), EDTA (1 mM) and oxidized glutathione (1 mM) at 340 nm, the activity of the glutathione reductase (GR) was obtained following the method optimized by Hasanuzzaman et al. (2011). The amount required to catalyze the reduction of oxidized glutathione per min was defined as one unit of GR enzyme.
The procedure of Hasanuzzaman et al. (2012) was applied to quantify the activity of glyoxalase (Gly) I enzyme by reading the reaction mixture (the enzyme assay solution, K–P buffer (100 mM, pH 7.0), MG (3.5 mM), glutathione (1.7 mM) and MgSO4 (15 mM)) at 240 nm.
A reaction mixture of enzyme extract, Tris − HCl buffer (100 mM, pH), 5,5′-dithio-bis(2-nitrobenzoic acid) (0.2 mM) and SD-lactoylglutathione (1 mM) was employed to determine the activity of Gly II enzyme following Hossain et al. (2010) method by recording the absorbance changes at 240 nm.
Gene Expression
After extraction of the total RNA from roots and leaves using TRIzol reagent (Invitrogen, USA), first-strand cDNA synthesis was performed by the RevertAid™ Reverse Transcriptase kit (Fermentase, Germany) (Ghasemi-Omran et al. 2021). qPCR reactions were performed using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific). The specific primers (Table S1) used for GSH1, PCS, ABCC1 and Actin (internal control) genes were designed by an online program (Primer3). The 2−ΔΔCT method was utilized to analyze qPCR data (Ghorbani et al. 2019a).
Statistical Analysis
Five biological replications were used for morphological and biochemical traits (at least 3 technical repeats for each replication), while gene expression was analyzed based on three biological replications (at least 3 technical repeats for each replication). All data were analyzed by SAS 9.1 software (Ghorbani et al. 2019b). In the tables and figures, the means (± standard division) were compared at a 0.05% level of confidence by the LSD test.
Results
Morphological and Photosynthetic Attributes
Arsenic treatment (50 μM) significantly diminished height and total dry weight by 22.6 and 25.8%, respectively, compared to untreated plants. In As-stressed plants, MJ, MJ + SNP, and MJ + ST + SNP treatments enhanced plant height by 14.2, 20.3 and 3.4% and total dry weight by 16, 25.8 and 14.3%, respectively, however, MJ + ST treatment diminished height and total dry weight by 11.7 and 4.9%, respectively, over As-exposed plants alone. Furthermore, all treatments (MJ, MJ + ST, MJ + SNP and MJ + ST + SNP) did not induce a significant effect on height and total dry weight in control plants (CP) (Table 1).
In CP, the application of MJ, MJ + ST, MJ + SNP, and MJ + ST + SNP treatments did not induce significant differences in the content of chlorophyll a, b, carotenoids or Fv/Fm values. The addition of 50 μM As lessened the contents of chlorophyll a, b, carotenoids, and Fv/Fm by 54.6, 60.7, 28, and 37.2%, respectively, over CP. In As-exposed plants, the application of MJ, MJ + SNP and MJ + ST + SNP treatments significantly improved the photosynthetic pigments and Fv/Fm value in comparison with As-treated plants alone, which recorded the highest increase under MJ + SNP treatment. However, MJ + ST treatment did not cause a significant difference in the photosynthetic pigments or Fv/Fm value in As-stressed plants (Table 1).
The Contents of NO and As
A significant rise in root and leaf contents of NO was recorded by 58 and 68.5%, respectively, under As stress over CP. In plants treated with 50 μM As, the exogenous application of MJ, MJ + SNP, and MJ + ST + SNP treatments caused a significant increase in root and leaf NO content, and the highest improvement was observed in plants treated with MJ + SNP. However, MJ + ST treatment lessened root and leaf NO levels in As-stressed plants (Table 2).
The addition of 50 μM As caused the accumulation of 462.7, 88.57, and 551.2 μg/gDW of As in the roots, shoots and whole plant, respectively. However, MJ, MJ + SNP and MJ + ST + SNP treatments diminished As accumulation in roots (50.5, 57.5 and 21.3%), shoots (68.2, 71.2 and 45.5%) and whole plants (53.3, 59.7 and 25.2%) in As-treated plants. MJ + ST treatment increased the concentration of As in the roots and the whole plant by 4 and 4%, respectively, in As-treated plants (Table 2).
Oxidative Stress Markers
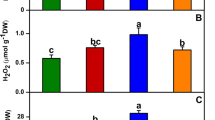
The application of MJ, MJ + ST, MJ + SNP, and MJ + ST + SNP treatments did not induce significant differences in the leaf levels of H2O2, MG, MDA, and EL in CP; however, the application of As significantly increased the leaf levels of H2O2, MG, MDA, and EL by 4.3, 2, 3.1, and 2.4-fold, respectively, over CP. In plants treated with As, the application of MJ and MJ + SNP significantly diminished the levels of H2O2, MG, MDA, and EL over As-treated plants alone, and the lowest levels of these traits were observed under MJ + SNP treatment. The use of MJ + ST significantly enhanced the levels of MG and MDA, while it did not induce significant differences in H2O2 and EL levels in As-stressed plants. MJ + ST + SNP treatment declined the levels of H2O2 and MDA, and increased the level of MG in the leaves of As-treated plants (Fig. 1A, B, C and D).
Levels of hydrogen peroxide (H2O2, A), methylglyoxal (MG, B), malondialdehyde (MDA, C) and electrolyte leakage (EL, D) in rice leaves sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
Antioxidant Enzymes and the Glyoxalase Pathway
The results showed that MJ, MJ + ST, MJ + SNP, and MJ + ST + SNP treatments did not induce significant differences in the activity of CAT, SOD, APX, and GR enzymes in CP. However, As treatment increased the activity of CAT, SOD, APX, and GR in the leaves of rice plants by 57.4, 37.4, 64.2, and 51.5%, respectively over CP. In As-treated plants, MJ and MJ + SNP treatments upregulated the activity of these enzymes compared to As-treated plants alone. Although MJ + ST did not have a significant effect on SOD activity, it decreased the activity of CAT, APX, and GR in As-treated plants. MJ + ST + SNP treatment enhanced the activity of SOD and GR enzymes in As-treated plants, while it did not cause a significant difference in CAT and APX (Fig. 2A, B, C and D).
The activities of catalase (CAT, A), superoxide dismutase (SOD, B), ascorbate peroxidase (APX, C) and glutathione reductase (GR, D) in rice leaves sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
When rice plants were treated with 50 μM As, the activity of Gly I and II enzymes was elevated by 35.8 and 47.6%, respectively, over CP. When As-treated plants were supplied with MJ and MJ + SNP, a further increase in the activity of these enzymes was recorded. However, MJ + ST treatment significantly declined Gly I and II activity in As-treated plants. MJ + ST + SNP treatment did not induce a significant difference in Gly I activity but decreased Gly II activity in As-exposed plants (Fig. 3A and B).
The activities of glyoxalase I (Gly I, A) and Gly II (B) in rice leaves sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
The Activity of NR Enzyme
In CP, the application of MJ and MJ + SNP treatments did not cause significant differences in NR activity. However, MJ + ST and MJ + ST + SNP treatments lessened NR activity by 56 and 56.2%, respectively, over CP. The application of As significantly enhanced NR activity over CP. The application of MJ and MJ + SNP further enhanced NR activity in As-stressed plants. However, MJ + ST and MJ + ST + SNP treatments lowered NR activity in As-stressed plants compared to plants treated with As alone (Fig. 4).
The activity of nitrate reductase in rice leaves sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
Root and Leaf Contents of PCs and GSH
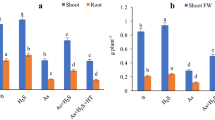
The application of MJ, MJ + ST, MJ + SNP, and MJ + ST + SNP treatments did not cause a significant difference in the root and leaf contents of PCs in CP. Arsenic treatment elevated the root and leaf contents of PCs by 52.4 and 74%, respectively over CP. In As-treated plants, MJ, MJ + SNP, and MJ + ST + SNP applications significantly enhanced the root accumulation of PCs by 38.8, 51.4, and 17.5%, respectively, while MJ + ST treatment did not induce significant effects on PCs content in roots compared to As-exposed plants alone. In the leaves of As-treated plants, MJ + ST treatment lessened the content of PCs by 9.4% and MJ + ST + SNP treatment increased the content of PCs by 8.7%. However, MJ and MJ + SNP treatments did not induce significant effects on the leaf content of PCs in As-exposed plants (Fig. 5A and B).
The root and leaf contents of phytochelatins (PCs, A and B) and glutathione (GSH, C and D) in rice plants sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
Significant increases in root and leaf content of GSH were observed under As treatment by 20.1 and 31.1%, respectively over CP. In As-treated plants, applications of MJ, MJ + SNP, and MJ + ST + SNP significantly enhanced root and leaf accumulation of GSH over As-exposed plants alone. The MJ + ST treatment did not induce a significant difference in GSH accumulation in the roots, however, it significantly declined GSH content in the leaves in As-exposed plants (Fig. 5C and D).
Root and Leaf Expression of GH1, PCS and ABCC1 Genes
The results of gene expression revealed that the application of MJ, MJ + ST, MJ + SNP, and MJ + ST + SNP treatments did not induce significant effects on root and leaf expression of the GSH1 gene in CP. However, a significant upregulation in GSH1 expression was found in the roots and leaves of As-treated rice plants over CP. In As-treated plants, the application of MJ, MJ + SNP, and MJ + ST + SNP treatments upregulated GSH1 expression in the roots and leaves over As-treated plants alone, and the highest increase was found under MJ + SNP treatment. When As-treated plants were exposed to MJ + ST treatment, GSH1 expression in the roots and leaves revealed a significant downregulation over As-treated plants alone (Fig. 6A and B).
The relative expression of GSH1 (A and B), PCS (C and D) and ABCC1 (E and F) in the roots and leaves of rice plants sprayed with methyl jasmonate (MJ, 0.5 mM) under arsenic (As, 50 µM) treatment combined with sodium tungstate (ST, 0.1 mM) and sodium nitroprusside (SNP, 0.1 mM). Different letters in each column show significant differences at the p < 0.05 based on the LSD test (means ± SD, n = 5)
When rice plants were treated with 50 μM As, PCS expression in roots and leaves was upregulated by 2.7- and 2.1-fold, respectively over CP. In As-treated plants, MJ, MJ + SNP, and MJ + ST + SNP treatments significantly upregulated PCS expression in the roots and the highest increase was recorded in plants treated with MJ + SNP. However, MJ + ST treatment lessened PCS expression in the roots of As-treated plants. MJ, MJ + SNP, and MJ + ST + SNP treatments did not induce significant differences in PCS expression in the leaves of As-exposed plants, while MJ + ST treatment declined PCS expression (Fig. 6C and D).
A significant upregulation in ABCC1 expression was found in the roots and leaves of rice plants treated with 50 μM As over CP. In the roots, MJ, MJ + SNP, and MJ + ST + SNP treatments increased ABC1 expression in As-exposed plants, with the highest rise observed under MJ + SNP treatment, while MJ + ST treatment did not induce a significant difference in ABCC1 expression. In the leaves, MJ + ST + SNP treatment upregulated ABCC1 expression and MJ + ST treatment downregulated ABCC1 expression in As-treated plants. However, MJ and MJ + SNP treatments did not induce significant effects on ABCC1 expression (Fig. 6E and F).
Discussion
Here, the possible role of the NR enzyme in As tolerance induced by MJ in rice plants was evaluated. Although the positive impacts of MJ on improving the tolerance of plants under As stress have been confirmed (Mousavi et al. 2020; Verma et al. 2020), no report of the possible role of NR in MJ-mediated As tolerance has been recorded. The results revealed that 50 μM As treatment diminished the growth and biomass of the rice plant, as previously recorded on rice (Ghorbani et al. 2020), oilseed rape (Farooq et al. 2016) and maize (Kaya et al. 2020). Arsenic stress has been shown to reduce plant growth by disrupting ionic homeostasis, inducing oxidative stress, and dysfunction of the photosynthetic apparatus (Hasanuzzaman and Fujita 2013; Ghorbani et al. 2020; Ahmad et al. 2020). However, the application of MJ significantly improved the growth and biomass of rice plants under As stress, which is consistent with the results obtained on rice (Mousavi et al. 2020; Verma et al. 2020) and oilseed rape (Farooq et al. 2016). These results confirm that MJ, as a growth regulator, can effectively improve the tolerance of plants to As toxicity. The exogenous application of MJ has been shown to reduce As uptake and diminish oxidative stress by modulating the expression of As transporters and augmenting the antioxidant defense system, thereby improving plant growth under As phytotoxicity (Mousavi et al. 2020; Verma et al. 2020). To investigate the possible role of NR enzyme in improving plant tolerance to As stress induced by MJ, ST was used as an inhibitor of NR activity in this study to prevent the synthesis or accumulation of NO during MJ application. The results showed that ST application prevented the positive effects induced by MJ, indicating that MJ improves the tolerance of rice plants under As toxicity by inducing NR activity. Kaya et al. (2020) affirmed that inhibition of NR activity abolished salicylic acid-induced As stress tolerance, indicating the important role of NR-synthesized NO in plants' tolerance to As stress. In the current study, SNP was used as a NO donor to confirm the participation of NR-synthesized NO in MJ-induced As tolerance. As previously indicated by Singh et al. (2016) and Hasanuzzaman and Fujita (2013), the application of SNP restored the ameliorative effects of MJ in MJ + ST-treated plants and enhanced the positive effects of MJ in MJ-treated plants under As stress, indicating the role of NO in improving plant tolerance to As stress. Thus, these results reveal that NR-synthesized NO as a signal molecule is activated by MJ and plays an important role in inducing MJ-induced As tolerance in rice plants.
Arsenic treatment adversely affected the chlorophylls and carotenoids contents and Fv/Fm value in rice, which indicates the destructive effect of As toxicity on the efficiency of photosynthetic apparatus. Arsenic stress has been reported to diminish photosynthetic pigments and disrupt photosynthetic centres by inducing chlorophyll-degrading enzymes and reducing the activity of enzymes involved in chlorophyll synthesis, as well as increasing the accumulation of toxic radicals and inducing oxidative stress (Zemanová et al. 2020; Ghorbani et al. 2020). The application of MJ alleviated the negative effects of As stress on photosynthetic attributes, indicating the protective effects of MJ on the photosynthetic pigments. The protective effects of MJ on the photosynthetic pigments of rice under As stress can be due to a reduction in the levels of reactive oxygen species (ROS) and an increase in the activity of antioxidant enzymes, as previously affirmed by Coelho et al. (2020) and Mousavi et al. (2020). ST treatment reversed the protective effects of MJ on photosynthetic pigments, indicating the participation of NR and NO in MJ-induced protective responses on the photosynthetic pigments of rice under As stress. The use of SNP abolished the inhibitory effects of ST on the protective role of MJ on the photosynthetic apparatus, which confirms the role of NO in MJ-induced defense responses. Increased photosynthetic pigments and protection of the photosynthetic apparatus by NO under As stress have been previously reported (Singh et al. 2016; Ahmad et al. 2020). Thus, MJ protects the photosynthetic pigments of rice plants under As toxicity by activating NR and increasing the endogenous level of NO.
The results indicated that MJ lessened the uptake of As by the roots and its translocation to the shoots and, consequently, diminished the accumulation of As in the whole plant, which could play an important role in mitigating the toxic effects of As on rice. The MJ-mediated decline in As content has been announced in Brassica napus (Farooq et al. 2016) and rice (Verma et al. 2020). MJ has been demonstrated to diminish the uptake and translocation of As in As-stressed rice plants by modulating the expression of As transporters (Mousavi et al. 2020). However, the application of ST completely reversed the MJ-mediated decrease in As accumulation, suggesting that NR-synthesized NO may be involved in modulating the expression of As transporters induced by MJ. Kaya et al. (2020) indicated that NR-mediated NO is implicated in reducing brassinosteroid-induced cadmium accumulation in pepper plants under cadmium toxicity. As a result, SNP application eliminated the inhibitory effects of ST and, as a result, reduced the accumulation of As in MJ + ST-treated plants under As toxicity, which affirms the role of MJ-induced endogenous NO in reducing As uptake in As-stressed rice. Similar results of an exogenous NO-induced decrease in As accumulation in Vicia faba (Ahmad et al. 2020) and rice (Singh et al. 2016) under As stress have been previously published.
Arsenic treatment enhanced the accumulation of H2O2 and MG, resulting in damage to bio-membranes and induction of EL, indicating the toxic effects of As on rice, as reported by Ghorbani et al. (2020) and Mousavi et al. (2020). However, MJ diminished the levels of H2O2 and MG by increasing the activity of antioxidant enzymes (CAT, SOD, GR, and APX) and the glyoxalase system (Gly I and II), thereby protecting bio-membranes (reducing MDA and EL). Farooq et al. (2016) revealed that MJ raised the tolerance of Brassica napus under As stress by upregulating the expression of CAT, SOD, APX, and peroxidase enzymes. ST treatment eliminated the upregulation in the activity of antioxidant enzymes and the glyoxalase cycle mediated by MJ, which increased H2O2, MG, MDA and EL in the leaves of rice plants under As stress. Kaya et al. (2020) indicated that NR and NO are involved in increasing the activity of antioxidant enzymes and reducing oxidative stress by brassinosteroid in cadmium-stressed pepper plants, indicating the essential role of NR-mediated NO in the defense responses of plants under HM stress. To confirm the role of exogenous NO in the induction of the defense mechanism triggered by MJ, SNP was used to replace ST-eliminated endogenous NO. The results revealed that the use of SNP reversed the mitigating effects of MJ on As-induced oxidative stress in MJ + ST-treated rice plants, indicating the significant role of NR-synthesized endogenous NO in inducing the MJ-mediated antioxidant defense mechanism. The participation of NR-synthesized NO in the induction of salicylic acid-mediated antioxidant defense systems under drought stress has also been reported by Kaya (2021) in pepper plants. It has previously been shown that the external application of NO augmented the antioxidant defense system and the glyoxalase cycle, thereby improving plant tolerance to As stress (Hasanuzzaman and Fujita 2013; Singh et al. 2016). However, our results suggest for the first time in the literature that MJ, by activating NR and enhancing the internal level of NO, augments the antioxidant defense system and the glyoxalase cycle, thereby improving the tolerance of rice plants to As phytotoxicity.
Phytochelatins are important defense compounds synthesized from GSH that, by chelating toxic metal ions, protect metabolic organelles against HM toxicity (Hall 2002; Ghorbani et al. 2018a). The results indicated that MJ enhanced the root and leaf expression of GSH1 and the root expression of PCS and ABCC1 in As-treated rice plants, which is consistent with increasing the root contents of PCs and GSH and the leaf content of GSH. It has been shown that increasing the expression of genes implicated in the sequestration of toxic metal ions can play an important role in enhancing plant tolerance under HM toxicity (Hasan et al. 2015). Thus, MJ protects metabolic organelles against toxic As by increasing the accumulation of GSH and PCs and the sequestration of As in vacuoles, which can play an important role in increasing the tolerance of rice plants under As stress. However, the positive effects of MJ on the induction of the As sequestration mechanism was abolished by ST treatment, suggesting the role of NR-synthesized NO as a signaling molecule downstream of MJ. The hindering effects of ST on the MJ-induced sequestration mechanism were reversed by SNP, which confirms the role of NR and NO in the induction of MJ-mediated sequestration mechanism. The role of NR-synthesized NO downstream of the phytohormones brassinosteroid and salicylic acid under cadmium and drought stress, respectively, has been previously announced. Thus, our findings show for the first time that MJ, by activating NR and increasing the endogenous level of NO, upregulated the expression of GSH1, PCS and ABCC1 and increased the accumulation of GSH and PCs compounds to promote plant tolerance under As toxicity.
Conclusion
The results revealed that MJ, by activating NR and increasing endogenous NO, increased rice plant tolerance to As toxicity, suggesting that NO may be involved as a signaling molecule downstream of the MJ-induced defense mechanism in As-stressed rice plants. To confirm the role of NR and NO in the induction of MJ-mediated defense mechanism, ST was used as an NR inhibitor, which revealed that ST reversed the positive effects of MJ on the antioxidant defense system, the glyoxalase cycle and As sequestration mechanism in As-stressed rice plants. To provide further evidence, SNP as a NO donor abolished the inhibitory effects of ST on the defense mechanism induced by MJ, which confirms the contribution of MJ-induced internal NO in improving the tolerance of rice plants under As stress. Thus, by inducing NR enzyme and increasing the internal level of NO, MJ augmented the antioxidant defense system and the glyoxalase cycle, as well as modulating As sequestration mechanism, which effectively reduced As accumulation and mitigated oxidative stress, protected the photosynthetic apparatus and bio-membranes, and thus promoted the growth and biomass of rice plants under As stress. In the future, the association of other signaling molecules or enzymes in inducing phytohormones-mediated abiotic stresses tolerance requires to be investigated.
Data Availability
All data used or analyzed during this study are available from the corresponding author on reasonable request.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59
Adams DO, Liyanage C (1991) Modification of an enzymatic glutathione assay for determination of total glutathione in grapevine tissues. Am J Enol Vitic 42:137–140
Ahmad P, Abdel Latef AA, Hashem A, AbdAllah EF, Gucel S, Tran LSP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P (2018) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93
Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M (2020) Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 244:125480
Asghari M (2019) Impact of jasmonates on safety, productivity and physiology of food crops. Trends Food Sci Technol 91:169–183
Bari R, Jones JD (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chance B, Maehly C (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Coelho DG, de Andrade HM, Marinato CS, Araujo SC, de Matos LP, da Silva VM, de Oliveira JA (2020) Exogenous jasmonic acid enhances oxidative protection of Lemna valdiviana subjected to arsenic. Acta Physiol Plant 42:97
De Vos RCH, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
del Amor FM, Cuadra-Crespo P (2011) Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: analysis of growth, gas exchange, and isotope composition. Plant Growth Regul 63:55–62
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–10
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fan J, Chen K, Amombo E, Hu Z, Chen L, Fu J (2015) Physiological and molecular mechanism of nitric oxide (NO) involved in bermudagrass response to cold stress. PLoS ONE 10(7):e0132991
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W (2016) Methyl Jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468
Fu YF, Zhang ZW, Yuan S (2018) Putative connections between nitrate reductase S-nitrosylation and NO synthesis under pathogen attacks and abiotic stresses. Front Plant Sci 9:474
Gerami M, Ghorbani A, Karimi S (2018) Role of salicylic acid pretreatment in alleviating cadmium-induced toxicity in Salvia officinalis L. Iran J Plant Biol 10(1):81–95
Ghasemi-Omran VO, Ghorbani A, Sajjadi-Otaghsara SA (2021) Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. In Vitro Cell Dev Biol- Plant 57:319–331
Ghorbani A, Zarinkamar F, Fallah A (2009) The effect of cold stress on the morphologic and physiologic characters of tow rice varieties in seedling stage. J Crop Breed 1:50–66
Ghorbani A, Zarinkamar F, Fallah A (2011) Effect of cold stress on the anatomy and morphology of the tolerant and sensitive cultivars of rice during germination. J Cell Tissue 2(3):235–244
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018a) Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol 65:898–907
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018b) Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol 20:729–736
Ghorbani A, Ghasemi Omran VO, Razavi SM, Pirdashti H, Ranjbar M (2019a) Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep 38:1151–1163
Ghorbani A, Razavi SM, Ghasemi Omran V, Pirdeshti H (2019b) Effects of endophyte fungi symbiosis on some physiological parameters of tomato plants under 10 day long salinity stress. J Plant Proc Func 7(27):193–208
Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C (2020) Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf 209:111793
Ghorbani A, Pishkar L, Roodbari N, Pehlivan N, Wu C (2021) Nitric oxide could allay arsenic phytotoxicity in tomato (Solanum lycopersicum L.) by modulating photosynthetic pigments, phytochelatin metabolism, molecular redox status and arsenic sequestration. Plant Physiol Biochem 167:337–348
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hasan MK, Ahammed GJ, Yin L, Shi K, Xia X, Zhou Y, Yu J, Zhou J (2015) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6:601
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22(3):584–96
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycine betaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 26:259–272
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot. https://doi.org/10.1155/2012/872875
Kaya C (2021) Nitrate reductase is required for salicylic acid-induced water stress tolerance of pepper by upraising the AsA-GSH pathway and glyoxalase system. Physiol Plant 172:351–370
Kaya C, Ashraf M, Alyemeni MN, Corpas FJ, Ahmad P (2020) Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J Hazard Mater 399:123020
Khalid S, Shahid M, Niazi NK, Rafiq M, Bakhat HF, Imran M, Dumat C (2017) Arsenic behaviour in soil-plant system: biogeochemical reactions and chemical speciation influences. In: Anjum NA, Gill SS, Tuteja N (eds) Enhancing Cleanup of Environmental Pollutants. Springer, Cham., pp 97–140
Kohli SK, Khanna K, Bhardwaj R, Allah EFA, Ahmad P, Corpas FJ (2019) Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8:641
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Mehmood T, Bibi I, Shahid M, Niazi NK, Murtaza B, Wang H, Murtaza G (2017) Effect of compost addition on arsenic uptake, morphological and physiological attributes of maize plants grown in contrasting soils. J Geochem Explor 178:83–91
Montilla-Bascón G, Rubiales D, Hebelstrup KH, Mandon J, Harren FJ, Cristescu SM, Prats E (2017) Reduced nitric oxide levels during drought stress promote drought tolerance in barley and is associated with elevated polyamine biosynthesis. Sci Rep 7(1):13311
Mousavi SR, Niknejad Y, Fallah H, Tari DB (2020) Methyl jasmonate alleviates arsenic toxicity in rice. Plant Cell Rep 39(8):1041–1060
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Park S, Lee DE, Jang H, Byeon Y, Kim YS, Back K (2013) Melatonin- rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J Pineal Res 54:258–263
Per TS, Khan NA, Masood A, Fatma M (2016) Methyl Jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in Mustard. Front Plant Sci 7:1933
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Mill sp. seedlings under copper stress. Am J Plant Sci 4:817–823
Ramezani M, Enayati M, Ramezani M, Ghorbani A (2021) A study of different strategical views into heavy metal (oid) removal in the environment. Arab J Geosci 21:1–16
Reyes-Díaz M, Lobos T, Cardemil L, Nunes-Nesi A, Retamales J, Jaakola L, Alberdi M, Ribera-Fonseca A (2016) Methyl jasmonate: an alternative for improving the quality and health properties of fresh fruits. Molecules 21:567–576
Salavati J, Fallah H, Niknejad Y, Barari Tari D (2021) Methyl jasmonate ameliorates lead toxicity in Oryza sativa by modulating chlorophyll metabolism, antioxidative capacity and metal translocation. Physiol Mol Biol Plants 27:1089–1104
Singh AP, Dixit G, Kumar A, Mishra S, Singh PK, Dwivedi S, Trivedi PK, Chakrabarty D, Mallick S, Pandey V, Dhankher OP, Tripathi RD (2016) Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa L.). Front Plant Sci 6:1272
Singh PK, Indoliya Y, Chauhan AS, Singh SP, Singh AP, Dwivedi S, Chakrabarty D (2017) Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci Rep 7:3592
Sun C, Lu L, Liu L, Liu W, Yu Y, Liu X, Lin X (2014) Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol 201(4):1240–1250
Verma G, Srivastava D, Narayan S, Shirke PA, Chakrabarty D (2020) Exogenous application of methyl jasmonate alleviates arsenic toxicity by modulating its uptake and translocation in rice (Oryza sativa L.). Ecotoxicol Environ Saf 201:110735
Weisany W, Sohrabi Y, Heidari G, Siosemardeh A, Ghassemi-Golezani K (2012) Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L). Plant Omics J 5:60–67
Wild R, Ooi L, Srikanth V, Münch G (2012) A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetylL-cysteine assay. Anal Bioanal Chem 403:2577–2581
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30(9):955–963
Zemanová V, Popov M, Pavlíková D, Kotrba P, Hnilička F, Česká J, Pavlík M (2020) Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol 20:130
Zhou B, Guo Z, Xing J, Huang B (2005) Nitric oxide is involved in abscisic acid induced antioxidant activities in Stylosanthes guianensis. J Exp Bot 56:3223–3228
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization and Methodology, A.G., C.W.; Validation and Investigation, A.G., L.P., E.M.J.; Analysis, A.G., C.W.; Resources, L.P., N.R., S.A.T.; Writing original, A.G; Review and editing, A.G., C.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Durgesh Kumar Tripathi .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghorbani, A., Pishkar, L., Roodbari, N. et al. Nitrate Reductase is Needed for Methyl Jasmonate-Mediated Arsenic Toxicity Tolerance of Rice by Modulating the Antioxidant Defense System, Glyoxalase System and Arsenic Sequestration Mechanism. J Plant Growth Regul 42, 1107–1119 (2023). https://doi.org/10.1007/s00344-022-10616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10616-2