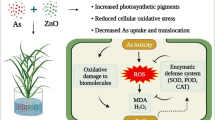
Abstract
The toxic and non-essential metalloid arsenic (As) is ubiquitous in the environment with its absorption from the soil into the plants’ roots posing detrimental effects on the crop plants and hence the food availability and food security are also threatened. The present study was intended to reduce the As-induced toxicity in rice seedlings (Oryza sativa L.) by phosphate (PO43−). For this, three concentrations of potassium phosphate (KH2PO4), 50, 100 and 150 μM were supplemented along with 50 μM As exposure to hydroponically grown 7-day-old rice seedlings. Supplementation of PO43− significantly recovered arsenic-induced diminutions in growth parameters and photosynthetic pigment contents which were due to the significant increase in superoxide radical (SOR, O2•¯) and hydrogen peroxide (H2O2). Supplementation of 50 μM PO43− could significantly increase the activity of APX (ascorbate peroxidase) and GR (glutathione reductase) while 100 μM PO43− could increase the activity of DHAR (dehydroascorbate reductase) and monodehydroascorbate reductase (MDHAR). As the amount of PO43− was increased, the ratio of AsA/DHA (reduced to oxidized ascorbate) and GSH/GSSG (reduced to oxidized glutathione) was increased significantly due to increase in the reduced form of the non-enzymes i.e. AsA and GSH. The activity of SOD (superoxide dismutase) and GPX (guaiacol peroxidase) decreased significantly after a substantive increase in their activities due to As stress while the CAT (catalase) activity further enhanced after the supplementation of 50 and 100 μM PO43−. Thus, the As-induced oxidative stress in the rice seedlings was managed by concerted modulations in the activities of SOD, GPX, CAT and AsA-GSH cycle enzymes and metabolites.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) has long been viewed as the synonymous of toxicity, not only for human beings but for other animals and plants too. In recent decades, As has fascinated plant biologists since As-contaminated natural water is now a worldwide problem (Argos et al. 2010). Arsenic-loaded groundwater used for agricultural purpose is considered the major source of As penetration into the food chain (Adomako et al. 2009). Arsenic-induced toxicity has been reported in many plants (Jin and Huang 2010; Talukdar 2013; Ahmad et al. 2020; Singh et al. 2020). Arsenic severely intoxicates plants by reducing their biomass and plant height i.e. root and shoot length. Wilting and necrosis on leaves decrease leaf area and photosynthesis culminating into decrease in plant productivity and total death of the plant may occur (Garg and Singla 2011; Bhattacharya et al. 2012; Imran et al. 2013; Ahmad et al. 2020). The As-induced inhibitory effects on seed germination and degradation of enzymes have been well recognized in many plant species like tall fescue (Jin and Huang 2010), bean (Talukdar 2013) and Vicia faba(Ahmad et al. 2020).
There are two mineral forms of As in natural water: arsenite (AsO33−) and arsenate (AsO43−), denoted as As (III) (trivalent) and As (V) (pentavalent), respectively (Rai et al. 2011). They are considered to be the phytoavailable forms (Neidhardt et al. 2015). As (V) is dominant and stable in aerobic environments while As (III) dominates in O2 deficient or reducing environments as in groundwater. Organic forms of As in soil may include its monomethyl and dimethyl acid derivatives (MMAA and DMAA) and trimethylarsine oxide (TMAO)(Zakhar et al. 2018).
Arsenic (III) and As (V) are inter-convertible to each other (Fuhua et al. 1994). Arsenic (III) has the same fate inside the cell, whether it comes from outside or converted from pentavalent arsenic. It has been demonstrated that the expression of more genes and proteins takes place when plants come into the contact of As (V) in comparison to As (III). As (V) is the main phytoavailable form of As in O2-rich environment. It competes with PO43− during many cellular functions due to its analogy with PO43−. It may replace PO43− in ATP to form a weak compound ADP-As(Wu et al. 2011). As (V) is also responsible for the PO43− starvation and thus, hampers the PO43− signaling mechanism. Besides, As (V) has much affinity to the PO43− transporters as compared to PO43− itself (Zvobgo et al. 2018) and thus, As (V) will easily get entry into the cell whenever the outer concentration As (V) becomes high. It can easily be absorbed by roots and transported to the aboveground parts of plants through xylem where it is readily reduced to As (III) or other forms (Su et al. 2008).
As is a redox metalloid. There are significant experimental evidence that the exposure of plants to inorganic arsenic (Asi) does result in the over-production of active oxygen species (like singlet oxygen, 1O2; superoxide radical, O2•¯; and hydrogen peroxide, H2O2) which is concerned with the valance change from As (V) to As (III)(Talukdar 2013). A certain amount of ROS is always needed for the cell signaling process but when they are present in greater amount, they damage various components of cells, and the situation is termed as oxidative stress (Saed-Moucheshi et al. 2014b). Higher concentration of ROS than the certain threshold level breaks photosynthetic pigments, organic components of membrane system and nucleic acids. Therefore, usual cellular metabolism is disturbed (Talukdar 2013; Saed-Moucheshi et al. 2014a; Hossain et al. 2015). The equilibrium between the rate of ROS generation and their quenching decides the successful survival of an organism. Quenching of ROS is performed by a pervasive antioxidant system having several enzymes such as superoxide dismutase (SOD) and guaiacol peroxidase (GPX), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and non-enzymes such as ascorbate (AsA), glutathione (GSH) and proline, and thus the redox status of the cells is retained (Shakeri et al. 2019; Singh et al. 2020).
Studies have shown that PO43− prevents As uptake and reduces its translocation in wheat plants. Inorganic phosphate upregulated arsenate reductase in wheat (Triticum durum L.) (Pigna et al. 2009). This is an important mechanism in As reduction and its consequent sequestration in vacuoles in the form of an As (III)–PC complex.
In India, many rice-producing regions fall under severely As-polluted areas. Therefore, an approach must be developed to reduce As toxicity in rice plants. Furthermore, very few reports are available about the effects of PO43− on As-stressed rice seedlings. Therefore, it becomes plausible to assess that if exogenous PO43− could reduce As uptake in rice plants via competitive interaction. Simultaneously, amelioration by PO43− in As-stressed rice plants via changes in photosynthetic pigment contents and biochemical and antioxidant enzyme parameters would be other dimensions to know the mechanism of As toxicity. Keeping above facts into consideration, rice plant was taken as a plant material.
Material and methods
Experimental conditions
Rice (Oryza sativa L.) seeds cv. PAN 814-Swadesh obtained from Pan Seeds Pvt. Ltd., Suite No. 15, 2nd Floor, 2, N. C. Dutta Sarani, Kolkata, India were screened in hydroponics. Seeds were subjected to HgCl2 (0.1%, w/v) for surface sterilization and washed thoroughly for 30 s and soaked in deionized water for 24 h. These seeds were now transferred to culture room keeping them in Petri plates for 3–4 days at 25 ± 2 °C in dark to allow their proper germination. Then, uniform germinated seedlings were selected and transferred to plastic tray having fixed PVC cups (6.25 cm diameter and 8.5 cm height, 5 seedlings per pot) and grown in modified 1/3 strength Hewitt nutrient medium (Hewitt 1966) under hydroponic conditions for 7 days. They were further exposed to 50 μM As (V) (henceforth abbreviated as AsV) (source: sodium arsenate, Na2HAsO4) and three PO43− concentrations (50, 100 and 150 μM (henceforth abbreviated as P1, P2 and P3, respectively) (source: potassium dihydrogen phosphate, KH2PO4) for 10 days. P and As have similar hybridization state (sp3) in KH2PO4 and Na2HAsO4, respectively. Furthermore, the K content in KH2PO4 is lesser than K2HPO4, so KH2PO4 was preferred over K2HPO4. Furthermore, to investigate the PO43−-mediated biochemical changes, the experimental design consisted of a total of five samples i.e. control (no added AsV and PO43−) and treatments, As 50 μM, As 50 μM + PO43− 50 μM, As 50 μM + PO43− 100 μM and As 50 μM + PO43− 150 μM abbreviated as AsV, AsV+P1, AsV+P2 and AsV+P3, respectively. The arsenate concentration studied is equivalent to soil conditions and is environmentally relevant (please see review by Singh et al. 2015). KCl was added to the nutrient medium of control to compensate the reduction in K concentrations. They were arranged in a randomized block designed with three replicates. Now the plastic tray was placed inside the growth chamber (CDR Model GRW-300 DGe, Athens) under photosynthetically active radiation (PAR) of 350 μmol photons m−2 s−1 with 16:8 h day-night regime and 60% relative humidity maintained by humidifier at 25 ± 2 °C for a period until the secondary leaves emerged. During growth, seedlings were sprayed with water whenever required. All the nutrient solutions were changed twice per week (but not either As or PO43−), and the pH was adjusted to 5.5 using 0.1 KOH or HCl. After that, the uniform sized rice seedlings having secondary leaves were harvested, washed with deionized water, and used for the study of various biochemical and enzymatic parameters. All the physiological and biochemical experiments were done on the secondary leaves.
Measurement of morphological characters
Untreated and treated seedlings were uprooted and washed with distilled water and water was removed by smooth blotting. A meter scale was used to measure root and shoot lengths. Digital electronic balance (Model CA 223, Contech, India) was used to measure fresh mass of the seedling samples. For this, 17-day-old rice seedlings were used.
Estimation of chlorophylls and carotenoid contents
For the estimation of chlorophyll (Chl), 20 mg fresh leaves from each seedling sample was crushed in acetone having 80% (V/V) concentration. They were centrifuged at 10,000g for 10 min. Optical density of the supernatant was recorded at 663, 645, 510 and 480 nm. Lichtenthaler (1987) formula was used to calculate Chl a, b and carotenoid (Car) contents.
Determination of protein content
Protein content was determined according to the method of Bradford (1976) where Coomassie Blue G250 is used in the blue ionic form. The O.D. was read at 595 nm and bovine serum albumin was used to prepare the standard curve.
Determination of whole-cell O2 evolution
Whole-cell O2 evolution was measured in terms of oxygen evolution from the leaf discs in the presence of light using a Clark-type oxygen electrode (Digital Oxygen System, Model-10, Rank Brothers, UK) and was expressed as μmol O2 evolved g−1 FW h−1.
Measurement of hydrogen peroxide and lipid peroxidation
Measurement of H2O2 was done by the method of Velikova et al. (2000) while lipid peroxidation (in terms of malondialdehyde, MDA content) was determined by the method of Heath and Packer (1968).
Histochemical staining of ROS and indices of damage
Histochemical staining of O2•¯ was performed by the method of Castro-Mercado et al. (2009). Similarly, histochemical staining of H2O2 in the leaves of treated and untreated seedlings was performed by the method of Thordal-Christensen et al. (1997). For more details, our group’s previously published paper Mishra et al. (2016) can be consulted.
Determination of antioxidant enzymes
For the measurement of antioxidant enzyme activities, shoots and roots were homogenized in 3 ml of 100 mM potassium phosphate buffer (pH 7.5) containing 1 Mm EDTA and 1% (W/V) polyvinylpyrrolidone in a pre-chilled mortar and pestle and then centrifuged at 12000g for 15 min at 4 °C. Superoxide dismutase (SOD) activity was determined spectrophotometrically according to the method of Beyer and Fridovich (1987) at 560 nm and 1 unit (U) of SOD activity is defined as the quantity of protein required to cause 50% inhibition in reduction of NBT and shown as U mg−1 protein. Guaiacol peroxidase (GPX; EC 1.11.1.7) activity was determine according to the method of Kato and Shimizu (1987) at 470 nm and shown as μmole of guaiacol oxidized min−1 mg−1 protein. Catalase (CAT; EC 1.11.1.6) activity was determined by the method of Aebi (1974) and the absorbance was recorded at 240 nm. Enzyme activity was shown as μmole min−1 mg−1 protein.
The ascorbate peroxidase (APX) activity was ascertained according to the method of Nakano and Asada (1981). Monodehydroascorbate reductase (MDHAR) activity was determined by the method of Drazkiewicz et al. (2003) by monitoring NADPH oxidation at 340 nm (€ = 6.2 mM−1 cm−1) in a 3 ml of reaction mixture containing 0.1 mM NADPH, 2.5 mM ASC, 50 mM Na-phosphate buffer (pH 7.6) and 100 μg protein. The rate of enzyme activity was showed as μmole MDHA reduced min−1 mg−1 protein. DHAR (EC 1.8.5.1) activity was measured by the method of Tullio et al. (1998). The enzyme activity was calculated by using an extinction coefficient of ε = 14 mM−1 cm−1 at 265 nm. The rate of enzyme activity was showed as μmoles DHA reduced min−1 mg−1 protein.
Estimation of total ascorbate (AsA+DHA), reduced ascorbate (AsA) and dehydroascorbate (DHA) contents
Reduced ascorbate (AsA), dehydroascorbate (DHA) and total ascorbate (AsA+DHA) contents were determined by the method of Gossett et al. (1994).
Estimation of total glutathione (GSH+GGSG), reduced glutathione (GSH) and oxidized glutathione (GSSG) contents
Rice leaves (500 mg) were homogenized in 3 ml ice-cold 5% m-phosphoric acid containing 1 mM EDTA using a mortar and pestle. The homogenates were centrifuged at 11500g for 15 min at 4 °C and the collected supernatants were used according to the method of Brehe and Burch (1976) with some modifications.
For the details of methodologies pertinent to the estimation of H2O2, lipid peroxidation, ascorbate peroxidase, total, reduced and oxidized ascorbate, and total, reduced and oxidized glutathione contents, our group’s previous published paper Srivastava et al. (2012) can be consulted.
Statistical analysis
The results shown are means ± standard error of three independent experiments with three replicates in each experiment. Two-way ANOVA was carried out to compare control and treatment’s means by using Duncan’s multiple range test (DMRT) at P< 0.05. For this, the SPSS-16 software was used.
Results
Growth
The toxic expression of 50 μM As (V) on the growth of plants resulted in significant reduction in the fresh mass (31%), root length (30%) and shoot length (19%) as compared to the control (Table 1; Fig. 1a). Conversely, simultaneous exposures of 50, 100 and 150 μM PO43− (P1, P2 and P3) along with 50 μM AsV significantly enhanced (P<0.05) the fresh mass by 5, 14 and 9%, length of the roots by 8, 14 and 9%, and that of shoots by 7, 12 and 9%, respectively as compared to the seedlings grown under AsV 50 μM alone. Protein content also showed the similar trend, which was 16% decline after AsV treatment and recovered by the percentages of 7, 12 and 9% after the supplementation of P1, P2 and P3 doses PO43−. Thus, the decline in protein content was the least decline among the four parameters of growth.
Photosynthetic pigments
The 50 μM AsV-treated seedlings showed significant reduction of 27 and 26 % in Chl a and b, respectively while Car contents attained an increase of 26% as compared to control (Table 2). Moreover, under the combined treatments of AsV+P2, seedlings showed the maximum enhancement of 25% in the level of Chl a while 46 % in the level of Chl b. However, Car contents showed a maximum enhancement of 17% after the AsV+P2 treatment as compared to AsV alone treatment.
Whole-cell O2 evolution
Results pertaining to the whole-cell O2 evolution have been presented in Table 1. Rice seedlings showed a decline of 32% in photosynthesis when treated with As, while after supplementation with P1, P2 and P3 doses, the recoveries in photosynthesis were 15, 26 and 27%, respectively in the test rice seedlings.
H2O2 and MDA content
In the present study, the level of H2O2 and MDA content increased significantly i.e. 32% and 33%, respectively in the seedlings receiving 50 μM AsV as compared to those in control (Table 2). Conversely, only the simultaneous exposure of P1 and P3 along with 50 μM AsV to rice seedlings, H2O2, was decreased by a significant percentage of 10 and 13% while MDA content was decreased by 12 and 14% in comparison to the seedlings exposed with 50 μM AsV only.
Histochemical staining of ROS and indices of damage
Results of ROS were verified by the histochemical staining of O2•¯ and H2O2 in the leaves of the rice seedlings. A dense blue formazan formed due to the reduction of NBT by O2•¯ and a significant accumulation of H2O2-mediated brown spot appeared and spread throughout the leaf after 50 μM AsV exposure to the test seedlings. Applying PO43− to the AsV-treated seedlings considerably reduced the spots of O2•¯ and H2O2 in comparison to AsV alone treated seedlings and maximum reduction was observed in 50 μM AsV+P3(Fig. 1b).
Response of SOD, GPX and CAT in rice seedlings
The rice seedlings revealed boosts in responses for SOD, GPX and CAT when exposed to 50 μM AsV(Fig. 2). The SOD, GPX and CAT activities increased significantly by 33, 43, and 24%, respectively in 50 μM AsV-treated seedlings as compared to the control. The activities of SOD, GPX and CAT were decreased by 10% each by AsV+P1 treatment in comparison to those of only As-treated values. Moreover, in AsV+P2-treated rice seedlings, the SOD, GPX and CAT activities were 26, 28 and 18% lesser than those of the singly As-treated seedlings. Similarly, in AsV+P3-treated rice seedlings, the SOD, GPX and CAT activities were 14, 15 and 5% lesser than those of the singly As-treated seedlings.
Changes in the level of SOD (A), CAT (B) and GPX (C) in response to AsV alone and in combination with three different doses of PO43− in the rice seedlings. All the values are means ± standard error of three independent experiments. Bars having the same letter(s) are not significantly different according to DMRT at P ≤ 0.05 significance level
Responses of APX, GR, DHAR and MDHAR in rice seedlings
Pentavalent arsenic at its 50 μM concentration increased the activity of APX, GR, DHAR and MDHAR by 27, 28, 19 and 21%, respectively when they were compared with the control seedlings (Fig. 3). The activity of APX was calculated 7% lesser in AsV+P1-treated plants; however, 3% and 12% enhancements were recorded in its activity after the exposures of P2 and P3 simultaneously with AsV in comparison to those of AsV alone treated seedlings. In the case of DHAR and MDHAR, AsV+P2 brought about 9 and 15% decline in their activities in comparison to those of As alone treated seedlings. In the case of GR, AsV+P1 and AsV+P2 had the similar normalizing effect as on the activities of DHAR and MDHAR, whereas AsV+P3 had caused 18% decline in GR, and 7 and 9% decline in DHAR and MDHAR in comparison to those of AsV alone treated seedlings.
Changes in the level of APX (A), MDHAR (B), DHAR (C) and GR (D) in response to AsV alone and in combination with three different doses of PO43− in the rice seedlings. All the values are means ± standard error of three independent experiments. Bars having the same letter(s) are not significantly different according to DMRT at P ≤ 0.05 significance level
Responses of AsA and GSH
The 50 μM AsV alone increased the amount of AsA+DHA and DHA by 84% and 16%, respectively. However, under the combined treatment, AsA+DHA content was declined by 4% in AsV+P2 and by 24% in AsV+P3-treated seedlings as compared to those of AsV alone treated seedlings. Moreover, the values of AsA/DHA were substantially declined by AsV alone, respectively; however, a gradual increase was seen in the ratio of AsA/DHA as the amount of PO43− was increased in successive treatments (Table 3). The 50 μM AsV alone treated seedlings caused 8% decline in GSH+GSSG in comparison to that of the control seedlings. Similar to AsA/DHA ratio, the values of GSH/GSSG were substantially declined by AsV alone, respectively; furthermore, a gradual increase was seen in the ratio of GSH/GSSG as the amount of PO43− was increased in successive treatments.
Discussion
In the present study, arsenic-exposed rice seedlings showed a significant (p<0.05) decline in fresh weight, root length and shoot length, which is in conformity with few previous studies (Shri et al. 2009; Sinha et al. 2010). In our experiments too, AsV toxicity was lessened in rice seedlings under the combined exposure of AsV and PO43− in the terms of fresh mass of root and shoot, pigment contents, indices of oxidative stress, antioxidants and metabolites (Tables 1, 2 and 3, Figs. 1, 2 and 3) and provided better compatibility to tolerate AsV toxicity (Table 4). Identical physical and chemical properties endow AsV the ability to compete with PO43¯ (i.e., similar electronic configuration, valence shells and atomic radii) and whenever the concentration of AsV increases than a threshold limit, it overpowers PO43¯ (Zhao et al. 2010; Saifullah et al. 2018). Another reason is being the competitive inhibition in the uptake of PO43− by AsV across the same kind of transporter OsPT1 and OsPT13(Muehe et al. 2014). Consequently, the absorption of PO43− through tissues and membranes of different organelles would have been depressed by AsV. PO43− has certain role in cellular metabolism, comprising methylation, reduction uptake and conjugation with reduced glutathione (GSH). The observed recovery with 100 μM PO43− (P2) in growth parameters is a noteworthy finding, which substantiates the above notion.
Another dimension of AsV toxicity is decline in Chl a, b and Car contents (Table 2). This is in agreement with some of the earlier studies on rice seedlings (Shri et al. 2009; Sinha et al. 2010; Kumar et al. 2013; Muehe et al. 2014), duckweed (Duman et al. 2010) and black gram (Srivastava and Sharma 2013). Stoeva and Bineva (2003) opined that the decline in Chl a, b and Car under the As exposure is due to the disruption of the chloroplast structure, while according to Jain and Gadre (2004), this decrease is due to alteration in the action of sulfhydryl requiring enzymes, viz. ALA (δ-aminolevulinate synthase) and ALA dehydratase (important for tetrapyrrole biosynthesis which is required for chlorophyll formation) and RuBisCO. Furthermore, recovery in Chl a and b by PO43¯ in AsV-intoxicated rice seedlings is in harmony with the findings of Naeem and Khan (2009) in coffee senna and Shri et al. (2009) and Li-gang et al. (2012) on rice seedlings. Improvement in Chl a and b by the addition of PO43− is synchronized by enhancement in plant growth and decline in the levels of TBARS. Therefore, our results emphasize the crucial role of PO43−. The significant fall in O2 evolution by AsV could be due to the change in the configuration of chlorophyll molecules by the replacement of the central atom (Mg) of chlorophyll by As (Yadav et al. 2014). Similar results were also observed by Sanglard et al. (2016) who also reported a decreased net photosynthetic rate in rice seedlings under As stress. In another findings, Ahsan et al. (2010) reported a significant fall in RuBisCO and chloroplast 29-kDa ribonucleoproteins in rice plants after As treatment. Since the RuBisCO and chloroplast 29-kDa ribonucleoproteins constitute a major portion, the decline in their level significantly suppressed the photosynthetic activity. Recovery in photosynthesis might be due to the improvement in carbon sink and higher efficiency in the use of nutrients in photosynthetic events. Phosphate molecules must have stimulated Calvin cycle; thereby, the rate of CO2 fixation in the leaves has increased. Apart from this, phosphate supplementation might have resulted into higher gas exchange and photochemical and non-photochemical efficiencies, improving the photosynthetic capacity of the leaves, which is due to the improved electron transport, thereby improving the efficiency of PS II photochemistry.
It is a well-accepted fact that the oxidative stress causes lipid peroxidation as well as damage to various biomolecules (Patra et al. 2004; Saed-Moucheshi et al. 2014a). The extent of cell membrane disintegration (in terms of lipid peroxidation) is enhanced when plants are exposed to different biotic and abiotic stresses, including As (Meharg and Hartley-Whitaker2002). In current study too, AsV-exposed seedlings showed increased level of MDA content. This is the proof that scavenging mechanism of plant is not properly functioning and can easily be related to the excess formation of free radical i.e. H2O2. It may start uncontrolled oxidation and free radical chain reactions which ultimately result in more stressful environment to the plant. Results of this study are in conformity with earlier studies on mung bean and Pteris sp. (Srivastava et al. 2005).
However, whenever calibrated amount of PO43− was given to AsV-stressed rice seedlings, the H2O2 and hence MDA content were significantly decreased. It clearly points out that PO43− is able to lower the AsV-induced toxicity. This could be interrelated to the significant increment in the activity of GPX, the enzyme responsible for the degradation of H2O2. GPX protects plants against oxidative stress and acts as a substrate in H2O2 scavenging and lipid hydroperoxides (Arthur 2000). On the other hand, there are indications that ROS might have directly been scavenged by PO43− and changed to lesser toxic products and thus the oxidative damage was effectively prevented. These results are synchronous with the findings of Choudhury et al. (2011), Kumar et al. (2013) and Mi et al. (2014) on rice seedlings. These results suggest that AsV toxicity can be ameliorated, in general in plants and specifically in rice by the supplementation of PO43−.
Along with GPX, SOD is the major antioxidant enzyme, which scavenges ROS and plays a pivotal role in defense against oxidative stress (Ighodaro and Akinloye 2017). In the present study, the synchronized action of SOD and GPX constituted the frontline enzymatic picket by converting O2•¯ and H2O2 consecutively into H2O in As-stressed rice seedlings (Table 2; Fig. 1b). Our results are in agreement with those of Shri et al. (2009) where As-stressed rice seedlings showed increase in the activities of SOD and GPX. These results were further confirmed by in vivo staining of O2•− and H2O2(Fig. 1b).
CAT is a heme-containing tetrameric protein and possibly one of the best known enzymes that splits H2O2 into H2O and O2. In the present study, the level of H2O2 was increased in As-treated seedlings which could be correlated with the lesser activity of CAT; therefore, the toxic symptoms appeared in the seedlings. On the other hand, the CAT activity became higher when PO43− was additionally supplemented to the seedlings. Differential behavior of various antioxidants against H2O2 relies on the fact that different antioxidant enzymes reside in different cell organelles. The maximum CAT activity was recorded in the case of AsV+P2-treated rice seedlings which proves the significant amelioration of the As-induced oxidative stress CAT by the P2 concentration of PO43−(Choudhury et al. 2011).
The coordinated functions of APX, MDHAR, DHAR and GR together with AsA and GSH in the AsA-GSH cycle split H2O2 into H2O and O2 and further recycle AsA and GSH (Singh et al. 2020). The increment in APX activity over the control indicates that the plant was responding against AsV-induced toxicity. Further rise in APX activity after P2 addition was seen which is corroborated by the increase in biomass and decrease in MDA content. On the other hand, the APX activity was lesser in comparison to those seedlings which were additionally supplemented with PO43− along with AsV, perhaps due to higher accumulation of AsV in the root and shoot tissues. These results are corroborated by the increase in MDA content and decrease in plant growth.
AsA is recycled by MDHAR and DHAR, which is necessary to maintain the supply of AsA and thereby the ROS scavenging process (Hasanuzzaman et al. 2017). In our experiments, the activities of GR, MDHAR and DHAR were also enhanced during the combined exposure of AsV+PO43− as compared to those of singly AsV-treated seedlings. This observation might be due to significant decrease in the GSH content in rice seedlings as it was converted to phytochelatins. Greater demand of GSH in response to arsenic-induced oxidative stress might be fulfilled through the increased GR, MDHAR and DHAR activity. This result suggests that GR, MDHAR and DHAR play a central role in AsA-GSH cycle in the defense against As toxicity when supplemented with PO43−. In this cycle, APX decomposes H2O2 via the oxidation of AsA, and then AsA is recycled from DHA by the consumption of GSH as an electron donor, followed by regeneration of GSH by GR activity. Similar results have been reported in rice and Pteris spp. (Abedin and Meharg 2002; Srivastava et al. 2005). Thus, the activities of AsA-GSH cycle enzymes were reduced by AsV-led oxidative stress on one hand and an enhancement in SOD activity on the other. This may result in uncontrolled cellular H2O2 production, whereas higher activities of MDHAR, DHAR and GR in rice seedlings under the combined treatment of AsV+PO43− suggest that ROS could be quenched through the SOD–CAT pathway or/and by the AsA-GSH cycle. This idea of a “two-way defense system” was propounded by Mittler (2002) on the basis of differential affinities of APX and CAT for H2O2 and coordinated upregulation of H2O2-scavenging enzymes in cell which confers tolerance of rice seedlings against As stress, at least partially.
Oxidative stress caused by AsV in rice seedlings and protection by PO43− may further be associated with the redox status of the key non-enzymaticantioxidants—ascorbate and glutathione, which are the two major non-enzymatic antioxidants that play significant roles in the quenching of ROS to maintain a redox potential inside the cell (Smith et al. 1988; Sharma et al. 2012). In the AsA-GSH cycle, AsA detoxifies ROS and makes the basis of its antioxidant action (Hasanuzzaman et al. 2017). In this study, the AsA+DHA content and the AsA/DHA ratio decreased (Table 3) with 50 μM AsV concentration. AsA/DHA ratio indicates the redox status of a cell and its high ratio is supposed to be crucial for the successful survival of the organism under the stress. Thus, it is used as an important indicator for assessing the degree of oxidative stress. Moreover, these results were supported by Jung et al. (2019) who confirmed that the reduced ascorbate scavenges ROS directly, or indirectly by the means of APX. However, in our study, PO43− supplementation resulted in a lower AsA+DHA content and a higher AsA/DHA ratio (Table 3) as compared to those of AsV treatments alone, and hence protected the rice seedlings against the oxidative damage. These results are in agreement with Kumar et al. (2013) who showed that PO43− supplementation restored AsA content in plants with arsenic-induced damage.
Conclusion
The outcome of the present study indicates that the application of As (V) to rice seedlings interrupted the growth as well as the photosynthetic process by causing chlorophyll degradation. Yet, there is also an induced oxidative stress and deterioration of membranes through increased extent of lipid peroxidation, consequently inhibiting the growth of rice seedlings. Moreover, our study also provides strong evidence that PO43− could successfully alleviate As (V)-induced growth inhibition, which may be attributed by (i) increased amount of photosynthetic pigments and (ii) reduced oxidative stress by redox homeostasis with the assistance of a compatible antioxidant system including AsA-GSH cycle enzymes. Alleviation of As (V) toxicity requires a coordinated action of physiological and biochemical processes. This study, therefore, prospers our understandings about the responses of rice seedlings against As (V) stress and other environmental stresses too. However, molecular studies using PO43− mutants would be more promising to ascertain the exact role of PO43− in modulating the crop responses against As stress.
Availability of data and materials
Not applicable.
References
Abedin MJ, Meharg AA (2002) Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa L.). Plant Soil 243:57–66
Adomako EE, Solaiman ARM, Williams PN, Deacon C, Rahman GKMM, Meharg AA (2009) Enhanced transfer of arsenic to grain for Bangladesh grown rice compared to US and EU. Environ Int 35:476–479
Aebi H (1974) Catalase. In: Bergmayer HU (ed) Methods of enzymatic analysis Verlag Chemie. Academic Press Inc, Weinheim/ NewYork, pp 673–680
Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M (2020) Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 244:125480
Ahsan N, Lee D-G, Kim K-H, Alam I, Lee S-H, Lee K-W, Lee H, Lee B-H(2010) Analysis of arsenic stress-induced differentially expressed proteins in rice leaves by two-dimensional gel electrophoresis coupled with mass spectrometry. Chemosphere. 78:224–231
Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, VanGeen A, Graziano J, Ahsan H (2010) Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet 376:252–258
Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57:1825–1835
Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in condition. Anal Biochem 161:559–566
Bhattacharya S, Sarkar ND, Banerjee P, Banerjee S, Mukherjee S, Chattopadhyay D, Mukhopadhyay A (2012) Effects of arsenic toxicity on germination, seedling growth and peroxidase activity in Cicer arietinum. Int J Agric Food Sci 2:131–137
Bradford MM (1976) A dye binding assay for protein. Anal Biochem 72:248–254
Brehe JE, Burch HB (1976) Enzymatic assay for glutathione. Anal Biochem 74:189–197
Castro-Mercado E, Martinez-Diaz Y, Roman-Tehandon N, Garcia-Pineda E (2009) Biochemical analysis of reactive oxygen species production and antioxidative responses in unripe avocado (Persea Americana Mill var Hass) fruits in response to wounding. Protoplasma 235:67–76
Choudhury B, Chowdhury S, Biswas AK (2011) Regulation of growth and metabolism in rice (Oryza sativa L.) by arsenic and its possible reversal by phosphate. J Plant Interact 6:15–24
Drazkiewicz M, Skorzynska-Polit E, Krupa Z (2003) Responses of the ascorbate glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci 164:195–202
Duman F, Ozturk F, Aydin Z (2010) Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): effects of concentration and duration of exposure. Ecotoxicology 19:983–993
Fuhua C, Weiqi C, Shugui D (1994) Toxicities of four arsenic species to Scenedesmus obliquus and influence of phosphate on inorganic arsenic toxicities. Environ Toxicol Chem 41:1–7
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321
Gossett DR, Millhollon EP, Cran LM (1994) Antioxidant response to NaCl stress in salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Hasanuzzaman M, Mahmud JA, Nahar K, Anee TI, Inafuku M, Oku H, Fujita M (2017) Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In: Khan MIR, Khan NA (eds) Reactive oxygen species and antioxidant systems in plants: role and regulation under abiotic stress. Springer, Singapore, pp 257–281
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hewitt EJ (1966) Sand and water culture methods used in the study of plant nutrition. Technical Communication No. 22 of the Commonwealth Bureau of Horticulture and Plantation Crops. Farnham Royal, Bucks, England: The Commonwealth Agricultural Bureau. London.
Hossain MA, Bhattacharjee S, Armin S-M, Qian P, Xin W, Li H-Y, Burritt DJ, Fujita M, Tran L-SP(2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420
Ighodaro OM, Akinloye OA (2017) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med 54(4):287–293
Imran MA, Khan RM, Ali Z, Mahmood T (2013) Toxicity of arsenic (As) on seed germination of sunflower (Helianthus annuus L.). Int J Phys Sci 8:840–847
Jain M, Gadre RP (2004) Inhibition of 5-amino levulinic acid dehydratase activity by arsenic in excised etiolated maize leaf segments during greening. J Plant Physiol 161:251–255
Jin JWX, Huang YF (2010) Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr J Biotechnol 9:1619–1627
Jung HI, Kong MS, Lee BR, Kim TH, Chae MJ, Lee EJ, Jung GB, Lee CH, Sung JK, Kim YH (2019) Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate–glutathione homeostasis in rice seedlings. Front Plant Sci 10:1089
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic dependent peroxidative degradation. Can J Bot 65:729–735
Kumar N, Mallick S, Yadava RN, Singh AP, Sinha S (2013)Co-application of selenite and phosphate reduces arsenite uptake in hydroponically grown rice seedlings: toxicity and defence mechanism. Ecotoxicol Environ Saf 91:171–179
Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Li-gang H, Wen-fuchen Guo-chen Z, Wei M, Chun-yan Q, Liang L, Hong-jia S (2012) Effects of phosphate fertilizer on cold tolerance and its related physiological parameters in rice under low temperature stress. J Northeast Agric Univ 4:1–10
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and non-resistant plant species. New Phytol 154:29–43
Mi L, Niu X, Lu M, Ma J, Wu J, Zhou X (2014)Phosphine-induced physiological and biochemical responses in rice seedlings. Chemosphere 100:77–82
Mishra RK, Kumar J, Srivastava PK, Bashri G, Prasad SM (2016) PS II Photochemistry, oxidative damage and anti-oxidative enzymes in arsenate-stressedOryza sativa L. Seed Chem Ecol 165:58–70
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Muehe EM, Eisele JF, Daus B, Kappler A, Harter K, Chaban C (2014) Are rice (Oryza sativa L.) phosphate transporters regulated similarly by phosphate and arsenate? A comprehensive study. Plant Mol Biol 85:301–316
Naeem M, Khan MMA (2009) Phosphorus ameliorates crop productivity, photosynthesis, nitrate reductase activity and nutrient accumulation in Coffee senna (Senna Occidentalis L.) under phosphorus-deficient soil. J Plant Interact 4:145–153
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Neidhardt H, Kramar U, Tang GH, Norra S (2015) Arsenic accumulation in the roots of Helianthus annuus and Zea mays by irrigation with arsenic-rich groundwater: insights from synchrotron X-ray fluorescence imaging. Chem der Erde Geochem 75:261–270
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Ecotoxicol Environ Saf 52:199–223
Pigna M, Cozzalino V, Violante A, Meharg AA (2009) Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut 197:371–380
Rai A, Tripathi P, Dwivedi S, Dubey S, Shri M, Kumar S, Tripathi PK, Dave R, Kumar A, Singh R, Adhikari B, Bag M, Tripathi RD, Trivedi PK, ChakrabartyD TR (2011) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:986–995
Saed-Moucheshi A, Pakniyat H, Pirasteh-Anosheh H, Azooz MM (2014a) Role of ROS as signaling molecules in plants. In: Parvaiz Ahmad (Ed.), Oxidative damage to plants: antioxidant networks and signaling. Academic Press, pp. 585-620.
Saed-Moucheshi A, Shekoofa A, Pessarakli M (2014b) Reactive oxygen species (ROS) generation and detoxifying in plants. J Plant Nutr 37:1573–1585
Saifullah, Dahlawi S, Naeem A, Iqbal M, Farooq MA, Bibi S, Rengel Z (2018) Opportunities and challenges in the use of mineral nutrition for minimizing arsenic toxicity and accumulation in rice: a critical review. Chemosphere 194:171–188
Sanglard LMVP, Detmann KC, Martins SCV, Teixeira RA, Pereira LF, Sanglard ML, Fernie AR, Araújo WL, DaMatta FM (2016) The role of silicon in metabolic acclimation of rice plants challenged with arsenic. Environ Exp Bot 123:22–36
Shakeri E, Mozafari AA, Sohrabi F, Saed-Moucheshi A (2019) Role of proline and other osmoregulatory compounds in plant responses to abiotic stresses. In: Pessarakli M (ed) Handbook of plant and crop stress, 4th edn. CRC Press Boca Raton, Boca Raton, pp 165–173
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Singh R, Parihar P, Prasad SM (2020) Sulphur and calcium attenuate arsenic toxicity in Brassica by adjusting ascorbate–glutathione cycle and sulphur metabolism. Plant Growth Regul 91:221–235
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Sinha S, Sinam G, Mishra RK, Mallick S (2010) Metal accumulation, growth, antioxidants and oil yield of Brassica juncea L. exposed to different metals. Ecotoxicol Environ Saf 73:1352–1361
Smith IK, Vierheller TL, Thurne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5 dithiobis2-nitrobenzoic acid. Anal Biochem 175:408–413
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyperaccumulator and sensitive fern species to arsenic. J Exp Bot 56:1332–1342
Srivastava PK, Singh VP, Prasad SM (2012) Compatibility of ascorbate-glutathione cycle enzymes in cyanobacteria against low and high UV-B exposures, simultaneously exposed to low and high doses of chlorpyrifos. Ecotoxicol Environ Saf 74:79–88
Srivastava S, Sharma YK (2013) Impact of arsenic toxicity on black gram and its amelioration using phosphate. ISRN Toxicol 2013:340925
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bulgarian J Plant Physiol 29:87–95
Su YH, McGrath SP, Zhu Y, Zhao FJ (2008) Highly efficient xylem transport of arsenite in the arsenic hyperaccumulator Pteris vittata. New Phytol 180:434–441
Talukdar D (2013)Arsenic-induced oxidative stress in the common bean legume, Phaseolus vulgaris L. seedlings and its amelioration by exogenous nitric oxide. Physiol Mol Biol Plants 19:69–79
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley— powdery mildew interaction. Plant J 11:1187–1194
Tullio MCD, Gara LD, Paciolla C, Arrigoni O (1998) Dehydroascorbate reducing proteins in maize are induced by the ascorbate biosynthesis inhibitor lycorine. Plant Physiol Biochem 36:433–440
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Plant Sci 151:59–66
Wu C, Ye Z, Shu W, Zhu Y, WongM (2011) Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J Exp Bot 62:2889–2898
Yadav G, Srivastava PK, Singh VP, Prasad SM (2014) Light intensity alters the extent of arsenic toxicity in Helianthus annuus L. Seed Biol Trace Elem Res 158:410–421
Zakhar R, Derco J, Čacho F (2018) An overview of main arsenic removal technologies. Acta Chim Slov 11:107–113
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zvobgo G, WaLwalaba JL, Sagonda T, Mapodzeke JM, Muhammad N, Shamsi IH, Zhang G (2018) Phosphate alleviates arsenate toxicity by altering expression of phosphate transporters in the tolerant barley genotypes. Ecotoxicol Environ Saf 147:832–839
Acknowledgements
The Head, Department of Botany, University of Allahabad, India is gratefully acknowledged for providing necessary laboratory facilities.
Funding
The authors are obliged to the University Grants Commission, New Delhi for the award of Dr. D. S. Kothari Postdoctoral Fellowship Scheme-F 4-2/2006 (BSR)/13-113/2013 (BSR) to Rohit Kumar Mishra.
Author information
Authors and Affiliations
Contributions
Rohit Kumar Mishra designed the experiment, analyzed the data, interpreted the results and wrote the manuscript. Gitanjali Mishra sketched the graphical abstract and helped in manuscript preparation. Parul Parihar, Rachana Singh and Jitendra Kumar accomplished the experiment. Prabhat Kumar Srivastava and Sheo Mohan Prasad reviewed and improved the manuscript. The final version of the manuscript has been seen and agreed by all the authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Arsenic stress induces diminutions in growth attributes and photosynthetic pigments.
• Arsenic stress provokes oxidative burst in rice seedlings.
• The histochemical analysis of ROS proves the severe oxidative stress experienced by rice seedlings.
• The supplementation of PO43− strengthened the compatibility of ascorbate-glutathione(AsA-GSH) cycle.
• The application of PO43− fortified the antioxidative defence system against ROS.
Supplementary information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Mishra, R.K., Mishra, G., Singh, R. et al. Managing arsenic (V) toxicity by phosphate supplementation in rice seedlings: modulations in AsA-GSH cycle and other antioxidant enzymes. Environ Sci Pollut Res 29, 14418–14429 (2022). https://doi.org/10.1007/s11356-021-16587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16587-3