Abstract
Arsenic (As) toxicity and its biochemical effects have been mostly evaluated in ferns and a few higher plants. In this study, we investigated the effect of As (10.0 and 50.0 μM) on seedling growth, root anatomy, lipid peroxidation (malondialdehyde and conjugated dienes), electrolyte leakage, H2O2 content, root oxidizability and the activities of antioxidant enzymes in mung bean (Phaseolus aureus Roxb.). Arsenic significantly enhanced lipid peroxidation (by 52% at 50.0 μM As), electrolyte leakage and oxidizability in roots. However, there was no significant change in H2O2 content. Arsenic toxicity was associated with an increase in the activities of superoxide dismutase (SOD), guaiacol peroxidase (GPX) and glutathione reductase (GR). In response to 50.0 μM As, the activities of SOD and GR increased by over 60% and 90%, respectively. At 10.0 μM As, the activity of ascorbate peroxidase (APX) increased by 83%, whereas at 50.0 μM it declined significantly. The catalase (CAT) activity, on the other hand, decreased in response to As exposure, and it corresponded to the observed decrease in H2O2 content. We conclude that As causes a reduction in root elongation by inducing an oxidative stress that is related to enhanced lipid peroxidation, but not to H2O2 accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a toxic heavy metal dispersed in the environment through a variety of industrial, mining and agricultural activities (Mahimairaja et al. 2005). In the natural environment, it pollutes the soil and contaminates water, thus posing a serious threat to biota, including plants, animals and humans (Mahimairaja et al. 2005). The contamination of groundwater due to As is a serious problem in Bangladesh and parts of West Bengal in India, where millions of humans are at the risk of As-poisoning due to drinking of As-contaminated water (Pearce 2003). Further, irrigation of soils with As-contaminated water significantly elevates the As levels in the soil. It severely affects the growth and development of plants, and causes toxicity resulting in various biochemical and physiological disorders (Li et al. 2006 and references therein). However, As toxicity depends on the concentration of As, duration of its exposure and the physiological state of the plant. Recently, some ferns, like Pteris vittata (brake fern), P. cretica, P. longifolia and P. umbrosa were found to grow well in As-contaminated soils and accumulate high levels of As in their fronds (Ma et al. 2001; Meharg 2003).
Because As is a highly toxic metal and its contamination poses a serious threat to aquatic and terrestrial plants and animals, including humans, efforts are underway world wide to remediate As-contaminated soils and water using biological organisms, particularly plants. Bioremediation, including biosorption and biomethylation by microorganisms and phytoremediation using As-hyperaccumulator species, is of great significance in cleansing As from contaminated soils (Mahimairaja et al. 2005 and references therein). Among As-hyperaccumulators, fern species, Pteris vittata and Pityrogramma calomelanos, have extraordinary potential to accumulate As in their fronds and, therefore, appear to be very promising for phytoremediation (Gonzaga et al. 2006). In fact, phytoremediation is an efficient, viable and economical option for cleansing As-polluted soils and water.
Heavy metals, in general, cause toxicity and cellular disruption in plant species through induction of oxidative stress via increased generation of ROS, like1O2 (singlet oxygen), O −2 (superoxide radical), OH· (hydroxyl radical) and H2O2 (Gratäo et al. 2005). Plants have developed a well-organized scavenging machinery involving antioxidant enzymes (APX, GPX, CAT, SOD and GR) and antioxidant molecules (glutathione and ascorbate) that play a protective role by preventing heavy metal-induced damages (Gratäo et al. 2005).
In fact, plants also show a great variation in their response to As toxicity (Meharg 2003). In hyperaccumulator species, As increases antioxidant mechanisms, both enzymatic and non-enzymatic, leading to its detoxification and subsequent hyperaccumulation in the tissue (Srivastava et al. 2005; Singh et al. 2006). In contrast, in non-hyperaccumulators, As induces an oxidative stress resulting in cellular damages in terms of enhanced lipid peroxidation, H2O2 accumulation and up-regulation of several scavenging enzymes (Hartley-Whitaker et al. 2001; Mascher et al. 2002).
Most of the studies till date have investigated the levels of As-induced oxidative damage and antioxidant responses in fern species; however, there are very few reports in other sensitive species, like pea (Päivöke and Simola 2001) and red clover (Mascher et al. 2002). Since there are both inter- and intra-specific variations in As-induced toxicity profiles, it is pertinent to evaluate As toxicity in a wide variety of plant species. In the present study, we investigated As-induced toxicity in mung bean (Phaseolus aureus Roxb.), a non-accumulator species, by studying the degree of oxidative damage and associated changes in biochemical attributes. We selected mung bean because it is regarded as an important bioindicator species for As contamination (Van den Broeck et al. 1998). Further, it is an important widely grown pulse crop in India. However, there appears to be no information about its sensitivity vis-a-vis tolerance to As. Therefore, in the present investigation, we studied how As, at two concentrations, viz., 10.0 μM (low and non-toxic) and 50.0 μM (high and toxic), affected the seedling growth, oxidative stress markers (MDA, H2O2, CDs, EL and RO) and antioxidant enzyme machinery in mung bean.
Materials and methods
Plant material and arsenic treatments
Mung bean (Phaseolus aureus Roxb. cv. ML-5) seeds purchased locally from the market were surface-disinfested with NaOCl (0.1%, w/v) and washed under running tap water followed by distilled water. Thirty seeds were placed in a Petri dish (ϕ 15 cm) on Whatman No. 1 filter paper moistened with 7.0 ml of 10.0 (≅21.8 μg As) or 50.0 (≅109.0 μg As) μM As solution or distilled water (as a control); As was supplied in the form of sodium arsenate (MW = 312.01; GR; purity = 98.5%; Loba-Chemie, Mumbai, India). These As concentrations were environmentally relevant and comparable to those present under natural conditions (Sheppard 1992). It has been shown that As concentration is usually <10 mg kg−1 in non-contaminated soils, but its concentration increases in contaminated soils (Matshullat 2000).
There were three treatments including the control (0.0, 10.0 and 50.0 μM As), with five replicates in each. The experiment was laid out in a completely randomized design (CRD) in an environmentally controlled growth chamber under a 16 h photoperiod (240 μmol m−2 s−1 PFD) at 28/18 (±2)°C and 75 ± 2% RH.
Morphological observations and arsenic content
After a week, root and shoot lengths of the growing seedlings were determined. Because As-induced detrimental effects were found to be more pronounced in roots, they were harvested, stored at −40°C and subsequently used for further biochemical studies and the assessment of oxidative damage.
The amounts of As in root and shoot tissues were determined using an atomic absorption spectrophotometer (Model EC 4139; ECL, Hyderabad, India) equipped with a hydride-generation assembly. The plant material was oven-dried at 70°C followed by digestion with concentrated HNO3. An aliquot of the digest (2.0 ml) was analyzed for As by hydride formation, using sodium tetraborohydrate (NaBH4) as a reducing agent at pH 6.0. Arsenic concentration was measured at 193.7 nm, using a hollow cathode lamp as a radiation source and acetylene-air mixture as an oxidant fuel; the spectral bandwidth was 1 nm. A reagent blank together with a known standard were run along with the sample to maintain the analytical quality, and the measurements were based on the peak area.
To examine As-induced anatomical changes in roots, we prepared the sections (5–10 μm) of the treated as well as control roots and observed them under a light microscope. Briefly, the sections were cut with a razor from freshly harvested roots and stained with 0.25% (w/v, dissolved in 50% ethanol) safranin for tissue differentiation. These sections were mounted in 20% glycerin to prepare temporary mounts, observed under a Trinocular Stereo Zoom Microscope (Model RSM-9; Radical Instruments, Ambala, India) and photographed with a digital imaging system, Nikon Coolpix 4500, fitted to the microscope.
Lipid peroxidation
Oxidative damage to lipids was determined as lipid peroxidation in terms of malondialdehyde (MDA) and conjugated dienes (CDs). MDA, a major TBARS, was determined as per Heath and Packer (1968). Roots (100 mg) were homogenized in 5.0 ml of TCA (0.1%, w/v) and centrifuged at 10,000 g for 10 min. One ml of the supernatant was mixed with 4.0 ml of 0.5% TBA in 20% TCA. The mixture was heated at 95°C for 30 min, cooled over ice and centrifuged at 10,000 g for 10 min. The absorbance of the supernatant was recorded at 532 nm and corrected for non-specific absorbance at 600 nm. MDA content was calculated using an extinction coefficient (ε) of 155 mM−1 cm−1 and expressed as nmol g−1 FW. For CDs, the roots (100 mg) were homogenized in 5.0 ml of 96% (v/v) ethyl alcohol. The absorbance was measured at 234 nm (ε = 26.5 mM−1 cm−1) following Boveris et al. (1980); the CD content was expressed as μmol g−1 FW.
Electrolyte leakage (EL)
Roots (200 mg) were incubated in distilled water at 25°C for 2 h in test tubes and initial conductivity (E1) of the bathing medium was measured. The tubes containing the root material were boiled for 30 min to release all the electrolytes, cooled to 25°C, and the conductivity (E2) was measured again. The electrolyte leakage was calculated as follows: EL = (E1/E2) × 100.
H2O2 content
Roots (100 mg) were extracted with 5.0 ml of TCA (0.1%, w/v) in an ice bath, and the homogenate was centrifuged at 12,000 g for 15 min (Velikova et al. 2000). To 0.5 ml of the supernatant, 0.5 ml of phosphate buffer (pH 7.0) and 1.0 ml of potassium iodide (1 M) were added. The absorbance of the mixture was measured at 390 nm. H2O2 content was determined using an extinction coefficient (ε) of 0.28 μM−1 cm−1 and expressed as nmol g−1 FW.
Root oxidizability (RO)
RO, a measure of roots’ oxidizing ability and thus viability, was determined in terms of red triphenyl formazan formed through TTC reduction assay (Batish et al. 2007). To 100 mg roots, 5.0 ml of TTC (0.4%, w/v) and 5.0 ml of phosphate buffer (1/15 M, pH 7) were added, and the mixture was incubated for 3 h at 40°C. After adding 2.0 ml of 2 N H2SO4, the roots were homogenized in 10.0 ml of ethyl acetate to extract formazan. The absorbance of the extract was measured at 485 nm, and RO was expressed as A 485 g−1 h−1.
Enzyme extraction
Roots (250 mg) were homogenized in 10.0 ml of pre-chilled 0.1 M phosphate buffer (pH 7.0) under ice-cold conditions. The homogenate was filtered through four layers of cheesecloth and centrifuged at 15,000 g for 30 min. The supernatant was stored at 4°C until used for assaying the enzyme activities. An aliquot (0.5 ml) of the supernatant was used for protein estimation, using bovine serum albumin as a calibration standard (Lowry et al. 1951). All steps of the enzyme extraction including centrifugation were done at 4°C, and the assays were performed at 25°C using a UV-VIS spectrophotometer (Model UV 190; Shimadzu Corporation, Tokyo, Japan).
Enzyme assays
The activity of superoxide dismutase (SOD) was assayed based on its ability to inhibit NBT photochemical reduction (Beauchamp and Fridovich 1971). The reaction mixture (4.0 ml) contained 63 μM NBT, 13 mM l-methionine, 0.1 mM EDTA, 13 μM riboflavin, 0.05 M sodium carbonate and 0.5 ml enzyme extract (0.5 ml distilled water in the control). Reaction tubes were kept under two 15W fluorescent lamps for 15 min, incubated in the dark for 15 min, and the absorbance was recorded at 560 nm. One unit of SOD represented the amount that inhibited NBT photoreduction by 50% at 25°C.
The activity of APX was determined by measuring the decrease in absorbance at 290 nm (ε = 2.8 mM−1 cm−1) due to oxidation of ascorbic acid to dehydroascorbate (Nakano and Asada 1981). The reaction mixture (2.0 ml) consisted of 25 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbic acid, 1.0 mM H2O2 and 0.2 ml enzyme extract. For CAT assay, the reaction mixture (2.0 ml) contained 25 mM phosphate buffer (pH 7.0), 10 mM H2O2 and 0.2 ml of enzyme extract, and the activity was measured as the disappearance rate of H2O2 at 240 nm (ε = 39.4 mM−1 cm−1), according to Cakmak and Marschner (1992). The activity of GR was determined by following the oxidation of NADPH at 340 nm (ε = 6.224 mM−1 cm−1), according to Foyer and Halliwell (1976). The reaction mixture (2.0 ml) contained 25 mM phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM GSSG, and 0.12 mM NADPH. For GPX assay, the reaction mixture (2.0 ml) contained 25 mM phosphate buffer (pH 7.0), 0.05% guaiacol (w/v), 1.0 mM H2O2, 0.1 mM EDTA and 0.2 ml enzyme extract, and the activity was determined by measuring the increase in absorbance at 470 nm (ε = 26.6 mM−1 cm−1) due to oxidation of guaiacol, as described by Egley et al. (1983).
The activities of APX, GPX, CAT and GR were expressed as enzyme unit (EU) mg−1 protein, where 1 EU is the amount required to decompose 1.0 μM substrate (ascorbate or H2O2 or NADPH or guaiacol) min−1 at 25°C.
Statistical analyses
The experiment was conducted in a completely randomized design (CRD) with five replications; each replication comprised a single Petri dish, containing 30 seeds. For enzyme assays, there were five replicated (independent) tissue samples. The data were analyzed by one-way ANOVA followed by the comparison of mean values using post-hoc Tukey’s test at P ≤ 0.05.
Results
Effect of As on seedling growth
An exposure to As caused a significant reduction in root and shoot lengths of mung bean seedlings (Table 1). This inhibitory effect was more pronounced on root length than on shoot length. The root length decreased by over 63% and 82% in response to 10.0 and 50.0 μM As, respectively. In contrast, the reduction in shoot length was comparatively lesser. This was also evident from a declining root/shoot ratio upon As treatment. Further, the accumulation of As was more in roots than in shoots. Roots accumulated 0.37 ± 0.025 μg As g−1 DW, as compared to 0.17 ± 0.013 μg As g−1 DW in shoots when the seedlings were treated with 50.0 μM As. However, at 10.0 μM As, roots and shoots accumulated 0.20 ± 0.013 and 0.12 ± 0.009 μg As g−1 DW, respectively (data not shown).
The results showed that As also induced anatomical changes in mung bean roots (Fig. 1). In comparison to untreated control roots where all the epidermal cells were intact and the root hairs were turgid, As exposure resulted in a severe decrease (at 10.0 μM) in, or a complete absence (at 50.0 μM) of root hairs. Arsenic caused damage to the epidermal cells and the cortex, with these cells losing their shape and showing signs of shriveling and disintegration. Further, as compared to the control roots where the stele was in a tetrarch condition, there was a lack of complete differentiation and pith formation in response to As treatment (Fig. 1).
Cross-sections of mung bean roots showing anatomical changes upon exposure to 0.0 (A, D, G), 10.0 (B, E, H) and 50.0 μM (C, F, I) arsenic. First column, cross-section of the root; middle column, close-up of the epidermal region showing epiblema, root hairs and cortical cells; last column, close-up of the central stellar portion
As-induced oxidative damage
Arsenic increased the MDA content in mung bean roots, thereby indicating enhanced lipid peroxidation. The MDA content increased by about 20% and 52% over the control at 10.0 and 50.0 μM As, respectively (Table 2). There was not much difference in the amount of conjugated dienes (CDs) at 10.0 μM As; however, at 50.0 μM As, there was about 28% decline in CDs (Table 2). In contrast to MDA content, As did not cause any accumulation of H2O2. The H2O2 content showed a trend similar to CDs, with a significant reduction at 50.0 μM As. Unlike H2O2, RO increased significantly when the seedlings were treated with As. It almost doubled at 10.0 μM As and increased nearly by 10-fold in response to 50.0 μM As. The results further showed that As caused an electrolyte leakage (EL) from the roots, and there was a significant increase in EL at 50.0 μM As (Table 2).
As-induced antioxidant enzyme activities
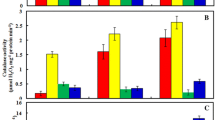
We found that As significantly altered the activities of scavenging enzymes, such as SOD, CAT, APX, GPX and GR in mung bean roots. At 10.0 μM As, SOD activity enhanced by about 60% over the control (Table 3). Likewise, there was a significant increase in the activities of GPX and GR in As-treated mung bean roots. At 50.0 μM As, GPX activity increased by about 26% over the control, whereas GR activity was more than 90% of the control (Table 3). In contrast, APX activity increased by about 84% over the control at 10.0 μM As; however, at 50.0 μM As, it significantly declined. Likewise, the activity of CAT decreased in response to As treatment, with about 70% decline over the control at 50.0 μM As.
Discussion
The inhibitory effect of As on root growth is consistent with earlier reports in other plant species (Kapustka et al. 1995; Van den Broeck et al. 1998; Hartley-Whitaker et al. 2001). It is also evident from the reported higher rates of As accumulation in roots than in shoots (Van den Broeck et al. 1998; Shaibur et al. 2006). However, this is in contrast to an As-accumulator, like Pteris vittata, which accumulates As in its fronds (Ma et al. 2001). In the present study, a reduction in root elongation was accompanied by anatomical changes in mung bean roots. Though the exact reasons for such an effect are unknown, yet a heavy metal like Cd has been shown to induce changes in the root anatomy and vascular bundles (Rodríguez-Serrano et al. 2006). Recently, Vitória et al. (2006) have reported Cd-induced ultrastructural alterations in radish mesophyll cells.
Enhanced lipid peroxidation and reduced content of conjugated dienes in mung bean roots in response to As toxicity (50.0 μM) indicate increased generation of ROS vis-a-vis oxidative stress. This is in agreement with earlier reports that As causes severe lipid peroxidation in Holcus lanatus (Hartley-Whitaker et al. 2001), red clover (Mascher et al. 2002), bean (Stoeva et al. 2005) and Pteris spp. (Srivastava et al. 2005; Singh et al. 2006). An increase in MDA levels indicates the occurrence of membrane damage due to peroxidation of polyunsaturated fatty acids, resulting in the generation of ROS and subsequent oxidative stress (Montillet et al. 2005). Arsenic-induced membrane damage was also evident from an enhanced electrolyte leakage (EL) from the roots, particularly at 50.0 μM As. Earlier it has been shown that As, at 100–800 mg kg−1 soil, damages the chloroplast membrane structure, thus disrupting the cellular integrity (Li et al. 2006). It is known that EL is an indicator of membrane damage and occurs due to membrane peroxidation resulting from an oxidative burst (Bajji et al. 2002).
Increased root oxidizability (RO) indicates a greater diffusion of oxygen from the roots, primarily to counter the toxic materials in the soil environment (Armstrong 1967). Indeed, enhanced RO in mung bean in response to As toxicity, as observed in this study, is consistent with earlier observations that roots increase their oxidizing ability to avoid toxicity by heavy metals (Doyle and Otte 1997). Kedrova et al. (2003) also correlated Al resistance of some rye (Secale cereale L.) cultivars with their higher capacity of root oxidizability. TTC salt used to measure RO actually absorbs electrons from the mitochondrial transport chain and correlates positively with the respiratory activity (Batish et al. 2007). A higher respiratory activity is, in turn, associated with enhanced ROS generation (Tiwari et al. 2002). In other words, enhanced RO is also an indicator of higher ROS generation.
There was no significant increase in H2O2 level in mung bean roots at 10.0 μM As, whereas there was about 54% decline in its level at 50.0 μM As. The observed changes in H2O2 level corresponded to changes in the amounts of CDs. In contrast, MDA content enhanced significantly at 10.0 μM As, thus indicating oxidative damage despite no apparent changes in H2O2 content. It indicates that, at low concentrations, As-induced oxidative damage does not involve any increase in endogenous H2O2 content. Possibly, a small increase in H2O2 content, as observed at 10.0 μM As, serves as a signal and triggers the defense response. Earlier studies have also shown that H2O2 acts as a signal molecule and serves a dual role in plants’ defense mechanism against abiotic stresses (Stone and Yang 2006). At low concentrations, H2O2 helps in stress-acclimation by providing tolerance, whereas at high concentrations it causes cellular damage leading to cell death (Vandenabeele et al. 2003; Stone and Yang 2006). Although H2O2 did not accumulate in response to As treatment, but its stimulation or increased production in mung bean roots cannot be ruled out. An increased activity of SOD in response to As treatment indicates a rapid dismutation of O −2 into H2O2.
We observed that an increased generation of ROS due to As treatment was also associated with enhanced activities of scavenging enzymes, such as SOD, GPX and GR. It is known that a balance is required between these scavenging enzymes to detoxify ROS in the cell. When this balance is disrupted, a compensatory mechanism is induced that increases the activities of other enzymes. SODs are the major O − 2 scavenger and provide a first line of defense against the cellular injury by environmental stresses (Gratäo et al. 2005). They are metalloproteins and exist in three isoforms, viz., Mn-SOD, Fe-SOD and Cu/Zn-SOD localized in different cell organelles depending upon their prosthetic groups. Though we did not assess the activities of their individual isoforms by native PAGE, yet any change (deficiency or excess of metal ions) is likely to affect their specific activities (Gajewska et al. 2006). Rodríguez-Serrano et al. (2006) reported that Cd treatment (50.0 μM) inhibited Cu/Zn SOD, but up-regulated Mn-SOD and Fe-SOD in pea roots. However, such observations in response to As toxicity are lacking. Excess H2O2 generated due to SOD activity is detoxified by CAT. However, a significant decline in CAT activity, as observed in this study, indicates that this enzyme is no way involved in providing protection against As toxicity. Earlier, a similar reduction in CAT activity in response to As was reported in Taxithelium nepalense (Choudhury and Panda 2004).
Probably, excess H2O2 was further detoxified by peroxidases, particularly GPX, and GR, which were substantially up-regulated in response to As. GPX acts upon H2O2 and forms GSSG that is further reduced to GSH by GR. In the present study, GPX activity increased by about 26% at 50.0 μM As. A similar increase in GPX activity (48–72%) was also reported in beans at 2.0–5.0 mg l−1 As (Stoeva et al. 2005). Enhanced GR activity that results in higher amount of GSH has been shown to be associated with an increase in ascorbate content and thus better protection against oxidative stress (Gratäo et al. 2005). Despite up-regulation of SOD and GPX activities, there was a significant decline in mung bean root growth. It indicates that GPX is not involved in providing protection against oxidative damage by H2O2. Furthermore, enhanced GPX activity can be correlated with higher lignification and consequent stunted growth, as an acclimation to stress (Gajewska et al. 2006).
APX is mainly located in chloroplasts and any damage to chloroplast is likely to affect its activity. Therefore, a decrease in APX activity at a higher concentration of As (50.0 μM), as obtained in the study, is not surprising. Li et al. (2006) reported that As damaged the chloroplast ultrastructure, affected its integrity and interfered with other associated metabolic activities. Arsenic also interferes with the activities of enzymes and proteins by binding to intracellular thiols (−SH), thus inactivating them (Meharg and Hartley-Whitaker 2002). The decreased activities of CAT and APX indicate their inactivation/ degeneration due to As-induced oxidative stress. Earlier, it has been demonstrated that superfluous superoxides inactivate cytosolic APX (Halliwell and Gutteridge 1989). In the present study, the total activities of different scavenging enzymes in the crude homogenates were determined. However, we did not measure the compartment-specific activities of theses enzymes, which might reflect their specific importance in antioxidant response (Foyer and Noctor 2005). Anyhow, a localized increase in ROS alters the redox balance at the whole plant level because there is an extensive network of defense system, with cross-talks between different compartments that could be triggered by ROS (Foyer and Noctor 2005). In response to heavy metal-induced oxidative stress, the antioxidant machinery, which is induced within a few hours after exposure, plays a significant role during the initial seed germination process (Ma et al. 2007). However, in our study, analyses were conducted 7 days after As exposure, and, therefore, we could not measure such a response.
In conclusions, our results showed that As at ecologically relevant concentrations affected the early root growth, induced root anatomical changes and altered the antioxidant scavenging machinery through induction of oxidative stress due to lipid peroxidation, but not H2O2 accumulation. However, the exact sequence of reactions at the molecular level and the genes, which are activated to bring about such a response, are largely unknown.
Abbreviations
- APX:
-
Ascorbate peroxidase
- As:
-
Arsenic
- CAT:
-
Catalase
- CD:
-
Conjugated diene
- DW:
-
Dry weight
- EL:
-
Electrolyte leakage
- FW:
-
Fresh weight
- GPX:
-
Guaiacol peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione reduced
- GSSG:
-
Glutathione oxidized
- H2O2 :
-
Hydrogen peroxide
- LP:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reduced
- NBT:
-
Nitro blue tetrazolium
- RO:
-
Root oxidizability
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
- TCA:
-
Trichloroacetic acid
- TTC:
-
2,3,5-Triphenyl tetrazolium chloride
References
Armstrong W (1967) The oxidizing activity of roots in waterlogged soils. Physiol Plant 20:920–926
Bajji M, Kinet J-M, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul 36:61–70
Batish DR, Lavanya K, Singh HP, Kohli RK (2007) Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul 51:119–128
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–286
Boveris A, Cadenas E, Chance B (1980). Low level chemiluminescence of the lipoxygenase reaction. Photobiochem Photobiophys 1:175–182
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Choudhury S, Panda SK (2004) Induction of oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under lead and arsenic phytotoxicity. Curr Sci 87:342–348
Doyle MO, Otte ML (1997) Organism-induced accumulation of iron, zinc and arsenic in wetland soils. Environ Pollut 96:1–11
Egley GH, Paul RN, Vaughn KC, Duke SO (1983) Role of peroxidase in the development of water impermeable seed coats in Sida spinosa L. Planta 157:224–232
Foyer CH, Halliwell B (1976) Presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gajewska E, Slaba M, Andrzejewska R, Sklodowska M (2006) Nickel-induced inhibition of wheat root growth is related to H2O2 production, but not to lipid peroxidation. Plant Growth Regul 49:95–103
Gonzaga MIS, Santos JAG, Ma LQ (2006) Arsenic phytoextrcation and hyperaccumulation by fern species. Sci Agric 63:90–101
Gratäo PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494
Halliwell B, Gutteridge JM (1989) The chemistry of oxygen radicals and other derived species. In: Halliwell B, Gutteridge JM (eds) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 22–85
Hartley-Whitaker J, Ainsworth G, Meharg AA (2001) Copper and arsenate induced oxidative stress in Holcus lanatus L. clones with differential sensitivity. Plant Cell Environ 24:13–22
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kapustka LA, Lipton J, Galbraith H, Cacela D, Lejeune K (1995) Metallic and arsenic impacts to soils, vegetation communities and wildlife habitat in southwest Montana uplands contained by smelter emissions: II. Laboratory phytotoxicity studies. Environ Toxicol Chem 14:1905–1912
Kedrova L, Saveljev J, Sheshegova T, Shirokhih I, Lisitsyn E (2003) Selection of winter rye (Secale cereale L.) for aluminum and acid resistance. Plant Breed Seed Sci 48:163–168
Li W-X, Chen T-B, Huang Z-C, Lei M, Liao X-Y (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809
Lowry OH, Rosebrough NT, Farr AL, Randall RJ (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Ma B, Wan J, Shen Z (2007) H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regul (in press). DOI 10.1007/s10725-007-9183-1
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelly ED (2001) A fern that hyperaccumulate arsenic. Nature 409:579
Mahimairaja S, Bolan NS, Adriano DC, Robinson B (2005) Arsenic contamination and its risk management in complex environmental settings. Adv Agron 86:1–82
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Matshullat J (2000) Arsenic in the geosphere—a review. Sci Total Environ 249:297–312
Meharg AA (2003) Variation in arsenic accumulation—hyperaccumulation in ferns and their allies. New Phytol 157:25–31
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic-resistant and nonresistant plant species. New Phytol 154:29–43
Montillet J-L, Chamnongpol S, Rustérucci C, Dat J, Van de Cotte B, Agnel J-P, Battesti C, Inzé D, Van Breusegem F, Triantaphylidès C (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol 138:1516–1526
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Päivöke AEA, Simola LK (2001) Arsenate toxicity to Pisum sativum: Mineral nutrients, chlorophyll content, and phytase activity. Ecotoxicol Environ Safety (Environ Res Section B) 49:111–121
Pearce F (2003) Arsenic’s fatal legacy grows. New Sci 179:4–5
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Shaibur MR, Kitajima N, Sugawara R, Kondo T, Huq SMI, Kawai S (2006) Physiological and mineralogical properties of arsenic-induced chlorosis in rice seedlings grown hydroponically. Soil Sci Plant Nutr 52:691–700
Sheppard SC (1992) Summary of phytotoxic levels of soil arsenic. Water Air Soil Pollut 64:539–550
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Srivastava M, Ma LQ, Singh N, Singh S (2005) Antioxidant responses of hyperaccumulator and sensitive fern species to arsenic. J Exp Bot 56:1335–1342
Stoeva N, Berova M, Zlatev Z (2005) Effect of arsenic on some physiological parameters in bean plants. Biol Plant 49:293–296
Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxidant Redox Signal 8:243–270
Tiwari BS, Belenghi B, Levine A (2002) Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol 128:1271–1281
Vandenabeele S, Van Der Kelen K, Dat J, Gadjev I, Boonefaes T, Morsa S, Rottiers P, Slooten L, Van Montagu M, Zabeau M, Inzé D, Breusegem FV (2003) A comprehensive analysis of H2O2-induced gene expression in tobacco. Proc Natl Acad Sci USA 100:16113–16118
Van den Broeck K, Vendecasteele C, Geuns JMC (1998) Speciation by liquid chromatography-inductively coupled plasma-mass spectrometry of arsenic in mung bean seedlings used as a bio-indicator for the arsenic contamination. Anal Chim Acta 361:101–111
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Vitória AP, Da Cunha M, Azevedo RA (2006) Ultrastructural changes of radish leaf exposed to cadmium. Environ Exp Bot 58:47–52
Acknowledgement
Komal Arora is thankful to Council of Scientific and Industrial Research (CSIR), New Delhi for financial assistance in the form of a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.P., Batish, D.R., Kohli, R.K. et al. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53, 65–73 (2007). https://doi.org/10.1007/s10725-007-9205-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-007-9205-z