Abstract
Objectives
The purpose of this study was to evaluate the long-term efficacy and safety of radiofrequency ablation (RFA) for low-risk papillary thyroid microcarcinoma (PTMC) in a large population.
Methods
From June 2014 to December 2017, 414 patients (323 females, 91 males, mean age 43.56 ± 9.79 years, range 18–73 years) with unifocal low-risk PTMC confirmed by core-needle biopsy (CNB) were treated by RFA. Patients were followed up at 1, 3, 6, and 12 months and every 6–12 months thereafter by ultrasound and contrast-enhanced ultrasound (CEUS). The volume and the volume reduction ratio (VRR) were calculated. Recurrence and lymph node or distant metastasis were evaluated.
Results
The mean initial volume was 92.74 ± 83.43 mm3 (range 4.19–490.07 mm3), which decreased significantly to 1.37 ± 7.94 mm3 (range 0–67.97 mm3) at a mean follow-up time of 42.15 ± 11.88 months (range 24–69 months) with a mean VRR of 98.81 ± 6.41% (range 50–100%). No life-threatening or delayed complications occurred. After RFA, 366 tumors (88.41%) completely disappeared. The overall incidence of local tumor progression rate was 3.62%. Among them, one patient (0.24%) was diagnosed to have residual cancer by CNB and underwent additional RFA. Four patients (0.97%) developed metastatic lymph node, and 10 patients (2.42%) had recurrent PTMC. A total of 13 patients underwent additional RFA, and 11 lesions completely disappeared during the follow-up.
Conclusions
RFA is an effective and safety treatment for low-risk PTMC after a long-term follow-up period for a large cohort with careful patient enrollment evaluation.
Key Points
• Radiofrequency ablation is an effective and safe alternative for low-risk PTMC.
• The overall incidence of local tumor progression rate was low.
• No life-threatening or delayed complications occurred.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global incidence of thyroid cancer has been increasing over the last 30 years, accounting for 3.4% of all cancers diagnosed annually worldwide [1], which has been the ninth most common cancer in the world [2]. According to the National Cancer Center of China, the thyroid cancer incidence rate is 14.6 per 100,000 person-years, ranking in seventh place incidence in both sexes and fourth place in women. Most of this increase is attributable to the identification of papillary thyroid microcarcinoma (PTMC), which is a form of papillary thyroid cancer with a maximum diameter of 1 cm or less in size regardless of the presence or absence of high-risk features [3, 4]. Most PTMC are low risk with excellent prognosis [2, 4]. The recurrence rate and the rate of cause-specific survival at 10 years are only 3% and 100%, respectively [4].
Thyroid surgery was the current standard treatment for PTMC [5]. Given the indolent behavior and favorable prognosis of low-risk PTMC, surgery may result in more harm than benefit, because of some permanent complications, cosmetic problems, and lifelong thyroid hormone replacement [6]. Active surveillance was a new option recommended for patients with low-risk PTMC by the 2015 American Thyroid Association (ATA) guidelines [5]. Studies from Japan have revealed that it was a safe and more cost-effective long-term management option than immediate surgery [7,8,9]. However, patient preference and compliance were important elements for this strategy [10]. Most of them would choose immediate surgery instead of active surveillance because of the anxiety about cancer progression [11]. Anxiety was also the most common reason for the decision to perform delayed surgery during active surveillance [12]. Additionally, the definition of PTMC does not depend on the presence of high-risk features such as lymph node metastasis (LNM) and/or distant metastasis. The prognosis of a minority PTMC could be poor with a 30% recurrence rate and a 74.1% rate of cause-specific survival at 10 years [4]. Unfortunately, no clinical or imaging method could reliably identify of predict the small percent of these invasive PTMC and prevent a large majority of PTMC patients from active surveillance [6].
Radiofrequency ablation (RFA), as a commonly used thermal ablation technique, has emerged as a new and promising treatment for many tumor types, including liver, kidney, and bone cancers, as well as soft-tissue tumors of the breast, head, and neck [13]. It has been recommended as a treatment for benign thyroid nodule and recurrent thyroid cancer by guidelines [5, 14]. After ablation, the volume reduction is significant with improvement of clinical concerns [15, 16]. For the last few years, studies from multiple centers suggested that RFA, as well as microwave ablation (MWA) and laser ablation (LA), were effective and safe alternatives for low-risk PTMC patients who were anxious about living with cancer or post-operative complications [17,18,19,20,21,22,23,24,25,26,27]. Comparative studies also indicated that thermal ablation techniques were not inferior to surgery with lower incidence of complications and higher quality of life [28,29,30]. Although studies showed favorable therapeutic responses, the applications still remained controversial because of the small sample size and short-term follow-up periods. The largest population of PTMC patients who underwent ablation was 185; however, the follow-up time was only 20.7 months [18]. Our previous study reported the longest follow-up period over 5 years, but the sample size was only 94 [28]. To our knowledge, no study has investigated the long-term efficacy and safety of RFA for low-risk PTMC in a large cohort.
Therefore, the purpose of this study was to evaluate the efficacy and safety of RFA for low-risk PTMC with a large cohort and a long-term follow-up period.
Materials and methods
This retrospective study was approved by the Institutional Review Board of Chinese PLA General Hospital. Written information consent was obtained from all the patients prior to RFA.
Patients
All the enrolled patients fulfilled the following criteria: (1) patients with PTC were confirmed by core-needle biopsy (CNB); (2) a single tumor with maximum diameter no larger than 10 mm; (3) absence of capsular infiltration and extrathyroidal invasion on US;(4) no lymph node metastasis on US; (5) no distant metastasis on chest CT; (6) patients who were unsuitable for surgery or rejected surgical treatment clearly; (7) no history of neck irradiation; and (8) follow-up time was larger than 24 months. The exclusion criteria were (1) patients with aggressive histological PTC (e.g., tall cell, insular, columnar cell carcinoma) confirmed by CNB; (2) the maximum diameter of the tumor was more than 10 mm; (3) patients with multiple PTMC; (4) capsular infiltration and extrathyroidal invasion on US; (5) cervical lymph node metastasis or distant metastasis was found; (6) conscious disturbance or neck extension disorder that could not tolerate RFA procedure; (7) coagulation disorder or serious heart failure/respiratory failure/liver failure/renal failure; (8) contralateral vocal cord paralysis; (8) follow-up time less than 24 months.
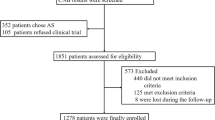
From June 2014 to December 2017, there were 651 patients who underwent RFA for low-risk PTMC in this institution. Among them, patients with multiple lesions (N = 139) and follow-up time less than 24 months (N = 98) were excluded. Finally, a total of 414 patients (323 females, 91 males, mean age 43.56 ± 9.79 years, range 18–73 years) with unifocal low-risk PTMC were enrolled in this study. The flowchart of patient enrollment is shown in Fig. 1.
Pre-ablation evaluation
Before RFA, each tumor underwent US to evaluate the size, location, margin, shape, echogenicity, calcification, and vascularity. The volume of the tumor was calculated with the following equations: V = πabc/6 (V is the volume, while a is the largest diameter, b and c are the other two perpendicular diameters).
US was performed using a Siemens Acuson Sequoia 512 Ultrasound System (Siemens, Mountain View) or a Philips iU22 Ultrasound System (Philips Healthcare) or a Mindray M9 Ultrasound System (Mindray). CNB and RFA were all performed using a Siemens Acuson Sequoia 512 Ultrasound System. Contrast-enhanced ultrasound (CEUS) was used to evaluate the blood supply region of the tumor before and immediately after RFA. Sulfur hexafluoride (SonoVueR) was used as ultrasound contrast agent. CEUS was performed after bolus injection of SonoVue (2.4 ml), followed by 5 ml of normal saline flush. Real-time microbubble perfusions within the tumor and surrounding tissues were observed for a minimum of 2 min.
Ablation procedures
All RFA procedures were performed by an experienced US physician (YKL, with more than 20 years’ experience in thyroid US and interventional US). A bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18-gauge bipolar RF applicator with 0.9 cm active tip were used (CelonProSurge micro 100-T09, Olympus Surgical Technologies Europe) in this study. Patients lay on an operating table in the supine position with the neck extended. Local anesthesia with 1% lidocaine was administered. If the distance between the tumor and critical cervical structures (trachea, carotid artery, jugular vein, esophagus, and recurrent laryngeal nerve) was less than 5 mm, hydrodissection technique was used. Normal saline was injected using another needle (23 gauge) to separate the target tumor from critical structures in order to prevent thermal injury. RFA was performed using the trans-isthmic approach and moving-shot technique. The RFA power was 3 W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the radiofrequency power was increased to 5–9 W. We enlarged the ablation area which exceeded the tumor edge (at least 3–5 mm) to prevent marginal residue and recurrence [21, 22]. CEUS was performed immediately after the RFA procedure to evaluate the ablation area. If any enhancement existed, a complementary ablation could be performed.
During the procedure, special attention was given to the protection of critical cervical structures in order to prevent significant complications such as hematoma or nerve injury. Each patient was observed for 1–2 h in the hospital while any complication occurring during and immediately after ablation was carefully evaluated according to the clinical signs and symptoms.
Post-ablation assessment
Clinical follow-ups were performed at 1, 3, 6, and 12 months and every 6–12 months thereafter. The ablation areas were evaluated by US, CEUS, chest CT (once a year), and clinical evaluation. The development of metastatic LNs (enlargement, loss of the fatty hilum, a rounded rather than oval shape, hyperechogenicity, cystic change, calcifications, and peripheral vascularity [5]) and the suspicious new lesions were submitted to biopsy. The volume reduction rate (VRR) was calculated as follows: VRR = ([initial volume − final volume] × 100)/initial volume.
We considered RFA to be successful if one of the following criteria was met: (1) the ablation area of low-risk PTMC completely disappeared on US; (2) the ablation area remained scar-like on US but there was absence of enhancement on both arterial and venous phases on CEUS; and (3) if the ablation area did not disappear, negative result was confirmed by CNB, which was performed at the central zone, the peripheral zone, and the surrounding thyroid parenchyma at 3 or 6 months after RFA.
Local tumor progression was defined to include three situations [28]: (1) persistent detected lesions confirmed to be PTMC by CNB; (2) new recurrent lesions confirmed to be PTMC by CNB; and (3) cervical LNM confirmed by biopsy. Distant metastasis was detected by CT, positron emission tomography, or bone scan if there were suspicious symptoms.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software (Version 25.0). Continuous data were expressed as mean ± SD (range). Wilcoxon signed rank tests were used to compare the mean volume before RFA and at each follow-up point after RFA. A difference with p < 0.05 was considered as statistically significant.
Results
Clinical characteristics of patients are presented in Table 1. A total of 414 patients (323 females, 91 males) with 414 tumors were enrolled in this study. The mean age was 43.56 ± 9.79 years (range 18–73 years). The mean initial volume was 92.74 ± 83.43 mm3 (range 4.19–490.07 mm3). The mean follow-up time was 42.15 ± 11.88 months (range 24–69 months).
The power used was as follows: 3 W for 204 patients, 4 W for 158 patients, 6 W for 15 patients, and 7 W for 10 patients, respectively. The mean power was 3.71 ± 0.91 W (range 3–7 W). The mean RFA time was 217.29 ± 117.53 s (range 35–766 s), and the mean energy was 803.84 ± 489.30 J (range 150–4520 J).
Efficacy
The mean volume and VRR at each follow-up point after RFA are shown in Table 2. The changes of volume and VRR at each follow-up point are shown in Figs. 2 and 3. Due to the enlarged ablation, the mean volume of the ablation areas at 1 and 3 months was significantly larger than the initial volume (p < 0.001). However, 3 months after RFA, the mean volume was gradually decreased. At the last follow-up point, the mean volume was 1.37 ± 7.94 mm3 (range 0–67.97 mm3) and the mean VRR was 98.81 ± 6.41% (range 50.00–100%). A total of 366 tumors (88.41%) completely disappeared. The numbers of complete disappearance were 21 (5.07%), 77 (18.60%), 158 (38.16%), 45 (10.87%), 54 (13.04%), and 11 (2.66%) at 3, 6, 12, 18, 24, and 36 months after RFA, respectively. A representative case of RFA treatment and follow-up is shown in Fig. 4.
US image of a 31-year-old female with a low-risk PTMC. a Before RFA, US image showed a tumor (arrow) located in the right thyroid lobe with an initial volume of 131.94 mm3. b Immediately after RFA, the volume of the ablation area (arrow) was 1231.47 mm3 on CEUS. c, d At 1 month after RFA, the volume of the ablation area (arrow) was 593.74 mm3. e, f At 3 months after RFA, the volume of the ablation area (arrow) was 146.60 mm3. g, h At 6 months after RFA, the volume of the ablation area (arrow) was 52.36 mm3. i, j At 12 months after RFA, the ablation area could not be identified on the longitudinal US image. There was only a focal concavity in the capsule caused by shrinkage of the scar (arrow) on the transverse US image. k, l At 18 months after RFA, the ablation area completely disappeared
Local tumor progression
The clinical characteristics and outcomes of tumors with local tumor progression are summarized in Table 3. The overall rate of local tumor progression was 3.62% (15/414). One ablation area was found positive by CNB in the peripheral zone and diagnosed to have residual cancer (Fig. 5). Before RFA, abundant vascularity was shown in the peripheral zone of this tumor. During the follow-up, the volume was decreased but vascularity was still shown in the peripheral zone and appeared inside the ablation area. Therefore, CNB was performed at 6 months after RFA for evaluation and the result was positive in the peripheral zone. This patient underwent additional RFA. CNB was performed at 6 months after additional RFA and the result was negative.
US image of a patient with the residual cancer diagnosed by CNB after RFA. a Before RFA, the US image showed a tumor (arrow) located in the right thyroid lobe with an initial volume of 175.92 mm3. b There was abundant vascularity in the peripheral zone of the tumor on CDFI. c, d At 1 month after RFA, the volume of the ablation area (arrow) was 560.76 mm3. Vascularity was shown in the peripheral zone of the ablation area on CDFI. e, f At 3 months after RFA, the volume of the ablation area (arrow) was 167.55 mm3. Vascularity was shown in the peripheral zone and was also found inside the ablation area on CDFI. g, h At 6 months after RFA, the volume of the ablation area (arrow) was 109.95 mm3. Vascularity appeared both in the peripheral zone and inside the ablation area on CDFI. CNB was performed and the result was positive in the peripheral zone. The ablation area was diagnosed to have residual cancer and underwent additional RFA
Four patients (0.97%) developed metastatic LN after RFA, and three of them were in the ipsilateral neck. The mean volume was 131.81 ± 44.37 mm3 (range 70.68–175.92 mm3). Among them, 3 metastatic LNs were located in lateral compartments and one in central compartments. The mean time of metastatic LN development was 19.50 ± 5.74 months (range 12–24 months). The time-point of metastatic LN development was 2 at 12 months and 2 at 2 years after RFA, respectively. All the metastatic LNs underwent additional RFA and completely disappeared during the follow-up. A total of 10 patients (2.42%) had recurrent PTMC after RFA. The mean volume was 54.57 ± 76.09 mm3 (range 9.43–263.89 mm3). The mean time of recurrent PTMC development was 27.60 ± 12.71 months (range 6–48 months). The time-point of recurrent PTMC development was as follows: 1 at 6 months, 2 at 1 years, 3 at 2 years, 3 at 3 years, and 1 at 4 years after RFA. Among them, 7 were in the contralateral lobe and 3 in the ipsilateral lobe. One patient chose active surveillance and the volume was stable during 1-year follow-up. Nine patients underwent additional RFA. All the lesions were successfully treated, and 7 of them disappeared during the follow-up. No distant metastasis was detected.
Safety
All patients were tolerable to the RFA procedure. Only 16 patients underwent local pain and discomfort which resolved spontaneously within 2 or 3 days. None of the patients experienced any life-threatening or delayed complications related to RFA during the follow-up.
Discussion
This large cohort study demonstrated the long-term effectiveness and safety of RFA for low-risk PTMC. During a mean follow-up time of 42.15 ± 11.88 months, the mean VRR was 98.81 ± 6.41% and 88.41% of the tumors completely disappeared. The overall rate of local tumor progression was 3.62%, and most of them were successfully treated by additional RFA. No life-threatening complications or sequelae occurred after RFA. These results demonstrated that RFA was a safe and effective alternative for low-risk PTMC during a long-term follow-up.
For the last few years, studies from multiple centers indicated RFA, as well as MWA and LA, were effective and safe alternatives for low-risk PTMC patients who were anxious about living with cancer or post-operative complications [17,18,19,20,21,22,23,24,25,26,27]. RFA has also been considered as an alternative for patients with PTMC who refused surgery or were ineligible for surgery by guideline [14]. After ablation, the volume reduction was significant, and some ablation areas even disappeared during the follow-up. According to recent meta-analyses, the pooled proportion of VRR after ablation was 98.1% [31] and the pooled proportion of complete disappearance was 57.6–76.2% [31, 32]. Although studies showed favorable therapeutic responses, there was a lack of effectiveness data with the large cohort and long-term follow-up periods to evaluate the role of RFA for low-risk PTMC. Our previous study about RFA for low-risk PTMC reported the longest follow-up period of over 5 years, but the sample size was only 94 [28]. The study with the largest population that underwent ablation was reported by Teng et al [18], which enrolled 185 PTMC patients for MWA. The VRR was 98.65% and the complete disappearance rate was 84.5%. However, the follow-up time was only 20.7 months. In this study, a total of 414 patients underwent RFA with a long-term follow-up time of 42.15 ± 11.88 months, which was the largest cohort of patients with low-risk PTMC treated by RFA to date. In this study, the VRR was 98.81 ± 6.41% and 88.41% of tumors completely disappeared. These results were consistent with above-mentioned studies, which indicated the efficacy of RFA for low-risk PTMC with a large sample size was satisfactory and sustainable after a long-term follow-up.
In this study, all the patients were tolerant to the RFA procedure and no major complications occurred. Only 16 patients underwent local pain and discomfort which resolved spontaneously within 2 or 3 days. According to recent meta-analysis, the pooled proportion of overall and major complications after ablation was 3.2% and 0.7%, respectively [31]. The pooled proportions of complications after RFA were only 1.7% [32]. Several reasons were associated with the low complications. First, during the RFA procedure, real-time US imaging could allow the physician to ablate the targeted tumor accurately and monitor the critical structure carefully. Second, the moving-shot technique, trans-isthmic approach, and hydrodissection technique were used during the RFA procedure, which have been recommended as safe techniques for preventing thermal injury [14]. Third, the RFA procedure was performed by an experienced US physician, which was also an important factor to minimize the incidence of complication [14].
The rate of recurrence and metastasis after RFA for low-risk PTMC were low. Previous studies reported that the incidence of LNM after ablation was 0.6–3.1% [19, 22, 29, 32], and the incidence of recurrent PTMC was 0.5–2.78% [18, 29, 32, 33]. A similar result was observed in this study; the incidence of LNM and recurrent PTMC was 0.97% and 2.42%, respectively. Previous comparative studies indicated that there were no statistically significant differences between the thermal ablation group and surgical group for recurrent PTMC and LNM [28, 29, 33]. Furthermore, 70% recurrent PTMC in this study were found in the contralateral lobe and could not be prevented by thyroid lobectomy which was recommended for low-risk PTMC. There were several treatment managements for the recurrent tumor. Surgery was the traditional treatment, but it was hard for patients who refused surgery to accept. RFA and other thermal ablation techniques have been recommended as a safe and effective alternative to surgery for recurrent tumor by guidelines [5, 14]. Chung et al [34] showed a longer-term efficacy of RFA for locally recurrent PTMC, with a mean tumor VRR of 99.5% and the complete disappearance of 91.3% of treated tumors. In this study, most patients underwent additional RFA and the recurrent tumors were successfully and safely treated.
This study indicated the existence of persistent disease following ablation treatment was possible. Recently, Ma et al [35] reported surgical confirmation of residual tumor in one PTMC patient after previous ablation. In this study, one ablation area was diagnosed to have residual tumor by CNB. The reason might be related to the vascularity. Studies indicated that the blood vessels could act as a heat sink and dissipate the hyperthermia which decreased the efficacy of RFA [13, 36, 37]. Because of the influence of peripheral vascularity, the positive CNB was found in the peripheral zone in this study. Similarly, studies found that vascularity was also a factor related to the regrowth of benign nodules [38, 39]. However, unlike benign nodules, the primary purpose of RFA for low-risk PTMC was not to improve the symptom or cosmetic problem but to obtain complete ablation. Therefore, caution should be taken in the patient enrollment and pre-ablation evaluation. In addition, during the RFA procedure, the tumor vasculature should be totally damaged. Park et al [40] introduced vascular ablation techniques to solve this problem, which were artery-first ablation technique and the marginal venous ablation technique. These two techniques could ablate the tumor-feeding artery and draining veins along the peripheral zone to decrease the heat-sink effect during the procedure and lead to complete ablation.
There were some limitations in this study. First, it was a single-center retrospective study. Further prospective multicenter studies are needed. Second, the sensitivity of US to detect central metastatic LN and multifocality was low, and their presences could not be completely excluded although for low-risk PTMC, the effect of hidden metastasis and occult lesions on overall survival was small [5]. Third, we only evaluated the efficacy and safety of RFA for patients with unifocal low-risk PTMC. Although some studies enrolled patients with multifocality PTMC, the sample size was too small to draw a conclusion. Further studies about the efficacy and safety of RFA in multifocality PTMC are needed.
In conclusion, RFA is an effective and safe treatment for low-risk PTMC after a long-term follow-up period for a large cohort with careful patient enrollment evaluation.
Abbreviations
- CEUS:
-
Contrast-enhanced ultrasound
- CNB:
-
Core-needle biopsy
- LA:
-
Laser ablation
- MWA:
-
Microwave ablation
- PTMC:
-
Papillary thyroid microcarcinoma
- RFA:
-
Radiofrequency ablation
- US:
-
Ultrasound
- VRR:
-
Volume reduction rate
References
Chmielik E, Rusinek D, Oczko-Wojciechowska M et al (2018) Heterogeneity of thyroid cancer. Pathobiology 85:117–129
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
La Vecchia C, Malvezzi M, Bosetti C et al (2015) Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136:2187–2195
Miyauchi A, Ito Y, Oda H (2017) Insights into the management of papillary microcarcinoma of the thyroid. Thyroid 28:23–31
Haugen BR, Alexander EK, Bible KC et al (2015) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133
Zanocco KA, Hershman JM, Leung AM (2019) Active surveillance of low-risk thyroid cancer. JAMA 321:2020–2021
Ito Y, Uruno T, Nakano K et al (2003) An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 13:381–387
Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y (2010) Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg 34:1222–1231
Ito Y, Miyauchi A, Inoue H et al (2010) An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35
Nickel B, Brito JP, Barratt A, Jordan S, Moynihan R, McCaffery K (2017) Clinicians’ views on management and terminology for papillary thyroid microcarcinoma: a qualitative study. Thyroid 27:661–671
Oh HS, Kwon H, Song E et al (2019) Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid 29:642–649
Oh HS, Ha J, Kim HI et al (2018) Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid 28:1587–1594
Chu KF, Dupuy DE (2014) Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14:199
Kim JH, Baek JH, Lim HK et al (2018) 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol 19:632–655
Lang BHH, Woo YC, Chiu KW (2019) Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol 29:93–101
Korkusuz Y, Gröner D, Raczynski N et al (2018) Thermal ablation of thyroid nodules: are radiofrequency ablation, microwave ablation and high intensity focused ultrasound equally safe and effective methods? Eur Radiol 28:929–935
Zhang M, Luo Y, Zhang Y, Tang J (2016) Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid 26:1581–1587
Teng DK, Li HQ, Sui GQ et al (2019) Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine 64:109–117
Ji L, Wu Q, Gu J et al (2019) Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging 19:16
Ding M, Tang X, Cui D et al (2019) Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol 74:712–717
Chen J, Cao J, Qiu F, Huang P (2019) The efficacy and the safety of ultrasound-guided ablation therapy for treating papillary thyroid microcarcinoma. J Cancer 10:5272–5282
Zhang L, Zhou W, Zhan W, Peng Y, Jiang S, Xu S (2018) Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg 42:2476–2484
Teng D, Sui G, Liu C, Wang Y, Xia Y, Wang H (2018) Long-term efficacy of ultrasound-guided low power microwave ablation for the treatment of primary papillary thyroid microcarcinoma: a 3-year follow-up study. J Cancer Res Clin Oncol 144:771–779
Zhou W, Jiang S, Zhan W, Zhou J, Xu S, Zhang L (2017) Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol 27:2934–2940
Yue W, Wang S, Yu S, Wang B (2014) Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 30:150–157
Lim HK, Cho SJ, Baek JH et al (2019) US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol 20:1653–1661
Zhang Y, Zhang MB, Luo YK, Li J, Zhang Y, Tang J (2019) Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med 00:1–9
Zhang M, Tufano RP, Russell J et al (2020) Ultrasound-guided radiofrequency ablation versus surgery for low risk papillary thyroid micro-carcinoma: results of over 5 years follow-up. Thyroid 30:408–417
Li J, Liu Y, Liu J, Yang P, Hu X, Qian L (2019) A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperthermia 36:640–646
Li J, Liu Y, Liu J, Qian L (2018) Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperthermia 34:653–659
Choi Y, Jung SL (2020) Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid 30:720–731
Tong M, Li S, Li Y, Li Y, Feng Y, Che Y (2019) Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperthermia 36:1278–1286
Zhou W, Ni XF, Xu SY, Zhang L, Chen YD, Zhan WW (2019) Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperthermia 36:897–904
Chung SR, Baek JH, Choi YJ, Lee JH (2019) Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol 29:4897–4903
Ma B, Wei W, Xu W et al (2018) Surgical confirmation of incomplete treatment for primary papillary thyroid carcinoma by percutaneous thermal ablation: a retrospective case review and literature review. Thyroid 28:1134–1142
Shin JH, Baek JH, Ha EJ, Lee JH (2012) Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012:919650–919650
Ahmed M, Brace CL, Lee FT, Goldberg SN (2011) Principles of and advances in percutaneous ablation. Radiology 258:351–369
Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH (2013) Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 23:1044–1049
Sim JS, Baek JH, Lee J, Cho W, Jung SI (2017) Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia 33:905–910
Park HS, Baek JH, Park AW, Chung SR, Choi YJ, Lee JH (2017) Thyroid radiofrequency ablation: updates on innovative devices and techniques. Korean J Radiol 18:615–623
Funding
This study has received funding from the National Natural Science Foundation of China (No. 81771834).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yukun Luo.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, L., Lan, Y., Xiao, J. et al. Long-term outcomes of radiofrequency ablation for unifocal low-risk papillary thyroid microcarcinoma: a large cohort study of 414 patients. Eur Radiol 31, 685–694 (2021). https://doi.org/10.1007/s00330-020-07128-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07128-6