Abstract
Objectives
The purpose of this study was to evaluate the longer-term efficacy of ultrasound (US)-guided radiofrequency ablation (RFA) for treating locally recurrent papillary thyroid cancer (PTC).
Methods
We retrospectively reviewed 29 patients who underwent RFA for 46 recurrent PTC between September 2008 and April 2012 and were subsequently followed up for at least 5 years. Follow-up included size change on US and thyroglobulin (Tg) level at 1, 3, 6, and 12 months and every 6–12 months thereafter. Any complications were reported during follow-up.
Results
The mean follow-up duration after RFA was 80 ± 17.3 months (range, 60–114 months). Tumor volume decreased significantly, from 0.25 ± 0.42 mL before ablation to 0.01 ± 0.08 mL at the final evaluation (p < 0.001), with a mean volume reduction of 99.5% ± 2.9%. Forty-two of the 46 treated tumors (91.3%) had completely disappeared by the final evaluation. The mean serum Tg level decreased from 2.55 ± 4.7 to 0.75 ± 1.83 ng/dL (p < 0.001). There were no delayed complications associated with RFA during the follow-up period.
Conclusions
RFA seems to be an effective minimally invasive therapy for the treatment of locally recurrent PTC even in the longer-term period.
Key Points
• RFA is an effective local control treatment option for recurrent PTCs even in the longer-term period with mean tumor VRR of 99.5% and the complete disappearance of the treated tumors in 91.3%.
• The mean serum Tg level decreased significantly after RFA and biochemical remission rate was 51.7%.
• No delayed complication after RFA for local recurrent PTC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid cancer (PTC) is the most common subtype of thyroid cancer, representing approximately 80% of all thyroid cancers [1]. Although the prognosis of patients with PTC is generally good [1], up to 13% of localized PTCs completely excised during initial surgery were found to recur over 40 postoperative years [2]. The standard treatment for recurrent tumors consists of repeat operation, followed by treatment with radioactive iodine and thyroid hormone. However, repeated neck operations are usually challenging due to the distortions of normal tissue planes and fibrosis caused by scar tissue formation in the surgical bed, thus increasing the risk of complications [3]. Additionally, small recurrent lesions may be difficult to detect without ultrasound (US) examination [3].
Nonsurgical localized therapies, including thermal ablation [4,5,6,7,8] and ethanol ablation (EA) [9,10,11], may be an effective alternative to repeat operation in patients with recurrent PTC. Recent guidelines recommend these localized treatments for patients with a single or a few metastases or with metastases at high risk of local complications [12, 13]. A meta-analysis of the efficacy and safety of radiofrequency ablation (RFA) for treating locally recurrent thyroid cancer shows that these treatments have therapeutic success rates (volume reduction rate [VRR] > 50%) of 89.5–100% [14], with 68.8% of these lesions completely disappearing after RFA. However, this meta-analysis was limited by the relatively short-term follow-up periods of the included studies. To our knowledge, no study to date has investigated the longer-term efficacy of RFA in patients with locally recurrent PTC. These findings suggested the need to evaluate the longer-term efficacy of RFA to verify the completeness of treatment. Previously, our institution reported the outcome of RFA for locally recurrent PTC after a mean follow-up period of 26.4 months [15]. The purpose of this study was to evaluate the longer-term efficacy of US-guided RFA for treating locally recurrent PTC.
Materials and methods
The retrospective study protocol was approved by our Institutional Review Board for human investigation, and written informed consent was obtained from all patients prior to US-guided fine-needle aspiration biopsy (FNAB) and RFA.
This data were retrospectively collected from the cohort of our previous short-term follow-up study (mean, 26.4 months) for treating recurrent PTC in the neck using RFA between September 2008 and April 2012 [15]. Previous study included 61 recurrent PTCs of 39 patients. This retrospective study finally included 29 patients who underwent RFA for 46 recurrent PTCs and were followed up for at least 5 years. Two of 10 patients who lost to follow-up died from lung cancer and pneumonia, respectively, and another eight patients were followed up at another hospital nearby their home. All patients undergone total thyroidectomy and central compartment node dissection, and 20 had also undergone lateral neck dissection. All patients received postoperative radioiodine therapy and supplemental levothyroxine thyrotropin-suppressing therapy. All RFA procedures were performed according to the request of physician for three reasons: (1) radioiodine refractory PTC (n = 17), (2) history of repeat operation more than two times (n = 11), and (3) high risk for general anesthesia (n = 1).
All patients were evaluated by US examination, US-guided FNAB of the recurrent tumors, contrast-enhanced neck CT, and laboratory tests before RFA. The presence of a recurrent tumor was confirmed by cytological smears and/or by a finding of increased thyroglobulin (Tg) levels in FNAB samples. Tumor diameters prior to RFA were measured by US examination, and the volume (V) of each tumor was calculated as V = πabc / 6, where a is the largest diameter and b and c are the two perpendicular diameters [16].
US-guided RFA was performed by one of two radiologists with 11 and 16 years of experience, respectively. We used a radiofrequency generator (Cool-Tip RF system, Radionics; SSP-2000, Taewoong Medical; VIVA RF system, STARmed) and an 18- or 19-gauge internally cooled electrode with 0.5-, 0.7-, and 1-cm active tips (Cool-Tip, Radionics; Well-point RF electrode, STARmed; VIVA, STARmed), depending on the size of the targeted tumor. Moving-shot and hydrodissection techniques were used, and the surrounding normal tissue was also ablated to prevent marginal recurrence [15, 17]. Ablation was started with 10 W of power in a 0.5-cm active tip, 15 W in a 0.7-cm active tip, and 30 W in a 1-cm active tip. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the radiofrequency power was increased in 5- to 10-W increments up to 50 W. The ablation was terminated when all portions of the treated tumor had changed to transient hyperechoic zones.
The patients were followed up for at least 5 years, by US and serum Tg level at 1, 3, 6, and 12 months and every 6–12 months thereafter. US examinations were performed by the two radiologists who had performed RFA. The percentage reduction in volume was calculated as VRR = ([initial volume − final volume] × 100) / initial volume [18].
All patients underwent contrast-enhanced computed tomography (CT) scans after ablation. Additional RFA was performed if the 1-month follow-up US showed the presence of power Doppler signals, suggesting that a remnant of the treated nodule was viable, or if immediate follow-up CT detected an enhanced portion of the treated tumor.
All data were analyzed using SPSS 23 for Microsoft Windows version 23.0 statistical software (SPSS). The Wilcoxon signed-rank test was used to compare the largest tumor diameter, tumor volume, and serum Tg levels before RFA and at final follow-up. Differences were considered statistically significant when the p value was < 0.05.
Results
Table 1 shows the baseline characteristics of the 29 patients, which included 12 men and 17 women, mean age 51.8 years (range, 21–84 years). Of the 46 recurrent tumors, 23 (50%) were occurred in the thyroid operation bed or neck level 6 and 23 (50%) were occurred in the lateral neck lymph node. The mean number of treatment sessions for each tumor was 1.1 ± 0.4 (range, 1–3), and the mean follow-up duration after RFA was 80 ± 17.3 months (range, 60–114 months).
Tumor volume, largest tumor diameter, and serum Tg concentrations observed at each follow-up evaluation are shown in Figs. 1 and 2. The volume of the recurrent tumors decreased significantly, from 0.25 ± 0.42 mL before ablation to 0.01 ± 0.08 mL (p < 0.001) at final evaluation, with a mean tumor VRR of 99.5% ± 2.9% (range, 81%–100%). The mean serum Tg level also decreased significantly, from 2.55 ± 4.7 ng/dL before ablation to 0.75 ± 1.83 ng/dL (p < 0.001) at final follow-up, and was below the level of detection (0.08 ng/dL) in 15 (51.7%) of the 29 patients at the last follow-up.
The 35 tumors that had completely disappeared in our previous study [15] did not recur after longer-term follow-up. Previous study reported 11 incompletely disappeared lesions on US images in short-term follow-up [15]. We summarized the longer-term results of these 11 lesions in Table 2. Of the 11 such lesions described in our previous study, 10 were followed up for at least 5 years. In addition, one lesion, which had been missed previously, was added (no. 11). Of these 11 recurrent tumors, seven completely disappeared after RFA (Fig. 3); however, four were still visible on US and/or CT. Overall, of the 46 tumors, 42 (91.3%) completely disappeared. Of the remaining four tumors, one presented as a macrocalcification on US examination and CT, with no change in size for 110 months and absence of malignant cells on FNAB; one gradually increased in size on follow-up CT despite three sessions of RFA and was confirmed as an anaplastic carcinoma after incisional biopsy; one was located too deep in the paratracheal area for complete RFA and was resected 4 months after RFA; and one showed a residual enhanced lesion adherent to trachea on follow-up CT. Of the 29 patients, 8 had new locoregional recurrent lesions, two had distant metastatic lesions, and the other 19 showed no evidence of diseases during follow-up. None of these patients experienced any delayed complications related to RFA during a mean follow-up period of 80 months.
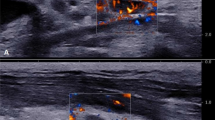
A 48-year-old female with recurrent papillary thyroid cancer at left level 6 after thyroidectomy and RI therapy. Transverse ultrasound image shows a 1.8-cm-sized hypoechoic mass at left level 6 (arrows) (a). An internally cooled electrode with a 1-cm-sized active tip is inserted into the recurrent tumor (b). After completion of RFA, the ablated zone is larger than the initial tumor size (c). 18 months after RFA, the transverse and longitudinal US scans show the treated tumor is decreased in size (d, e). 84 months after RFA, the treated tumor is not observed on US (f). RI radioactive iodine, RFA radiofrequency ablation, US ultrasound
Discussion
This study shows the longer-term effectiveness of US-guided RFA for controlling locally recurrent PTC. The efficacy of RFA was indicated by the 99.5% VRR, the complete disappearance of 91.3% of the treated tumors, and the significantly decreased serum Tg concentrations. Moreover, RFA shows no delayed complications during a mean follow-up period of 80 months. These results suggest that RFA is an acceptable nonsurgical treatment option for local control of recurrent PTCs.
Previous studies report the short-term effectiveness of RFA in the treatment of recurrent thyroid cancer [4,5,6,7,8, 15, 19]. To our knowledge, however, our study is the first to present longer-term (> 5 years) follow-up data. Previous shorter-term RFA studies reported acceptable local tumor control effect with a mean tumor VRR of 50.9–96.4% and the complete disappearance of 25–94% of treated tumors [5,6,7,8,9, 15]. Our longer-term study revealed similar efficacy and safety. RFA induces coagulation necrosis, hyaline sclerosis, and scarring within the tumor [20]. This process is slow, with a long period of time required for the tumor to decrease in size. Of the 29 patients in this study, four had residual lesions after RFA. Residual tumors are induced by incomplete treatment because of anaplastic transformation or deep location of tumor. In addition, mean serum Tg level decreased abruptly at 1 month after RFA, and the level remained low until the last follow-up. Recent meta-analysis and current RFA guideline revealed that the serum Tg decreased after RFA [14, 18]. The meta-analysis revealed that the pooled proportion of reduction in serum level of Tg after RFA was 71.6% ([CI 63.5–79.7%]; I2 = 0.0%) [14]. Furthermore, previous studies revealed various early complications of RFA [15] for recurrent cancers. In our study population, the early complication rate was reported in three patients. All three complications were voice change which had recovered in all patients within 2 months after RFA. Four patient complained self-limiting local pain during ablation. During longer-term follow-up period, no patient experienced delayed complications. A recent meta-analysis compared the efficacy of EA and RFA for recurrent thyroid cancers [14]. That study reported that RFA was more effective, with a higher tumor VRR ≥ 50%, a higher rate of complete lesion disappearance after treatment, and a lower rate of local tumor recurrence. Moreover, RFA required fewer treatment sessions than EA. However, the complication rate was lower for EA than for RFA. These differences may be due to differences in treatment areas, with RFA treating a larger area, including the tumor and surrounding normal soft tissue [14, 15]. When we compared our results with the long-term effects of EA for recurrent PTC [21], we found that the rate of tumor size increase (17.1% vs. 2.2%) and the mean number of ablations (2.7 vs. 1.1) were higher after EA than after RFA, suggesting that RFA is more effective than EA for local control of recurrent PTC.
Repeat operation is undoubtedly a definitive curative treatment for recurrent tumors. Current guidelines recommend evaluation of lesions > 0.8 cm because of the indolent nature of thyroid cancer, the technical difficulties of repeat operation, and the risks of general anesthesia [10, 12, 22, 23]. Although repeat operation may be delayed [5, 24], untreated tumors may suddenly transformed to aggressive subtype [25]. By contrast, RFA can be performed in outpatient clinics without general anesthesia and can be applied relatively easily, repeatedly, and safely. The reported rates of permanent recurrent laryngeal nerve paralysis and hypocalcemia after repeat surgery range from 0 to 12% and from 0 to 7%, respectively [8, 26, 27]. By comparison, the incidence of voice change after RFA for recurrent thyroid cancers was reported to be 7.95%, but most of these were transient, and there have been no reports of hypoparathyroidism [28]. The lower incidence of complications with RFA might be associated with the ability of high-resolution US imaging to target the tumor exactly, knowledge of US-based nerve anatomy [29], thyroid-dedicated small active tips (0.38 and 0.5 cm) [21], and the use of hydrodissection techniques [21, 30].
Concerns associated with RFA include recurrent tumors that may not be found by imaging modalities and are only detected in pathologic specimens after surgical dissection; however, the clinical significance of these undetected tumors remains unknown [8]. In our study, eight patients had new locoregional recurrent lesions and two had distant metastatic lesions during follow-up among 29 patients. Furthermore, less than 50% of patients showed decreased but still elevated serum Tg level after treatment. Thus, RFA is effective to the local control of ablated metastatic lymph node, but has a limited role in controlling the disease and its recurrences at other regions.
This study had several limitations. First, it is a retrospective design and included only patients followed for > 5 years, which may have resulted in a selection bias. However, because this study included 11 of the 12 patients with residual lesions in our previous study [15], the efficacy of treatment was likely not overestimated due to selection bias. Second, this study was based on data from a single, specialist center; different results may occur in studies of patients treated at community hospitals with less expertise.
In conclusion, this study demonstrated the longer-term efficacy of RFA for locally recurrent PTC, with a mean tumor VRR of 99.5% and the complete disappearance of 91.3% of treated tumors. These results suggest that RFA seems to be an effective minimally invasive therapy for the treatment of locally recurrent PTC even in the longer-term period.
Abbreviations
- CT:
-
Computed tomography
- EA:
-
Ethanol ablation
- FNAB:
-
Fine-needle aspiration biopsy
- PTC:
-
Papillary thyroid carcinoma
- RFA:
-
Radiofrequency ablation
- Tg:
-
Thyroglobulin
- US:
-
Ultrasound
- VRR:
-
Volume reduction rate
References
Siegel R, DeSantis C, Virgo K et al (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241
Hay ID, Thompson GB, Grant CS et al (2002) Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg 26:879–885
Samaan NA, Schultz PN, Hickey RC et al (1992) The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab 75:714–720
Baek JH, Kim YS, Sung JY, Choi H, Lee JH (2011) Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. AJR Am J Roentgenol 197:W331–W336
Park KW, Shin JH, Han BK, Ko EY, Chung JH (2011) Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 18:2564–2568
Lee SJ, Jung SL, Kim BS et al (2014) Radiofrequency ablation to treat loco-regional recurrence of well-differentiated thyroid carcinoma. Korean J Radiol 15:817–826
Wang L, Ge M, Xu D et al (2014) Ultrasonography-guided percutaneous radiofrequency ablation for cervical lymph node metastasis from thyroid carcinoma. J Cancer Res Ther 10(Suppl):C144–C149
Kim JH, Yoo WS, Park YJ et al (2015) Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology 276:909–918
Kim BM, Kim MJ, Kim EK, Park SI, Park CS, Chung WY (2008) Controlling recurrent papillary thyroid carcinoma in the neck by ultrasonography-guided percutaneous ethanol injection. Eur Radiol 18:835–842
Heilo A, Sigstad E, Fagerlid KH et al (2011) Efficacy of ultrasound-guided percutaneous ethanol injection treatment in patients with a limited number of metastatic cervical lymph nodes from papillary thyroid carcinoma. J Clin Endocrinol Metab 96:2750–2755
Hay ID, Lee RA, Davidge-Pitts C, Reading CC, Charboneau JW (2013) Long-term outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery 154:1448–1454 discussion 1454-1445
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133
Yi KH, Lee EK, Kang HC et al (2016) 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol 9:59–126
Suh CH, Baek JH, Choi YJ, Lee JH (2016) Efficacy and safety of radiofrequency and ethanol ablation for treating locally recurrent thyroid cancer: a systematic review and meta-analysis. Thyroid 26:420–428
Lim HK, Baek JH, Lee JH et al (2015) Efficacy and safety of radiofrequency ablation for treating locoregional recurrence from papillary thyroid cancer. Eur Radiol 25:163–170
Jeong WK, Baek JH, Rhim H et al (2008) Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–1250
Park HS, Baek JH, Choi YJ, Lee JH (2017) Innovative techniques for image-guided ablation of benign thyroid nodules: combined ethanol and radiofrequency ablation. Korean J Radiol 18:461–469
Kim JH, Baek JH, Lim HK et al (2018) 2017 thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol 19:632–655
Guenette JP, Monchik JM, Dupuy DE (2013) Image-guided ablation of postsurgical locoregional recurrence of biopsy-proven well-differentiated thyroid carcinoma. J Vasc Interv Radiol 24:672–679
Dobrinja C, Bernardi S, Fabris B et al (2015) Surgical and pathological changes after radiofrequency ablation of thyroid nodules. Int J Endocrinol 2015:576576
Kim SY, Kim SM, Chang H et al (2017) Long-term outcomes of ethanol injection therapy for locally recurrent papillary thyroid cancer. Eur Arch Otorhinolaryngol 274:3497–3501
Shaha AR (2012) Recurrent differentiated thyroid cancer. Endocr Pract 18:600–603
Forrest JB, Rehder K, Cahalan MK, Goldsmith CH (1992) Multicenter study of general anesthesia. III. Predictors of severe perioperative adverse outcomes. Anesthesiology 76:3–15
Lim CY, Yun JS, Lee J, Nam KH, Chung WY, Park CS (2007) Percutaneous ethanol injection therapy for locally recurrent papillary thyroid carcinoma. Thyroid 17:347–350
Burman KD (2012) Treatment of recurrent or persistent cervical node metastases in differentiated thyroid cancer: deceptively simple options. J Clin Endocrinol Metab 97:2623–2625
Kim MK, Mandel SH, Baloch Z et al (2004) Morbidity following central compartment reoperation for recurrent or persistent thyroid cancer. Arch Otolaryngol Head Neck Surg 130:1214–1216
Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT (2010) Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab 95:2187–2194
Chung SR, Suh CH, Baek JH, Park HS, Choi YJ, Lee JH (2017) Safety of radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: a systematic review and meta-analysis. Int J Hyperthermia 33:920–930
Ha EJ, Baek JH, Lee JH (2015) Ultrasonography-based thyroidal and perithyroidal anatomy and its clinical significance. Korean J Radiol 16:749–766
Shin JH, Baek JH, Ha EJ, Lee JH (2012) Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012:919650
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jung Hwan Baek.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
We previously reported the outcome of RFA for locally recurrent PTC after a mean follow-up period of 26.4 months, finding that RFA appears to effectively control locoregional recurrence. This study, an extension of our previous report, analyzed the outcomes of RFA after > 5 years of follow-up.
Lim HK et al. Eur Radiol. 2015;25:163–170
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, S.R., Baek, J.H., Choi, Y.J. et al. Longer-term outcomes of radiofrequency ablation for locally recurrent papillary thyroid cancer. Eur Radiol 29, 4897–4903 (2019). https://doi.org/10.1007/s00330-019-06063-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06063-5