Abstract
Objectives
Assessing the efficacy of single high-intensity focused ultrasound (HIFU) ablation in benign thyroid nodules beyond 12 months.
Methods
One hundred and eight patients underwent single HIFU treatment. Extent of nodule shrinkage [by volume reduction ratio (VRR)] and obstructive symptom score [by 0-10 visual analogue scale (VAS)] were evaluated for 24 months after treatment. VRR (%) was calculated based on the formula: [baseline volume – volume at visit] / [baseline volume] × 100. Binary logistic regression was performed to evaluate factors associated with 24-month treatment success (VRR ≥ 50%).
Results
After treatment, the mean (± SD) VRR at 3, 6, 12 ,18 and 24 months were 51.32 ± 20.71%, 62.99 ± 22.05%, 68.66 ± 18.48%, 69.76 ± 17.88% and 70.41 ± 17.39%, respectively, while the median (IQR) VAS at baseline, 6, 12 and 24 months was gradually lowered from 4.0 (2.0), 2.0 (1.0), 2.0 (1.0) to 1.0 (2.0), respectively. Sixty-three (58.3%) nodules had a further volume reduction (i.e. > 4.5%) from 12 to 24 months, while 22 (20.4%) nodules had a volume increase of > 4.5% from 12 to 24 months. Small pre-ablation nodule volume was a significant determinant for treatment success at 24 months (OR=1.045, 95% CI=1.021–1.092, p = 0.038).
Conclusions
A majority of nodules had further volume reduction beyond 12 months after single HIFU ablation, but since one-fifth of nodules had a notable volume increase beyond 12 months, a longer period of surveillance would be necessary. Small pre-ablation nodule volume was a significant factor determining 24-month treatment success.
Key Points
• Small but significant nodule shrinkage continues beyond 12 months after single treatment.
• Obstructive symptom continues to improve beyond 12 months after single treatment
• Smaller-sized nodules have a greater chance of treatment success at 24 months
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodules are common. Although most are benign and will remain relatively unchanged over time, some do cause symptoms necessitating surgical resection [1,2,3]. However, surgery is not without risks and requires a general anaesthesia and hospitalisation. As a result, there has been an increasing interest in developing less invasive, non-surgical techniques in treating benign thyroid nodules [4, 5]. For predominantly solid or wholly solid nodules, several image-guided thermal ablation techniques like laser ablation (LA), microwave and radiofrequency ablation (RFA) have been shown to be effective [4, 5].
High-intensity focused ultrasound (HIFU) is one of the newer ablation techniques that has been shown to be effective in not only causing significant nodule shrinkage but also alleviating obstructive symptoms shortly after single treatment [6,7,8,9]. Interestingly, apart its thermal effect, less intense HIFU energy is capable of causing mechanical or non-thermal ablation that could lead to various important pathological, immunological and therapeutic consequences both locally and systemically [10, 11].
However, despite being a promising form of ablation, the medium to long-term efficacy following a single HIFU treatment remains unclear. Although studies have shown that nodules generally continue to shrink while obstructive symptoms continue to improve over time [8, 9, 12,13,14], it is uncertain whether these results can continue beyond 12 months. Cases of nodule regrowth and recurrence have been reported following other forms of ablation technique when treated nodules were followed beyond 12 months [12,13,14,15]. Also, to ensure treatment success in the long-term, it would be important to identify patient or disease factors that may affect long-term treatment efficacy. With these issues, therefore, the present study aimed to evaluate the amount of physical shrinkage and symptom improvement beyond 12 months and also to identify what factors might influence treatment success at 2 years from single HIFU treatment.
Methods
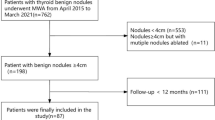
This retrospective analysis was approved by the local institutional review board (UW 17-234). All relevant clinical and treatment data were recorded prospectively after obtaining informed consent. Consecutive patients who underwent HIFU ablation for a symptomatic, solid or predominantly solid (<30% cystic areas) benign thyroid nodule from 2015 to 2016 were analysed. At our institution, only patients who were indicated but unwilling to undergo thyroidectomy were considered for ablation. Details on the eligibility for ablation were previously described [9]. In brief, the nodule had to be proven benign on fine-needle aspiration cytology (Bethesda category II) and to have a low or very low suspicion sonographic pattern together with its centre located within the treatable depth of 7–30 mm from the skin surface. Also, the swelling (which could either be a solitary nodule or a dominant nodule in a multinodular gland) had to be causing obstructive symptoms and the longest diameter of the nodule had to be ≥20 mm but ≤60 mm on ultrasonography (US). For the present study, any patients with incomplete or less than 24 months of follow-up or had received two or more treatments to the same nodule within 24 months were excluded.
At each visit (baseline and 3, 6, 12, 18 and 24 months), the three orthogonal dimensions of the index nodule were measured on US by an independent experienced sonographer. Nodule dimensions were measured using the LOGIQ e (GE Healthcare, Chicago, IL, USA) scanner equipped with a 10- to 14-MHz linear matrix transducer. Three orthogonal diameters of the index nodule (its longest diameter and two other perpendicular diameters) were recorded. In general, the longest diameter was the cranio-caudal dimension (length) of the nodule, while the other two perpendicular diameters were the medio-lateral (width) and antero-posterior (depth) dimensions of the nodule. All measurements were to the nearest 0.1 mm. To estimate nodule volume, we used the formula: volume (mL) = [width (in cm) × depth (in cm) × length (in cm)] × (π/6) where π was taken as 3.1416. The volume reduction ratio (VRR) (%) was calculated based on the formula: [(baseline volume – volume at visit) / (baseline volume)] × 100. Since our intra-observer coefficient of variation for sonographic volume was around 4.5% [16], only a change in nodule volume >4.5% between time-points was taken as an actual change. Treatment success was defined as VRR >50%. Nodule regrowth was defined as a >20% increase in nodule volume relative to the 12-month volume given that the 12-month volume is normally the lowest measured volume on US in the first 24 months.
Clinical assessments
In addition to US assessments, the thyroid swelling was clinically graded by the WHO goitre grade system [17] before treatment. Also, at each clinic visit (baseline, 6-, 12- and 24-month) patients were asked to rate their obstructive symptoms on a visual analogue scale (VAS) (0–10) (0 = no obstructive symptoms; 10 = most significant obstructive symptoms).
Application of HIFU ablation
All treatments were carried out in a similar matter by one person (B.H.L.) using the same commercially available US-guided HIFU device (see later). All patients were instructed to be fasted overnight and to admit to the hospital in the early morning where baseline blood tests including serum thyroid function tests [free T4 and thyroid stimulating hormone (TSH) levels], thyroglobulin (Tg) and anti-thyroid autoantibodies were taken.
At treatment, all patients were placed in a supine position with neck slightly extended and received a bolus of intravenous Diazepam (Actavis, Dublin, Ireland) (10–15 mg) and Pethidine (Martindale Pharma, Wooburn Green, Buckinghamshire, UK) (50–100 mg). Patients’ heart rate, blood pressure, respiration rate and peripheral oxygenation were monitored throughout the procedure. Patients were asked to show a hand sign without moving the neck if the pain became too severe during treatment. In that situation, either the energy setting was lowered or more medications were administrated.
The US-guided HIFU device comprised an energy generator, a treatment head, a skin-cooling device and a touch-screen interface for planning. The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter) (Fig. 1). The treatment head was placed on the neck of the patient on the side of the index nodule and was finely adjusted until the nodule was within the treatable depth of 7-30mm from skin surface. Once marked on the treatment screen, the device computer (Beamotion version no. TUS 3.2.2; Theraclion, Malakoff, France) automatically divided the nodule into multiple ablation voxels. Each voxel measured approximately 7.3 mm in thickness and 5 mm in width and received a continuous 8-s pulse of HIFU energy followed by 20–30 s of cooling time before the beam was moved to the adjacent voxel (Fig. 2). To ensure safety, nearby structures like the carotid artery, trachea and skin were marked out on the treatment screen before the start of treatment by the operator. To avoid inadvertent heat injury to important surrounding structures, the device automatically selected the safety margins for the skin, the trachea and recurrent laryngeal nerve and from the ipsilateral carotid artery. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10oC liquids) at the tip of the treatment head. Both the total amount of energy delivered to the nodule (in kilojoules) and the “on-beam” (sonification) time taken (in minutes) were automatically recorded by the device’s computer. The “on-beam” treatment time was the duration between the first to the last pulse (in minutes). Oral diet was resumed immediately afterwards and patients were discharged home 2–3 h after treatment. Afterwards, a transcutaneous laryngeal US was done to assess the mobility of both vocal cords [18]. Vocal cord palsy (VCP) was defined as having an impaired or absent movement in one of the vocal cords corresponding to the ablated side. Any other possible complications including skin burn or Horner’s syndrome were recorded.
Statistical analysis
Continuous variables were expressed as mean ± SD and/or median and interquartile range (IQR) when appropriate. For comparison between groups, either t-test or the Mann-Whitney U test was used. Chi-squared tests were used to compare categorical variables. For correlation between continuous variables, the Pearson correlation test was performed. Both the univariate and multivariate analyses were done by logistic regression analysis. For multivariate analysis, only parameters considered to be associated with ablation success were entered together using the step-down procedure. Obstructive symptom was rated on a VAS of 0-10 at baseline and at 6, 12 and 24 months. All statistical analyses were performed using SPSS version 18.0 (SPSS, Chicago, IL, USA). All significance tests were two-tailed and those with a p value less than 0.05 were considered statistically significant.
Results
A total of 136 patients underwent HIFU ablation of a symptomatic, benign thyroid nodule. Of these, 4 (2.9%) patients were lost to follow-up within the first 24 months, while 24 (17.6%) patients either received two or more treatments in the same session or two or more treatments within 24 months. After excluding these 28 patients, 108 (79.4%) patients were eligible for analysis. This cohort included outcomes of 22 patients previously published [9]. At the time of analysis, the mean (± SD) follow-up was 28.7 ± 4.0 months. Baseline characteristics and treatment parameters are shown in Table 1. In terms of complications, three (2.8%) patients developed unilateral VCP afterwards but they all recovered (i.e. regained normal movement) within the first 3 months. One other patient (0.9%) suffered Horner’s syndrome on the side of the treatment. Her mild ptosis improved gradually over a period of 6 months.
Extent of nodule shrinkage (VRR) and rate of treatment success in the first 24 months
Following a single HIFU ablation, the overall nodule shrinkage rate or VRR at 3, 6, 12, 18 and 24 months were 51.32 ± 20.71%, 62.99 ± 22.05%, 68.66 ± 18.48%, 69.76 ± 17.88% and 70.41 ± 17.39%, respectively (Fig. 3). The rate of treatment success at 3, 6, 12, 18 and 24 months was 42/108 (41.7%), 66/108 (61.1%), 73/108 (67.6%), 75/108 (69.4%) and 76/108 (70.4%), respectively. In the first 12 months, all of the nodules became progressively smaller in volume and the 12-month nodule volume was the lowest recorded volume relative to baseline. However, interestingly, despite the overall volume reduction over the 24 months, 63 (58.3%) of nodules had a further volume reduction (i.e. >4.5%) from 12 to 24 months. In this subgroup, the mean volume reduction (%) was 9.9 ± 9.5%. There were 23 (21.3%) nodules which remained static in volume from 12 to 24 months and there were 22 (20.4%) nodules which had a volume increase (i.e. >4.5%) during this period. In this latter subgroup, the mean volume increase (%) was 13.62 ± 9.60%. In terms of absolute volume increase, the mean was 0.45 ± 0.67 mL and the largest absolute increase was 3.10 mL (a nodule which measured 18.01 mL at 12 months became 21.11 mL at 24 months or a 17.2% increase in volume). Nevertheless, none of these treated nodules fulfilled the criteria for nodule regrowth (i.e. >20% from lowest recorded volume) in the first 24 months of follow-up.
Change in symptom score (by VAS) in the first 24 months
At baseline, the mean (± SD) symptom score by VAS was 4.12 ± 1.22 (median = 4.0; IQR = 2.0). Following treatment, the mean symptom scores at 6, 12 and 24 months were lowered relative to the baseline [2.57 ± 1.35 (median = 2.0; IQR = 1.0), 1.56 ± 1.05 (median = 2.0; IQR = 1.0), 1.27 ± 1.04 (median = 1.0; IQR = 2.0), respectively] (Fig. 4). At 6 months, 85 (78.7%) patients had a VAS score lower than that at baseline. Among those who did not have a lower VAS score, 20 (18.5%) patients had the same VAS score as that of baseline, while only 3 (2.8%) patients reported a VAS score higher than that of baseline. At 24 months, 103 (95.4%) patients had a lower VAS score than that of baseline and only 5 (4.6%) patients had the same VAS score as the baseline.
Association between VRR and symptom score (by VAS) over time
At 6 months, patients reporting an improved symptom score (i.e. reporting a lower 6-month VAS score than baseline) (n = 85) had a significantly greater VRR than those who reported a similar (n = 20) or a higher VAS (n = 3) than baseline (67.45 ± 17.87% vs 51.71 ± 25.71%, p < 0.001 and 67.45 ± 17.87% vs 11.90 ± 17.13%, p = 0.001, respectively). Similarly, at 24 months, patients reporting an improved symptom score (i.e. a lower VAS than that of baseline) (n = 103) had a significantly greater VRR than those with the same VAS as baseline (n = 5) (71.50 ± 16.85% vs 48.07 ± 13.99%, p = 0.005). There was a significant correlation between VRR at 24 months and the absolute difference in VAS score at 24 months from baseline (r = -0.820, p = 0.040).
Factors determining treatment efficacy at 24 months
Table 2 compares the baseline characteristics and treatment parameters between those who had VRR >50% (i.e. treatment success) at 24 months (group I) and those who had VRR ≤50% at 24 months (group II). Age, sex ratio, body mass index, thyroid function, side and location of index nodule, as well as number of other nodules within the thyroid gland, were not significantly different between the two groups. However, group II had a significantly higher proportion of WHO grade 3 nodules than group I (46.2% vs 23.2%, p = 0.039). Also, in concordance with this, the nodule width, length and depth were all significantly larger in group II than group I (3.01 cm vs 2.55 cm, p = 0.037; 4.25 cm vs 3.29 cm, p = 0.001; 2.46 cm vs 2.00 cm, p = 0.003, respectively). As a result, the nodule volume at baseline in group II was almost twice of that in group I (20.34 mL vs 10.80 mL, p = 0.003) and also, the “on-beam” treatment time was significantly longer in group II than group I (55.20 min vs 38.51 min, p = 0.038). Despite having similar baseline symptom score (by VAS), the scores at 12 and 24 months were significantly lower in group I (p = 0.003 and p = 0.005, respectively). Relative to baseline, VAS scores at 6, 12 and 24 months were significantly less in group I (p < 0.001), while in group II only VAS scores at 12 and 24 months were significantly less (p < 0.001) (Table 3).
In the univariate analysis by logistic regression, only larger pre-ablation nodule (OR = 0.945, 95% CI = 0.911–0.980, p = 0.003) and longer total “on-beam” time (OR = 0.972, 95% CI = 0.946–0.998, p = 0.035) were significant factors for treatment success at 24 months. In the multivariate analysis, none of the factors turned out to be significant after the step-down procedure (Table 4).
Discussion
To our knowledge, this was the first study to evaluate the clinical efficacy of HIFU ablation beyond 12 months. In terms of major findings, first, our data showed that there was a small but significant overall reduction in nodule volume (i.e. physical shrinkage) from 12 to 24 months (from 68.66 ± 18.48% to 70.41 ± 17.39% p < 0.001) and the rate of treatment success increased incrementally from 67.6 to 70.4% over this period. These findings were consistent with the experience from other forms of ablation technique [12,13,14,15]. One previous study reported that with a single application of RFA treatment, there was still an incremental reduction in nodule volume from 78.6% at 12 months to 79.4% at 24 months relative to baseline volume [14]. Another study reported a similar amount of reduction from 59% at 12 months to 60% at 24 months with a single LA application [14]. Regarding why the actual shrinkage rate varied between studies, one explanation might be due to the differences in case selection, operator experience and delivered energy [19].
However, it is interesting to note that when the volume of each nodule was looked at individually, only less than two-thirds (or 58.3%) of nodules had an actual volume reduction from 12 to 24 months, with another one-fifth (or 21.3%) remaining static in volume (± 4.5%). Therefore, the actual amount of shrinkage was relatively small when compared to that in the first 12 months. Also, worth noting was the fact that another one-fifth (or 20.4%) of nodules actually had a volume increase of >4.5%, with the largest absolute volume increase of 3.10 mL or 17.2% from 12 to 24 months. Therefore, even though none of the 108 nodules observed fulfilled the criteria for nodule regrowth of 20% relative to volume at 12 months, our data implied that perhaps a longer and closer follow-up is indicated and might prove to be worthwhile. This is why some authors have advocated the use of multiple treatments to optimise the long-term treatment efficacy and success [9, 20].
In addition to physical nodule shrinkage, our data showed that over the 24-month period, the severity of obstructive symptom became less or milder as reported by patients. This was despite the fact that the physical nodule volume appeared to plateau after 12 months. There was an overall decrease in mean symptom score (by VAS) from 12 to 24 months [from 1.56 ± 1.05 (median = 2.0; IQR = 1.0) to 1.27 ± 1.04 (median = 1.0; IQR = 2.0)]. In terms of association with nodule shrinkage, our data found that those with an improved symptom score had significantly greater nodule shrinkage (i.e. VRR) than those with similar symptom score at 6 and 24 months (p < 0.001 and p = 0.005, respectively). Also, there was a significant correlation between those with larger VRR and greater improvement in symptom score relative to baseline (p = 0.040). These findings had previously been reported [8,9,10,11,12].
In terms of factors determining treatment success at 24 months, the initial or pre-ablation nodule volume was a significant factor in the univariate analysis (OR = 0.945, 95% CI = 0.911–0.980, p = 0.003). Relative to those who did not achieve treatment success (or VRR >50%) at 24 months, those who did achieve treatment success had significantly smaller-volume nodules (10.80 ± 9.97 mL vs 20.34 ± 16.73 mL, p = 0.003). This finding was consistent with the experience of others and ours [21]. We believe this might be due to the fact that a more complete ablation could be achieved with a single ablation in smaller nodules, while perhaps more than a single ablation might be required for larger nodules. As a result, our group have begun to treat larger nodules (volume >15-20 mL) with two sequential treatments in the same session using a similar technique as the one for multinodular goitre [22]. Although ablation time was also found to be significant in the univariate analysis, this was simply because larger nodules needed longer ablation time than smaller nodules. As a result, it did not turn out to be an independent factor for treatment success.
Despite these findings, we would like to acknowledge several shortcomings. First, our study was only a moderately sized study and so some of the findings were prone to type II errors. Second, despite being the first study to evaluate longer-term efficacy after single HIFU treatment, an even longer period of follow-up would be necessary for the proper evaluation of events like nodule growth and recurrence.
Conclusions
The majority of patients had a further nodule volume reduction and symptom improvement beyond 12 months following a single HIFU ablation, but since about one-fifth of them had a notable nodule volume increase beyond 12 months, a longer period of follow-up would seem to be necessary. Pre-ablation nodule volume was a significant factor for treatment success at 24 months.
Abbreviations
- HIFU:
-
High-intensity focused ultrasound
- TSH:
-
Thyroid stimulating hormone
- VCP:
-
Vocal cord palsy
- VRR:
-
Volume reduction ratio
References
Gharib H, Papini E, Garber JR et al (2016) AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules—2016 update. Endocr Pract 22:622–639
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133
Durante C, Costante G, Lucisano G et al (2015) The natural history of benign thyroid nodules. JAMA 313:926–935
Mauri G, Cova L, Monaco CG et al (2016) Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperth 15:1–5
Pacella CM, Mauri G, Achille G et al (2015) Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–3910
Pacella CM, Mauri G, Cesareo R et al (2017) A comparison of laser with radiofrequency ablation for the treatment of benign thyroid nodules: a propensity score matching analysis. Int J Hyperth 33:911–919
Korkusuz H, Fehre N, Sennert M, Happel C, Grünwald F (2015) Volume reduction of benign thyroid nodules 3 months after a single treatment with high-intensity focused ultrasound (HIFU). J Ther Ultrasound 3:4. https://doi.org/10.1186/s40349-015-0024-9
Kovatcheva RD, Vlahov JD, Stoinov JI, Zaletel K (2015) Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology 276:597–605
Lang BH, Woo YC, Wong CKH (2017) High-intensity focused ultrasound for treatment of symptomatic benign thyroid nodules: a prospective study. Radiology 284:897–906
Mauri G, Nicosia L, Xu Z et al (2018) Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol 91:20170641
Hoogenboom M, Eikelenboom D, den Brok MH, Heerschap A, Fütterer JJ, Adema GJ (2015) Mechanical high-intensity focused ultrasound destruction of soft tissue: working mechanisms and physiologic effects. Ultrasound Med Biol 41:1500–1517
Lim HK, Lee JH, Ha EJ, Sung JY, Kim JK, Baek JH (2013) Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 23:1044–1049
Papini E, Rago T, Gambelunghe G et al (2014) Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 99:3653–3659
Spiezia S, Garberoglio R, Milone F et al (2009) Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–225
Valcavi R, Riganti F, Bertani A, Formisano D, Pacella CM (2010) Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–1261
Lang BH, Wong CKH, Wong KP, Chu KK, Shek TWH (2017) Effect of thyroid remnant volume on the risk of hypothyroidism after hemithyroidectomy: a prospective study. Ann Surg Oncol 24:1525–1532
Zimmermann M, Saad A, Hess S, Torresani T, Chaouki N (2000) Thyroid ultrasound compared with World Health Organization 1960 and 1994 palpation criteria for determination of goiter prevalence in regions of mild and severe iodine deficiency. Eur J Endocrinol 143:727–731
Wong KP, Lang BH, Ng SH, Cheung CY, Chan CT, Lo CY (2013) A prospective, assessor-blind evaluation of surgeon-performed transcutaneous laryngeal ultrasonography in vocal cord examination before and after thyroidectomy. Surgery 154:1158–1164
Baek JH (2017) Factors related to the efficacy of radiofrequency ablation for benign thyroid nodules. Ultrasonography 36:385–386
Huh JY, Baek JH, Choi H, Kim JK, Lee JH (2012) Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session: prospective randomized study. Radiology 263:909–916
Lang BH, Woo YC, Chiu KW (2017) Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid 27:714–721
Lang BHH, Woo YC, Chiu KW (2018) Sequential high intensity focused ultrasound (HIFU) ablation in the treatment of benign multinodular goitre: an observational retrospective study. Eur Radiol. https://doi.org/10.1007/s00330-018-5333-2
Acknowledgements
We would like to thank Mr. Hill Yu for conducting all the symptom assessments for the entire study period and Professor Stephen Cheng (Head of Department of Surgery, University of Hong Kong) for agreeing to be the guarantor of this paper.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Stephen Cheng (Head of Department).
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The 6- and 12-month outcomes of 22 patients were previously reported in a small prospective study [9].
Methodology
• Retrospective
• Observational
• Single institution
Rights and permissions
About this article
Cite this article
Lang, B.H.H., Woo, YC. & Chiu, K.WH. Two-year efficacy of single-session high-intensity focused ultrasound (HIFU) ablation of benign thyroid nodules. Eur Radiol 29, 93–101 (2019). https://doi.org/10.1007/s00330-018-5579-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5579-8