Abstract
Objective
To investigate and compare the clinical outcomes of radiofrequency ablation (RFA) for multifocal papillary thyroid microcarcinoma (PTMC) versus unifocal PTMC in a large cohort.
Methods
Patients with low-risk PTMC (n = 487) who underwent RFA were included in this retrospective study and divided into the unifocal group (U group) (n = 432) and the multifocal group (M group) (n = 55) according to the number of lesions. After 1:1 propensity score matching (PSM), volume, volume reduction ratio (VRR), the development of local tumor progression including lymph node metastasis (LNM), recurrent PTMC and persistent lesions, and recurrence-free survival (RFS) rate were evaluated and compared between the two groups. The different impacts of multifocality on recurrence after RFA for PTMC were investigated by Cox analysis.
Results
During a mean follow-up time of 49.25 ± 12.98 months, the overall VRR was 99.40 ± 4.43% and the overall incidence of local tumor progression was 3.70% (18/487). No complications occurred after RFA. After PSM, no significant differences were found in volume (0.11 ± 0.69 mm3 vs 0 mm3, p = 0.441), VRR (99.87 ± 0.78% vs 100%, p = 0.441), complete disappearance rate (95.61% vs 89.09%, p = 0.201), incidence of local tumor progression (5.45% vs 5.45%, p = 1.000), LNM (1.82% vs 0%, p = 0.317), recurrent PTMC (1.82% vs 5.45%, p = 0.611), persistent lesions (1.82% vs 0%, p = 0.317), and RFS rate (96.36% vs 94.55%, p = 0.632) between the M group and U group. The association between multifocality and local tumor recurrence also remained nonsignificant (p = 0.619). No distant metastasis or delayed surgery occurred.
Conclusions
The impact of multifocality on the prognosis after RFA for low-risk PTMC was little. RFA might be a promising treatment for both unifocal and multifocal PTMC in properly selected patients after sufficient preoperative evaluation.
Key Points
• No significant differences are found in the local tumor progression between the unifocal PTMC and multifocal PTMC.
• Multifocality is not associated with higher recurrence after RFA for low-risk PTMC.
• RFA is a promising alternative for both unifocal and multifocal PTMC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of thyroid cancer has increased worldwide at a rate higher than that of any other cancer, ranking in ninth place for incidence of cancer [1]. Roughly 50% of the increase is attributable to papillary thyroid microcarcinoma (PTMC), most of which are low risk with favorable prognosis [2, 3]. Tumor multifocality is observed in 20–40% PTMC [4] and the first-line treatment is surgery [2]. However, it has several drawbacks, including invasive procedure, cosmetic problems, and lifelong thyroid hormone replacement [5], which may not be accepted by all the patients. Active surveillance (AS) is a new option for low-risk PTMC [2], and multifocal PTMC is not listed as an exclusion because it is not a risk factor for tumor enlargement by ≥ 3 mm or the appearance of lymph node metastasis (LNM) [6]. However, acceptance of this management seems to be varied and low [6].
Radiofrequency ablation (RFA) and other thermal ablation have been applied effectively and safely for low-risk PTMC patients who rejected surgery or AS [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23]. The pooled proportion of volume reduction rate (VRR) and complete disappearance rate after ablation were 98.1% [24] and 57.6–76.2% [24, 25]. However, these results were mainly based on the efficacy of unifocal PTMC, not multifocal PTMC. To date, the study with the largest population of multifocal PTMC underwent ablation was reported by Teng et al [11], which evaluated 167 patients with unifocal PTMC and 18 patients with 33 multifocal PTMC for a follow-up time of 20.7 ± 8.8 months, even though the sample size was relatively small and clinical outcomes of multifocal PTMC were not evaluated separately. Intrathyroidal multifocal PTMC is low risk [2], and the clinical outcomes of ablation for multifocal PTMC should be evaluated to better understand the clinical value of this treatment for PTMC. However, little research has reported the efficacy and safety of RFA for multifocal PTMC.
Therefore, the purpose of this study was to evaluate the clinical outcomes of RFA for multifocal PTMC and compare the results with unifocal PTMC.
Materials and methods
The Institutional Review Board of our institution approved this retrospective study. All the patients were provided written informed consent before RFA. The RFA informed consent emphasized that surgery was the routine treatment recommended by guidelines.
Patients
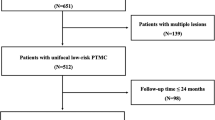
The inclusion criteria were as follows: (1) PTMC lesion was confirmed by core-needle biopsy (CNB) or fine-needle aspiration (FNA); (2) multiple PTMC lesions present in the ipsilateral lobe [26]; (3) no imaging evidence of extrathyroidal extension (ETE), LNM, and distant metastasis on ultrasound and chest CT; (4) patients who were unsuitable for surgery or rejected surgery clearly; (5) follow-up time was > 24 months. The exclusion criteria were (1) no convincing evidence of aggressive disease by biopsy [2]; (2) bilateral PTMC (at least one lesion within the contralateral lobe [26]); (3) imaging evidence of ETE or LN/distant metastasis; (4) consciousness disorder or neck extension disorder; (5) coagulation disorder or serious organ failure; (6) follow-up time ≤ 24 months.
From June 2014 to March 2018, 681 patients with low-risk PTMC underwent RFA in this institution, and 487 patients with 546 low-risk PTMCs met the inclusion criteria were included in this study. Among them, 432 patients with 432 unifocal PTMC were in the unifocal group (U group) and 55 patients with 114 multifocal PTMC in the multifocal group (M group) (Figure 1).
Per-treatment ultrasound evaluation
The patients all underwent thorough examinations, including complete blood count, thyroid function tests, coagulation tests, and imaging evaluation, including ultrasound and chest CT [7, 10, 16]. The volume of tumor was calculated with the equations: V = πabc/6 (V is the volume, while a is the largest diameter on ultrasound, b and c are the other two perpendicular diameters). The largest tumor of multifocal PTMC was taken as the primary tumor, and its diameter and volume were used for further analysis.
RFA procedure
All RFA procedures were performed by an experienced ultrasound radiologist with more than 20 years’ experience in thyroid US and interventional US. A bipolar RFA generator (CelonLabPOWER, Olympus Surgical Technologies Europe) and an 18-gauge bipolar RF applicator with 0.9-cm active tip were used (CelonProSurge micro 100-T09, Olympus Surgical Technologies Europe) in this study. The targeted tumor was evaluated by multi-angle scanning to determine a practical and proper approach. Doppler ultrasound was used to access the detailed vascular anatomy along the approach route to prevent bleeding. Local anesthetic (1% lidocaine) was injected at the subcutaneous puncture site and the thyroid anterior capsule. RFA was performed using the trans-isthmic approach and moving-shot technique. The hydrodissection technique was used if the distance between the tumor and critical cervical structures (trachea, carotid artery, jugular vein, esophagus, and recurrent laryngeal nerve) was less than 5 mm. Normal saline was injected using another needle (23 gauge) to form at least a 1-cm distance between the tumor and the critical structure in order to prevent thermal injury [7, 16]. If the fluid was absorbed, it was replenished. If the multifocal lesions were located in the same plane, the smaller one was ablated first. Otherwise, the deeper-located lesion was ablated first. We enlarged the ablation area which exceeded the tumor edge (at least 3–5 mm) to prevent marginal residue and recurrence [7]. The RFA power was 3W. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the power was increased to 5–9 W. Contrast-enhanced ultrasound (CEUS) was performed immediately after the RFA procedure to evaluate the ablation area. If any enhancement existed, a complementary ablation could be performed.
Follow-up
Clinical follow-ups were performed at 1, 3, 6, and 12 months and every 6–12 months thereafter and consisted of ultrasound, CEUS, chest CT (once a year), and clinical evaluation. VRR was calculated as follows: VRR = ([initial volume-final volume] × 100)/initial volume. The development of suspicious LN or new lesion was submitted to biopsy.
Primary and secondary outcomes
Primary outcomes were local tumor progression and recurrence-free survival (RFS). Local tumor progression was defined to include three situations [8]: (1) persistent lesion was found at the previously treated site confirmed to be PTMC by biopsy; (2) new recurrent lesion which separated from the ablated tumor confirmed to be PTMC by biopsy; (3) cervical LNM confirmed by biopsy. Distant metastasis was detected by CT, positron emission tomography, or bone scan if there were suspicious symptoms. RFS was calculated from the beginning of RFA to the time of tumor recurrence or the last date of follow-up.
Secondary outcomes were complications and efficacy. Complications during follow-up were assessed using the reporting standards of the Society of Interventional Radiology [27]. The efficacy of ablation included the VRR and complete disappearance rate. We considered RFA to be successful if one of the following criteria was met: (1) the ablated area of PTMC completely disappeared on ultrasound; (2) the ablated area remained as scar-like on ultrasound but absence of enhancement on both arterial and venous phase on CEUS; (3) if the ablation area did not disappear, negative result was confirmed by CNB, which was performed to the central zone, the peripheral zone, and the surrounding thyroid parenchyma at 3 or 6 months after RFA.
Statistical analysis
Statistical analysis was performed using the SPSS statistical software (Version 25.0) and R software version 3.6.2 (www.r-project.org). To control the inherent potential biases of any retrospective study, a 1:1 propensity score matching (PSM) was applied in the two groups based on age, sex, primary tumor diameter, primary tumor volume, chronic lymphocytic thyroiditis (CLT), and the follow-up time. Patient characteristics and outcomes after RFA were compared between the groups before and after PSM. Categorical variables are presented as numbers with percentages and compared using Chi-square or Fisher’s exact test. Continuous data were expressed as mean ± SD and analyzed using Mann-Whitney U test. Kruskal-Wallis H test was used for comparison of the variables between the groups. Wilcoxon signed rank tests were used to compare the volume before RFA and at each follow-up period. RFS curves were generated using the Kaplan-Meier method and compared by the log-rank test. Univariable and multivariable analyses were performed by using a Cox proportional hazard model to identify the variables associated with recurrence. A difference with p < 0.05 was considered as statistically significant.
Results
A total of 487 patients with unifocal PTMC (N = 432) or multifocal PTMC (N = 55) who underwent RFA were enrolled in this study. Before PSM, the mean and median follow-up times were 49.25 ± 12.98 months and 48.03 months, respectively. In the M group, 51 patients had two tumors and 4 patients had three tumors. No significant differences in the primary tumor size were found in the U and M groups (94.73 ± 83.28 mm3 vs 108.05 ± 85.99 mm3, p = 0.151). However, the RFA times in the U group were significantly longer than those in the M group (218.23 ± 127.86 s vs 187.74 ± 164.06 s, p = 0.010). After 1:1 PSM, a good balance was achieved (Table 1). The mean and median follow-up times were 47.27 ± 11.87 months and 47.05 months, respectively.
Primary outcomes
The overall incidence of local tumor progression was 3.70% (18/487). The incidences of LNM, recurrent PTMC, and persistent lesions were 1.03% (5/487) and 2.26% (11/487) and 0.41%(2/487), respectively. Because 38 tumors disappeared in the first 3 months and another 26 tumors disappeared at 6 months, 482 tumors underwent post-ablation CNB and two were diagnosed as persistent lesions. These two ablated tumors underwent additional RFA and CNB after additional RFA were negative. Among 16 patients with recurrent lesions, one patient with recurrent PTMC in the U group chose AS and the volume of tumor was stable. The other 15 recurrent lesions underwent additional RFA, and 12 of them completely disappeared. No distant metastasis was detected. No patient underwent delayed surgery because of local tumor progression or anxiety.
Before PSM, there were no significant differences in the incidence of local tumor progression (5.45% vs 3.47%, p = 0.723), LNM (1.82% vs 0.93%, p = 0.452), recurrent PTMC (1.82% vs 2.31%, p = 1.000), and persistent lesions (1.82% vs 0.23%, p = 0.213) between the M group and U group. A total of 80.00% LNM was found in the lateral compartment and 72.73% recurrent PTMC were detected in the contralateral lobe. After PSM, no significant differences were found in the incidence of local tumor progression (5.45% vs 5.45%, p = 1.000), LNM (1.82% vs 0%, p = 0.317), recurrent PTMC (1.82% vs 5.45%, p = 0.611), and persistent lesions (1.82% vs 0%, p = 0.317) between the M group and U group (Table 2).
The RFS rates at 1 and 3 years were 99.31% and 96.99% in the U group, and 98.18% and 96.36% in the M group. After PSM, the RFS rates at 1 and 3 years were 100.00% and 94.55% in the U group. There were no significant differences in the RFS rates between the groups before or after PSM (p = 0.858; p = 0.632) (Figure 2). Cox regression analysis revealed that multifocality was not significantly associated with recurrence after adjusting for age, sex, size of tumor, and CLT (HR = 0.630, 95% CI: 0.102–3.890, p = 0.619) (Table 3).
Secondary outcomes
The overall VRR was 99.40 ± 4.43%. Before PSM, the volume and VRR in the M group were significantly larger than those in the U group in the first 6 months after RFA (all p < 0.05). However, no significant differences were found after 6 months (all p > 0.05) (Tables 4 and 5). A total of 499 tumors completely disappeared (91.39%). Among them, 109 disappeared tumors were in the M group (95.61%) and 390 were in the U group (90.28%) (p = 0.071). After PSM, only the volumes at 1 month in the M group were significantly smaller than those in the U group (p < 0.05). There were no significant differences in the VRR between the groups during the follow-up (all p > 0.05) (Figure 3). Forty-nine tumors completely disappeared in the U group. The complete disappearance rate in M group and U group remained nonsignificant (95.61% vs 89.09%, p = 0.201) (Figure 4).
The US and CEUS images of a 25-year-old female with multifocal PTMC before and after RFA. a Two PTMC tumors located in the right thyroid lobe before RFA. One tumor was in the superior pole (arrow) with an initial volume of 52.36 mm3. The other one was in the inferior pole (arrowhead) with an initial volume of 31.42 mm3. b At 1 month after RFA, the volumes of ablation areas in the superior (arrow) and inferior pole (arrowhead) were 368.60 mm3 and 175.92 mm3, respectively. c At 3 months after RFA, the ablation area located in the inferior pole disappeared. The volume of ablation area located in the superior pole (arrow) was 4.71 mm3 with a VRR of 91%. d At 6 months after RFA, the ablation area in the superior pole also disappeared
All patients were tolerable to the RFA procedure. No patients experienced complications related to RFA during the follow-up. Moreover, 20 patients in the U group (4.67%) and 3 patients in the M group (5.45%) underwent local pain or discomfort, which resolved spontaneously within 7 days.
Discussion
This study evaluated the long-term clinical outcomes of RFA for multifocal PTMC and compared the results with unifocal PTMC by PSM. No significant differences of volume, VRR, incidence of local tumor progression, and RFS rate were found between the multifocal PTMC with unifocal PTMC after a mean follow-up period of 49.25 ± 12.98 months. The association between multifocality and local tumor recurrence also remained nonsignificant. Moreover, no patients underwent delayed surgery because of anxiety or local tumor progression. No complications occurred after RFA. It indicated that RFA could achieve the same long-term therapeutic outcomes for multifocal PTMC as those for unifocal PTMC.
The current standard treatment for low-risk PTMC is surgery [2]. However, it has several drawbacks, including general anesthesia, risk of complications, cosmetic problems, and lifelong thyroid hormone replacement [5]. AS is recommended as a new conservative option for low-risk PTMC [2], which shows favorable results [6, 28,29,30]. The recent meta-analysis found that during the 5-year AS, the pooled proportion of tumor size enlargement was 5.3% and the pooled proportion of LMN was 1.6% [28]. However, patient preference and compliance were important elements for this strategy [31]. Because of anxiety, most patients would choose immediate surgery instead of AS [32]. Anxiety was also the most common reason for the decision to perform delayed surgery during AS [29]. For low-risk patients who were unsuitable for surgery or rejected surgery/AS, RFA and other thermal ablation techniques could be considered [33]. The incidences of LNM, recurrent PTMC, and persistent lesions after thermal ablation were 0.6–3.1% [15, 20, 22, 23, 25, 34], 0.5–4.05% [10, 11, 20, 22, 25, 34], and 1.42% [14], respectively. A recent meta-analysis included three studies reporting outcomes in patients with PTMC treated with thermal ablation and followed up for at least 5 years [35]. It found that of 207 patients with 219 PTMCs, only 5 recurrent PTMCs were observed in 4 patients, and no patients experienced local tumor recurrence, LNM, or distant metastasis or underwent delayed surgery due to anxiety during a mean pooled follow-up of 67.8 months. Comparative studies showed that no significant differences in the incidence of LNM (0–3.1% vs 1.3–4.44%) and recurrent PTMC (1.1–2.78% vs 0.7–2.22%) between thermal ablation and surgery were found [20, 22, 34]. This study included 487 patients who underwent RFA for the treatment of low-risk PTMC, and the results showed that during a mean follow-up time of 49.25 ± 12.98 months, the overall incidences of LNM, recurrent PTMC, and persistent lesions were 1.03%, 2.26%, and 0.41%, respectively. These results were not only in accordance with those from previous studies [10, 11, 14, 15, 23, 25], but also consistent with those from surgery or AS [20, 22, 28, 34]. Moreover, no patient underwent delayed surgery because of anxiety or local tumor progression. It suggested that RFA could be an effective local tumor control alternative for low-risk PTMC.
There are several reasons why the clinical outcomes of thermal ablation for multifocal PTMC are needed. First, multifocality is a common feature in PTMC patients with an incidence of 20–40% [4] and intrathyroidal multifocal PTMC is low risk according to risk stratification [2]. Although surgery and AS are recommended for low-risk PTMC [2], for patients who refuse surgery or AS, thermal ablation can be considered as alternatives [33]. Second, the indications of ablation for primary thyroid cancer have not yet been clearly established [33]. The long-term results of ablation for multifocal PTMC should be evaluated to better understand the clinical value of this treatment for primary thyroid cancer. Third, thermal ablation techniques have been performed to multifocal PTMC [7, 8, 10,11,12,13,14, 36]. However, the efficacy and safety have not been reported separately. It is the first study to evaluate the clinical outcomes between multifocal and unifocal PTMC following RFA in a large cohort. The results showed that VRR in the two groups were both over 99% with no significant differences. Complete disappearance rates in the two groups were also nonsignificant. The fact that long-term efficacy of RFA for multifocal PTMC was as satisfactory and sustainable as that for unifocal PTMC suggests RFA might be a potential treatment strategy for multifocal PTMC.
With the advance of modern molecular techniques, multifocal PTMC has been considered as multiple synchronous primary tumors arising from independent clones instead of intraglandular metastasis from a single primary tumor [37, 38], but its prognostic value still remains controversial. Several studies found multifocal PTMC at diagnosis had an increased risk of LN/distant metastasis and persistent local disease after initial treatment, and total thyroidectomy should be performed [37,38,39,40,41]. However, other studies reported that multifocality might have prognostic impact in PTC but less or none in PTMC and suggested its indiscriminative use as an independent risk factor prompting overtreatments should be avoided [42,43,44,45,46]. Jeon et al [26] reported that the incidence of loco-regional recurrence in patients with multifocal PTMC after unilateral lobectomy and total thyroidectomy was nonsignificant (3.15% vs 0.78%, p = 0.244). Kim et al [42] found that among PTMC patients who receive hemithyroidectomy, no significant differences of recurrence rate were found between multifocal PTMC and unifocal PTMC (1.5% vs 1.8%, p = 1.000). Zhou et al [44] also reported that although multifocal PTMC were significantly related to central LNM in the univariate analysis, it was the independent factor after multivariate analysis. Furthermore, no significant differences in tumor enlargement rates [47] or 10-year progression rates [6] were found between multifocal and unifocal PTMC patients undergoing AS. In this study, the incidences of LNM and recurrent PTMC for multifocal PTMC were both 1.82%, showing no significant differences from those for unifocal PTMC. In addition, no significant differences in the incidence of local tumor progression and the RFS rates were found between the two groups. The association between multifocality and recurrence also remained nonsignificant. The little impact of multifocality on the recurrence after RFA for PTMC might be explained by the indolent nature and the early treatment of multifocal lesions which might not yet demonstrate aggressive behavior. It suggested that multifocality might not increase the risk of local tumor progression after RFA for PTMC, and patients with multifocal PTMC might be treated following different strategies according to their different situations or preference.
This study showed that RFA for multifocal PTMC was equally safe. No patients experienced complications related to RFA. In contrast, the incidence of transient hypocalcemia and transient vocal fold paralysis after surgery for multifocal PTMC was 4.3% and 5.5%, respectively [26]. Several strategies were used in this study to minimize the complications. First, sufficient pre-ablation evaluation was very important, particularly for multifocal PTMC patients, which could not only identify the numbers and locations of lesions, but also determine an appropriate ablation route. Second, during RFA, the deeper-located lesion was ablated first because the high-echoic area caused by RFA procedure could shield the structure beneath, which made it impossible to ablate lesion in the deeper area [12]. Third, to prevent thermal injury, the moving-shot technique, trans-isthmic approach, and hydrodissection technique were used [33]. In addition, the RFA procedure was performed by an experienced physician because knowledge of ultrasound-based neck anatomy was necessary to prevent nerve injury [33].
Although considerable long-term clinical outcomes of RFA for both unifocal and multifocal PTMC were observed in this study, some concerns could not be ignored. First, the sensitivity of ultrasound to detect the multifocal tumors and microscopic central metastatic LN was low [2]. Studies showed that preoperative multifocal PTMC was a significant risk factor associated with bilateral thyroid lobe involvement at presentation, most of which were incidentally diagnosed by surgical pathology [37, 40, 41]. Accordingly, the existence of hidden metastasis and occult lesions could not be completely excluded. However, for low-risk PTMC, their effects on overall survival were small [2]. Second, the diagnostic accuracy of ultrasound to detection ETE was limited. Although microscopic ETE identified only on histological examination had no impact on AJCC/TNM risk of mortality system, gross ETE was still an unfavorable prognostic factor [48]. Moreover, multifocal PTMC with ETE were intermediate risk with a loco-regional recurrent rate varying from 4 to 32% depending on different situations [2]. Therefore, the indication of RFA should be strict with thorough preoperative evaluation. Multiple modalities including ultrasound, CEUS, and CT could be used to evaluate the PTMC lesions, which were reported to improve the diagnostic performance of ETE and LNM [2, 49,50,51]. Only patients with intrathyroidal PTMC without clinically evident metastases or local invasion who refuse surgery or AS could be considered as a candidate for RFA.
There were some limitations in this study. First, this was a single-center retrospective study. Second, the sample size of multifocal PTMC was relatively small. Third, given the good prognosis of PTMC, a longer follow-up period is still needed to confirm the results. Fourth, this study did not compare RFA with surgery or RFA with AS for the treatment of multifocal PTMC. Further studies about the comparison between RFA, surgery, and AS for the treatment of low-risk PTMC are needed. Despite these limitations, no previous study has addressed the clinical outcomes of RFA for multifocal PTMC. Our finding may help in understanding the indication of RFA for primary PTMC.
In conclusion, the impact of multifocality on the prognosis after RFA for low-risk PTMC was little. RFA might be a promising treatment for both unifocal and multifocal PTMC in properly selected patients after sufficient preoperative evaluation.
Abbreviations
- AS:
-
Active surveillance
- CEUS:
-
Contrast-enhanced ultrasound
- CLT:
-
Chronic lymphocytic thyroiditis
- CNB:
-
Core-needle biopsy
- ETE:
-
Extrathyroidal extension
- FNA:
-
Fine-needle aspiration
- LNM:
-
Lymph node metastasis
- M group:
-
Multifocal group
- PSM:
-
Propensity score matching
- PTMC:
-
Papillary thyroid microcarcinoma
- RFA:
-
Radiofrequency ablation
- RFS:
-
Recurrence-free survival
- U group:
-
Unifocal group
- VRR:
-
Volume reduction rate
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Haugen BR, Alexander EK, Bible KC et al (2015) 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133
Miyauchi A, Ito Y, Oda H (2017) Insights into the management of papillary microcarcinoma of the thyroid. Thyroid 28:23–31
Zheng WH, Wang KJ, Wu JZ, Wang WD, Shang JB (2018) Multifocality is associated with central neck lymph node metastases in papillary thyroid microcarcinoma. Cancer Manag Res 10:1527–1533
Zanocco KA, Hershman JM, Leung AM (2019) Active surveillance of low-risk thyroid cancer. JAMA 321:2020–2021
Sugitani I, Ito Y, Takeuchi D et al (2021) Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery Task Force on Management for Papillary Thyroid Microcarcinoma. Thyroid 31:183–192
Zhang M, Luo Y, Zhang Y, Tang J (2016) Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid 26:1581–1587
Teng DK, Li WH, Du JR, Wang H, Yang DY, Wu XL (2020) Effects of microwave ablation on papillary thyroid microcarcinoma: a five-year follow-up report. Thyroid 30:1752–1758
Zhou W, Jiang S, Zhan W, Zhou J, Xu S, Zhang L (2017) Ultrasound-guided percutaneous laser ablation of unifocal T1N0M0 papillary thyroid microcarcinoma: Preliminary results. Eur Radiol 27:2934–2940
Cho SJ, Baek SM, Lim HK, Lee KD, Son JM, Baek JH (2020) Long-term follow-up results of ultrasound-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: more than 5-year follow-up for 84 tumors. Thyroid 30:1745–1751
Teng DK, Li HQ, Sui GQ et al (2019) Preliminary report of microwave ablation for the primary papillary thyroid microcarcinoma: a large-cohort of 185 patients feasibility study. Endocrine 64:109–117
Wu R, Luo YK, Tang J et al (2020) Ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma: a retrospective analysis of 198 patients. Int J Hyperth 37:168–174
Lim HK, Cho SJ, Baek JH et al (2019) US-guided radiofrequency ablation for low-risk papillary thyroid microcarcinoma: efficacy and safety in a large population. Korean J Radiol 20:1653–1661
Yan L, Luo Y, Zhang Y et al (2020) The clinical application of core-needle biopsy after radiofrequency ablation for low-risk papillary thyroid microcarcinoma: a large cohort of 202 patients study. J Cancer 11:5257–5263
Ji L, Wu Q, Gu J et al (2019) Ultrasound-guided percutaneous laser ablation for papillary thyroid microcarcinoma: a retrospective analysis of 37 patients. Cancer Imaging 19:16
Zhang Y, Zhang MB, Luo YK, Li J, Zhang Y, Tang J (2019) Effect of chronic lymphocytic thyroiditis on the efficacy and safety of ultrasound-guided radiofrequency ablation for papillary thyroid microcarcinoma. Cancer Med 8:5450–5458
Yue W, Wang S, Yu S, Wang B (2014) Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperth 30:150–157
J-h K, Baek JH, Sung JY et al (2017) Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperth 33:212–219
Li J, Liu Y, Liu J, Qian L (2018) Ultrasound-guided percutaneous microwave ablation versus surgery for papillary thyroid microcarcinoma. Int J Hyperth 34:653–659
Zhou W, Ni XF, Xu SY, Zhang L, Chen YD, Zhan WW (2019) Ultrasound-guided laser ablation versus surgery for solitary papillary thyroid microcarcinoma: a retrospective study. Int J Hyperth 36:897–904
Yue WW, Qi L, Wang DD et al (2020) US-guided microwave ablation of low-risk papillary thyroid microcarcinoma: longer-term results of a prospective study. J Clin Endocrinol Metab 105
Zhang M, Tufano RP, Russell JO et al (2020) Ultrasound-guided radiofrequency ablation versus surgery for low-risk papillary thyroid microcarcinoma: results of over 5 years' follow-up. Thyroid 30:408–417
Zhang L, Zhou W, Zhan W, Peng Y, Jiang S, Xu S (2018) Percutaneous laser ablation of unifocal papillary thyroid microcarcinoma: utility of conventional ultrasound and contrast-enhanced ultrasound in assessing local therapeutic response. World J Surg 42:2476–2484
Choi Y, Jung SL (2020) Efficacy and safety of thermal ablation techniques for the treatment of primary papillary thyroid microcarcinoma: a systematic review and meta-analysis. Thyroid 30:720–731
Tong M, Li S, Li Y, Li Y, Feng Y, Che Y (2019) Efficacy and safety of radiofrequency, microwave and laser ablation for treating papillary thyroid microcarcinoma: a systematic review and meta-analysis. Int J Hyperth 36:1278–1286
Jeon YW, Gwak HG, Lim ST, Schneider J, Suh YJ (2019) Long-term prognosis of unilateral and multifocal papillary thyroid microcarcinoma after unilateral lobectomy versus total thyroidectomy. Ann Surg Oncol 26:2952–2958
Sacks D, McClenny TE, Cardella JF, Lewis CA (2003) Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Cho SJ, Suh CH, Baek JH et al (2019) Active surveillance for small papillary thyroid cancer: a systematic review and meta-analysis. Thyroid 29:1399–1408
Oh HS, Ha J, Kim HI et al (2018) Active surveillance of low-risk papillary thyroid microcarcinoma: a multi-center cohort study in Korea. Thyroid 28:1587–1594
Ito Y, Miyauchi A, Oda H (2018) Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol 44:307–315
Nickel B, Brito JP, Barratt A, Jordan S, Moynihan R, McCaffery K (2017) Clinicians' views on management and terminology for papillary thyroid microcarcinoma: a qualitative study. Thyroid 27:661–671
Oh H S, Kwon H, Song E et al (2019) Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid 29(5):642–649
Kim JH, Baek JH, Lim HK et al (2018) 2017 Thyroid radiofrequency ablation guideline: Korean Society of Thyroid Radiology. Korean J Radiol 19:632–655
Li J, Liu Y, Liu J, Yang P, Hu X, Qian L (2019) A comparative study of short-term efficacy and safety for thyroid micropapillary carcinoma patients after microwave ablation or surgery. Int J Hyperth 36:640–646
Cho S J, Baek S M, Na D G, Lee K D, Shong Y K and Baek J H (2021) Five-year follow-up results of thermal ablation for low-risk papillary thyroid microcarcinomas: systematic review and metaanalysis. Eur Radiol. https://doi.org/10.1007/s00330-021-07808-x
Ding M, Tang X, Cui D et al (2019) Clinical outcomes of ultrasound-guided radiofrequency ablation for the treatment of primary papillary thyroid microcarcinoma. Clin Radiol 74:712–717
So YK, Kim MW, Son YI (2015) Multifocality and bilaterality of papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol 8:174–178
Kaliszewski K, Diakowska D, Wojtczak B, Migon J, Kasprzyk A, Rudnicki J (2019) The occurrence of and predictive factors for multifocality and bilaterality in patients with papillary thyroid microcarcinoma. Medicine (Baltimore) 98:e15609
W Z, K W, J W, W W, J S (2018) Multifocality is associated with central neck lymph node metastases in papillary thyroid microcarcinoma. Cancer Manag Res 10:1527–1533
Karatzas T, Vasileiadis I, Charitoudis G, Karakostas E, Tseleni-Balafouta S, Kouraklis G (2013) Bilateral versus unilateral papillary thyroid microcarcinoma: predictive factors and associated histopathological findings following total thyroidectomy. Hormones (Athens) 12:529–536
Connor MP, Wells D, Schmalbach CE (2011) Variables predictive of bilateral occult papillary microcarcinoma following total thyroidectomy. Otolaryngol Head Neck Surg 144:210–215
Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS (2015) Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol 22:125–131
Choi WR, Roh JL, Gong G et al (2019) Multifocality of papillary thyroid carcinoma as a risk factor for disease recurrence. Oral Oncol 94:106–110
Zhou YL, Gao EL, Zhang W et al (2012) Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol 10:67
Iacobone M, Jansson S, Barczynski M, Goretzki P (2014) Multifocal papillary thyroid carcinoma-a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg 399:141–154
Feng JW, Qu Z, Qin AC, Pan H, Ye J, Jiang Y (2020) Significance of multifocality in papillary thyroid carcinoma. Eur J Surg Oncol
Ito Y, Miyauchi A, Inoue H et al (2010) An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg 34:28–35
Tuttle RM, Haugen B, Perrier ND (2017) Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): what changed and why? Thyroid 27:751–756
Lee DY, Kwon TK, Sung MW, Kim KH, Hah JH (2014) Prediction of extrathyroidal extension using ultrasonography and computed tomography. Int J Endocrinol 2014:351058
Liu Y, Liu H, Qian CL, Lin MS, Li FH (2017) Utility of quantitative contrast-enhanced ultrasound for the prediction of extracapsular extension in papillary thyroid carcinoma. Sci Rep 7:1472
Wei X, Li Y, Zhang S, Gao M (2014) Prediction of thyroid extracapsular extension with cervical lymph node metastases (ECE-LN) by CEUS and BRAF expression in papillary thyroid carcinoma. Tumour Biol 35:8559–8564
Funding
No funding
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yukun Luo.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, L., Zhang, M., Song, Q. et al. Clinical outcomes of radiofrequency ablation for multifocal papillary thyroid microcarcinoma versus unifocal papillary thyroid microcarcinoma: a propensity-matched cohort study. Eur Radiol 32, 1216–1226 (2022). https://doi.org/10.1007/s00330-021-08133-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08133-z