Abstract
Purpose
Patellar maltracking due to incorrect component alignment is considered as a main reason for anterior knee pain after total knee arthroplasty (TKA). In contrast to coronal and axial component placement, the influence of sagittal component alignment on patellar kinematics has not been investigated so far.
Methods
In ten lower cadaveric limbs, TKAs were implanted using a commercial computer navigation system. In six knees, the femoral component was aligned in 5° and in four knees in 0° of flexion, respectively. Patellar kinematics were registered by means of a computer navigation system using an additional patella tracking array and correlated with femoral and tibial sagittal component alignment.
Results
Sagittal component alignment significantly altered patellar mediolateral shift (p < 0.05). In contrast, patellar epicondylar distance, rotation and tilt were not significantly influenced.
Conclusions
Sagittal component alignment in TKA has a major impact on patellar kinematics and should therefore be considered while addressing tibiofemoral kinematics intraoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implant alignment is an issue of high importance in total knee arthroplasty (TKA) [7, 17, 32], and malalignment can cause premature component loosening [3, 27], abnormal wear [10, 33] and patellofemoral complications such as anterior knee pain [5, 6, 12]. The appearance of anterior knee pain after TKA is reported in up to 20 % of the cases, and patellar maltracking is considered as one of the main reason [4, 6, 11, 12, 21, 28]. Several investigations state that patellar maltracking is mainly caused by rotational malalignment of the femoral and tibial components [19]; especially, internal rotation of the femoral component seems to contribute to altered patellofemoral kinematics and consequently to the occurrence of anterior knee pain [6, 11]. In a radiological investigation, Berger et al. [6] showed that combined internal rotation of the femoral and tibial components directly correlates with severity of post-operative patellofemoral complications. Lüring et al. [24] demonstrated that rotation of the femoral component according to the transepicondylar axis leads to a more physiological patellar tracking than 3° of external rotation relative to the posterior condylar line. Miller et al. [26] also stated most physiological patellar tracking in knees with a femoral component rotation parallel to the transepicondylar axis. In a previous investigation, we were able to demonstrate the major impact of rotational component alignment on patellofemoral kinematics in TKA by means of a computer navigation system [20]. In contrast to coronal and axial component alignment, just a few studies have investigated the role of sagittal component alignment in TKA in general, as reported in a current review by Gromov et al. [16]. In one recent clinical investigation, a correlation between femoral component flexion and the appearance of painful patellar crepitus could be found [9]. However, the influence of sagittal component alignment on patellar kinematics has not been investigated so far. We hypothesized that changes in sagittal component alignment in TKA significantly alter patellar kinematics and should therefore be considered while addressing tibiofemoral kinematics intraoperatively. The evaluation and quantification of the effect of sagittal component changes on patellar kinematics might help the surgeon to reconsider extensive femoral or tibial component changes.
Materials and methods
Ten lower limbs of five whole-body specimens were used for this investigation. All knees were free of patella or trochlea dysplasia, severe arthritic deterioration or varus/valgus alignment deviations more than 3° and had no history of prior surgical procedures or injuries at the lower limbs. Patellar kinematics relative to the femur were measured using a commercial optical imageless computer navigation system including patellar tracking (Knee Patella Tracking Software, BrainLAB; Feldkirchen, Germany) before and after standard TKA. To achieve standardized conditions, the limbs were placed onto a passive motion machine during each measurement and an extension/flexion cycle was performed, while no muscle force was applied.
Surgical procedure and experimental setup
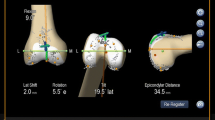
Before performing a standard medial parapatellar approach, the capsule of each knee was marked at four defined locations (3 cm proximal to the superior patellar tip, at the medial superior patellar edge, centrally at the medial patellar edge and at the medial distal patellar edge) to ensure later anatomical closure. A passive optical reference array was secured into the distal medial femur via an additional 1-cm incision to avoid soft tissue tension during measurements and the proximal medial tibia. After referencing the hip centre by circumduction, the landmarks needed to assess femorotibial kinematics (femoral epicondyles, femoral knee centre, medial and lateral malleolus, tibial plateau centre) were digitized. The line connecting the middle of the posterior cruciate ligament to the medial border of the patellar tendon attachment according to Akagi et al. [1] was chosen as the tibial anterior/posterior axis. Additionally, a patella array was fixed onto the frontside of the patella by a small screw as recommended by the manufacturer. A central point at the medial edge, the superior and inferior tip and at the highest point of the posterior articular ridge of the patella were chosen to define the patella coordinate frame. After accurate anatomical closure of the joint capsule at the prior marked locations, natural patellar kinematics and the relative orientation between the femoral, tibial and patellar coordinate frame were recorded. Three full extension/flexion cycles were performed, while patellar tracking was measured between 30° and 90° of flexion. Due to missing quadriceps muscle load, values until 30° of flexion were irregular and excepted from the experimental protocol. The limbs were placed onto a passive motion machine in neutral position without fixation of either the femur or the tibia to simulate intraoperative conditions. Subsequently, the implantation of trial components (PFC Sigma, cruciate retaining, fixed bearing inlay; DePuy, Warsaw, Indiana, USA) was performed with the tibial and distal femoral cut perpendicular to the tibial and femoral mechanical axis. The femoral component consists of a “J-sign” design and a trochlea groove perpendicular to the posterior condylar line. In all knees, the femoral rotation was set to 3° of external rotation in relation to the femoral posterior condylar line. In the sagittal plane, the restoration of the preoperative tibia slope was aspired. In six cases, the femoral component flexion was set to 5°, while four knees were implanted with 0° of femoral component flexion in relation to the femoral mechanical axis. Due to distal femoral bowing, the fifth knee planned for the 0° femoral flexion group was switched to the 5° flexion group to avoid ventral notching. The rotational alignment of the tibial trays was set to the line connecting the middle of the posterior cruciate ligament to the medial border of the patellar tendon attachment according to Akagi et al. [1], using the navigation pointer, and was stabilized using screws to ensure stable conditions during measurements. No surgical patellar intervention such as resurfacing or patelloplasty was performed, and no ligament releases were conducted due to well-balanced healthy knees. A restoration of the joint line height could be achieved in every knee by means of the navigation system. A second measurement of patellar kinematics was taken closing the joint capsule again at the prior marked locations to achieve equal soft tissue tension. Prior patellar kinematics were measured, each time the accuracy of the desired position of the trial components was verified using the navigation system and adapted if required (Fig. 1). For measurement of patellar kinematics, patellar shift (mediolateral translation in millimetres, medial: +/lateral: −), patellar axial tilt (in degrees, medial: −/lateral: +) and patellar rotation (in degrees, medial: +/lateral: −) were chosen, which represent the most common patellar kinematic parameters in the literature. Additionally, epicondylar distance was chosen, which represent the anterior/posterior position of the patella in relation to the femur during the whole flexion cycle (in millimetres: perpendicular distance of the highest point of the posterior articular ridge of the patella to the anatomical transepicondylar axis). Subsequently, values for femoral and tibial sagittal component alignment were documented and correlated with patellar kinematics. The optical computer navigation system used has been verified to be a reliable measurement procedure to evaluate three-dimensional tibiofemoral and patellofemoral kinematics with an accuracy of 0.1 mm and 0.1 [15, 23]. Figure 2 shows patellar kinematics before and after trial component implantation.
Statistical analysis
Generalized linear models with intercept as random effect and tibiofemoral flexion as fixed effect were used to assess the influence of femoral component flexion and posterior tibial component slope on patellar kinematic parameters. The student’s t test was used to assess significant differences between the groups. A p value <0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.3.
Results
Influence of sagittal component alignment
Both femoral and tibial sagittal component alignment significant changed patellar mediolateral shift. A change of femoral component flexion from 0° to 5° with a 1° increase in posterior tibial component slope in the investigated range of 4°–6.5° (mean 5.3°) leads to a patellar lateral shift of 17.3 mm (9.8 + 7.5). Sagittal component alignment did not significantly influence patellar epicondylar distance, patellar rotation or tilt. However, an increase in femoral flexion and tibial slope decreased epicondylar distance and increased patella internal rotation. Furthermore, an increase in femoral component flexion led to an increase in patellar lateral tilt, while a decreased by higher posterior tibial component slope was observed (Table 1).
Restoration of preoperative patellar kinematics
Patellar mediolateral shift, rotation and tilt showed a closer to normal restoration between 30° and 90° of flexion in knees implanted with 5° of femoral component flexion with statistically significant values for mediolateral shift and tilt (p < 0.001). Interestingly, for epicondylar distance better values in the 0° femoral flexion group at 30° of flexion were found, while the 5° femoral flexion group was closer to normal at 80° and 90° of flexion. However, this was not found statistically significant. In all knees, the patella shifted more medially over tibiofemoral flexion. In the 5° flexion group as well as in the 0° flexion group, the patellae shifted more laterally at all flexion angles and tilted medially from 50° to 90° of flexion, in the opposite direction, compared to the preoperative knee. In general, the patellae rotated externally over tibiofemoral flexion, while the epicondylar distance decreased in all knees. Compared to the preoperative knee, the 5° and 0° flexion group showed more externally rotated patellae and a decrease in epicondylar distance during the whole motion cycle. Figure 3 shows patellar kinematics of the preoperative knee and after implantation of the TKA with the femoral component in 5° and 0° of flexion. For a better visualization over tibiofemoral flexion, the additional influence of tibial component slope on patellar kinematics was not considered in this figure.
Patellar kinematics in different tibiofemoral flexion angles of the preoperative knee and after TKA with 5° and 0° of femoral component flexion (mediolateral shift, medial: +/lateral: −; axial tilt, medial: −/lateral: +; rotation, medial: +/lateral: −; epicondylar distance in mm) without consideration of the influence of tibial component slope
Discussion
The most important finding of this investigation was that in addition to rotational component alignment, sagittal femoral and tibial component position has a significant influence on patellar kinematics. Thus, our hypothesis could be confirmed. In general, patellar kinematics of the preoperative knees could be restored closer to normal with the femoral component flexed to 5° compared to 0°. Alignment changes in the sagittal profile significantly altered mediolateral patellar shift. However, no significant influence of both femoral component flexion and posterior tibial component slope on patellar tilt, rotation and epicondylar distance could be found in this investigation. The appropriate sagittal component alignment in TKA and its impact on kinematic behaviour and functional outcome has been studied relatively little [8, 29]. Some recent investigations reported a negative influence of both forced femoral component extension, which leads to ventral notching, and forced femoral flexion, which results in an overstuffing of the ventral prosthetic shield and reduces the contact surface of the component. Both can cause severe post-operative complications such as fractures, post-operative anterior knee pain or early prosthetic loosening [9, 14]. Furthermore, it is known that increased femoral component flexion decreases the flexion gap and alters condylar lift-off and tibiofemoral kinematics in TKA [2, 13]. In the current literature, a femoral component flexion between 0° and 3° is recommended to achieve satisfying tibiofemoral kinematics [16]. Kim et al. [22]. found that sagittal alignment of the tibial component less than 0° or greater than 7° posterior slope had a higher failure rate as compared to a neutrally aligned control group. Cadaver investigations have reported that an increase in posterior tibial component slope may lead to increased flexion following TKR, while a clinical study by Kansara and Markel [18] could not find any difference in post-operative flexion. Stoddard et al. [31] could not find any difference in patellar tracking between a symmetrical and an asymmetrical femoral component. However, in the current literature no study concerning the influence of sagittal component alignment on patellar kinematics in TKA could be found. For that reason, there is no comparable data available. The strong impact on patellar mediolateral shift might be explained by changed parapatellar soft tissue tension caused by changed femoral component flexion. Interestingly, both femoral component flexion groups showed an increase in patellar medial tilt from 50° to 90° of flexion. This might indicate a too prominent lateral femoral condyle which cannot be balanced by femoral component flexion, but rather femoral component rotation as described in our previous investigation [20]. We assume that the better restoration of epicondylar distance in the 0° flexion group at 30° might be ascribed to the more prominent proximal femoral component tip of the used “J-curved” prosthesis caused by the 5° of femoral component flexion. However, we could not find statistically significant results for this effect. The influence of tibial component slope on patellar kinematics might be ascribed to an increase in tibial anterior shift with increased posterior tibial slope as previously published [13, 27]. Previous investigations report altered tibiofemoral contact points after both cruciate-retaining and cruciate-substituting TKA compared to a healthy control group using fluoroscopy [9, 30]. By means of the computer navigation system, a parallel orientation of the trochlea groove to the mechanical axis was generated which might play an important role in terms of muscle load and applied soft tissue tension. Other femoral component designs with an anatomically orientated trochlea groove might better restore the preoperative Q-angle and consecutively patellar tracking.
The present investigation has some limitations. Patellar kinematics were measured without muscle load and through passive range of motion from 30° to 90° of flexion to avoid irregular data in early flexion angles. Thus, the tibiofemoral “screw home mechanism” and its influence on patellofemoral kinematics could not be investigated in the present study. However, the data were collected using healthy cadaveric knees still attached to the torso to simulate intraoperative conditions, and can therefore be used for further clinical investigations. Moreover, Masri and Mc Cormack [25] reported that patellar kinematics are not strongly influenced by quadriceps contractions compared to passive motion. In the preoperative knee, reference points on the patella need to be registered after arthrotomy. Hence, patellar tracking of the natural knee was measured after anatomical closure of the capsule and might differ from untouched knee joints. However, defined marks were set to achieve same anatomical closure before and after TKA and again intraoperative conditions were tested. The closure and reopening of the arthrotomy as well as the motion cycle on the passive motion machine was conducted with great care, due to possible deterioration of the capsule. The tibial trial components were fixed by screws to ensure stable conditions during measurements. To evaluate changes in patellar kinematics, the femoral component was set to 0° and 5° of flexion and posterior tibial component slope ranged from 4° to 6.5° relative to the mechanical axis. Thus, results for different sagittal component alignment are just valid if a linear change of patellar kinematics beside this range can be expected. Because of different sagittal femoral component alignment and therefore resulting complex experimental set-up, further measurements in different posterior tibial component alignments were renounced. Due to a cadaveric investigation and the small group sizes, results should not be overestimated. Furthermore, the usage of components from one manufacturer might have produced unique patellar kinematics, not transferable to knees resurfaced with other implants. However, due to the great impact of sagittal component alignment on patellofemoral kinematics, we believe that the results of the present investigation are widely applicable. Finally, the patella was not resurfaced in order to compare patellar kinematics between the preoperative knee and after TKA. Therefore, a resurfaced patella might have shown a different tracking behaviour in some extent.
Pertaining to the present data, surgeons should be aware of altering patellar kinematics, especially mediolateral shift, while increasing femoral component flexion and/or posterior tibial component slope to a great extent. If possible, the preoperative sagittal profile should be restored and changes by balancing the tibiofemoral flexion gap should be kept to a minimum until the clinical relevance has been investigated.
Conclusion
The hypothesis that sagittal component alignment significantly alters patellar kinematics could be confirmed. Thus, sagittal component alignment should be considered with regard to patellar kinematic changes during TKA. Further biomechanical and clinical investigations seem to be necessary to analyse the clinical relevance of this issue regarding post-operative anterior knee pain in TKA.
References
Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C (2004) An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res 420:213–219
Baier C, Fitz W, Craiovan B, Keshmiri A, Winkler S, Springorum R, Grifka J, Beckmann J (2014) Improved kinematics of total knee replacement following partially navigated modified gap-balancing technique. Int Orthop 38:243–249
Bargren J, Blaha J, Freeman M (1983) Alignment in total knee arthroplasty. Correlated biomechanical and clinical observations. Clin Orthop Relat Res 173:178–183
Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L (2001) Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop Relat Res 392:46–55
Belvedere C, Ensini A, Leardini A, Dedda V, Feliciangeli A, Cenni F, Timoncini A, Barbadoro P, Giannini S (2014) Tibio-femoral and patello-femoral joint kinematics during navigated total knee arthroplasty with patellar resurfacing. Knee Surg Sports Traumatol Arthrosc 22:1719–1727
Berger RA, Crossett LS, Jacobs JJ, Rubash HE (1998) Malrotation causing patellofemoral complications after total knee arthroplasty. Clin Orthop Relat Res 356:144–153
Colle F, Lopomo N, Bruni D, Visani A, Iacono F, Zaffagnini S, Marcacci M (2014) Analysis of knee functional flexion axis in navigated TKA: identification and repeatability before and after implant positioning. Knee Surg Sports Traumatol Arthrosc 22:694–702
Dennis DA, Komistek RD, Colwell CEJ, Ranawat CS, Scott RD, Thornhill TS, Lapp MA (1998) In vivo anteroposterior femorotibial translation of total knee arthroplasty: a multicenter analysis. Clin Orthop Relat Res 356:47–57
Dennis D, Kim R, Johnson D, Springer B, Fehring T, Sharma A (2011) The John Insall Award: control-matched evaluation of painful patellar crepitus after total knee arthroplasty. Clin Orthop Relat Res 469:10–17
Eckhoff DG, Metzger RG, Vandewalle MV (1995) Malrotation associated with implant alignment technique in total knee arthroplasty. Clin Orthop Relat Res 321:28–31
Farrokhi S, Keyak JH, Powers CM (2011) Individuals with patellofemoral pain exhibit greater patellofemoral joint stress: a finite element analysis study. Osteoarthr Cartil 19(3):287–294
Figgie H, Goldberg V, Figgie M, Inglis A, Kelly M, Sobel M (1989) The effect of alignment of the implant on fractures of the patella after condylar total knee arthroplasty. J Bone Joint Surg 71:1031–1039
Fitz W, Sodha S, Reichmann W, Minas T (2012) Does a modified gap-balancing technique result in medial-pivot knee kinematics in cruciate-retaining total knee arthroplasty? A pilot study. Clin Orthop Relat Res 470:91–98
Giffin JR, Vogrin TM, Zantop T, Woo SL-Y, Harner CD (2004) Effects of increasing tibial slope on the biomechanics of the knee. Am J Sports Med 32:376–382
Griffin FM, Insall JN, Scuderi GR (2000) Accuracy of soft tissue balancing in total knee arthroplasty. J Arthroplasty 15:970–973
Gromov K, Korchi M, Thomsen MG, Husted H, Troelsen A (2014) What is the optimal alignment of the tibial and femoral components in knee arthroplasty? Acta Orthop 85(5):480–487
Howell SM, Papadopoulos S, Kuznik KT, Hull ML (2013) Accurate alignment and high function after kinematically aligned TKA performed with generic instruments. Knee Surg Sports Traumatol Arthrosc 21:2271–2280
Kansara D, Markel DC (2006) The effect of posterior tibial slope on range of motion after total knee arthroplasty. J Arthroplasty 21:809–813
Keshmiri A, Maderbacher G, Baier C, Sendtner E, Schaumburger J, Zeman F, Grifka J, Springorum HR (2015) The influence of component alignment on patellar kinematics in total knee arthroplasty: an in vivo study using a navigation system. Acta Orthop 86:1–7
Keshmiri A, Springorum H, Baier C, Zeman F, Grifka J, Maderbacher G (2014) Is it possible to re-establish pre-operative patellar kinematics using a ligament-balanced technique in total knee arthroplasty? A cadaveric investigation. Int Orthop 39(3):441–448
Kienapfel H, Springorum H-P, Ziegler A, Klose K-J, Georg C, Griss P (2003) Der Einfluss der Femur- und Tibiakomponentenrotation auf das patellofemorale Versagen beim künstlichen Kniegelenkersatz. Orthop 32:312–318
Kim Y-H, Park J-W, Kim J-S, Park S-D (2014) The relationship between the survival of total knee arthroplasty and postoperative coronal, sagittal and rotational alignment of knee prosthesis. Int Orthop 38:379–385
Lee D-H, Park J-H, Song D-I, Padhy D, Jeong W-K, Han S-B (2010) Accuracy of soft tissue balancing in TKA: comparison between navigation-assisted gap balancing and conventional measured resection. Knee Surg Sports Traumatol Arthrosc 18:381–387
Luring C, Perlick L, Bathis H, Tingart M, Grifka J (2007) The effect of femoral component rotation on patellar tracking in total knee arthroplasty. Orthopedics 30(11):965–967
Masri BA, McCormack RG (1995) The effect of knee flexion and quadriceps contraction on the axial view of the patella. Clin J Sport Med 5(1):9–17
Miller MC, Berger RA, Petrella AJ, Karmas A, Rubash HE (2001) Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res 392:38–45
Moreland JR (1988) Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res 226:49–64
Ranawat CS (1986) The patellofemoral joint in total condylar knee arthroplasty: pros and cons based on five-to ten-year follow-up observations. Clin Orthop Relat Res 205:93–99
Shelburne KB, Kim H-J, Sterett WI, Pandy MG (2011) Effect of posterior tibial slope on knee biomechanics during functional activity. J Orthop Res 29:223–231
Stiehl J, Komistek R, Dennis D, Paxson R, Hoff W (1995) Fluoroscopic analysis of kinematics after posterior-cruciate-retaining knee arthroplasty. J Bone Joint Surg 77(6):884–889
Stoddard J, Deehan D, Bull A, McCaskie A, Amis A (2014) No difference in patellar tracking between symmetrical and asymmetrical femoral component designs in TKA. Knee Surg Sports Traumatol Arthrosc 22:534–542
Vandenneucker H, Labey L, Victor J, Vander Sloten J, Desloovere K, Bellemans J (2014) Patellofemoral arthroplasty influences tibiofemoral kinematics: the effect of patellar thickness. Knee Surg Sports Traumatol Arthrosc 22:2560–2568
Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG (1994) Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res 299:31–43
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Additional information
The work was performed at the Department of Orthopedic Surgery at the Medical University of Regensburg/Germany.
Rights and permissions
About this article
Cite this article
Keshmiri, A., Springorum, H.R., Baier, C. et al. Changes in sagittal component alignment alters patellar kinematics in TKA: an in vitro study. Knee Surg Sports Traumatol Arthrosc 24, 823–829 (2016). https://doi.org/10.1007/s00167-016-4004-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4004-6