Abstract
Sustainability is the development which meets the needs of the present without compromising the ability of future generations to fulfill their needs. Environmental sustainability respects and cares for all kinds of life forms existence without affecting the sustenance of natural resources. The best method of sustaining the environment is paying back all the components of ecosystem services in a recyclable mode. Where in biotic and abiotic harmony of environment restores aesthetic values and ecosystem services of the nature. This in turn maintains intricate equilibrium required for resurrecting the natural ecosystems. Environmental biotechnology is the branch of biotechnology that addresses environmental issues removal of pollutants, renewable energy generation or biomass production, by involving biological entities and their process. Environmental biotechnology has its greatest contribution to agriculture, especially by improving crop yields for environment sustenance. It offers opportunities to create designer crops of specific environments and to make crops more efficient producers of food and energy. Thus, biotechnology can manipulate primary energy flows; it can also reduce fossil-fuel energy inputs into agricultural systems. Moreover, it contributes to the mitigation of environmental problems such as deforestation and soil erosion. Green energy methods/biofuels are urgently needed to replace fossil fuels in order to battle pollution and the threat of global warming. Biotechnology constitutes a vehicle for the improved manipulation of biogeochemical cycles, wherein bioremediation and biodegradation alleviate conditions of polluted soil and degraded water ecosystems. Industrial biotechnology aims to alter the manufacturing process by reducing wastes generation-conserving natural resources, trimming costs, and speeding new “greener” market products. Emerging biotechnologies having low-input techniques involving microbes, plants and animals offering novel approaches (genetic manipulation or ‘engineering’) for striking a balance between developmental needs and environmental conservation. This chapter reviews the issues relating to the use of biotechnological methods vis-à-vis biotools in solving the problems of environmental degradation and sustainable development .
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
For centuries, humans are in hope that soil, water, and air components of the environment are sufficient enough to sink and assimilate wastes generated by population centers, industry, and farming. While we now know that it’s not true. Pollution occurs as a result of improper management of domestic, municipal, and industrial wastes/xenoibiotics (unnatural or synthetic; from the Greek xenons, meaning “foreign) and their rapid accumulation in the environment beyond carrying/assimilating limits of natural ecosystem causes hazard and/or nuisance to mankind and environment (Adriano 1986; Alloway 1990; Raskin et al. 1997). Anthropogenic activities due to urbanization, unsafe agricultural practices, rampant industrialization, mining, and exploration are at the forefront of global environmental pollution (Samant et al. 2018; Dharupaneedi et al. 2019). Environmental pollution is not a new phenomenon, yet it remains the world’s greatest problem facing by mankind, and the leading environmental causes of morbidity and mortality.
Currently, human life and environment is greatly affected by three problems: Food security, health problems due to pollutants, and environmental deterioration. Food security and health problems are directly or indirectly linked with the sustenance of clean and safe environment/ecosystem services. These problems need to be attended with eco-friendly approaches so that the biosphere retain, assimilate, restore itself within the carrying capacity, the so-called resilience power of environment. Environment/ecological resilience is the capacity of damage that an ecosystem could withstand without altering its intricate processes and components of functioning. Both developed and developing nations share this burden together, though awareness and imposing stricter laws have contributed to a larger extent in protecting their environment. Today the abuse and overuse of natural resources that result from the pressures of development, population, and poverty have reached a point where the problems of how to sustain the human environment has become a major concern of most governments. The antipollution regulations instituted by most governments remain unforced. However, governments have been enforcing the three Rs: Reduce, Reuse, and Recycle a means to prevent further environmental deterioration. In spite of the global attention to check pollution, the impact is still being felt due to its severe long-term consequences (Arthur et al. 2005). In spite of governments enforcing laws, it is foremost important responsibility lies on mankind in protecting and maintaining the environment in long way.
Sustainability of environment is vital as human progresses into the future. “Sustainability relies on the principle that meeting the requirements of the present needs without compromising the trend of passing the current available resources to future generations.” Magnification of environmental problem can be reduced greatly by the use of alternative (renewable) energy resources (biofuels/pesticides/herbicides) along with the reclamation of degraded natural ecosystems (Khan and Fu 2020; Pande et al. 2020). It is very crucial to conserve diverse life forms (species) existing on the biosphere (biodiversity), which maintains intricate relations operating at each tropic level of different ecosystems, as loss of single biotic or abiotic factors will drastically affect the biogeochemical cycling/ecological functioning required for environmental sustainability. Biodiversity is the pool from which human race derives food, fodder, fuel, fiber, shelter, medicine, and raw materials for industrial goods required for the ever-increasing aspirations of humans. Biodiversity somehow controls and maintains the stability of physical and chemical factors of the environment. Both food and fuel energy resources need to be served in a sustainable way. Resource recovery and recycling, and hazardous waste disposal, are environmentally-beneficial facets of biotechnology. Anthropogenic activities are accounting for loss of species, habitat destruction, and fragmentation, which are the part of biggest issues faced by the mankind on the road map to environmental sustainability. Environmental sustainability broadly deals with the sustenance (support and maintenance) of the environment to continue life on earth in a normal way, where human development and environmental maintenance go hand in hand.
The need of hour is to address these issues through modern technologies unlike traditional approaches to overcome agriculture/food, health, and environmental issues through breeding, traditional medicines, and pollutants degradation. Biotechnology is an interdisciplinary science and technology whose principles are based on the use of biological entities or its processes for the generation of reliable products and services which are being environmental benign (Haq et al. 2020). Biotechnology concepts centered on biodiversity and its gene pool availability, conservation and later controlled redesign, manipulation, and services offered for the environment and mankind’s well-being. Its approaches have implications to cut short the use of fossil fuels and greenhouse emissions by the generation novel means of biofuel s and thus produce biodegradable products, recycle wastes, thus making manufacturing process “greener.” The extraordinary achievements of biotechnology over past three decades enormous to use its modern tools and approaches in many crucial areas of agriculture, food, health care (medicines, vaccines, diagnostics, gene therapy), bioenergy, environmental protection (Yan et al. 2020; Tang et al. 2020; Peng et al. 2020; Pande et al. 2020; Khan and Fu 2020). The current chapter summarized biotechnology latest trends (Fig. 2.1) in agriculture for global food security, conservation, restoration of RET (Rare Endangered Threatened) and endemic plant species, mitigation of environmental pollutants by bioremediation and phytoremediation, cleaner bioenergy/biofuels production by microbes and microbes in modern food production and processing. In line with this, the fears and limitations of biotechnology approaches toward attaining safe environment and possible future directions surmount limitations via the advances mentioned in this chapter.
The low-input biotechnological strategies use biological entities/organisms (microbes, animals, and plants) or bioprocesses aimed at cleaner and more effective ways to maintain the natural and esthetic value of environment thus catering to resurrect the ecosystems. The bioprocesses of the biological organisms are designed, manipulated to produce: (1) Biological alternatives (Bioinsecticides, herbicides, etc.) to replace ruthless use of harmful chemicals in agro-ecosystems. (2) GMOs (Genetically modified organisms) in bioremediation (Kumar et al. 2013; Janssen Dick and Gerhard 2020) and phytoremediation (Ilker et al. 2020) process to alleviate adverse effects of polluted soil and water bodies and for their restoration. (3) Production of Bio-fuels replaces the use fossil fuels thus overcoming environmental pollution & global warming (Tang et al. 2020; Khan and Fu 2020). (4) Genetically engineered microorganisms (GEMs) improved strains of microorganisms having high efficacy and efficiency for modern nutritive (biofortified) food production and process (Zhang et al. 2019; Hanlon and Sewalt 2020). Aforesaid are emerging, reliable, and effective strategies put forward by biotechnology to maintain human health, development , and environment sustenance.
2 Applications of Biotechnology
2.1 Transgenic Crops/Plants
Climate change and food crisis are pressing problems to scientists and policy makers worldwide. Crop production as of now is extremely vulnerable to climate change leading to low yields and diseases, making difficult to guaranty the food security by 2050. Food crisis due to low crop yields/diseases is increasing at an alarming rate due to adverse effects of biotic and abiotic environmental factors and it is difficult to keep pace of current agriculture production to the demands of raising world population. According to United Nations (UN) Food and Agricultural Organization (FAO) estimate, the world population occupancy by 2050 is expected to reach ten billion people (FAO 2018). If we continue to consume energy and food resources at the current pace, we may need more than 3.5 times the current energy supply and 1.7 times the current food supply in 2050 (Kim and Kwak 2020). Exacerbation of plant growth and productivity due to a wide range of stresses has been significantly affecting future global food security needs. In order to bridge the gap between the supply and demand of the ever-increasing global population, it is indispensable to foster new breeds of stress-tolerant crops with traits conferring higher yields in spite of several environmental abiotic and biotic stresses. Thus, understanding how and to what extent climate change will affect agricultural productivity is crucial. Agricultural biotechnology has been addressing the challenge of the food security and nutraceuticals to increasing population without effecting environmental harmony (Van Montagu 2020). Genetically modified (GM) crops represent the most rapidly adopted technology in the history of agriculture, having now reached 25 years of commercial production (Smyth 2019). These crops grown by millions of farmers, many in developing countries, the technology is providing significant economic and environmental benefits, such as reduction in chemical use of 37%, increased yields of 22% and improved farm profits of 68% (Klumper and Qaimm 2014). This area of research has contributed environmentally friendly crops such as insect resistant, herbicide resistant species; oxidative, salt stress as well crops that can fix nitrogen available in the environment (Bloch et al. 2020).
2.1.1 Crops Tolerant to Biotic Stresses
The goals of breeding genetically modified plants correspond to those of conventional plant breeding: on the one hand quantitative (increase in yield) and qualitative improvements (taste, color of the blooms, shelf-life, raw materials), and, on the other hand, developing crop resistance against biotic (fungi, pests, viruses, bacteria, nematode worms) and abiotic stress factors (cold, heat, wet, drought, salt content) of the environment. Plants being sessile, vulnerable to various biotic and abiotic stresses, torment them at each level (morphological, physiological, biochemical, and molecular) that intricate severe repercussions on their growth and productivity.
Agriculture biotechnology aims at sustainable agriculture by solving issues related to food production in an ecological way. Genetic engineering is an effective tool to develop climate-smart crops that can grow in adverse environmental conditions with sustainable yield and productivity (Dhankher and Foyer 2018). One of the early successes of biotechnology is insertion of genes from a naturally occurring soil bacterium, Bacillus thuringiensis (Bt), into maize, cotton, and other crops to impart internal protection from insect feeding. For many farmers, Bt crops are proving to be viable tool for integrated pest management programs by giving growers new pest control choices. In the recent past, several promising microbial insecticides were developed as environmentally friendly biological substitutes replaced the use of noxious chemical pesticides (Samada and Tambunan 2020). Several subspecies of the bacterium B. thuringiensis produce parasporal crystals that after ingestion, kills specific insects. In the gut region of targeted insect, conversion of insecticidal pro-toxin to toxin mediated by the alkaline pH and digestive proteases. The death of the insect is the consequence of the formation of membrane channels in the gut cells. Thus lead to futile use of ATP, in turn lead to decreased cellular metabolism, cessation of feeding, dehydration, and eventually insect dies. The B. thuringiensis toxins are found to be highly specific for a limited number of insect species, non-toxic to non-target insects and biodegradable in nature. Consequently, they are highly unlikely to cause significant biological selection to develop resistant forms under normal conditions (Azizoglu et al. 2020).
In order to control mosquitoes, B. thuringiensis toxin genes were cloned into various microorganisms that live near pond surfaces and thus made to engulf and target mosquito larvae (Laurence et al. 2011). This strategy appears to be an effective means of delivering the B. thuringiensis toxin to the targeted insect/pest. Apart from Cry genes, several other genes such as trypsin inhibitor, protease inhibitors, and cysteine inhibitors have also been cloned in various crop plants to enhance their tolerance against several insect pest diseases (Tanpure et al. 2017). In another study rhizosphere bacteria has been engineered with B. thuringiensis toxin genes lessen the damage caused by insects that attack the roots of plants (Azizoglu et al. 2020). In addition, applied potential of chitinases of B. thuringiensis subsp. Isralensis cryt1A protein has been studied, a means of gene stacking to confer both fungal and pest resistance (Martínez-Zavala et al. 2020).
Root-knot nematode (Meloidogyne incognita) is another group of plant pests that commonly affects the growth and yield of many horticultural crops. Transgenic plants overexpressing several proteinase inhibitor genes (Cysteine proteinase) have been considered as the most effective means of controlling yield losses as they block the metabolic process of Meloidogyne incognita by activating the proteolytic activity of several other important proteinases (Zhang et al. 2015a). Resistance against root-knot nematode has been developed in transgenic brinjal, tomato, and potato wherein 70–80% less nematode lesions noticed when compared to their non-transgenic plants (Seow-Neng et al. 2017). Chitinases are another major class of genes imparting disease resistance they catalyze the hydrolysis of chitin, an important insoluble constituent of the cell wall of fungi (Chen et al. 2018). Similarly, overexpression of other non-plant antimicrobial genes such as phytoalexins, defensin in transgenic plants was expressed to confer resistance against several fungal diseases (Levy et al. 2018).
The plant viruses do significantly affect crop growth and productivity, and till date conventional breeding has failed to generate crop plants resistant viral diseases (Trębicki et al. 2015; Bakhsh and Hussain 2015). Transgenic papaya plants cloned with the mutated replicase gene resulted in the improved resistance against Papaya Ringspot Virus (PRSV) disease under field condition when compared to wild plants (Fragoso et al. 2017). Moreover, transgenic banana plants overexpressing replicase-associated gene exhibited improved tolerance to the Banana bunchy top virus (BBTV); on the contrary, non-transgenic banana plants showed severe bunchy top symptoms (Ghag et al. 2015). Advanced genome engineering technologies, RNA interference and CRISPR Cas9, have received much attention owing to their simplicity and high reproducibility. RNA interference has been reported to play a significant role in tailoring plants with enhanced virus resistance by facilitating the formation of self-complementary hairpin RNA structure under the control of rolC promoter, thereby controlling the systemic spread of viral disease (Leus 2018). Plant-dependent RNA interference (RNAi) plays a pivotal role in improving plant tolerance against various biotic stresses (Fang and Qi 2016). In a study, overexpression of dsRNAs in transgenic tobacco plants conferred resistance against H. armigera (Tanpure et al. 2017). RNAi in conjunction with transgenic technology has been widely employed to enhance the resistance of crop plants against various stresses (Mamta and Rajam 2018). Furthermore, overexpression of CYP6AE14 and cysteine protease gene in Gossypium hirsutum (35GhCP1) transgenic cotton enhanced their resistance against cotton bollworm disease (Mamta and Rajam 2018).
Baculoviruses are pathogenic to many different species of insects, but each strain of baculoviruses is specific to narrow range of insect species. Although baculoviruses kills their host (insect) organisms, the process is usually considered to be too slow in controlling insects that attack crop plants. However, when certain genes are cloned into different strains of baculovirus, the virus can act as a delivery system for a gene that produces an insecticidal protein during the viral life cycle. Several tests of this strategy have been successful in laboratory trails. Recently, when a gene for a neurotoxin that kills insects was cloned into a baculovirus, the construct worked effectively under field trails (Pazmiño-Ibarra et al. 2019).
2.1.2 Crops Tolerant to Abiotic Stresses
The world agriculture is in the midst of developing high yielding, disease resistant eco-friendly crop plants and during this changing era of global climate. Environmental stresses such as water logging, drought, salinity, high and low temperature (abiotic stresses), and elevated CO2 levels affect plant development and pose threat to sustainable agriculture. For sustainable agriculture, it is important to critically investigate abiotic stress physiology and differential gene expression (functional genomics) studies. Plant biotechnology tools are viable enough to maximize plant productivity by introducing stress tolerance genes and key metabolic genes from wild germplasm into adapted cultivars (Kwak 2019). Environmental stresses have become a matter of contention due to concerns about the outcomes of climate change and its negative effects on plant resources, genetic diversity, and ultimately on world food safety (Trębicki et al. 2015). Plant responses to these stress factors are highly intricate and show modifications at the cellular, molecular, and genetic levels. Now, it has been scientifically proven that plant responds differently to multiple stresses as compared to individual stresses. As the changing climate will expose the plants to interactive effects simultaneously, therefore there is a dire need for further research in plant developmental responses to these stress factors; otherwise, this will have a negative effect on sustainable agriculture.
As plants are immobile, they have to tolerate fluctuating environmental conditions in order to survive. Naturally, plants are endowed with the ability to sense climate change and adapt accordingly. With changing environment, plants do evolve and develop precise molecular and cellular mechanisms that enable them to survive under harsh conditions. Unfortunately, little to no research done on how plants cope up under such circumstances, this knowledge gap should be minimized to develop the plant species, which can tolerate individual to multiple stresses (Ahuja et al. 2010). Luckily, several genes encoding stress-tolerant compounds, metabolites, and antioxidants have been identified in related distantly related plants that being exploited for engineering-sensitive plants for multiple stress tolerance. Since conventional breeding approaches which mainly involve varietal cross, mutation breeding and transfer of undesirable genes with desirable genes are supreme limitations. In conclusion, application of transgenic technology is the only viable option to engineer abiotic stress-tolerant plants by altering the expression levels of various genes of stress defense pathway. Recent studies “omics” approach led to better understanding of transcriptome, proteome, and metabolomics of plants by linking with stress perceptions and responses. These approaches are also studied in other crop and woody plant species besides a model plant Arabidopsis (Coolen et al. 2016; Varoquaux et al. 2019; Razzaq et al. 2019; Zhang et al. 2019; You et al. 2019).
Biotechnology concepts centred on biodiversity and its gene pool availability, conservation and later controlled redesign, manipulation, and services offered for the environment and mankind's well-being (Zhu 2016). Overexpression of ROS-scavenging enzymes in plants is an effective way to overcome heat stress-induced oxidative damages in plants. In transgenic apple plants, overexpression of cytosolic ascorbate peroxidase (cAPX) developed heat tolerance by reducing the membrane damage and improved the photosynthetic efficiency (Zandalinas et al. 2018). Recently, genetic engineering approaches for the development of genotypes with enhanced tolerance to drought stress have been reviewed by Shinwari et al. (2020). Moreover, Ali and Yun (2020) reviewed the role of Arabidopsis HOS15 in negatively regulating abscisic acid (ABA) signaling and drought stress tolerance.
Agricultural land salinization due to overuse of fertilizers, receiving scanty annual rainfall altered the soil compositions and pH. Thus, soil salinity is increasingly becoming the most important abiotic stress factor influencing plant growth, development, and agricultural productivity worldwide (Bless et al. 2018). Bulle et al. (2016) demonstrated overexpression of Na+/H+ antiporter gene (TaNHX2) in chili as well as in tomato plants wherein resulted increased ability of transgenic chili and tomato plants to adverse effect of salinity stress, improved plant water contents, enhanced accumulation of osmolytes like proline and glycine betaine, and affected the expression of other downstream stress-responsive genes. Akyol et al. (2020) reviewed novel insights into the root microbiome of halophytes to improve salinity tolerance of crops. Li et al. (2020) reviewed biotechnology perspectives on dryland agriculture environment for sustainable productivity in China. In addition, Paeng et al. (2020) demonstrated the function of the molecular chaperone NPR1 in protecting Arabidopsis plants from heat stress.
Moreover, the expression of heterologous genes in plant systems allows enhanced production of a wide range of valuable compounds/metabolites of high commercial value. Plants as bioreactors, it is reported that plants in this context on large scale produce monoclonal antibodies and antibody fragments for cancer treatment (Mu et al. 2020); the polymer polyhydroxyalkanoates (PHAs) to make a biodegradable plastic-like material (Dobrogojski et al. 2018). Many biopharmaceuticals including recombinant vaccine antigens, monoclonal antibodies, and other commercially viable proteins are produced in plants, some of which are in the pre-clinical and clinical pipeline (Shanmugaraj et al. 2020).
Environmental Safety
Plant stresses due to global climate changes threatening the crop productivity and food security. In view of global food demand, breeders and genetic engineers have to aim up to improve the yield and quality of major crops plants without neglecting the environmental safety. Several countries, including the USA, have adapted the principle of “substantial equivalence” when evaluating the safety of genetically engineered foods. This implies that genetically modified plant or animal food products must be similar in composition to the corresponding conventional food. Levels of nutrients, anti-nutrients, and natural toxins must not be different, and animal-feeding trials must not show differences in the development, health or performance of the animals that would indicate reduced nutritional or increased toxicity or allergenicity of the genetically modified food. If the genetically engineered product is not substantially different from the conventional product, labeling them as genetically engineered is not required.
With the introduction of (genetically modified)GM technology, particularly Bt cotton, have resulted in significant reductions in pesticide poisoning cases due to reduced applications and reduced levels of insecticide exposure (Smyth 2019). Reductions in farmer pesticide poisonings have been quantified in China, India, Pakistan, and South Africa. In South Africa, farmers reduced pesticide applications from 11.2 per year to 3.8%, in addition reported cases of pesticide poisoning declining from over 50 per year to <10 over the first 4 years of Bt cotton adoption (Bennet et al. 2003). It is reported in China that one-third of non-Bt cotton farmers got pesticide poisoning, while this is very less (9%) in comparison to Bt cotton producing farmers (Hossain et al. 2004). Following an assessment of the health effects in India, it was discovered that there was a 2.4–9 million case reduction in pesticide poisoning every year (Kouser and Qaim 2011). Cumulatively, since 2003, when Bt cotton was first commercialized in India, a minimum of 38 million fewer instances of pesticide poisoning have occurred, with an upper potential of 144 million. Farmers in Pakistan growing non-Bt cotton reported up to seven instances of pesticide poisoning in the growing season with 35% reporting no instances, versus Bt cotton farmers reporting up to six poisonings with 45% reporting none (Kouser and Qaim 2011).
In fact, GM crops with traits for insect resistance and herbicide tolerance have contributed to reduce agriculture’s environmental footprint by facilitating environmentally friendly farming practices. The adoption of GM insect resistant and herbicide tolerant technology has reduced pesticide spraying by 775.4 million kg (8.3%) and, as a result, decreased the environmental impact associated with herbicide and insecticide use (Brookes and Barfoot 2020). Klumper and Qaimm (2014) conducted a meta-analysis based on primary data from farm surveys or field trials in different regions worldwide. This comprehensive study demonstrates that GM insect resistant (IR) traits have reduced pesticide usage by 36.9% on average. According to a medical evaluation of Chinese farmers that included health indicators, fungicides used in non-Bt cotton production were linked to liver dysfunction, while insecticides used in non-Bt cotton production were linked to severe nerve damage (Zhang et al. 2016).
The release of insect resistant crop varieties in the fields has begun to have a noticeable potential to improve human health by the reduction in cancer rates. Prior to the commercialization of Bt crops, chemical spray to control insect crop damage in maize increased the potential for the development of harmful health effects. With existing food security challenges in many developing countries, corn containing mycotoxins are consumed as part of the household diet due to the lack of any other option. A meta-analysis after 21 years of maize production quantified that Bt maize has lower concentrations of mycotoxins (29%), fumonisins (31%), and thricotecens (37%) (Pellegrino et al. 2018). Mycotoxins are toxic and carcinogenic to humans and animals as well. Fumonisins are correlated to being the cause of higher rates of neural tube defects in high maize-based diets (Missmer et al. 2006). Several crop plants which are grown at large scale are engineered to resistant to weeds, thus the crop productivity has been increased dramatically by reducing the agronomic competition for nutrients and space in between main crop and weeds. This approach has become most successful and is the basis for the largest number of transgenic plants that are used in the field (Gesine et al. 2017).
Genetically modified crops have made significant contributions to address the United Nations Sustainable Development Goals, in particular goals in reducing poverty and hunger. Moreover, increased yields have contributed to higher household incomes, reduce poverty; the increased yields witnessed household food security (Smyth 2019). In many developing countries, plant-based nutrient intake accounts for 100% of an individual’s nutrient diet, thus highlighting the importance of nutritionally enhanced crop-derived foods. Nutritionally fortified foods improve an individual’s nutrient intake, preventing leading causes of death due to morbidities (cancer, diabetes, cardiovascular disease, and hypertension). Biofortified GM crops have been adopted for increasing micronutrient availability in human nutrition (Hefferon 2015).
Production of transgenic plants, at large scale happens via transformation and expression of transgenes in the nuclear genomes. Among the ecological concerns raised about genetically engineered organisms is that transgenes could move (“transgene flow,” the process of containing transgene by recurrent hybridization) via pollen from the crop and into related wild varieties growing in natural or semi-natural communities. Such concerns are addressed by new field of transgene containment called as Transplastomic technology or Plastid transformation technology. Since plastids are inherited maternally in the majority of angiosperm species, they would therefore not be found in pollen grains of corps. Insertion of transgenes, therefore, into the plastid genome has the potential of preventing gene flow via pollen. Hence, the plastid transformation technology is considered as environmentally friendly method because plastid and their genetic information are maternally inherited in many species due to consequent lack of transmission of plastid DNA by pollen (Wani et al. 2010). In addition, it is important to ease public concern and increase public acceptance production of marker free transplastomic plants. As chloroplast genome is capable of expressing more than 120 foreign genes originated from different organisms (bacteria, animals, viruses, fungi, and humans), addressing other barriers will make chloroplast genome very attractive site to avail biotechnological applications with incredible impact on human life and environment (Srinivas et al. 2016; Adem et al. 2017).
Genome editing is the alteration of the genomic DNA of an organism with high accuracy and efficiency using site-specific genome targeting methods. Unlike conventional mutagenesis methods, Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) CRISPR-associated protein 9 (CRISPR-Cas9), emerged as the popular strategy currently employed for genome editing. The CRISPR-Cas9 principle based on the adaptive immune response in archaea/bacteria which protect themselves from invading foreign DNA by catalyzing sequence-specific cleavage of nucleic acids which result in the generation of double-stranded breaks (DSBs). Once generated, these DSBs are repaired either by nonhomologous end-joining (NHEJ) method or by homologous recombination (HR), thus causing modification of target site within the plant genome, and uses three types of engineered nuclease, viz., zinc finger nuclease (ZFN), transcription activator-like effector nuclease (TALENS), and CRISPR-Cas, for directing site-specific cleavage (Bortesi and Fischer 2015). CRISPR-Cas9 has been widely used in a variety of agricultural crops such as in tomato, citrus, grape, etc. for activation or repression of certain target genes. Numerous investigations have reported that CRISPR-Cas9 is extremely helpful in developing virus-resistant crops for, e.g., researchers have successfully developed virus resistance cucumber plants with nonfunctional eukaryotic translation initiation factor 4E (eIF4E) using CRISPR-Cas9 technology. The developed transgenic cucumber plants exhibited enhanced tolerance to Cucumber vein yellowing virus and Papaya ring spot mosaic virus (Chandrasekaran et al. 2016) as well. In another study, transgenic grape cultivar in which ribonucleoproteins were mutated using CRISPR-Cas9 approach exhibited enhanced tolerance to powdery mildew disease, and transgenic apple cultivar mutated for the same gene using CRISPR-Cas9 showed enhanced resistance against fire blight disease (Malnoy et al. 2016). Genome editing system (CRISPR)/CRISPR-associated protein 9 (CRISPR/Cas9) has revolutionized crop improvement by enabling robust and precise targeted genome modifications for the development of transgene-free disease resistant crops (Ahmad et al. 2020; Chen et al. 2019) In Fig. 2.2 genome editing with CRISPR-Cas9 for transgene-free disease resistant crops has been depicted.
Genome editing with CRISPR-Cas9 for transgene-free disease resistant crops: Plant trait improvement through crispr-cas9 system of genome editing found to be efficient, robust, time saving, less laborious, and cost-effective. Construct with sgRNA and cas9 are assembled and when transformed to generate crops plants tolerant to biotic and abiotic stresses could help to meet the food security in a sustainable mode
Concluding Remarks
Millions of farmers producing Bt cotton are less likely to be poisoned by pesticides; worldwide, 17 million farmers growing GM crops have reduced chemical exposures (Smyth 2019). Environmental stresses are responsible for reversible and irreversible changes which minimize plant plasticity leading to reduced growth and agricultural productivity. To overcome the food shortage, it is very important to generate more climate-resilient plants in years to come. Currently holistic approaches are needed from several biological kinds of research to study individual and multiple stress conditions effecting crop plants performance. Nevertheless, the integration of OMICS approaches could help to identify stress related genes and regulators, which aids in manipulation of candidate genes for the development of climate-resilient plants. Genome editing using artificial nucleases particularly,(CRISPR), CRISPR-associated protein 9 (Cas9), has revolutionized basic as well as applied research including plant breeding by accelerating the editing of target genome in precise and predictable manner. Genome editing will greatly facilitate the engineering of complex traits, such as stacked disease tolerance (stacked traits are insect resistance and herbicide tolerance as these traits may lead to lesser use of pesticides, higher yield, and efficient control of weeds), resilience to abiotic stress, as well as nutritional and organoleptic properties (Halewood et al. 2018). For example, the de novo domestication of wild tomato, a display on how to leverage the genetic variety of wild plants (Zsogon et al. 2018), demonstrates the promise of precision genome engineering for agricultural enhancement (Khanday et al. 2018).
The hallmarks of CRISPR/Cas9 are the development of transgene-free disease resistant crops. The production of transgene-free plants (e.g. in A. thaliana, rice, tobacco, lettuce, wheat, potato, etc.) via this technology may avoid the plants from GMO label. Thus, powerful genome editing tool—CRISPR/Cas system in conjunction with conventional and modern breeding technologies should be utilized to cope with environmental stresses and to secure the world food security by 2050. Genetic engineering and genome editing are ready to be deployed at the earliest to improve crop breeding and build a more sustainable agriculture.
2.1.3 Nitrogen Fixation in Cereal/Non-legumes
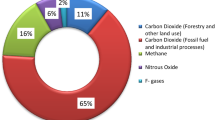
Nitrous oxide responsible for global warming potential of 296 implies that it has a global warming potential (GWP) about 300 times greater than carbon dioxide—this means that 1 lb of nitrous oxide is counted as 296 lb of CO2. Nitrous oxides persist in the atmosphere for more than 100 years. The formation and release of N2O from agricultural fields happens when excess nitrogen fertilizer applied to crops interacts with common soil bacteria. It is estimated that nitrogen fertilizer accounts for one-third of the GHGs (greenhouse gases) produced by agriculture, reduced fertilizer reduces nitrogen pollution of ground and surface waters.
Bacterial genus Rhizobium fixes up to five times more nitrogen globally per year, than the amount of nitrogen fixed industrial Haber-Bosch process. Deployment of synthetic nitrogen fuels in modern agriculture and its production is energy-intensive, need massive expansion in land use and its application leads to aquatic pollution and greenhouse gas emissions (Bloch et al. 2020). Sustainable intensification of agriculture to provide food for humans, feedstock’s for biobased fuels and materials requires alternative options for nitrogen management. One of the greatest challenges in agriculture is supplying sufficient plant-available nitrogen to cereal crops, which provide 45% of the world’s dietary energy consumed directly by humans (Zhang et al. 2015b). For nearly 50 years, nitrogen fixation in cereal crops has been pursued to address this challenge. Engineering the capacity to fix nitrogen in cereals, either by themselves or in symbiosis with nitrogen-fixing microbes, represent attractive future options that, nevertheless, require more intensive and internationally coordinated research efforts. Biological nitrogen fixation might be a viable option for long-term agriculture (Soumare et al. 2020). Biotechnology techniques have been trying to transfer nitrogen-fixing (nif) genes from Rhizobium strains to other organisms. Recent study reports on transferring nitrogen fixation (nif) genes to non-diazotrophic hosts. These advances have laid the groundwork to enable cereal crops to “fix” nitrogen themselves to sustain their growth and yield. Transfer of nif genes into rhizosphere microorganisms another avenue needs further research and field testing, particularly under stress conditions that impede the effective use of Rhizobium strains. Efforts to engineer plants for nitrogen fixation have made strides through eukaryotic nitrogenase expression and a deepened understanding of root nodulation pathways, but deployment of transgenic nitrogen-fixing cereals may be outpaced by population growth. By contrast, a root associated bacterium that can fix and supply nitrogen to cereals could offer a sustainable solution for nitrogen management on a shorter timescale (Bloch et al. 2020). While, research is also being carried out on nitrogen-fixing bacteria (Frankia & Azospirillum species) which has symbiotic association with trees or crops.
Conservation and Restoration of Plant Species
Due to anthropogenic and natural causes, endemic plant species are usually more vulnerable to higher rate of extinction risk. Unfortunately, many of these plants are under the threat of extinction due to habitat depletion, habitat destruction, and overexploitation, for which conservation efforts are required to ensure their long-term availability. In addition to climate change, invasion of alien/exotic species, biomagnifications of pollutants pushed native species to the verge of extinction. At global contest for preservation of such species in wild status, in situ conservation alone will not assure their conservation and restoration. Moreover, several active phytochemicals from different parts of rare, endemic, vulnerable medicinal plants were isolated and used as drugs either alone or in combination with other compounds. Moreover, ex situ conservation strategies must be undertaken for conservation of these species, for which establishment of seed banks are the more efficient and cost-effective methods. However, when seed banking is not an option, alternative approaches should be considered. Biotechnological tools are more viable and complementary option for plant conservation including short-, medium-, and long-term strategies, and their application for plant species conservation has increased considerably in the last years (Corlett 2017).
Biotechnology largely contributed to plant conservation and restoration biology this is not to replace the traditional conservation methods but to complement and improve the methods established (Engelmann 2011). For conservation, shoot tips and seeds are the most preferred explants, however, micropropagation/ clonal propagation using various explants is the most common in vitro culture technique for conservation of RET (Rare, Endangered, Threatened) and Endemic plant species. This involves the production and rapid multiplication of true to type plants using various explants under controlled and aseptic condition (Yarra et al. 2010; Aileni et al. 2011; Kokkirala et al. 2012). Plant conservation biotechnology comprises not only the conservation of plant genetic resources but also its management, characterization, and application (sustainable use) (Coelho et al. 2020). In particular, in vitro conservation, which is the maintenance of plant germplasm in culture collections using tissue culture technologies, can provide easy access for the evaluation, utilization, and safe exchange of plant material (Deepa and Dennis Thomas 2020). Because fast-growing trees are more efficient at exploiting CO2 for photosynthesis, micropropagation and synthetic seed generation using plant tissue culture techniques are recommended. The major problem is the continuous deforestation that is associated with a possible increase in atmospheric CO2. The conservation of 33% of forests and plantations coverage is the need of hour. Certain biotechnological approaches have also been made to improve the CO2 utilization for enhancing photosynthetic efficiency. The enzyme ribulose-bisphosphate carboxylase (RUBP-case) is closely associated with plants CO2 assimilation and fixation. In line with this attempts have been made to genetically manipulate this enzyme for increasing the biomass and photosynthetic efficiency. Recently, research was conducted to improve the photosynthetic capabilities of several C3 crops, either by the introduction of C4 genes or through the overexpression of C3 genes (Ashraf and Harris 2013).
Cryopreservation, when long-term (several years), uses liquid nitrogen at ultra-low temperature (−196aniC) to store plant materials, wherein cells metabolic process is slowed down almost completely. Retaining the genetic viability and timely retrieval of cell culture are very crucial during conservation studies. Cryopreservation of plant genetic resources of crop or economically important species is being carried out and similar conservation studies are applied from RET and endemic plant species (Haque and Ghosh 2016). A medium-term (1–15 years) in vitro conservation technique is slow growth culture, which is maintained at low temperature and low light intensity to reduce the growth of plants. Synthetic seed technology for the production of artificial seed using somatic embryos, meristems or adventitious shoots is another viable in vitro conservation technique in several plant species (Rihan et al. 2017). Low survival rate and genetic variations including epigenetic changes in cryopreserved plant materials are problems associated with ex situ conservation strategies. There is an urgent need to expand conservation research and particularly transfer of this academic knowledge to the actual implementation of conservation strategies and their restoration.
2.2 Mitigation of Environmental Deterioration
Over the past 20–30 years, large scale of industrialization and traditional agricultural practices contributed diverse and harmful loads of pollutants (organic halogen compounds, metal and sulphur containing compounds, inorganic and organic acids) to environment which has resulted in tremendous pollution of air, water, soil leading to their intervention in natural ecological process of organisms and environment. The problem is worldwide and possibly causing threat to both the environment and human health (Samant et al. 2018). The use of pesticides and herbicides helps to increase agricultural productivity; however, unremitting application of these noxious chemicals causes a huge loss of biodiversity and contamination of agricultural land, enter the food web, whereby get biomagnified (Pande et al. 2019). Such pollutants are prime causes of life-threatening human degenerative diseases and morbidity (cancer, Alzheimer’s disease, atherosclerosis, Parkinson’s disease, etc.). Conventionally, incineration and chemical treatment have been used to breakdown many toxic chemicals, but these methods are expensive and often create new environmental problems (Arthur et al. 2005).
2.2.1 Bioremediation
Environmental pollution is the major obstacle in the past several decades owing due to increasing human activities on natural reservoirs, unsafe agricultural practices and rapid industrialization. Bioremediation is the process involves plants and microbes natural capacity of cleaning the environment by degradation, detoxification, and sequestration of contaminants present in water and soil (Adriano 1986; Alloway 1990; Ojuederie and Babalola 2017). Bioremediation facilitates the restoration of contaminated and degraded habitat by removing up hazardous wastes is an innovative, environmentally safe, noninvasive process and results in safe and recyclable end forms (Shah 2011; Ojuederie and Babalola 2017). Bioremediation can be carried in situ or ex situ methods. In the in situ bioremediation, the contaminants are treated directly at the site, whereas in ex situ bioremediation the contaminants are collected from the site and are treated elsewhere. Till now little number of microbes (culturable microbes) have been exploited and a huge microbial biodiversity is still unexplored. In addition, to enhance the metabolic potential of the microbes for ecological restoration and degradation of recalcitrant pollutants, various forms of bioremediation approaches such as bioaccumulation, biosorption, biotransformation, biomineralization chemotaxis, biostimulation, bioaugmentation, biofilm formation, application of genetically engineered microorganisms (GEM) with improved catabolic activities, advanced omics have been widely employed (Girma 2015; Pande et al. 2020). In the past few years, the metabolic potential of microbes is being used for efficient degradation and remediation of environmental wastes (Janssen Dick and Gerhard 2020). Using bioremediation methods, highly toxic pollutants were converted into less non-toxic forms using the different metabolic potentials of microbes such as transformation, mineralization, and immobilization. However, xenobiotic compounds such as highly halogenated, nitrated aromatic compounds, and pesticides are not investigated on their microbial degradation till date (Gangola et al. 2019). It is tricky that the efficiency of microbial degradation relies on different factors, i.e. concentration, chemical nature of pollutants, physiological features of the environment, and nutritional requirements of microorganisms.
It is known that microbial genus Pseudomonas is the predominant group of soil microorganisms used to degrade xenobiotic compounds. Biochemical assays showed that various Pseudomonas strains capable break down and as a consequence, detoxify hundreds of different organic compounds. In many cases, one strain can use any of several different related compounds as its sole carbon source. The biodegradation of complex organic molecules generally requires the concerted efforts of several different enzymes. In some organisms the genes that contribute to the biodegradative pathway are found in both chromosomal and plasmid DNA as well. In general, degradative bacteria in most cases, enzymatically convert xenobiotic, nonhalogenated aromatic compounds to either catechol or protocatechuate. Later, through a series of oxidative cleavage reactions, catechol and protocatechuate are processed to yield either acetyl coenzyme A (acetyl-coA) and succinate or pyruvate and acetaldehyde, compounds that are readily metabolized by almost all organisms. Despite the ability of many naturally occurring microorganisms to degrade different xenobiotic chemicals, there are certain limitations to the biological treatment of those waste materials. For example, (1) no single microorganism can degrade all organic wastes; (2) high concentration of some organic compounds can inhibit the activity or growth of degradative microorganisms; (3) most contaminated sites contain mixtures of chemicals, and an organism that can degrade one or more of the components of the mixture may be inhibited by other components; (4) Many nonpolar compounds adsorb on to particular matter in soils or sediments and become less available to degrative microorganisms; and (5) microbial biodegradation of organic compounds is often quite slow.
One way to address these problems is to transfer plasmid genes carrying different degradative pathways by conjugation into a single bacterium. Way back, Chakrabarty and his coworkers for the first time genetically engineered bacterium (Pseudomonas) carrying plasmid genes of different pathway encoded genes involved to degrade four different organic molecules (camphor, octane, salicylate, and naphthalene). The first genetically engineered microbe was created by Chakrabarty group in 1971. The patent was approved in 1980 by the United States Supreme Court, later, variant of the genus Pseudomonas capable of degrading crude oil constituents has been investigated (Ezezika and Singer 2010). Azad et al. (2014) narrated a comprehensive review of the use of genetically engineered bacteria and plants on the bioremediation of contaminated soils having heavy metals and other organic pollutants.
Because native microorganisms are not capable of carrying out pollutant cleanup, the use of genetically modified microorganisms (GEMs) for in-situ bioremediation of damaged habitats is being studied. The aim of genetic engineering for bioremediation is to modify plants, microorganisms, and enzymes so that they would be useful tools for degradation of harmful substances (WolejKo et al. 2016). GEMs have been demonstrated successfully as they have better genetic makeup to lessen the effect of harmful pollutants in the surrounding environment (Azad et al. 2014; Shah and Pathak 2019). Dixit et al. (2015) reviewed that several engineered microbes are competent strains for bioremediation of contaminated environment as they have enhanced ability to breakdown a variety of contaminants. Some of the most widely used genes in bioremediation process include tmerA gene for Hg up take, phenol catabolic genes (pheA, pheB, pheC, pheD, and pheR) (Marconi et al. 1997) and the ArsM gene for the removal of As from contaminated soils (Liu et al. 2011). It is also reported by Dixit et al. (2015) that expression of mer operon (genes) from Escherichia coli encoding Hg2+ reduction into genetically engineered bacterium Deinococcus geothermalis gave that microorganism ability to reduce Hg contamination at high temperatures. In a study, site directed mutagenesis and rational design tools of biotechnology has been used in manipulations of enzymes involved to degrade organic compounds (Kumar et al. 2013). Moreover, deployment of GEMs in bioremediation process is found to be indispensable tools in the war to degrade noxious substances of polluted sites. In the same process, it is very important to check stability of GEMS to perform consistently under field conditions (Ghosal et al. 2016).
Bioremediation process necessitates the blending of diverse multifaceted variables that will provide us a better understanding and prediction of pollutant’s fate. Maintenance of controlled environmental factors and with standing opposition from indigenous microbes is the prerequisites need to be worked out for successful application of GEMs during bioremediation process (Dixit et al. 2015). In addition, novel molecular techniques should be explored to screen and isolate microorganisms for use in heavy metal bioremediation. With advancements in the areas of modern techniques of genetics and omics (genomics, proteomics, and metabolomics), it has enabled scientists to investigate and understand the physiology, ecology, and biochemistry of polycyclic aromatic hydrocarbon (PAH)-degrading microorganisms (Ghosal et al. 2016). These approaches also help us to assemble information on biodegrading genes, proteins, and metabolites. Moreover, with the advent of NGS (next-generation sequencing) and in silico techniques in this area facilitated to use metagenomic, proteomic, and bioinformatic studies of diverse eco-friendly microbes which render an exceptional understanding of major pathways for biodegradation.
Metagenomics studies proved to be significant in analyzing the functional range of the microbial consortia. Currently, several investigations on metagenomics have given key concepts aid to enhance bioremediation (Duarte et al. 2017; Tripathi et al. 2018). Furthermore, the use of GEMs in the bioremediation process has been discovered to be essential instruments in the battle to decompose noxious compounds at contaminated locations (Jaiswal et al. 2019; Malla et al. 2018). Metatranscriptomic investigations have profound interest in environmental restoration, as these are used to verify the gene activity within a specified environmental condition. The combined effects of omics based practices with environmental proteomics can help to give much better outcomes. Moreover, Niu et al. (2018) studies have explored the significance and success of comparative transcriptomic and proteomic analysis for microbial-mediated bioremediation.
Researchers (Bargiela et al. 2015; Malla et al. 2018) used metaproteomic and metabolomic approaches for the bioremediation of environmental sites which were severely contaminated with petroleum hydrocarbons. With the deployment of omics studies novel genes, transcripts, or enzymes engaged in xenobiotic bioremediation can be identified (Fig. 2.3).
Environmental Safety
Due to modernization (rampant industrialization), habitat degradation and related anthropogenic activities, it is the only bioremediation tool to clean and sustain the earth planet from the short term to long terms effects of noxious pollutants. The ability of the microorganisms used in bioremediation to compete with indigenous microbial population is essential for the success of bioremediation. Environmental constraints such as temperature and low nutrient concentrations, as well as other factors that are difficult to manage, impede their usage and the bioremediation process efficacy. GEMs are used in bioremediation of affected environmental sites by their detoxification and degradation but their sustainance is poorly understood in the processes of their action. In addition, the use of antibiotic genes as selectable markers should be discontinued and replaced with other scorable markers to avoid antibiotic resistance genes being unintentionally transferred to other soil microorganisms.
Bioremediation is eco-friendly and economical in restoring the biological and physicochemical properties of the degraded soil (Arora 2018), however, still these in situ techniques need to be improvised and advanced research and ethical issues need to be addressed for the use of efficient and safe GEM’s. Moreover, the development of pollutant degrading microbial consortium requires more studies to determine the catabolic potential of microbes both alone and in combination (Arora and Panosyan 2019). Thus, it may strengthen our understanding of microbial consortia-mediated remediation of contaminated sites. For utilizing the full potential of known as well as novel species, it is very essential to expand our knowledge to know the interaction between microbial communities and the polluted environment.
Indigenous microorganisms are found to be inefficient to remove noxious and refractory pollutants, which is a major challenge to environmental biotechnologists. Moreover, the developed GEMs In situ application found to be impactful but associated with the risk of horizontal gene transfer, which causes unrestrained propagation (of the GEMs) in the environment (Naik and Duraphe 2012). The impacts of these bacteria on the ability of indigenous microbes in the environment before being released into hazardous areas, would need to be researched. Nevertheless, for any microbial-based bioremediation process, it is crucial to monitor implanted recombinant strains of bacteria and design strategies to program cell death once the biocatalyst had completed its task (Jan et al. 2014). Induction of controlled suicidal gene system operates just after engineered microbes have completed the remediation of contaminated sites, an alternative to prevent horizontal gene transfer. In addition, horizontal gene transfer can also be prevented by the use of antisense technology which involves inserting antisense RNA-regulated plasmids and protein plasmids into the microbe which terminate or degrade after carrying out their work in remediation (Azad et al. 2014). Moreover, the use of GEMs in the ex situ bioremediation process in bioreactors is considered to be the best choice, as there is no competition with indigenous microorganisms and also they are maintained with controlled temperature and growth conditions (Urgun-Demirtas et al. 2006). Furthermore, field studies are important to check the efficiency and associated risk factors of GEM after incorporating into the natural ecosystems. Significant development of computational tools and omics based investigation has concepts to be gained to unravel microbial-mediated bioremediation pathways. Undoubtedly, the methodical application of microbial consortia and knowledge of well-defined molecular and biochemical mechanisms will facilitate successful implementation of bioremediation techniques. The operations of these strategies are still in nascent stage; however, application of omics strategies in the investigation of microbial molecular action would assist in trailing the desired organism and also effectively eradicating the environmental pollutants.
Conclusion Remarks
Once bioremediation process is optimized, it is considered safe, inexpensive, and environmentally amicable to eradicate the pollutants by speeding up the natural process of biodegradation and detoxification. Therefore, urgent need for better understanding the microbial genetics to widen the knowledge of microbial communities and their reaction toward the environmental pollutants will enhance pollutant degrading potential of a given microbe. Undoubtedly, bioremediation is rendering a pathway for a better pollution free planet leading to its sustainability. To investigate the functional makeup of microbial communities inside contaminated locations for metal resistance genes that may be exploited to improve particular heavy metal breakdown strains of microorganisms, metagenomic techniques and metabolic studies should be applied. Public perception of the use of gene technology for bioremediation will also need to change for its effective utilization; this will require cooperation between researchers and environmentalist.
2.2.2 Phytoremediation of Polluted Soils/Water Ecosystems
Rapid industrialization resulted in drastic rise in the waste discharge into the environment and led to the accumulation of major contaminants such as heavy metals, hazardous wastes, explosives, and petroleum products (Adriano 1986; Alloway 1990). Heavy metal (arsenic, cadmium, copper, chromium, mercury, zinc, selenium, nickel, and lead) accumulation in soil has been increased largely due to various natural catastrophes and human interventions (Suman et al. 2018; Ashraf et al. 2019). As heavy metals are non-biodegradable, they persist in the environment, have potential to enter the food chain via plant systems, and eventually may accumulate in large quantities (biomagnification) in human body as he is major tertiary consumers. Owing to their noxious nature, heavy metal contamination posed a serious threat to human health and environmental ecosystems (Yan et al. 2020). The total impact of degradation by heavy metals is an irreparable and irreversible threat for the environment with negative consequences. Using plants for bioremediation (called phytoremediation) is an effective in situ technology used in restoration of polluted water and soil (Pajević et al. 2016).
Phytoremediation is an eco-friendly approach that could be a successful mitigation measure to revegetate heavy metal-polluted soil in a cost-effective way. Phytoremediation has good public acceptance and shows a variety of advantages compared with other physicochemical techniques (Suman et al. 2018; DalCorso et al. 2019). There are a number of phytoremediation strategies that are applicable for the remediation of heavy metal-contaminated soils, including (1) phytostabilization—using plants to reduce heavy metal bioavailability in soil (Gerhardt et al. 2017; Burges et al. 2018), (2) phytoextraction—using plants to extract and remove heavy metals from soil (Sarwar et al. 2017), (3) phytovolatilization—using plants to absorb heavy metal from soil and release into the atmosphere as volatile compounds (Mahar et al. 2016), and (4) phytofiltration—using hydroponically cultured plants to absorb or adsorb heavy metal ions from groundwater and aqueous waste (Dhanwal et al. 2017).
Increasing levels of toxic metals and metalloids in the environment have led to identification of hyperaccumulator plants as they accumulate very high heavy metals concentrations in their aboveground tissues in their natural habitats. It is also known that plants are useful sensors to identify environmental contamination and potential exposures to pollutants (Henry et al. 2013). The application of heavy metal hyperaccumulators is the most straightforward approach for phytoremediation, and hundreds of hyperaccumulator plants have been identified so far (Yan et al. 2020). However, phytoremediation with these natural hyperaccumulators still suffers from a few limitations, as it is a time-consuming process, which takes a very long time to cleanup heavy metal-contaminated soil, particularly in moderately and highly contaminated sites. This may partially be due to slow growth rate and low biomass production of these hyperaccumulators. In order to enhance efficiency of phytoremediation, a better understanding of the mechanisms underlying heavy metal accumulation and tolerance in plant is indispensable. Fortunately, recombinant DNA approaches have been emerging as a powerful tool to modify plants with desired traits such as fast grow, high biomass production, high heavy metal tolerance and accumulation, and good adaption to various climatic and geological conditions. Hence, better understanding of the mechanisms of heavy metal uptake, translocation, and detoxification in plants, and identification and characterization of different molecules and signaling pathway, will be of great importance for the design of ideal plant species for phytoremediation via genetic engineering (Wu et al. 2010; Shah and Pathak 2019).
Notably, apart from exploiting of transgenic approach using functional genes, defined promoters were over expressed to encode accumulation/detoxification mechanisms for heavy metals remediation (Ilker et al. 2020). In study, transgenic canola (Brassica napus L. cv. Westar) developed using a rice gene, OsMyb4, under the control of Arabidopsis thaliana COR15a stress-inducible promoter (Raldugina et al. 2018). When cultivated under high levels of Cu (as 150 M CuSO4) and Zn (as 5000 M ZnSO4), such transgenic canola plants showed greater tolerance (more than 15 days) than natural kinds. This study has demonstrated that OsMyb4 is positive regulator of phenylpropanoid pathway and proline synthesis and may also have potential in phytoremediation. Zhang et al. (2014) reported that lignin biosynthesis was enhanced Jute CCoAOMT1 gene was over expressed in to A. thaliana. Ectopic expression of Vicia Sativa Caffeoyl-CoA O methyltransferase (VsCCoAOMT) increased the uptake and tolerance of cadmium in A. thaliana (Xia et al. 2018). In another study Wang et al. (2018) exploited heavy metal ATPases activity by its expression in model plant tobacco, wherein it has showed improved tolerance against cadium. With data available from Populus trichocarpa genome, its heavy metal ATPase gene, PtoHMA5 was cloned into N. tobaccum. Following which, the transgenic N. tabacum leaves exhibited increased Cd accumulation (25.05%) and tolerance (Wang et al. 2018). Transgenic plants carrying the genes from endophytic bacteria has significance of remediating heavy metals (Zn, Pb, Cd) from soils. In one of the study when CUP and bph C genes under control of cauliflower mosaic virus 35 promoter introduced into the stinging nettle plants (Urtica dioica) increased heavy metal accumulation for polychlorinated biphenyls (PCBs) in contaminated soils has been noticed. This finding claim that remediation by stinging nettle could have much wider range of applications than previously thought (Viktorova et al. 2017).
In a report transgenic sugar beets demonstrated to have dominant ability to retain Cd, Cu, and Zn ions and showed enhanced glutathione and phytochelatin activities under the applications of heavy metal stresses compared with wildtype sugar beets (Liu et al. 2015). In another study, bacterial γ-glutamylcysteine synthetase has been overexpressed which enhanced the cd detoxification in Populus tremula × P. alba (He et al. 2015). Notably, transgenic plants expressing bacterial reductases were reported to enhance volatilization of mercury, selenium, and arsenic accumulations in plant shoots (Mosa et al. 2012). Sun et al. (2018) investigated that poplar (genus Populus) were effectively used in remediation of Hg from soils using a class of ATP-binding cassette transporter gene. This was achieved by the transformation of ATP-binding cassette transporter gene (PtABCC1) from Populus trichocarpa to wildtype Arabidopsis.
Moreover, apart from metal transporter proteins, functional gene Arsenic reductase 2 gene from A. thaliana was cloned in tobacco to study its arsenic phytoremedial potential (Nahar et al. 2017). Recently, Basharat et al. (2018) reviewed that the simplicity, inexpensiveness, and capabilities of gene editing (CRISPR–mediated) technique could soon be used to enhance plants and bacteria involved in phytotechnologies, such as phytostabilization, phytoextraction, phytomining, phytovolatilization, and bioenergy generation.
Phylloremediation is a kind of phytoremediation where plant leaves and leaf associate microbes are engaged to clean the air in the environment to get rid of toxic pollutants (Wei et al. 2017). The ability of removal of heavy metal in the environment can be improved by genetically modified crops engaged in absorption and accumulation of heavy metals from both the soil and air. It is reported that genetically modified crops for the absorption of heavy metals from both air and soil carried out in agricultural areas by intercropping or mixed cropping practicing to increase the effectiveness of phytoremediation (Vamerali et al. 2010). Using genetically engineered organisms with appropriate distances in agricultural farming areas may reduce heavy metal entry into the food chain and will help to remove heavy metals from soil. Eventually, transgenic species are not suppose to competitors of agricultural plants and should be easily removed from the farming land after their tasks of phytoremediation attained. Moreover, there is an increasing worldwide economic interest and scientific focus on developing transgenic biofuel//bioenergy crops with increased biomass and high heavy metal accumulation ability to make phytoremediation feasible as well (Barbosa et al. 2015). Shah and Pathak (2019) reviewed various strategies to increase phytoremediation potential of hyperaccumulator plants whose biomass has high calorific value. In Fig. 2.4 explains the Methodologies to increase phytoremediation efficiency of hyperaccumulator plants rich in energy depicted.
Environmental Safety
The ability of removal noxious chemicals form environment achieved with identification of candidate wild plant species or by developing genetically modified crops for enhanced reclamation of pollutants from the environment. It is now alarming situation to maintain the environment resilience potency for the earth plant for its sustainability. Anh et al. (2017) reported that Pteris vittata, Pityrogramma calomelanos, and Vetiveria zizanioides were employed to remove heavy metals from soil in Vietnam under field conditions. In another study, pollutants from wastewater were successfully carried out using agricultural species including Medicago sativa, Zea mays, Helianthus annus, and Sorghum bicolor (Atia et al. 2019). Cameselle et al. (2019) showed good results with large-scale phytoremedial field treatments utilising Brassica rapa subsp. rapa and Lolium perenne. Phytoremedial experiments utilising Elodea canadensis for Co removal from wastewater and Plantago lanceolate for Cd and As removal from polluted locations were also conducted (Mosoarca et al. 2018). In addition, phytoremedial studies were performed using Elodea canadensis for Co removal from wastewater and Plantago lanceolate for removal of Cd and As from the contaminated sites (Mosoarca et al. 2018). Moreover, Salvia sclarea (Chand et al. (2015), Sedum plumbizincicola (Deng et al. (2016), and S. alfredii and P. vittata (Wan et al. 2015) were used for large-scale remediation from harmful heavy metal-contaminated sites. These studies witnessed eco-friendly approaches associated with the wild-type plant species, while there is dire need to speed up the plant earth recovery from burdened pollutants with the introduction of genetic engineering methods. However, owing to the great potential for the removal of pollutants, there are some serious concerns about the environmental applications of transgenic plants. One of the serious concerns is about the gene flow from the genetically modified plants to the corresponding wild relatives (Gunarathne et al. 2019). Most important prerequisite in phytoremediation for the effectiveness relies on their field applications, no matter how good and effective a method developed with employing a genetically modified organism it remains experimental and it is the greatest limitation for it. Environmental risk assessment of those transgenic plants introduced for effectual phytoremediation must be associated with complete and long-term field testing as a critical step in research and development. The research and development process must look closely at the prevention technologies for further spreading of modified genes from transgenic plants to natural plant communities. It is well documented that transgenic plants have exceptional capacity to contribute to the revitalization process of contaminated lands through the phytoremediation process. However, the development of further mechanisms such as genetic use restriction technologies, which can be utilized to control the dispersion of transgenic plants in natural habitats, should be facilitated.
Concluding Remarks
The use of hyperaccumulator plants to remediate contaminated sites depends on the quantity of metal at that site and the type of soil. Environmental factors play a major role in the success of bioremediation as the microbes used will be hampered if appropriate environmental conditions are not available. More rapidly growing plants with high phytoextraction ability should be identified for the remediation of pollutants from contaminated environmental sites. Moreover, assessment of metal stress on beneficial rhizospheric microorganisms and crops should be carried out and effective ways of enhancing the bioremediation process need to be worked out. Transgenic microbes and plants could effectively remediate contaminated sites of heavy metal and organic pollutants but its use should be subject to stringent biosafety procedures to ensure that there is no health or environmental hazards. More efficient ways of using transgenic plants and microbes should be identified that will enable effective remediation of polluted environments without horizontal transfer of recombinant plasmids or pollens to indigenous organisms, which is currently a major challenge (Gunarathne et al. 2019).
Phytoremediation is acknowledged as a cost-effective and environment-friendly alternative to traditional methods of environmental cleanup. It is a promising means of ameliorating heavy metal pollution from contaminated sites using transgenic hyperaccumulator plants. It is prioritized to consider aromatic plants, native wild plants or invasive plants as targets for creating transgenic plants for remediation of heavy metals, and also research on these targets should be carried out for identifying novel genes related to heavy metal accumulation and detoxification processes. Plants develop various cellular and molecular adaptations to tolerate the heavy metal stress. Some advancements are there in identification and understanding of metal transporters, hyperaccumulation, phytochelatins (PCs), and metallotioneins (MT) proteins that ensure heavy metal tolerance to plants. However, genes responsible for heavy metal uptake, translocation and sequestration need to be explored more to allow the development of new plant varieties that can be successfully exploited for phytoremediation. Practically, single approach is neither possible nor sufficient for effective cleanup of heavy metal-polluted soil. The combination of different approaches, including genetic engineering and microbe-assisted and chelate-assisted approaches, is essential for highly effective and exhaustive phytoremediation in the near future (Fig. 2.5).
2.3 Bioenergy for Eco-Friendly Fuels (Microbes and Biofuel: Toward a Brighter Future)
We are now seeing a warming earth with the increased incidence of extreme weather events, loss of habitat and species on a scale never seen before, and the problems due to overpopulated world. In the industrial era, fossil fuels are indispensable energy contributors; however, they are non-recyclable and less eco-friendly in nature as they accounting for the main cause of environmental pollution and its consequences. Current trend of population raise, overexploitation fossil fuels are increasing the demand for fuels availability and causing an upsurge in fuel prices and global warming effects (Tang et al. 2020). According to BP statistical review of world energy that fossil fuels including crude oil, coal, and natural gas are predicted to exhaust in the next 50 years (Dudley 2018). Long run reliance on fossil fuels is a major threat to world energy security, and its further mining from natural reserves poses serious environmental threats. Exploration of novel, economical and environmental benign and renewable sources of energy is very important to mitigate these concerns. Therefore, the need of the hour is equipping with an affordable, renewable, alternate, and sustainable energy source to conventional fossil fuels. In the recent times, biofuels are considered as inexhaustible and alternative source of energy. These biofuels categorized as first generation, second generation, and the third generation, corresponding to the feedstocks of food sources sugarcane, wheat, corn, soybean, potato, or sugar beet (Bringezu et al. 2007), lignocellulosic biomass and agricultural wastes (Eisentraut 2010), and microalgae and cyanobacteria (Demirbas 2011; Khan and Fu 2020), respectively. It is expensive to convert plant material into useable ethanol. Also, there are ethical considerations. Large amounts of land must be used to produce fuel, which reduces the land available for food production, which is a problem in developing countries with large populations to feed. The amount of land needed also threatens vital ecosystems. Microalgae and cyanobacteria are considered to the best attractive source for energy due to its high biomass productivity rate (dry weight per unit time per unit area) than those of higher plants. Thus, microbial system to produce biofuel is therefore less wasteful, more ethical, and cheaper, owing to their positive effect on the environment due to less to no greenhouse gas emissions. Microalgae- and cyanobacteria-based biofuel is considered as the promising one and has attracted more and more attention in the past years, owing to the characteristics such as fast growing rate, high-efficiency photosynthesis, and high lipid content for some species (Peng et al. 2016; Khan and Fu 2020). Microalgae and cyanobacteria are the current green molecular factories found to be effective and sustainable tools to grapple the pressing problem of climate change caused due to reliance on fossil fuels and based emission of anthropogenic CO2 (Urtubia et al. 2016; Choi et al. 2019).
Brazil is a world leader in sustainable biofuel production, as most automobiles of this country running on bioethanol or bioethanol blends. In view of this, currently it is demanding to isolation of new algae, with higher biomass production and oil accumulation. A schematic presentation of typical algal cell depicting biotechnological perspectives for boosting algal biofuel (biodiesel, bioethanol, and biohydrogen) production has been explained in Fig. 2.6.
Biotechnological perspectives for eco-friendly algal biofuel s production. (1) Knock-out and overexpression of genes involved in lipid biosynthesis. (2) Overexpression of native fermentation-related genes (PDC gene encoding pyruvate decarboxylase, ADH gene encoding alcohol dehydrogenase) in starch biosynthesis. (3) Genetic engineering of enzyme hydrogenase for improved biohydrogen (H2) photolysis, biogas (methane), and bioelectricity production
Recent investigation on microalgal strain improvement for biofuels (biodiesel, bioethanol, biogas, and biohydrogen) production has been carried out by mutagenesis of single target gene or engineering of multiple gene pathways involved in lipid, starch or pigment biosynthesis (Shin et al. 2019). In conjunction studies on knocking-out and overexpression of genes involved in lipid biosynthesis with the aim to modify microalgae and cyanobacteria for better ability to produce fatty acids and lipids (which upon transesterification generate biodiesel) has been documented (Urtubia et al. 2016). Previously, overexpression studies of native fermentation-related genes (for example, the PDC gene coding pyruvate decarboxylase and the ADH gene coding for alcohol dehydrogenase) have been attempted for the production of bioethanol (Dexter and Fu 2009). In the recent past, biogas (methane) produced via anaerobic digestion of algal biomass is a well-studied example of bioprocess configuration (Jankowska et al. 2017). Biohydrogen manufacturing has also been carried out, as biohydrogen is an appealing and alternative fuel because it creates no carbon byproducts during burning and is preferable for bio-electricity generation via fuel cells (Ban et al. 2018). Therefore, genetic manipulation of hydrogenases in algae has improved the yield of biohydrogen (H2) production more recently by photolysis (Yang et al. 2019), which is an attractive clean fuel helps in drastic reduction of carbon emission (Ban et al. 2018; Nagy et al. 2018). There has been an increasing interest in exploiting microalgae and cyanobacteria for the production biofuel precursors, such as triacyl-glycerol (TAG) and starch, which can be readily transformed into biodiesel and bioethanol (Rengel et al. 2018; Yunus and Jones 2018). Some species are better-suited for biofuel production as they are able to naturally biosynthesize and accumulate considerable amounts of intracellular lipids and starch which may be further improved with the advent of genetic manipulation studies (Urtubia et al. 2016). It is Chlamydomonas reinhardtii, Synechocystis sp. PCC 6803, Phaeodactylum tricornutum, and various Chlorella species are among the extensively studied engineered algal strains with regard to their biofuel producing potentials (Yang et al. 2019; Xue et al. 2017; Yunus et al. 2018).
Cyanobacteria are excellent biotechnological platforms to overproduce valuable compounds, with the advantages of comparatively simple genomes, easy transformation, diversity of suitable strains and availability of fully sequenced genomes and considered as model strains/systems (Yunus et al. 2018; Majidian et al. 2018). The biotechnological strategies used for enhancing algal biofuel production through genetic manipulation of these microorganisms are diverse, but the main themes are enhancing lipids and starch biosynthesis (Yunus and Jones 2018; Yunus et al. 2018; Takemura et al. 2019), increasing carbon-capture ability and lipids accumulation (Dinamarca et al. 2017; Gee and Niyogi 2017), and genetic engineering for biohydrogen production (Khetkorn et al. 2017; Yang et al. 2019; Bolatkhan et al. 2019). Immobilization studies of microalgae are capable of metabolizing the nutrients present in medium or wastewater into biomass and high-value cellular constituents (Bouabidi et al. 2019). The essential nutrients (required during the microalgae growth, such as salinity, nitrogen, pH, temperature, and light) are altered to promote the accumulation of lipid in microalgae under the concept of nutrient stress enhancement. Thus, lack of nitrogen, sulfur, phosphorus, and carbon will exert stress on the microalgae and result in the accumulation or enhanced production of energy storage metabolites, such as carbohydrates and lipids (Ran et al. 2019). In a study, Song et al. (2020) combined the nutrient stress approach and phytohormones fortification to enhance the microalgae lipid production, wherein it has promoted the biomass production.
Parsaeimehr et al. (2017) demonstrated the use two classes of low-cost exogenous bioactive molecules (phytohormones and antioxidants), to enhance the biomass and lipid content (alpha-linolenic acid fraction) of Chlorella protothecoides. Moreover, Bhuyar et al. (2020) used different types of phytohormones effect on Chlorella sp. within these, indole-3-butyric acid showed profound effect with enhanced outcome with regard to its biomass and lipid oil production. In a study conducted by Chen et al. (2020), within the range of phytohormones used, indoleacetic acid acted as positive stimulator and reported the enhanced coproduction of astaxanthin and lipid content in the green algae Chromochloris zofingiensis. Ryu et al. (2020) had produced Nannochloropsis salina mutant Mut68 via insertion mutation to increase the lipid production. After Mut68 was incubated for 8 days. When compare to wild type, the fatty acid methyl ester contents and productivity in the mutant increased by 34% and 75%, respectively.
Microalgae being simpler and its unicellular structure make the organism to more amenable to conduct genetic engineering or metabolic engineering studies. Fortunately, recent developments in microalgae omics together with the unlocking of its signaling and biosynthesis pathway have paved the way for understanding gene regulation, metabolic flux, proteins activity, and interaction (Guarnieri and Pienkos 2015). These technological perspectives were used to develop genetically modified microalgae strains engaged in enhanced lipid production/accumulation (Rastogi et al. 2018). It is evident that genetic engineering works to yield improved strains of an organism by overexpressing the key genes involved in fatty acid biosynthesis or inhibiting the lipid catabolism pathway such as starch synthesis and beta-oxidation. In a study, the microalga P. tricornutum was engineered at metabolic level to enhance the overproduction of omega-3 long chain polyunsaturated fatty acid docosahexaenoic acid (DHA). In second iteration, co-expression of anacyl-CoA-dependent Δ6-desaturase with the Δ5-elongase further increased DHA levels. (Hamilton et al. 2014). Apart from the genetic manipulation studies in Nannochloropsis oceania, Poliner et al. (2018) overexpressed different fatty acid desaturase genes (FAD), Δ5 and Δ12 fad encoding sequences, to increase the eicosapentaenoic acid production in microalgae. In another study, Li et al. (2019) overexpressed a novel bZIP1 transcription factor NobZIP1N that is involved in lipid metabolism transcription regulation in N. oceanic for concurrent lipid overproduction and secretion with no effect on microalgae growth.
The understanding of the lipid biosynthesis pathway of the microalgae provides a pathway to induce or accumulate the lipid in the microalgae for the production of biofuel and other bioproducts through the employment of precise genetic modifications. Investigations established CRISPR/Cas9 as a cutting-edge genome-editing technology, as CRISPR technique has relatively short duration, low cost, high accuracy and enhances the efficiency compared to other existing DNA editing tools (Doudna and Charpentier 2014). The application of these genome editing technologies amenable to enhance the quality and quantity of the valuable biofuel compounds in the microalgae. Shin et al. (2019) demonstrated the use of genome editing tool CRISPR-Cas9 for enhancing biodiesel production. This study shows they have knock out phospholipase A2 gene in Chlamydomonas reinhardtii to increase (64.25%) the overall lipid production. Chang et al. (2020) knocked out target gene ADP-glucose pyrophosphorylase (AGP) in marine algae Tetraselmis sp. using CRISPR-Cas9 RNP method to inhibit carbohydrate synthesis and thus to increase the lipid production by 274% as compared to the wild type.
Environmental Safety
Genetic engineering involves the modification or alteration of genes that were involved in lipid production and the effects are overwhelming and satisfactory. Genetic engineering offers the advantages by “tailor-made” microalgae species with faster maturation time and greater yield with reduced harvesting time. Despite this, genetic engineering should be monitored and examined closely, so that it does not become a possible ecological hazard. Therefore, the genetically modified microalgae have to pass thorough regulatory affairs for open cultivation system. Mutagenesis offer to design microalgae mutants, this may reduce the production cost, but it’s a chance event and tedious to identify the beneficial mutants of interest. However, this raises the concern of the scientist about the unknown side effects or outcomes by these microalgae mutants. In addition, the release of new genetically engineered microalgae may cause an imbalance in the ecology and limit the genetic diversity by disrupting the natural process of gene flow (Patra and Andrew 2015). Besides, the compounds produced from the genetically modified microalgae may pose a human health risk and additional regulations are required for the introduction of genetically modified microalgae for human consumption. In conclusion, the lipid accumulation machinery in the microalgae can be trigged by different approaches, such as stress conditions, genetic modification or the addition of exogenous stimulators, which in turn produce lipid in a large-scale and sustainable way. Biotechnology intervention for sustainable production of algal bioproducts has a great potential to produce next-generation eco-friendly biofuel s because of their higher productivity, ability to grow on non-arable land and high solar energy conversion efficiency (Ban et al. 2018).
Concluding Remarks
As on date, successful large-scale applications of algal-based biofuel production are rare, highlighting the scientific and applied challenges to integrate for the production of eco-friendly and sustainable biofuels. The recent development in biotechnology offers an effective CO2 capture, higher biofuel productivities, and more diverse biofuel molecules for sustainable development . Therefore, a shift of research paradigms is needed to introduce cutting edge biotechnologies, such as synthetic biology, gene editing, and artificial intelligence to promote the industrial scale production of algal biofuels. As a whole, the challenges must be tackled comprehensively for successful industrialization, sustainable, and economical production of microalgae biofuels and bioproducts. This goal may be accomplished by continuous research and development efforts by academia-industry linkages.
GEMs in Modern Food Production
Microorganisms contribution in the timeline of human history evident their remarkable role in the production/processing of food with nutritive values, even much before humans aware that these organisms accounted for the fermentation processes involved (Jay et al. 2005; Zhang et al. 2017). All enzymes are proteins that have catalytic functions at optimal conditions that are being leveraged in food processing. Natural food enzymes often have constrained for higher production due to extreme food processing conditions. Various food enzymes are commonly used in the food production to perform a number of different technical effects such as: reducing lactose content of foods (lactase), dough strengthening or starch modification in baking (amylases), vegetable oil refining (phospholipase), coffee production (mannanase), fruit- and vegetable processing (pectin esterase), conversion of starches into sugars and specialty products (carbohydrases such as amylase, glucoamylase, and transglucosidase), and hydrolysis of proteins (protease). Thus, genetically modified enzymes (gene sequence manipulation) are designed to improve the enzymological properties and to increase purity and yield after standardizing their gene expression studies. A given microorganism produces the enzyme as per the sequence of amino acid building blocks that define its properties and catalytic function. Various methods of genetic modification of microorganisms (strain improvement) are used to tailor enzyme functionality and stability via protein engineering, it also includes the controlled cultural conditions (e.g., pH and temperature) which differ from the natural conditions under which the organism develops and its improved strains are identified and commercialized.
Advances in the areas of biochemistry and molecular biology over the last several decades have led to largely use of GEMs in the production of medical and food substances, especially such processes are attributed to environmentally friendly, animal-friendly, and cost-effective means of production. For instance, insulin is currently produced using microbes rather than sacrificing pigs to harvest their pancreas, the only earlier source of insulin. Moreover, microbially-produced trypsin and chymosin are available as alternatives replacing the harvesting trypsin or rennet from animal sources such as pigs and cows. The applications of GEMs production are not only limited in replacing animal-based production methods but also extends to replace plant-based products. For example, alternatives to traditional agricultural production of plant-based substances, such as stevia extracts and vanilla, GEMs-based production of steviol glycosides (Philippe et al. 2014) and vanillin (Brochado et al. 2010) has many sustainability benefits including reduction in land usage, production of less waste, and provision of a more stable and affordable supply to meet the increasing growing population. Moreover, examples of food ingredients produced today by GEMs include vitamins, amino acids, functional proteins (e.g., texturants), nutritional proteins, oligosaccharides, flavors, and sweeteners (Adrio and Demain 2010). The industrial biosynthesis of riboflavin is one of the great success stories in biotechnology and metabolic engineering, till date around 100% of the commercially-produced riboflavin is manufactured using GEMs (Schwechheimer et al. 2016). Acevedo-Rocha Carlos et al. (2018) has review current biotechnological status of mircrobial cell factories for manufacturing of B vitamins in a sustainable way. Currently, cheese made with chymosin (the active milk protein coagulating enzyme in rennet) produced by GEMs, otherwise the earlier source is harvesting the enzyme from calf stomachs. Luckily, over the past several decades, the use of genetically modified microorganisms (GEMs) has become indispensable tools in food production and processing. Deployment of GEMs has improved food production by enhancing efficiency, reducing, resource requirements, and ultimately enabled innovations such as the cost-effective fortification of food with nutrients, vitamins, and amino acid, and delivery of tailored enzymes to achieve best food processing capabilities (Hanlon and Sewalt 2020).
Apart from genetic engineering much comprehensive understanding and integration of bioin-formatics, enzymology, biophysical chemistry, and molecular biology required for producing genetically modified food enzymes. Most genetically modified enzymes are expressed in well-established standard microbial model systems such as Escherichia coli and Pichia pastoris (Zhang et al. 2019). Briefly, following are the few important strategies employed while producing genetically modified enzymes. (1) Gene sequence optimization is commonly used for heterologous expression of recombinant enzymes to optimize codon bias of the host cell, as well as to limit mRNA secondary structure that could inhibit translation (Rosano and Ceccarelli 2014). Example: Genetically modified invertase with high transfructosylating activity was used for simple and efficient production of prebiotic fructooligosaccharides (Marin-Navarro et al. 2015). (2) Gene truncation or fusion requires knowledge of the relationship between amino acid sequence and enzyme function. (3) Site-directed mutagenesis is a rational design process based on in-depth knowledge of the target enzyme in terms of structure, catalytic mechanism, and active site. Example: Novel Bacillus licheni-formis α-amylases obtained using directed evolution by replacing active site domain His residues with Arg and Asp residues had higher activity at pH 4.5 than the wild type enzyme (Liu et al. 2017). In another study, the substrate specificity of Thermotoga maritima b-glucosidases after genetic modification was enhanced for quercetin glucosides (Sun et al. 2014). (4) Directed evolution, a non-rational design process that requires no prior knowledge of the target enzyme is an in vitro accelerated process that mimics natural evolution for generating enzymes. It consists of two key processes: genetic diversity generation for constructing combinatorial libraries and high-throughput screening or selection. Example: Bacillus spp. are the main producers of alkaline proteases with high thermal and pH stabilities. The cold activity at 10 °C and alkali-resistant properties of a Bacillus alcalophilus alkaline protease were improved through directed evolution using error-prone PCR (Liu et al. 2014) for use in cold-temperature food processing. Similarly, an alkaline serine protease from mesophilic Bacillus pumilus was engineered to have increased hydrolytic efficiency at 15 °C without compromising thermostability (Zhao and Feng 2018). (5) Semi-rational design involves mutations based on sequence, structure or computational models, followed by small-scale mutagenesis and screening methods (Hua et al. 2018). An example of semi-rational design is site saturation mutagenesis, in which a mutant library is created containing all possible substitutions at one or more target positions in a gene sequence (Ozgun et al. 2016). Figure 2.7 depicts various improved microbial strains methods used for sustainable food production.
3 Environmental Safety and Concluding Remarks
Various genetically modified food enzymes are currently produced from GEMs. Safety concerns have been raised regarding potential contamination of food with bacterial toxins or mycotoxins, allergens, or uncharacterized extraneous substances as impurities (De Santis et al. 2018). Genetic modification of enzymes may also change their allergenic properties, posing new potential health risks while processing or production of food additives/substances. For instance, type I sensitization was found in a study of 813 exposed industrial workers using genetically modified enzymes (Budnik et al. 2017). Thus, before marketing, genetically modified food enzymes need approval from various regulatory bodies such as the US Food and Drug Administration, the Association of Manufacturers and Formulators of Enzyme Products and the European Food Safety Authority, through processes that vary as per different countries guidelines. In addition, in several cultural and developing countries like India ethical and religious concerns have been raised for genetically modified enzymes. For instance, it has been suggested that raw materials or ingredients used in fermentation of microorganisms to produce enzymes should be halal (Ermis 2017). Currently, novel genetically modified food enzymes are largely used for applications in food processing involving carbohydrates, followed by lipids. Although there are challenges for safe use, genetic modification strategies for the development, whilst, novel food enzymes are highly promising for the future, and expected that more genetically modified enzymes will be introduced in various aspects of food processing.
In conclusion, most current production methods are unsustainable because they use nonrenewable sources and often generate hazardous waste. Many microorganisms produce vitamins naturally, but their corresponding metabolic pathways are tightly regulated since vitamins are needed only in catalytic amounts. Metabolic engineering is accelerating the development of microbial cell factories for vitamins/enzymes that could compete with chemical methods and they have been optimized and successful over decades, but scientific hurdles remain. Additional technological and regulatory issues need to be overcome/addressed for innovative bioprocesses to reach the market.
References
Acevedo-Rocha Carlos G, Gronenberg LS, Mack M, Commichau FM, Genee HJ (2018) Microbial cell factories for the sustainable manufacturing of B vitamins. Curr Opin Biotechnol 56:18–29
Adem M, Beyene D, Feyissa T (2017) Recent achievements obtained by chloroplast transformation. Plant Methods 13(30):2–11
Adriano DC (1986) Trace elements in the terrestrial environment. Seiten, 99 Abb., zahlr. Tab. Springer, New York. Preis: 228, DM
Adrio JL, Demain AL (2010) Recombinant organisms for production of industrial products. Bioengin Bugs 1(2):116–311
Ahmad S, Wei X, Sheng Z, Hu P, Tang S (2020) CRISPR/Cas9 for development of disease resistance in plants: recent progress, limitations and future prospects. Brief Funct Genomics 19(1):26–39
Ahuja I, de Vos RC, Bones AM, Hall RD (2010) Plant molecular stress responses face climate change. Trends Plant Sci 15(12):664–674
Aileni M, Kokkirala VR, Yarra R, Umate P, Anil Kumar V, Kasula K, Abbagani S (2011) In vitro regeneration, flowering and seed formation from leaf explants of Scoparia dulcis L. a medicinal plant. Med Aromat Plants 5(2):143–146
Akyol TY, Sato S, Turkan I (2020) Deploying root microbiome of halophytes to improve salinity tolerance of crops. Plant Biotechnol Rep 14:143–150
Ali A, Yun DJ (2020) Arabidopsis HOS15 is a multifunctional protein that negatively regulate ABA-signaling and drought stress. Plant Biotechnol Rep 14:163–167
Alloway BJ (1990) In: Alloway BJ (ed) Heavy metals in soils. Wiley, New York
Anh BTK, Ha NTH, Danh LT, Van Minh V, Kim DD (2017) Phytoremediation applications for metal-contaminated soils using terrestrial plants in Vietnam. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: management of environmental contaminants, vol 5, pp 157–181
Arora NK (2018) Bioremediation: a green approach for restoration of polluted ecosystems. Env Sustain 1:305–307
Arora NK, Panosyan H (2019) Extremophiles: applications and roles in environmental sustainability. Env Sustain 2:217–218
Arthur EL et al (2005) Phytoremediation—an overview. CRC Crit Rev Plant Sci 24(2):109–122
Ashraf M, Harris P (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox Environ Safe 174:714–727
Atia FAM, Al-Ghouti MA, Al-Naimi F, Abu-Dieyeh M, Ahmed T, Al-Meer SH (2019) Removal of toxic pollutants from produced water by phytoremediation: applications and mechanistic study. J Water Process Eng 32:1–15
Azad MAK, Amin L, Sidik NM (2014) Genetically engineered organisms for bioremediation of pollutants in contaminated sites. Chin Sci Bull 59(8):703–714
Azizoglu U, Jouzani GS, Yilmaz N, Baz E, Ozkok D (2020) Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: a review. Sci Total Environ 734:139169. https://doi.org/10.1016/j.scitotenv.2020.139169
Bakhsh A, Hussain T (2015) Engineering crop plants against abiotic stress: current achievements and prospects. Emir J Food Agric 27(1):24–39
Ban S, Lin W, Wu F, Luo J (2018) Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour Technol 251:350–357
Barbosa B, Boléo S, Sidella S (2015) Phytoremediation of heavy metal-contaminated soils using the perennial energy crops Miscanthus spp. and Arundo donax L. Bioenergy Res 8:1500–1511
Bargiela R, Herbst FA, Martínez-Martínez M, Seifert J, Rojo D, Cappello S (2015) Metaproteomics and metabolomics analyses of chronically petroleum-polluted sites reveal the importance of general anaerobic processes uncoupled with degradation. Proteomics 15:3508–3520
Basharat Z, Novo LAB, Yasmin A (2018) Genome editing weds CRISPR: what is in it for phytoremediation? Plan Theory 7(51):1–8
Bennet R, Buthelezi TJ, Ismael Y, Morse S (2003) Bt cotton, pesticides labour and health: a case study of smallholder farmers in the Makhathini Flats Republic of South Africa. Outlook Ag 32(2):123–128
Bhuyar P, Yusoff MM, Ab Rahim MH, Sundararaju S, Maniam GP, Govindan N (2020) Effect of plant hormones on the production of biomass and lipid extraction for biodiesel production from microalgae Chlorella Sp. J Microbiol Biotechnol Food Sci 9(4):671–674
Bless AE, Colin F, Crabit A, Devaux N, Philippon O, Follain S (2018) Landscape evolution and agricultural land salinization in coastal area: a conceptual model. Sci Total Environ 625:647–656
Bloch SE, Ryu M-H, Ozaydin B, Broglie R (2020) Harnessing atmospheric nitrogen for cereal crop production. Curr Opin Biotechnol 62:181–188
Bolatkhan K, Kossalbayev BD, Zayadan BK, Tomo T, Veziroglu TN, Allakhverdiev SI (2019) Hydrogen production from phototrophic microorganisms: reality and perspectives. Int J Hydrog Energy 44(2):5799–5811
Bortesi L, Fischer R (2015) The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol Adv 33(1):41–52
Bouabidi ZB, EI-Naas M, Zhang Z (2019) Immobilization of microbial cells for the biotreatment of wastewater: a review. Environ Chem Lett 17:241–257
Bringezu S, Ramesohl S, Arnold K, Fischedick M, Von Geibler J (2007) Towards a sustainable biomass strategy: what we know and what we should know. Wuppertal papers. https://www.researchgate.net/publication/237522564_Towards_a_sustainable_biomass_strategy
Brochado AR, Matos C, Møller BL, Hansen J, Mortensen UH, Patil KR (2010) Improved vanillin production in baker’s yeast through in silico design. Microb Cell Factories 9(84):1–15
Brookes G, Barfoot P (2020) Environmental impacts of genetically modified (GM) crop use 1996–2018: impacts on pesticide use and carbon emissions. GM Crops Food 11(4):215–241
Budnik LT, Scheer E, Burge PS, Baur X (2017) Sensitising effects of genetically modified enzymes used in flavour, fragrance, detergence and pharmaceutical production: cross-sectional study. Occup Environ Med 74(1):39–45
Bulle M, Yarra R, Abbagani S (2016) Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol Breed 36(36):1–12
Burges A, Alkorta I, Epelde L, Garbisu C (2018) From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int J Phytoremediation 20(4):384–397
Cameselle C, Gouveia S, Urréjola S (2019) Benefits of phytoremediation amended with DC electric field. Application to soils contaminated with heavy metals. Chemosphere 229:481–488
Chand S, Yaseen M, Rajkumari Patra DD (2015) Application of heavy metal rich tannery sludge on sustainable growth, yield and metal accumulation by clarysage (Salvia sclarea L.). Int J Phytoremediation 17(12):1171–1176
Chandrasekaran J, Brumin M, Wolf D, Leibman D, Klap C, Pearlsman M, Sherman A, Arazi T, Gal-On A (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol Plant Pathol 17(7):1140–1153
Chang KS, Kim J, Park H, Hong S-J, Lee C-G, Jin E (2020) Enhanced lipid productivity in AGP knockout marine microalga Tetraselmis sp. using a DNA-free CRISPR-Cas9 RNP method. Bioresour Technol 303:122932
Chen J, Piao Y, Liu Y, Li X, Piao Z (2018) Genome-wide identification and expression analysis of chitinase gene family in Brassica rapa reveals its role in clubroot resistance. Plant Sci 270:257–267
Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70:28.1–28.31
Chen J-H, Wei D, Lim P-E (2020) Enhanced coproduction of astaxanthin and lipids by the green microalga Chromochloris zofingiensis: selected phytohormones as positive stimulators. Bioresour Technol 295:1–41
Choi HI, Lee JS, Choi JW, Shin YS, Sung YJ, Hong ME, Kwak HS, Kim CY, Sim SJ (2019) Performance and potential appraisal of various microalgae as direct combustion fuel. Bioresour Technol 273:341–349
Coelho N, Gonçalves S, Romano A (2020) Endemic plant species conservation: biotechnological approaches. Plants 9(3):1–22
Coolen S, Proietti S, Hickman R, Davila Olivas NH, Huang PP, Van Verk MC, Van Pelt JA, Wittenberg AH, De Vos M, Prins M, Van Loon JJ (2016) Transcriptome dynamics of Arabidopsis during sequential biotic and abiotic stresses. Plant J 86(3):249–267
Corlett RT (2017) A bigger toolbox: biotechnology in biodiversity conservation. Trends Biotechnol 35:55–65
DalCorso G, Fasani DE, Manara A, Visioli G, Furini A (2019) Heavy metal pollutions: state of the art and innovation in phytoremediation. Int J Mol Sci 20(14):1–17
De Santis B, Stockhofe N, Wal J-M, Weesendorp E, Lalles J-P, van Dijk J, Kok E, De Giacomo M, Einspanier R, Onori R (2018) Case studies on genetically modified organisms (GMOs): potential risk scenarios and associated health indicators. Food Chem Toxicol 117:36–65
Deepa AV, Dennis Thomas T (2020) In vitro strategies for the conservation of Indian medicinal climbers. In Vitro Cell Dev Biol Plant 56:784–802. https://doi.org/10.1007/s11627-020-10084-x
Demirbas MF (2011) Biofuels from algae for sustainable development. Appl Energy 88(10):3473–3480
Deng L et al (2016) Long-term field phytoextraction of zinc/cadmium contaminated soil by Sedum plumbizincicola under different agronomic strategies. Int J Phytoremediation 18(2):134–140
Dexter J, Fu P (2009) Metabolic engineering of cyanobacteria for ethanol production. Energy Environ Sci 2(8):857–864
Dhankher OP, Foyer CH (2018) Climate resilient crops for improving global food security and safety. Plant Cell Environ 41:877–884
Dhanwal P, Kumar A, Dudeja S, Chhokar V, Beniwal V (2017) Recent advances in phytoremediation technology. In: Kumar R, Sharma AK, Ahluwalia SS (eds) Advances in environmental biotechnology. Springer, Singapore, pp 227–241
Dharupaneedi SP, Nataraj SK, Nadagouda M, Reddy KR, Shukla SS, Aminabhavi TM (2019) Membrane-based separation of potential emerging pollutants. Sep Purif Technol 210:850–866
Dinamarca J, Levitan O, Kumaraswamy GK, Lun DS, Falkowski PG (2017) Overexpression of a diacylglycerol acyltransferase gene in Phaeodactylum tricornutum directs carbon towards lipid biosynthesis. J Phycol 53(2):405–414
Dixit R, Malaviya D, Pandiyan K, Singh UB, Sahu A, Shukla R, Singh BP, Rai JP, Sharma PK, Lade H (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212
Dobrogojski J, Spychalski M, Luciński R, Borek S (2018) Transgenic plants as a source of polyhydroxyalkanoates. Acta Physiol Plant 40(162):1–17
Doudna JA, Charpentier E (2014) Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346(6213):1–14
Duarte M, Nielsen A, Camarinha-Silva A, Vilchez-Vargas R, Bruls T, Wos-Oxley ML (2017) Functional soil metagenomics: elucidation of polycyclic aromatic hydrocarbon degradation potential following 12 years of in situ bioremediation. Environ Microbiol 19(8):2992–3011
Dudley B (2018) BP statistical review of world energy. BP Stat Rev 6:2018
Eisentraut A (2010) Sustainable production of second-generation biofuels, potential and perspectives in major economies and developing countries. International Energy Agency
Engelmann F (2011) Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell Dev Biol Plant 47:5–16
Ermis E (2017) Halal status of enzymes used in food industry. Trends Food Sci Technol 64:69–73
Ezezika OC, Singer PA (2010) Genetically engineered oil-eating microbes for bioremediation: prospects and regulatory challenges. Technol Soc 32(4):331–335
Fang X, Qi Y (2016) RNAi in plants: an Argonaute-centered view. Plant Cell 28(2):272–285
FAO (2018) The future of food and agriculture: alternative pathways to 2050. Food and Agriculture Organization of the United Nations, Rome. https://www.fao.org/global-perspectivestudies/resources/detail/en/c/1157074/
Fragoso G, Hernández M, Cervantes-Torres J, Ramírez-Aquino R, Chapula H, Villalobos N, Segura-Velázquez R, Figueroa A, Flores I, Jiménez H, Adalid L (2017) Transgenic papaya: a useful platform for oral vaccines. Planta 245(5):1037–1048
Gangola S, Joshi S, Kumar S, Pandey SC (2019) Comparative analysis of fungal and bacterial enzymes in biodegradation of xenobiotic compounds. In: Smart bioremediation technologies: microbial enzymes. Academic Press, Cambridge, MA, pp 169–189
Gee CW, Niyogi KK (2017) The carbonic anhydrase CAH1 is an essential component of the carbon-concentrating mechanism in Nannochloropsis oceanica. Proc Natl Acad Sci U S A 114(17):4537–4542
Gerhardt KE, Gerwing PD, Greenberg BM (2017) Opinion: taking phytoremediation from proven technology to accepted practice. Plant Sci 256:170–185
Gesine S, Eckerstorfer M, Rastelli V, Reichenbecher W, Restrepo-Vassalli S, Ruohonen-Lehto M, Saucy A-GW, Mertens M (2017) Herbicide resistance and biodiversity: agronomic and environmental aspects of genetically modified herbicide-resistant plants. Environ Sci Eur 29:5
Ghag SB, Shekhawat UK, Hadapad AB, Ganapathi TR (2015) Stacking of host-induced gene silencing mediated resistance to banana bunchy top virus and fusarium wilt disease in transgenic banana plants. Curr Trends Biotechnol Pharm 9(3):212–221
Ghosal D, Ghosh S, Dutta TK, Ahn Y (2016) Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHS): a review. Front Microbiol 7:1–21
Girma G (2015) Microbial bioremediation of some heavy metals in soils: an updated review. EAJBS 7(1):29–45
Guarnieri MT, Pienkos PT (2015) Algal omics: unlocking bioproduct diversity in algae cell factories. Photosynth Res 123(3):255–263
Gunarathne V, Mayakaduwa S, Ashiq A, Weerakoon SR, Biswas JK, Vithanage M (2019) Transgenic plants: benefits, applications, and potential risks in phytoremediation. Transgenic plant technology for remediation of toxic metals and metalloids. Elsevier, Amsterdam, pp 89–10
Halewood M, Chiurugwi T, Sackville Hamilton R, Kurtz B, Marden E, Welch E, Michiels F, Mozafari J, Sabran M et al (2018) Plant genetic resources for food and agriculture: opportunities and challenges emerging from the science and information technology revolution. New Phytol 217:1407–1419
Hamilton ML, Haslam RP, Napier JA, Sayanova O (2014) Metabolic engineering of Phaeodactylum tricornutum for the enhanced accumulation of omega-3 long chain polyunsaturated fatty acids. Metab Eng 22:3–9
Hanlon P, Sewalt V (2020) GEMs: genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit Rev Food Sci Nutr 61(6):959–970. https://doi.org/10.1080/10408398.2020.1749026
Haq S, Bhatti AA, Dar ZA, Bhat SA (2020) Phytoremediation of heavy metals: an eco-friendly and sustainable approach. In: Bioremediation and biotechnology, pp 215–231
Haque SM, Ghosh B (2016) High-frequency somatic embryogenesis and artificial seeds for mass production of true-to-type plants in Ledebouria revoluta: an important cardioprotective plant. Plant Cell Tiss Organ Cult 127(1):71–83
He J et al (2015) Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol 205(1):240–254
Hefferon KL (2015) Nutritionally enhanced food crops; progress and perspectives. Int J Mol Sci 16:3895–3914
Henry HF, Burken JG, Maier RM, Newman LA, Rock S, Schnoor JL, Suk WA (2013) Phytotechnologies: preventing exposures, improving public health. Int J Phytoremediation 15(9):889–899
Hossain F, Pray C, Lu Y, Huang J, Fan C, Hu R (2004) Genetically modified cotton and farmers’ health in China. Int J Occ Env Health 10(3):296–303
Hua W, El Sheikha AF, Xu J (2018) Molecular techniques for making recombinant enzymes used in food processing. In: Molecular techniques in food biology: safety, biotechnology, authenticity and traceability, pp 95–112
Ilker OI, Can H, Dogan I (2020) Phytoremediation using genetically engineered plants to remove metals: a review. Environ Chem Lett 19:669–698
Jaiswal S, Singh DK, Shukla P (2019) Gene editing and systems biology tools for pesticide bioremediation: a review. Front Microbiol 10:1–13
Jan AT, Azam M, Ali A, Haq QMR (2014) Prospects for exploiting bacteria for bioremediation of metal pollution. Crit Rev Environ Sci Technol 44(5):519–560
Jankowska E, Sahu AK, Oleskowicz-Popiel P (2017) Biogas from microalgae: review on microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew Sust Energ Rev 75:692–709
Janssen Dick B, Gerhard S (2020) Perspectives of genetically engineered microbes for groundwater bioremediation. Environ Sci Process Impacts 22:487–499
Jay JM, Loessner MJ, Golden DA (2005) Modern food microbiology, 7th edn. Springer, New York
Khan S, Fu P (2020) Biotechnological perspectives on algae: a viable option for next generation biofuels. Curr Opin Biotechnol 62:146–152
Khanday I, Skinner D, Yang B, Mercier R, Sundaresan V (2018) A male-expressed rice embryogenic trigger redirected for asexual propagation through seeds. Nature 565:91–95
Khetkorn W, Rastogi RP, Incharoensakdi A, Lindblad P, Madamwar D, Pandey A, Larroche C (2017) Microalgal hydrogen production—a review. Bioresour Technol 243:1194–1206
Kim HS, Kwak SS (2020) Crop biotechnology for sustainable agriculture in the face of climate crisis. Plant Biotechnol Rep 14:139–141
Klumper W, Qaimm M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS One 9(11):1–15
Kokkirala VR, Kota S, Yarra R, Bulle M, Aileni M, Gadidasu KK, Teixeira da Silva JA, Abbagani S (2012) Micropropagation via nodal explants of Woodfordia fruticosa (L.) Kurz. Med Aromat Plants 6(1):50–53
Kouser S, Qaim M (2011) Impact of Bt cotton on pesticide poisoning in smallholder agriculture: a panel data analysis. Ecol Econ 70:3–10
Kumar S, Dagar VK, Khasa YP, Kuhad RC (2013) Genetically modified microorganisms (GMOS) for bioremediation. In: Biotechnology for environmental management and resource recovery. Springer, New Delhi, pp 191–218
Kwak SS (2019) Biotechnology of the sweetpotato: ensuring global food and nutrition security in the face of climate change. Plant Cell Rep 38:1361–1363
Laurence D, Christophe L, Roger F (2011) Using the bio-insecticide Bacillus thuringenesis isralensis in mosquito control pests control and pesticides exposure and toxicity assessment pesticides in the modern world, pp 93–126
Leus L (2018) Breeding for disease resistance in ornamentals. In: Ornamental crops. Springer, Cham, pp 97–125
Levy A, Conway JM, Dangl JL, Woyke T (2018) Elucidating bacterial gene functions in the plant microbiome. Cell Host Microbe 24(4):475–485
Li D-W, Balamurugan S, Yang Y-F, Zheng J-W, Huang D, Zou L-G, Yang W-D, Liu J-S, Guan Y, Li H-Y (2019) Transcriptional regulation of microalgae for concurrent lipid overproduction and secretion. Sci Adv 5:1–11
Li GX, Xu BC, Yin LN, Wang SW, Zhang SQ, Shan L, Kwak SS, Ke Q, Deng XP (2020) Dryland agricultural environment and sustainable productivity. Plant Biotechnol Rep 14:169–176
Liu S, Zhang F, Chen J, Sun G (2011) Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J Environ Sci 23(9):1544–1550
Liu Y, Zhang T, Zhang Z, Sun T, Wang J, Lu F (2014) Improvement of cold adaptation of Bacillus alcalophilus alkaline protease by directed evolution. J Mol Catal B Enzym 106:117–123
Liu D, An Z, Mao Z, Ma L, Lu Z (2015) Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus StGCS-GS in the presence of Cd, Zn and Cu alone or in combination. PLoS One 10(6):1–15
Liu Y, Huang L, Jia L, Gui S, Fu Y, Zheng D, Guo W, Lu F (2017) Improvement of the acid stability of Bacillus licheniformis alpha amylase by site-directed mutagenesis. Process Biochem 58:174–180
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q et al (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121
Majidian P, Tabatabaei M, Zeinolabedini M, Naghshbandi MP, Chisti Y (2018) Metabolic engineering of microorganisms for biofuel production. Renew Sustain Energy 82(3):3863–3885
Malla MA, Dubey A, Yadav S, Kumar A, Hashem A, Abd Allah EF (2018) Understanding and designing the strategies for the microbe-mediated remediation of environmental contaminants using omics approaches. Front Microbiol 9:1–18
Malnoy M, Viola R, Jung MH, Koo OJ, Kim S, Kim JS, Velasco R, Nagamangala Kanchiswamy C (2016) DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front Plant Sci 7:1–8
Mamta B, Rajam MV (2018) RNA interference: a promising approach for crop improvement. In: Biotechnologies of crop improvement, vol 2. Springer, Cham, pp 41–65
Marconi AM, Kieboom J, de Bont JA (1997) Improving the catabolic functions in the toluene-resistant strain pseudomonas putida S12. Biotechnol Lett 19:603–606
Marin-Navarro J, Talens-Perales D, Polaina J (2015) One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl Microbiol Biotechnol 99(6):2549–2555
Martínez-Zavala SA, Barboza-Pérez UE, Hernández-Guzmán G, Bideshi DK, Barboza-Corona JE (2020) Chitinases of Bacillus thuringiensis phylogeny, modular structure, and applied potentials. Front Microbiol 10:3032. https://doi.org/10.3389/fmicb.2019.03032
Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH Jr, Rothman KJ, Hendricks KA (2006) Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect 114(2):237–241
Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21(6):1265–1277
Mosoarca G, Vancea C, Popa S, Boran S (2018) Adsorption, bioaccumulation and kinetics parameters of the phytoremediation of cobalt from wastewater using Elodea canadensis. Bull Environ Contam Toxicol 100:733–739
Mu N, Rahman MA, Kabir Y (2020) Plant-produced monoclonal antibody as immunotherapy for cancer. Hindawi BioMed Res Int 2020:3038564., 10 pages. https://doi.org/10.1155/2020/3038564
Nagy V, Podmaniczki A, Vidal-Meireles A, Tengolics R, Kovacs L, Rakhely G, Scoma A, Toth SZ (2018) Water-splitting-based, sustainable and efficient H2 production in green algae as achieved by substrate limitation of the Calvin–Benson–Bassham cycle. Biotechnol Biofuels 11(69):1–18
Nahar N, Rahman A, Nawani NN, Ghosh S, Mandal A (2017) Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana. J Plant Physiol 218:121–126
Naik M, Duraphe M (2012) Review paper on–parameters affecting bioremediation. Int J Life Sci Pharm Res 2(3):1–4
Niu H, Wang J, Zhuang W, Liu D, Chen Y, Zhu C (2018) Comparative transcriptomic and proteomic analysis of Arthrobacter sp. CGMCC 3584 responding to dissolved oxygen for cAMP production. Sci Rep 8:1–13
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14:1–26
Ozgun GP, Ordu EB, Tutuncu HE, Yelboga E, Sessions RB, Gul Karaguler N (2016) Site saturation mutagenesis applications on Candida methylica formate dehydrogenase. Scientifica 3:1–7
Paeng SK, Chi YH, Kang CH, Chae HB, Lee ES, Park JH, Wi SD, Bae SB, Phan KAT, Lee SY (2020) Chaperone function of Arabidopsis NPR1. Plant Biotechnol Rep 14:227–233
Pajević S, Borišev M, Nikolić N, Arsenov DD, Orlović S, Župunski M (2016) Phytoextraction of heavy metals by fast-growing trees: a review. Phytoremediation. Springer, Cham, pp 29–64
Pande V, Pandey SC, Joshi T, Sati D, Gangola S, Kumar S, Samant M (2019) Biodegradation of toxic dyes: a comparative study of enzyme action in a microbial system. In: Smart bioremediation technologies, pp 255–287
Pande V, Pandey SC, Sati D, Pande V, Samant M (2020) Bioremediation: an emerging effective approach towards environment restoration. Environ Sustain 3:91–103
Parsaeimehr A, Mancera-Andrade EI, Robledo-Padilla F, Iqbal HM, Parra-Saldivar R (2017) A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res 26:312–322
Patra S, Andrew AA (2015) Human, social, and environmental impacts of human genetic engineering. J Biomed Sci 2(14):1–3
Pazmiño-Ibarra V, Mengual-Martí A, Targovnik AM, Herrero S (2019) Improvement of baculovirus as protein expression vector and as biopesticide by CRISPR/Cas9 editing. Biotechnol Bioeng 116:2823–2833
Pellegrino E, Bedini S, Nuti M, Ercoli L (2018) Impact of genetically engineered maize on agronomic, environmental and toxicological traits: a meta-analysis of 21 years of field data. Sci Rep 8:1–12
Peng L, Zhang Z, Cheng P, Wang Z, Lan CQ (2016) Cultivation of Neochloris oleoabundans in bubble column photobioreactor with or without localized deoxygenation. Bioresour Technol 206:255–263
Peng L, Dongdong F, Chu H, Wang Z, Qi H (2020) Biofuel production from microalgae: a review. Environ Chem Lett 18:285–297
Philippe RN, De Mey M, Anderson J, Ajikumar PK (2014) Biotechnological production of natural zero-calorie sweeteners. Curr Opin Biotechnol 26:155–161
Poliner E, Pulman JA, Zienkiewicz K, Childs K, Benning C, Farré EM (2018) A toolkit for Nannochloropsis oceanica CCMP 1779 enables gene stacking and genetic engineering of the eicosapentaenoic acid pathway for enhanced long-chain polyunsaturated fatty acid production. Plant Biotechnol J 16(1):298–309
Raldugina GN et al (2018) Expression of rice OsMyb4 transcription factor improves tolerance to copper or zinc in canola plants. Biol Plant 62:511–520
Ran W, Wang H, Liu Y, Qi M, Xiang Q, Yao C, Zhang Y, Lan X (2019) Storage of starch and lipids in microalgae: biosynthesis and manipulation by nutrients. Bioresour Technol 291:1–12
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8(2):221–226
Rastogi RP, Pandey A, Larroche C, Madamwar D (2018) Algal green energy–R&D and technological perspectives for biodiesel production. Renew Sust Energy Rev 82(3):2946–2969
Razzaq A, Sadia B, Raza A, Khalid Hameed M, Saleem F (2019) Metabolomics: a way forward for crop improvement. Meta 9(303):1–37
Rengel R, Smith RT, Haslam RP, Sayanova O, Vila M, Leon R (2018) Overexpression of acetyl-CoA synthetase (ACS) enhances the biosynthesis of neutral lipids and starch in the green microalga Chlamydomonas reinhardtii. Algal Res 31:183–193
Rihan HZ, Kareem F, El-Mahrouk ME, Fuller MP (2017) Artificial seeds (principle, aspects and applications). Agronomy 7(71):1–15
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:1–17
Ryu AJ, Kang NK, Jeon S, Hur DH, Lee EM, Lee DY, Jeong B-R, Chang YK, Jeong KJ (2020) Development and characterization of a Nannochloropsis mutant with simultaneously enhanced growth and lipid production. Biotechnol Biofuels 13(46):1–14
Samada LH, Tambunan USF (2020) Biopesticides as promising alternatives to chemical pesticides: a review of their current and future status. J Biol Sci 20(2):66–76
Samant M, Pandey SC, Pandey A (2018) Impact of hazardous waste material on environment and their management strategies. In: Microbial biotechnology in environmental monitoring and cleanup, pp 175–192
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A et al (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Schwechheimer SK, Park EY, Revuelta JL, Becker J, Wittmann C (2016) Biotechnology of riboflavin. Appl Microbiol Biotechnol 100(5):2107–2119
Seow-Neng C, Bakar NA, Mahmood M, Chai-Ling H, Shaharuddin NA (2017) Alternative strategy in crop protection: protease inhibitors from turmeric. In: Crop improvement. Springer, Cham, pp 253–270
Shah K (2011) Cadmium metal detoxification and hyperaccumulators. Detoxification of heavy metals. Springer, Berlin, pp 181–203
Shah K, Pathak L (2019) Transgenic energy plants for phytoremediation of toxic metals and metalloids. In: Prasad MNV (ed) Transgenic plant technology for remediation of toxic metals and metalloids. Academic Press, New York, pp 319–340. https://doi.org/10.1016/B978-0-12-814389-6.00015-8
Shanmugaraj B, Bulaon CJI, Phoolcharoen W (2020) Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants 9(7):1–19
Shin YS, Jeong J, Nguyen THT, Kim JYH, Jin E, Sim SJ (2019) Targeted knockout of phospholipase A2 to increase lipid productivity in Chlamydomonas reinhardtii for biodiesel production. Bioresour Technol 271:368–374
Shinwari ZK, Jan SA, Nakashima K, Yamaguchi-Shinozaki K (2020) Genetic engineering approaches to understanding drought tolerance in plants. Plant Biotechnol Rep 14:151–162
Smyth SJ (2019) The human health benefits from GM crops. Plant Biotechnol J Lett 18(4):887–888
Song X, Zhao Y, Han B, Li T, Zhao P, Xu J-W, Yu X (2020) Strigolactone mediates jasmonic acid-induced lipid production in microalga Monoraphidium sp. QLY-1 under nitrogen deficiency conditions. Bioresour Technol 306:1–10
Soumare A, Diedhiou AG, Thuita M, Hafidi M, Ouhdouch Y, Gopalakrishnan S, Kouisni L (2020) Exploiting biological nitrogen fixation: a route towards a sustainable agriculture. Plants (Basel) 9(8):1–22
Srinivas K, Muralikrishna N, Kumar KB, Ragu E, Aileni M, Kiranmayee K, Yashodhara V, Sadanandam A (2016) Biolistic transformation of Scoparia dulcis L. Physiol Mol Biol Plants 22(1):61–68
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci 9:1476
Sun H, Xue Y, Lin Y (2014) Enhanced catalytic efficiency in quercetin-4′-glucoside hydrolysis of Thermotoga maritima β-glucosidase A by site-directed mutagenesis. J Agric Food Chem 62(28):6763–6770
Sun et al (2018) Overexpression of PtABCC1 contributes to mercury tolerance and accumulation in Arabidopsis and poplar. Biochem Biophys Res Commun 497(4):997–1002
Takemura T, Imamura S, Tanaka K (2019) Identification of a chloroplast fatty acid exporter protein, CmFAX1, and triacylglycerol accumulation by its overexpression in the unicellular red alga Cyanidioschyzon merolae. Algal Res 38:1–8
Tang DYY, Yew GY, Koyande AK, Chew KW, Vo DVN, Show PL (2020) Green technology for the industrial production of biofuels and bioproducts from microalgae: a review. Environ Chem Lett 18:1967–1985
Tanpure RS, Barbole RS, Dawkar VV, Waichal YA, Joshi RS, Giri AP, Gupta VS (2017) Improved tolerance against Helicoverpa armigera in transgenic tomato over-expressing multi-domain proteinase inhibitor gene from Capsicum annuum. Physiol Mol Biol Plants 23(3):597–604
Trębicki P, Nancarrow N, Cole E, Bosque-Pérez NA, Constable FE, Freeman AJ, Rodoni B, Yen AL, Luck JE, Fitzgerald GJ (2015) Virus disease in wheat predicted to increase with a changing climate. Glob Chang Biol 21(9):3511–3519
Tripathi M, Singh D, Vikram S, Singh V, Kumar S (2018) Metagenomic approach towards bioprospection of novel biomolecule(s) and environmental bioremediation. Annu Res Rev Biol 22:1–12
Urgun-Demirtas M, Stark B, Pagilla K (2006) Use of genetically engineered microorganisms (GEMs) for the bioremediation of contaminants. Crit Rev Biotechnol 26(3):145–164
Urtubia HO, Betanzo LB, Vasquez M (2016) Microalgae and cyanobacteria as green molecular factories: tools and perspectives. IntechOpen, Algae-organisms for imminent biotechnology. https://doi.org/10.5772/100261
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17
Van Montagu M (2020) The future of plant biotechnology in a globalized and environmentally endangered world. Genet Mol Biol 43:1–11
Varoquaux N, Cole B, Gao C, Pierroz G, Baker CR, Patel D, Madera M, Jeffers T, Hollingsworth J, Sievert J, Yoshinaga Y (2019) Transcriptomic analysis of field-droughted sorghum from seedling to maturity reveals biotic and metabolic responses. Proc Natl Acad Sci 116:27124–27132
Viktorova J et al (2017) Native phytoremediation potential of Urtica dioica for removal of PCBs and heavy metals can be improved by genetic manipulations using constitutive CaMV 35S promoter. PLoS One 12(10):1–12
Wan X, Lei M, Chen T (2015) Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci Total Environ 563:796–802
Wang XT, Zhi JK, Liu XR, Zhang H, Liu HB, Xu JC (2018) Transgenic tobacco plants expressing a P1B-ATPase gene from Populus tomentosa Carr. (PtoHMA5) demonstrate improved cadmium transport. Int J Biol Macromol 113:655–661
Wani SH, Haider N, Kumar H, Singh NB (2010) Plant plastid engineering. Curr Genomics 11(7):500–512
Wei X, Lyu S, Yu Y, Wang Z, Liu H, Pan D, Chen J (2017) Phylloremediation of air pollutants: exploiting the potential of plant leaves and leaf-associated microbes. Front Plant Sci 8:1–23
Wolejko E, Wydro U, Loboda T (2016) The ways to increase efficiency of soil bioremediation. Ecol Chem Eng 23(1):155–174
Wu G, Kang H, Zhang X, Shao H, Chu L, Ruan C (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174(1–3):1–8
Xia Y, Liu J, Wang Y, Zhang XX, Shen ZG, Hu ZB (2018) Ectopic expression of Vicia sativa Caffeoyl-CoA O methyltransferase (VsCCoAOMT) increases the uptake and tolerance of cadmium in Arabidopsis. Environ Exp Bot 145:47–53
Xue J, Balamurugan S, Li D-W, Liu Y-H, Zeng H, Wang L, Yang W-D, Liu J-S, Li H-Y (2017) Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply. Metab Eng 41:212–221
Yan A, Wang Y, Tan SN, Yusof MLM, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land front. Plant Sci 11:1–15
Yang D-W, Syn J-W, Hsieh C-H, Huang C-C, Chien L-F (2019) Genetically engineered hydrogenases promote biophotocatalysis-mediated H2 production in the green alga Chlorella sp. DT. Int J Hydrogen Energy 44(5):2533–2545
Yarra R, Aileni M, Kokkirala VR, Umate P, Abbagani S (2010) Micropropagation of Cochlospermum religiosum (L.) Alston—a threatened and economically important medicinal tree. Tree For Sci Biotechnol 5(1):49–52
You J, Zhang Y, Liu A, Li D, Wang X, Dossa K, Zhou R, Yu J, Zhang Y, Wang L, Zhang X (2019) Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol 19:267
Yunus IS, Jones PR (2018) Photosynthesis-dependent biosynthesis of medium chain-length fatty acids and alcohols. Metab Eng 49:59–68
Yunus IS, Wichmann J, Woerdenweber R, Lauersen KJ, Kruse O, Jones PR (2018) Synthetic metabolic pathways for photobiological conversion of CO2 into hydrocarbon fuel. Metab Eng 49:201–211
Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A (2018) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 62(1):2–12
Zhang G, Zhang Y, Xu J, Niu X, Qi J, Tao A, Zhang L, Fang P, Lin LH, Jianguang S (2014) The CCoAOMT1 gene from jute (Corchorus capsularis L.) is involved in lignin biosynthesis in Arabidopsis thaliana. Gene 546(2):398–402
Zhang L, Davies LJ, Elling AA (2015a) A Meloidogyne incognita effector is imported into the nucleus and exhibits transcriptional activation activity in planta. Mol Plant Pathol 16(1):48–60
Zhang X, Davidson EA, Mauzerall DL, Searchinger TD, Dumas P, Shen Y (2015b) Managing nitrogen for sustainable development. Nature 528:51–59
Zhang C, Hu R, Huang J, Huang X, Shi G, Li Y, Yin Y et al (2016) Health effects of agricultural pesticide use in China: implications for the development of GM crops. Sci Rep 6:1–8
Zhang YP, Sun J, Ma Y (2017) Biomanufacturing: history and perspective. J Ind Microbiol Biotechnol 44(4–5):773–784
Zhang Y, Li D, Zhou R, Wang X, Dossa K, Wang L, Zhang Y, Yu J, Gong H, Zhang X, You J (2019) Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol 19:66
Zhao HY, Feng H (2018) Engineering Bacillus pumilus alkaline serine protease to increase its low-temperature proteolytic activity by directed evolution. BMC Biotechnol 18(34):1–12
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167(2):313–324
Zsogon A, Cermak T, Naves ER, Notini MM, Edel KH, Weinl S, Freschi L, Voytas DF, Kudla J, Prees LE (2018) Denovo domestication of wild tomato using genome editing. Nat Biotechnol 36:1211–1216
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Aileni, M. (2022). Environment Sustainability and Role of Biotechnology. In: Arora, S., Kumar, A., Ogita, S., Yau, Y.Y. (eds) Innovations in Environmental Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-4445-0_2
Download citation
DOI: https://doi.org/10.1007/978-981-16-4445-0_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-4444-3
Online ISBN: 978-981-16-4445-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)