Abstract
Here, we report for the first time, the optimized conditions for microprojectile bombardment-mediated genetic transformation in Vassourinha (Scoparia dulcis L.), a Plantaginaceae medicinal plant species. Transformation was achieved by bombardment of axenic leaf segments with Binary vector pBI121 harbouring β-glucuronidase gene (GUS) as a reporter and neomycin phosphotransferase II gene (npt II) as a selectable marker. The influence of physical parameters viz., acceleration pressure, flight distance, gap width & macroprojectile travel distance of particle gun on frequency of transient GUS and stable (survival of putative transformants) expressions have been investigated. Biolistic delivery of the pBI121 yielded the best (80.0 %) transient expression of GUS gene bombarded at a flight distance of 6 cm and rupture disc pressure/acceleration pressure of 650 psi. Highest stable expression of 52.0 % was noticed in putative transformants on RMBI-K medium. Integration of GUS and npt II genes in the nuclear genome was confirmed through primer specific PCR. DNA blot analysis showed more than one transgene copy in the transformed plantlet genomes. The present study may be used for metabolic engineering and production of biopharmaceuticals by transplastomic technology in this valuable medicinal plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant metabolic bioactive compounds have been explored in pharmaceuticals, cosmetics, dyes, and perfumes. Such compounds are produced in trace amounts usually having complex structures which are not essential for plant growth (Zhou et al. 2009). Due to difficulties in chemical synthesis it is expensive to produce them commercially (Ludwig-Müller et al. 2014). Pharmacological screening of medicinal plants used in traditional remedies has lead to development and discovery of numerous compounds (e.g Morphine, Cocanine, Digitalis, Qunine). One such plant known for its medicinal values is Scoparia dulcis L., which is popularly known as Sweet-broomweed or Vassourinha or Licorice weed, belonging to the family Plantaginaceae (earlier it was in Scrophulariaceae). The plant is widely found in Asia, Africa, Europe and also in America, with medicinal values (Satyanarayana 1969).
Phytochemical screening studies in S. dulcis has revealed several novel phytochemicals of flavone and terpene class (Hayashi et al. 1990; Mishra et al. 2011). Among which scopadiol, scopadulcic acid A and B, scopdulciol, betulinic acid, scoparic acid A and B, scopadulin and ammelin have been demonstrated to possess analgesic (De Farias Freire et al. 1993), anti-inflammatory (Ahmed et al. 2001), antitumor (Hayashi et al. 1992; Nishino et al. 1993), antiviral (De Clercq 2001), antidiabetic (Latha et al. 2009), antimalarial (Riel et al. 2002), antioxidant properties (Babincová et al. 2002) and scavenging activity (Krishnamurthy et al. 2004). Hydroxamic acid has been isolated from dried roots and aerial parts (Chiu-Ming and Ming-Tyan 1976), which exhibits insecticidal, antifungal and antibacterial activity. Moreover, our research group evaluated its efficacy towards reverse transcriptase (Porika et al. 2009). In view of its wide ethnomedical acceptance it has attracted the attention of scientific community for its therapeutical values and thus remained as subject of intensified research. More recently, compilations of its diverse chemical constituents and pharmacological studies were available (Mishra et al. 2011; Okhale et al. 2010).
Plant metabolite contents are affected by abiotic and biotic stresses and climatic variations (Tabata 2004) and thus their growth in controlled environment overcomes several of their production limitations. Plant genetic manipulation is more reliable for the production of specific metabolites in controlled (in vitro) conditions (Ludwig-Müller et al. 2014). Interestingly, highly efficient transformation (Aileni et al. 2011a; Yamazaki et al. 1996) along with profused in vitro response (Aileni et al. 2011b; Aileni et al. 2008) was noticed in S. dulcis, that found to be similar to model plants like Nicotiana tabacum and Arabidopsis thaliana.
The present research effort arose from the efficient in vitro & transformation response of S. dulcis L., as a potential medicinal plant making it necessary to develop physical means of transformation for this plant species. The particle bombardment is an important physical means of transformation, as it offers an effective method of DNA delivery into the plant cell. It also demonstrates considerable versatility and efficacy to attain chloroplast transformation in plants, which cannot be achieved by using Agrobacterium spp. because the nucleus is targeted by T-DNA complex (Altpeter et al. 2005). Further, transplastomic development technology in S. dulcis may open up exciting possibilities for metabolic engineering or transform and expression of novel genes in the transplastomes for pharmaceutical traits.
Since, there are no reports available on particle gun mediated genetic transformation in S. dulcis, therefore, a series of experiments were conducted to determine factors affecting biolistic mediated genetic transformation using leaf segments and optimized the physical parameters viz., acceleration pressure, flight distance, gap width & macroprojectile travel distances effecting nuclear transformation (transient and stable expression) by using Bio-Rad PDS-1000/He device.
Materials and methods
Explant material and culture conditions
Fully expanded leaves (~2 cm) were excised from 2 months old in vitro grown plants (Aileni et al. 2011b; Aileni et al. 2008) and used as explants for particle bombardment studies. The cultures were aseptically maintained at 25 ± 2 °C, white fluorescent light (65μE/m2/s) with 16/8 h photoperiod. MS (Murashige and Skoog 1962) media used in the present study contain 3 % sucrose (w/v) and 0.8 % agar used as solidifying agent (Himedia, Mumbai, India).
Transformation vector
Binary vector pBI121 harbouring β-glucuronidase gene (GUS) as a reporter and neomycin phosphotransferase II gene (npt II) as a selectable marker (Chen et al. 2003) was used to optimize various parameters of particle gun mediated transformation. The GUS gene is governed by NOS terminator and CaMV 35S promoter, while npt II gene is driven by NOS promoter and terminator. The vector was introduced and maintained in E.coli-DH5α. The plasmid DNA was isolated from the overnight bacterial culture according to manufacturers protocol (Genentix Biotech Asia Pvt. Lt, New Delhi, India) and used for the bombardment experiments.
Microcarrier preparation
The gold particle stock was prepared by dissolving 60 mg of gold particles (0.6 μ, Bio-Rad) in 1 ml of 50 % sterile glycerol and vortexed for 5 min to avoid agglomeration of particles. The coating of plasmid DNA (0.5 μg) onto gold particles was carried according to manufacturers protocol (Bio-Rad, Hercules, California, USA). This included vortexing plasmid DNA and gold particles for 2–3 min in a solution of 20 μl of 0.1 M spermidine and 50 μl of 2.5 M CaCl2 followed by centrifugation at 5000 rpm for 5 min, collected pellet was rinsed twice subsequently in 140 μl of 70 and 100 % ethanol. The coated microparticles were then resuspended in 48 μl of 100 % ethanol and kept on ice until bombardment. For each bombardment 8 μl of these microcarriers was used. Petri plates with target explants arranged at the center and were subjected to bombardment.
Optimization of selection pressure
Dose response assay was performed by culturing control (non-bombarded) leaf explants (1.0 cm2) on MS medium containing plant growth regulators (RMBI medium: Regeneration Medium with 4 mg/L BAP + 0.2 mg/L IAA) with different kanamycin concentrations: 0, 25, 50, 75 and 100 mg/L. The transformation efficiency was determined by calculative percent of the GUS- positive with bombarded leaf explants to number of explants responding on selective shoot regeneration medium RMBI-K (RMBI medium supplemented with 50 mg/L kanamycin).
Standardization of bombardment parameters
Using Bio-Rad PDS (Particle Delivery System)-1000/He (Bio-Rad, Hercules, California, USA), the leaf explants fired at different variables, includes three acceleration pressures (650, 900 and 1100 psi) and four flight distances (3, 6, 9 and 12 cm - microprojectile travel distances). 2 cm distance between rupture disc and macrocarrier, 3 mm macrocarrier travel distance were kept constant. While, for each bombardment the vacuum chamber of the particle gun was maintained at 25 mm Hg. The frequency of transient GUS expression was taken into account for evaluating physical parameters. Axenic leaf explants (~2 cm) of S. dulcis were placed adaxial side up in the center of target tissue plates containing RMBI medium. Each petri plate (9 cm) containing 5 explants was bombarded with each acceleration pressure and target distances for three replication experiments along with control.
Assay for β-glucuronidase (GUS) activity and statistical analysis
Transient GUS expression was assessed after 2 days of bombardment for 10 randomly selected explants of each parameter according to established protocol (Jefferson et al. 1987). Histochemical staining was done with GUS assay buffer containing (1 g/L − 1 X-Gluc −5-Bromo 4-chloro 3-indolyl β-glucuronide -cyclohexyl ammonium salt, Sigma; with 0.05 M Na2HPO4, 10 mM EDTA and 0.1 % (v/v) Triton X-100) incubating the bombarded tissue at 37 °C for 24–48 h in sterile polystyrene reaction tubes. The stained tissue samples were destained with glacial acetic acid: alcohol (1:3 v/v) to bleach chlorophyll. Indigogenic staining dye taken by incubated tissues in the form of blue foci (spots) were examined under a dissecting microscope and scored to test the efficiency of transient GUS expression. Non- bombarded explants were treated as control.
Each treatment consists of atleast three plates and was duplicated thrice. The percent GUS activity was calculated in terms of number of leaf explants showing transient GUS expression (amounted with blue foci) to the total number of explants stained after bombardment. All the data for mean comparison was analysed statistically by ANOVA and DMRT.
Regeneration of bombarded plants
The bombarded leaf explants (~2 cm) were incubated for 3 days on RMBI medium and were then cut into desirable size, each of 1.0 cm2 and transferred on to RMBI-K medium, which acts for induction and selection of putatively transformed shoots. Kanamycin resistant cultures were subcultured onto the same medium for 3 times/rounds at regular intervals of two weeks. Further, the responding cultures were transferred to elongation medium containing lethal dose of kanamycin (50 mg/L) and maintained for two weeks for developing resistant shoots. The elongated shoots were transferred on RMRI-K medium (MS medium supplemented with 1.0 mg/L IBA and 50 mg/L kanamycin) for rooting. In vitro rooted plantlets were rinsed to remove the medium adherents and transferred to acclimatization pots filled with pre-sterilized soil-vermiculite (1:1) and covered with transparent plastic covers to avoid loss of humidity. Plantlets of 10–15 cm were then transplanted into greenhouse soil and grown to maturity.
Total DNA extraction, PCR and southern blot analysis
The bombarded and control plants (non- bombarded) were subjected to molecular analysis in order to confirm the stable integration of transgenes into the plant genome. For molecular analysis the genomic DNA was extracted from the young leaf explants of transgenic lines and wild donor plant of S. dulcis following CTAB (Cetyl trimethyl ammonium bromide) method. PCR was performed in a C1000TM programmable thermal cycler (Bio-Rad, Hercules, California, USA) using a pair of primers for GUS (Forward-5′ CCCCAACCCGTGAAATCAAAAAACT3′ Reverse-5′CCCTGCTGCGGTTTTTCACCGAAGT3′) and for npt II (Forward-5′ GCGGAGAATTAAGGGAGTCACGTTAT3′; Reverse-5′-CACCGCGCGCGATAATTTATCCTAG -3′) genes for verification of integration into the bombarded plant genomes. The PCR reaction mixture (50 μl) contained 100 ng of DNA, 10× PCR buffer, 25 mM MgCl2, 2.5 mM dNTPs, 10 pmol of each specific primer and 1 U of Taq DNA polymerase (Fermentas). PCR conditions with 940 C for 5 mins as initial denaturation, followed by 30 cycles at 94 °C for 30 s, 55 °C for 45 s for annealing with primer extension at 72 °C for 2 mins and a final extension at 72 °C for 5 mins were setup. Visual confirmation was done by loading the PCR products onto 1 % agarose gel and photographed (Bio-Rad, Hercules, California, USA).
The npt II transgene intergration in PCR positive lines was analyzed by using Southern blot analysis (Sambrook et al. 1989). The total genomic DNA (20 μg) of the putative transgenic lines was subjected to digestion with Hind III and hybridized with DIG- labeled probe which is complementary to 1355 bp within the npt II gene region. Further, run on 1 % agarose gel by electrophoresis and subsequently transferred onto a Hybond+ nylon membrane. Labeling, hybridization and Chemiluminescent detection was done according to manufacturers’ guidelines (Roche Applied Science).
Results
Optimization of antibiotic concentration for selection
An efficient and reproducible protocol for biolistic transformation in S. dulcis was developed by optimizing the suitable concentration of kanamycin at which transgenic plants were recovered without escapes. After 2 weeks, control explants cultured on selection medium (RMBI medium containing 25 mg/L kanamycin), showed partial bleaching (data not shown). While, within a week control explants cultured on RMBI medium containing 50 mg/L kanamycin was completely bleached (Fig. 1b). Different media was used during selection of putative transformants starting from regeneration of shoots, elongation to rooting stages (described in Materials & Methods). 50 mg/L kanamycin concentration in RMBI medium which is defined as selective shoot regeneration medium (RMBI-K) was used for selection of transformants. Whereas control explants on RMBI medium resulted a maximum of 95 % shoot regeneration after 4 weeks of culture (Fig. 1a).
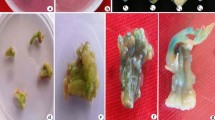
Production of transgenic Scoparia dulcis using axenic leaf explants via microprojectle -mediated genetic transformation. a Profuse shoot organogenesis from 95 % of axenic leaf explants after 4 weeks of culture on RMBI medium. b Complete inhibition of shoot organogenesis in all control explants within a week days of culture, on selective shoot regeneration medium (RMBI-K). c c1 Transient gus expression shown by bombarded explant at 650 psi with flight distance of 6 cm. c2 Transient gus expression shown by bombarded explant at 1100 psi with flight distance of 9 cm. c3 No transient gus expression shown by control (non-bombarded explants). d Putative transgenic Shoots developed from bombarded explants after three rounds of selection on selective shoot regeneration medium (RMBI-K). e Shoot cluster showing gus expression after three rounds of selection on selective shoot regeneration medium (RMBI-K). f Elongation of putative transgenic shoots after last round of selection on selective shoot regeneration medium (RMBI-K). g gus stained elongated putative transgenic shoots after last round of selection on selective shoot regeneration medium (RMBI-K). h gus -positive transgenic plantlet ready for rooting on RMRI-K medium. i Phenotypically normal trangenic plant hardening on soil in the greenhouse
Recovery of transformed plants, standardization of bombardment parameters & their molecular analysis
After 3 days following bombardment, explants (~2 cm) were cut into 1.0 cm2 size under aseptic conditions and transferred onto RMBI-K medium. Formation of kanamycin- resistant shoot buds from the cut ends of leaf explants was observed after 3 successive subcultures with time interval of 2 weeks (3 rounds of selection) on the same medium (Fig. 1d). While, non-bombarded explants gradually bleached within one week of culture on RMBI-K medium. However, putatively transformed portions remained green and exhibited slow regeneration. During the selection process on RMBI-K medium, gradually the number of responding cultures sorted out due to selection pressure. After 3 subcultures, an additional 2 weeks of culture on the same medium was required for elongation of shoots (Fig. 1f, h). Therefore, in the present study, elongated kanamycin resistant shoots were obtained after 4 rounds of selection. Such shoots were subsequently rooted on RMBI-K medium within another 2 weeks of culture. During the selection process, complete shoots were observed directly from bombarded leaf explants without intervening callus phase (Fig. 1e).
The parameters scoring the highest number of leaf explants with the highest number of blue foci was considered as optimal for calculating efficiency of transient GUS expression in S. dulcis. Different acceleration pressures and flight distances were found to have significant effect on transient GUS expression as well as transformation efficiency. Within the evaluated range of physical bombardment parameters (acceleration pressures & flight distances), the highest transient (80.0 %) and stable (52.0 %) expression of GUS gene was observed in explants bombarded at a flight distance of 6 cm and an acceleration pressure of 650 psi (Fig. 1c1 ). Under these conditions, the average number of blue foci amounted after transient GUS staining was, 40 to 50 per bombarded plate. Number of blue foci amounted was different under similar percent (42.0 %) transformation, observed at 650 psi acceleration pressure either with 3 and 9 cm of flight distances (Table 1). While, at flight distance 12 cm, only 20.0 % transformation efficiency was noticed. In another set of experiments, i.e., under acceleration pressure 900 psi the next highest transformation efficiency recorded again with 6 cm flight distance (64.0 %). While 22.0 %, 40.0 %, 20.0 % transformation efficiency was noticed at 3, 9, 12 cm flight distances respectively (Table 1). In the last set of experiments, i.e., under acceleration pressure 1100 psi, the highest (44.0 %) transformation efficiency observed with 6 cm flight distance. While, 20 % transformation efficiency noticed with 9 cm flight distance (Fig. 1c2 ). Whereas, no bombardment events were observed at both flight distances (3 and 12 cm) and under these conditions, corresponding blue foci amounted was only 5 to 10 which indicated the reduction in bombardment events.
Therefore, the highest transformation efficiencies was observed at 650 psi acceleration pressure with all the flight distances used (Table 1). A remarkable decrease in percentage of explant showing transient GUS expression and lower transformation efficiency was observed with acceleration pressure beyond 650 psi (Table 1). Therefore, rise in acceleration pressure (900 or 1100 psi) under similar flight distance (3, 6, 9 or 11 cm), did not show a remarkable increase in efficiency of GUS expression (Table 1). Among the flight distances tested, 6 cm was found to be optimal for efficient delivery cum integration of the transgene into the nuclear genome of S. dulcis. This is evident with % of GUS expression levels (60 or 40 %) even at high acceleration pressures (900 or 1100 psi). These effective factors were standardized and conditions were optimized at one step, were followed by the successive steps, which finally resulted in the efficient production of microprojectile mediated transgenic S. dulcis. No blue foci (spots) were amounted in the control cells that were shot with non-coated gold particles (Fig. 1c3 ). Leaves and elongated shoots from kanamycin-resistant clusters showed a typical dark blue colouration while control plant does not respond to GUS staining (Fig. 1g). Except, few of randomly selected kanamycin resistant plant lines, all other lines were stained upon GUS histochemical assay (Table 2). However, these lines were PCR positive to npt II gene. Putative transformants were successfully established under controlled greenhouse conditions (Fig. 1i).
PCR amplification of genomic DNA from putative transgenic lines resulted in banding pattern corresponding to 1812 bp of GUS gene (Fig. 2a) and 1355 bp of npt II (Fig. 2b), where as respective bands were absent in control plants. Stable integrative confirmation of npt II selectable marker gene in transgenic S. dulcis was verified using southern blot analysis. From the 4 transgenic lines tested, 1st and 4th lines showed single integration and other lines 2nd and 3rd were multiple integration patterns (Fig. 3). No integration was observed in the control plant. In the present study, the stable nuclear transformation efficiency of 52.0 % was established (Table 2) at the mean of 3.7 ± 0.24 transgenic plantlets per explant.
PCR analyses of transgenic plant lines of Scoparia dulcis produced via microprojectle -mediated genetic transformation. a PCR detection of uidA gene specific band (1812 bp) in transgenic line 1–4; No such band detected in wild (Wt) type; M, Molecular marker. b PCR detection of npt II (1355 bp specific band in transgenic line 1–4; No such band detected in wild (Wt) type; M, Molecular marker)
Discussion
Till date, there is no report available on direct gene transfer technology in S. dulcis. The present study was aimed to establish simple & reproducible biolistic-mediated genetic transformation using axenic leaf explants. In order to maximize the biolistic mediated gene delivery system in S. dulcis, different physical parameters like acceleration pressures, flight distances were tested with fixed gap width and macroprojectile travel distance. Moreover, we have optimized time period of culture, number of rounds of selection for each stage & dose assay to prevent transgenic escapes.
The availability of biolistic mediated-nuclear transformation in S. dulcis, has significant interest in developing transplastomic plants for metabolic engineering of certain regulatory gene traits and also in extensive development of molecular farming for production of biopharmaceuticals (Bock 2007). In previous transformation studies of S. dulcis 3.9 ± 0.39 transgenic plantlets per leaf explant were recorded (Aileni et al. 2011a). While there was no remarkable differences observed in the present investigation (3.7 ± 0.24 transgenic plantlets per leaf/explant). Regardless of type of transformation methods, the leaf explants of S. dulcis known to be one of the favorable sources of explants where shoot development was not accompanied by callus phase. It is also know that, leaf explants has high regeneration capacity which sustains the cell functions proficiently for prolonged regeneration cycles on selection pressure and which represents a high quality target tissue for transformation.
Among the variable physical parameters tested, highest transient (80.0 %) and stable (52.0 %) transformation efficiency was observed at a flight distance of 6 cm with acceleration pressure of 650 psi. This is in agreement that, transient expression occurs almost immediately after gene transfer and it occurs at higher frequency than stable integrations. Therefore, direct DNA transfer and expression cassette function can be assessed easily by transient expression (Altpeter et al. 2005). Variations in helium pressure have significant effect on biolistic gene transformation. Increased penetration force with higher pressures can cause injury of the cells that eventually reduce the efficiency of transformation (Joshi et al. 2011), similar consequences were observed in present study. While, low acceleration pressure (650 psi) has shown highest level of transient GUS expression at which the microcarriers were able to reach recipient tissue without causing injury, which indicates its suitability as most beneficial factor for S. dulcsis transformation.
The parameters such as distance between target tissue to macrocarrier and the velocity with which the microparticles could travel affect transformation rates, are to be optimized for effective transformation through biolistics that which clearly correlated with the results obtained in case of S. dulcis (Petrillo et al. 2008). These events facilitates the even distribution of DNA-coated microcarriers over the target leaf tissue that prevents damage (Tadesse et al. 2003). At least micro-projectile distances, tissue dislocation and mechanical damage was noticed and an increase in the particle velocity decreases the depth of their penetration that effects transformation rate. Maximum transient GUS expression rate and higher transformation efficiency was observed at 6 cm flight distance. In contrary to this, 9 cm microprojectile travel distance was found to be optimal parameter in various cases (Gharanjik et al. 2008; Ramesh and Gupta 2005; Singh et al. 2010). However, higher flight distances reduces transformation efficiencies was also reported (Ruma et al. 2009; Singh et al. 2010). Therefore, optimization of acceleration pressure and flight distance prerequisite for efficient DNA delivery in any system by biolistics, as the same varies from plant to plant system.
Every antibiotic shows its own impression on living tissue at certain concentrations which may effect the cell development and regeneration (Lin et al. 1995). Dose response studies conducted, revealed that 50 mg/L of kanamycin was found to be effective to regenerate bombarded plantlets without any escape. Non-transformed tissues gradually bleached; while putative green transformed portions showed slow growth during each round of selection and constitute expression of GUS gene was observed in transformed lines through histochemical assay. Bleached tissue resulted in decreased regenerative ability, poor growth and subsequent death. Moreover, PCR amplification was evident with integration of both GUS and npt II genes in the nuclear genome of S. dulcis. Few PCR confirmed kanamycin resistant lines did not express GUS, probably due to position effect of the genome, promoter deletion or part of GUS coding region or may be due to DNA methylation (Baulcombe 2004). In general microprojectile bombardment mediated transformation results in the insertion of desired gene sequences at multiple sites (Altpeter et al. 2005). Where as in the present investigation, 50 % of single copy insertion was confirmed by southern blotting.
In conclusion, we for the first time established a simple and efficient protocol for recovery of transgenic S. dulcis generated via biolistic- mediated nuclear transformation. Although the stable transformation efficiency (52.0 %) reported in the present study is less than that of earlier report (54.6 %) that which was attained by Agrobacterium mediated transformation (Aileni et al. 2011a). However, biolistic method is the most efficient way to adopt towards plastid transformation in S. dulcis. Inview of this genetic transformation approach by the particle bombardment process it has to be extended to a wide range of plants for introducing valuable trait characteristics.
References
Ahmed M, Shikha H, Sadhu S, Rahman M, Datta B (2001) Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis. Die Pharm 56:657
Aileni M, Abbagani S, Zhang P (2011a) Highly efficient production of transgenic Scoparia dulcis L. mediated by Agrobacterium tumefaciens: plant regeneration via shoot organogenesis. Plant Biotechnol Rep 5:147–156
Aileni M, Kokkirala VR, Yarra R, Vemunoori AK, Kasula K, Umate P, Abbagani S (2011b) In Vitro Regeneration, Flowering and Seed Formation from Leaf Explants of Scoparia dulcis L. Med Aromat Plant Sci Biotechnol 5(2);143-146
Aileni M, Rao Kokkirala V, Reddy Kota S, Umate P, Abbagani S (2008) Efficient in-vitro regeneration from mature leaf explants of Scoparia dulcis L., an ethnomedicinal. Plant J of Herbs, Spices & Med Plants 14:200–207
Altpeter F, Baisakh N, Beachy R, Bock R, Capell T, Christou P, Daniell H, Datta K, Datta S, Dix P, Fauquet C, Huang N, Kohli A, Mooibroek H, Nicholson L, Nguyen TT, Nugent G, Raemakers K, Romano A, Somers DA, Stoger E, Taylor N, Visser R (2005) Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breeding 15:305–327
Babincová M, Bacova Z, Machova E, Kogan G (2002) Antioxidant properties of carboxymethyl glucan: comparative analysis. J Med Food 5:79–83
Baulcombe D (2004) RNA silencing in plants. Nat 431:356–363
Bock R (2007) Plastid biotechnology: prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr Opin Biotechnol 18:100–106
Chen P-Y, Wang C-K, Soong S-C, To K-Y (2003) Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol Breeding 11:287–293
Chiu-Ming C, Ming-Tyan C (1976) 6-methoxybenzoxazolinone and triterpenoids from roots of Scoparia dulcis. Phytochem 15:1997–1999
De Clercq E (2001) Antiviral drugs: current state of the art. J Clin Virol 22:73–89
De Farias Freire SM, Da Silva Emim JA, Lapa AJ, Souccar C, Torres LMB (1993) Analgesic and antiinflammatory properties of Scoparia dulcis L. extracts and glutinol in rodents. Phytother Res 7:408–414
Gharanjik S, Moieni A, Mousavi A, Alizadeh H (2008) Optimization of transient expression of uidA gene in androgenic embryos of wheat (Triticum aestivum L. cv. Falat) via particle pombardment. Iran J Biotechnol 6:207–213
Hayashi K, Hayashi T, Morita N (1992) Cytotoxic and antitumour activity of scopadulcic acid B from Scoparia dulcis L. Phytother Res: 6:6–9
Hayashi T, Kawasaki M, Miwa Y, Taga T, Morita N (1990) Antiviral agents of plant origin. III. Scopadulin, a novel tetracyclic diterpene from Scoparia dulcis L. Chem Pharm Bull (Tokyo) 38:945–947
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. The EMBO J 6:3901
Joshi M, Mishra A, Jha B (2011) Efficient genetic transformation of Jatropha curcas L. by microprojectile bombardment using embryo axes. Ind Crop Prod 33:67–77
Krishnamurthy PT, Razdan R, Kasturirangan MN, Kishore G, Nanjan MJ (2004) Hepatoprotective activity of Scoparia dulcis linn. Against carbon tetrachloride-induced liver cirrhosis in rats Asian. J of Tradit Med 3:153–159
Latha M, Pari L, Ramkumar KM, Rajaguru P, Suresh T, Dhanabal T, Sitasawad S, Bhonde R (2009) Antidiabetic effects of scoparic acid D isolated from Scoparia dulcis in rats with streptozotocin-induced diabetes. Nat Prod Res 23:1528–1540
Lin J-J, Assad-Garcia N, Kuo J (1995) Plant hormone effect of antibiotics on the transformation efficiency of plant tissues by Agrobacterium tumefaciens cells. Plant Sci 109:171–177
Ludwig-Müller J, Jahn L, Lippert A, Püschel J, Walter A (2014) Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: strategies and applications. Biotechnol Adv 32:1168–1179
Mishra MR, Behera R, Jha S, Panda A, Mishra A, Pradhan D, Choudary P (2011) A Brief Review on phytoconstituents and Ethnopharmacology of Scoparia dulcis Linn.(Scrophulariaceae). Int J of Phytomedicine 3:422
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nishino H, Hayashi T, Arisawa M, Satomi Y, Iwashima A (1993) Antitumor-promoting activity of scopadulcic acid B, isolated from the medicinal plant Scoparia dulcis L. Oncol 50:100–103
Okhale SE, Amanabo MO, Jegede IA, Egharevba HO, Muazzam IW, Kunle OF (2010) Phytochemical and pharmacognostic investigation of antidiabetic scopariadulcis linn scrophulariaceae whole plant grown in Nigeria. Res 2:7–16
Petrillo CP, Carneiro NP, Purcino AÁC, Carvalho CHS, Alves JD, Carneiro AA (2008) Optimization of particle bombardment parameters for the genetic transformation of Brazilian maize inbred lines. Pesqui Agropecu Bras 43:371–378
Porika M, Aileni M, Kokkirala VR, Gadidasu K, Umate P, Rao AV, Devarakonda RK, Abbagani S (2009) In vitro HIV type-1 reverse transcriptase inhibitory activity from leaf extracts of Scoparia dulcis L. J of Herbs, spices & Med Plants 15:241–247
Ramesh M, Gupta AK (2005) Transient expression of β-glucuronidase gene in indica and japonica rice (Oryza sativa L.) callus cultures after different stages of co-bombardment. Afr J Biotechnol 4:596–600
Riel MA, Kyle DE, Milhous WK (2002) Efficacy of scopadulcic acid a against Plasmodium falciparum in vitro. J Nat Prod 65:614–615
Ruma D, Dhaliwal M, Kaur A, Gosal S (2009) Transformation of tomato using biolistic gun for transient expression of the β-glucuronidase gene. Indian J Biotechnol 8:363–369
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning vol 1, vol 7.58. Cold spring harbor laboratory press New York
Satyanarayana K (1969) Chemical examination of Scoparia dulcis (Linn): part I. J Ind Chem Soc 46:765–766
Singh N, Mishra A, Joshi M, Jha B (2010) Microprojectile bombardment mediated genetic transformation of embryo axes and plant regeneration in cumin (Cuminum cyminum L.). Plant Cell Tiss Org 103:1–6
Tabata Y (2004) Tissue regeneration based on tissue engineering technology. Congenit anom 44:111–124
Tadesse Y, Sagi L, Swennen R, Jacobs M (2003) Optimisation of transformation conditions and production of transgenic sorghum (Sorghum bicolor) via microparticle bombardment. Plant Cell Tiss Organ 75:1–18
Yamazaki M, Son L, Hayashi T, Morita N, Asamizu T, Mourakoshi I, Saito K (1996) Transgenic fertile Scoparia dulcis L., a folk medicinal plant, conferred with a herbicide-resistant trait using an Ri binary vector. Plant Cell Rep 15:317–321
Zhou ML, Shao JR, Tang YX (2009) Production and metabolic engineering of terpenoid indole alkaloids in cell cultures of the medicinal plant Catharanthus roseus (L.) G. Don (Madagascar periwinkle). Biotechnol Appl Biochem 52:313–323
Acknowledgments
The authors greatly acknowledge the financial assistance provided by the Department of Science and Technology-NEW DELHI (SR/SO/BB-011/2010, SERB), University Grants Commission-Rajiv Gandhi National Fellowship (No.F.14-2(ST)/2008(SA-III)) and Department of Science and Technology -INSPIRE (DST/INSPIRE Fellowship/2011/426), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Srinivas, K., Muralikrishna, N., Kumar, K.B. et al. Biolistic transformation of Scoparia dulcis L.. Physiol Mol Biol Plants 22, 61–68 (2016). https://doi.org/10.1007/s12298-016-0338-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-016-0338-2