Abstract
We describe a simple, efficient process for the production of 6-kestose, a trisaccharide with well-documented prebiotic properties. A key factor is the use of a yeast transformant expressing an engineered version of Saccharomyces invertase with enhanced transfructosylating activity. When the yeast transformant was grown with 30 % sucrose as the carbon source, 6-kestose accumulated up to ca. 100 g/L in the culture medium. The 6-kestose yield was significantly enhanced (up to 200 g/L) using a two-stage process carried out in the same flask. In the first stage, the culture was grown in 30 % sucrose at physiological temperature (30 °C) to allow overexpression of the invertase. In the second stage, sucrose was added to the culture at high concentration (60 %) and the temperature shifted to 50 °C. In both cases, 6-kestose was synthesized with high specificity, representing more than 95 % of total FOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
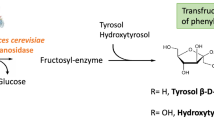
Commercial demand of prebiotic oligosaccharides is growing due to the well-documented health-promoting properties of these compounds (Roberfroid and Slavin 2000; Oozeer et al. 2013; Jeurink et al. 2013). Fructooligosaccharides (FOS) constitute one of the main types of prebiotic oligosaccharides (Sangeetha et al. 2005; Roberfroid 2007). Chemically, FOS are short-chains of fructosyl units (F) connected in most cases to a terminal sucrose molecule (GF) at their reducing end. They differ in their degree of polymerization (2 to 10) and structure. Fructosyl units may be interconnected through β-(2 → 1) linkages, as in 1-kestose (inulin type or 1F-FOS) or β-(2 → 6) linkages, as in 6-kestose (levan type or 6F-FOS). FOS with both linkage types (as bifurcose) are also present in nature. Alternatively, an (oligo) fructose branch may be connected through a β-(2 → 6) link to the glucose moiety of the terminal sucrose, as in neokestose (neoseries or 6G-FOS) (Vijn and Smeekens 1999). These differences in chemical structure are important for the biological function of FOS. While all three different types show prebiotic properties, FOS with β-(2 → 6) linkages have enhanced stability and better effect (Marx et al. 2000; Kilian et al. 2002; Jang et al. 2003; Semjonovs et al. 2004; Lim et al. 2007; Falony et al 2009).
The two main procedures for the industrial production of FOS are hydrolysis of inulin and synthesis from sucrose (Sangeetha et al 2005; Álvaro-Benito et al. 2007; Dominguez et al 2014). These two procedures yield products of different profile. FOS chains obtained from inulin by partial hydrolysis with inulinases generally lack a glucose-terminal residue, they are exclusively of the β-(2 → 1) type and have a longer degree of polymerization (>4 units) than those produced by the alternative procedure. FOS synthesis by enzymatic transfructosylation of sucrose yield mainly trisaccharides and tetrasaccharides whose chemical structure is determined by the product specificity of the enzyme. Hydrolytic enzymes (β-fructofuranosidases) such as invertase or inulinase and transglycosylating enzymes such as levansucrase, from different sources, are employed for the synthesis. The higher chemical versatility of FOS produced by enzymatic synthesis makes this procedure a more valuable choice.
Different processes for the enzymatic biosynthesis of FOS have been described (reviewed by Dominguez et al. 2014). In most cases, the production of the enzyme, a fructosyl hydrolase or transfructosidase, is carried out as a separate operation. Then, the enzyme is harvested and used to produce FOS from sucrose. Several “one-pot” processes have been reported, based on the addition of sucrose to fungal cultures of different species (e.g., Aureobasidium pullulans, Aspergillus japonicus, Penicillium expansum). In all these cases, the action of the fungal enzymes yielded FOS of the β-(2 → 1) type whose production could be optimized by experimental design tools (Dominguez et al. 2012; Mussatto et al. 2009, 2012; Prata et al. 2010). The aim of this study was to develop an efficient one-pot procedure to produce FOS of the β-(2 → 6) type (6-kestose), making use of a transformant culture of the baker’s yeast Saccharomyces cerevisiae which expresses an engineered version of the enzyme invertase. As a general feature of retaining glycosyl hydrolases, invertase shows some degree of transglycosylating activity which can be enhanced by fine-tuning of the enzyme’s catalytic site, as has been shown by the analysis of mutant versions of the enzyme expressed in Escherichia coli (Lafraya et al. 2011). One of these mutations N21S, which caused a high increase of transfructosylating activity, has been used in this study. The resolution of the three-dimensional structure of the yeast invertase (Sainz-Polo et al. 2013) provides a rational basis for further improvement of this enzyme. A new repertoire of invertase mutant forms could be assayed and eventually used for the synthesis of tailor-made FOS following a process analogous to what we describe here.
Materials and methods
Yeast strain, culture media, and growth conditions
S. cerevisiae CECT1624: MATa leu1 suc 0, obtained from ‘Colección Española de Cultivos Tipo’ (www.cect.org) was used as the host strain (Sc-suc 0 hereafter). Minimal medium, SC (0.17 % yeast nitrogen base, 0.5 % ammonium sulfate) was supplemented with 60 mg/L leucine. Complete medium, YP, was 1 % yeast extract, 2 % bacteriological peptone. Autoclaved glucose, or filter-sterilized raffinose, or sucrose were added at different final concentrations (2, 10, 30 %) as carbon sources. Solid media were prepared with 2 % agar.
DNA constructs
An EcoRI/HindIII fragment, containing a wild-type copy of the SUC2 gene from S. cerevisiae, was isolated from plasmid pLC6 (Del Castillo et al. 1992). This DNA fragment, which in addition to the coding sequence includes the gene promoter and 3′-UTR sequence, was subcloned into YEplac181 (Gietz and Sugino 1988) to generate plasmid pSUC-wt. A mutant version of the SUC2 gene encoding the N21S amino acid replacement was constructed by site-directed mutagenesis with primers AL706 and AL707 as previously described (Lafraya et al 2011). The plasmid harboring the mutant version of the gene was designated pSUC-N21S.
Yeast transformation
S. cerevisiae Sc-suc 0 was transformed with pSUC-wt or pSUC-N21S as described by Gietz and Woods (2002). The resulting transformants (Sc-wInv or Sc-mInv, respectively) were selected on YP-2 % raffinose solid media, as larger colonies grown on a background of small ones (untransformed). The larger colonies were subsequently purified by streaking them twice on SC-2 % raffinose plates. The transformant character of the isolated clones was confirmed by testing invertase activity.
Invertase and FOS production assays
Inocula of either Sc-wInv or Sc-mInv transformants freshly grown on SC-2 % raffinose plates were seeded in triplicate series of 250-mL Erlenmeyer flasks containing 50 mL of the following liquid media: YP-2 % sucrose, YP-10 % sucrose, and YP-30 % sucrose. As a negative control, the host strain was inoculated in the same media, in duplicate. As an additional control, transformants and host strain were grown in YP-2 % glucose. Aliquots were taken from the cultures at different times to measure cell density (A600), invertase activity and FOS content. Invertase was assayed with 1.75 M sucrose as substrate, in 100 mM acetate buffer pH 4.8 at 50 °C, by measuring the amount of glucose released with a commercial kit (Sigma-Aldrich). FOS were measured by anion exchange-chromatography through a CarboPac PA-100 column (4 × 250 mm), coupled to a pulsed amperometric detector (Dionex HPAEC-PAD), as previously described (Lafraya et al. 2011).
Additionally, we tested the effect of adding a supplementary amount of sucrose and increasing the temperature, in FOS synthesis. Cultures in the same liquid media described above, grown at 30 °C for 37 h, were diluted ten times and supplemented with 1.75 M sucrose in 100 mM acetate buffer pH 4.8. The temperature was then shifted to 50 °C. At different times, aliquots were taken and the reaction was stopped by heating at 95 °C, 10 min. FOS and glucose were determined as described above.
Structural modelling of enzyme-ligand complex
S. cerevisiae invertase (ScInv) atomic coordinates are available under PDB code 4EQV. 6-kestose was docked at the catalytic center by structural alignment with a previously modeled complex (Lafraya et al. 2011). In silico mutagenesis and structural analysis were performed with the program Pymol (2006; Delano Scientific, LLC). The distance between S21 and 6-kestose in the modeled mutant was calculated as an average from different sterically permitted orientations of the S21 side chain.
Results
Growth of the yeast transformants in sucrose media
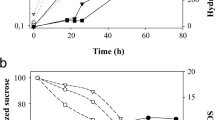
While the host strain lacks an invertase encoding gene (suc 0 genotype) and therefore is unable to metabolize sucrose, the two transformant strains expressing either wild-type or mutant (N21S) versions of the SUC2 gene (Sc-wInv and Sc-mInv, respectively) grew well in media with sucrose (Fig. 1). Both transformants reached higher cell densities in medium with 10 % sucrose than with 2 %, indicating that transition to the stationary phase in the latter medium was triggered by depletion of the carbon source. In medium with 30 % sucrose, the transformant cultures showed a somewhat longer lag-phase and maximum cell density was similar to that reached with 2 % sucrose. This likely reflects physiological constrains caused by increased osmotic pressure at high sucrose concentration and the activation of an osmoadaptative response (Hohmann 2002).
Growth of yeast transformants and host strain in different media. aYP-2 % glucose. b YP-2 % sucrose. c YP-10 % sucrose. d YP-30 % sucrose. Sc-wInv (triangles); Sc-mInv (circles); Sc-suc 0 (squares). Error bars represent standard deviation of triplicates (Sc-wInv and Sc-mInv) or duplicates (Sc-suc 0)
Analysis of invertase activity in the transformant strains
Expression of invertase was assayed at a high (1.75 M) sucrose concentration. Even though both, wild-type and N21S enzymes, have similar k cat values, substrate affinity of the N21S mutant is much lower, with a K m value (1.3 M) around 30-fold higher than that of the wild-type enzyme (Lafraya et al. 2011). Therefore, invertase activity values do not provide a direct estimation of the relative amounts of the two proteins. An indirect estimation can be done using the Michaelis-Menten equation and the reported values of K m and k cat for wild-type and N21S enzymes. According to this, the ratio of concentrations N21S/wild-type would be the ratio of the corresponding activities multiplied by ca. 1.5.
As expected, no invertase activity was detected in the host strain. Neither transformant strain showed activity in medium with glucose, as a result of catabolite repression. Figure 2 shows time-course expression of the enzymes in media with different sucrose concentrations. As an average, ca. 40 % of the enzyme activity was cell bound and the rest was secreted to the culture media (data not shown). In all cases, maximum invertase concentration was reached between 37 and 62.5 h after inoculation, and the enzymes were stable up to 87 h. Invertase was produced at similar levels in media with 2 or 10 % sucrose, but it increased significantly: 4.5-fold (Sc-wInv) or 2.5-fold (Sc-mInv), at 30 % sucrose. This suggests that maximal induction of the SUC2 gene requires sucrose at concentration between 10 and 30 %. Comparing the invertase activity of both transformants (Fig. 2a, b) and taking into account the correspondence between activity and enzyme concentration mentioned above, it can be calculated that in all three media the mutant enzyme was produced in higher amount than the wild type. This may be a consequence of the lower substrate affinity of the N21S enzyme which forces the Sc-mInv cells to maintain a high plasmid copy number and high enzyme production that allow them to grow at substrate concentrations (2 to 30 %) below the N21S enzyme K m (ca. 45 %).
Determination of FOS production in transformant cultures
We assayed the evolution of the sugar content in the transformant cultures. In all cases, the most abundant FOS was 6-kestose (Table 1 and data not shown). Usually FOS production kinetics shows net synthesis at the initial states of reaction and net degradation after prolonged incubations, since this product is also a substrate for hydrolysis. This was also the case for the 6-kestose synthesized in vivo by the yeast transformants (Fig. 3). Sc-mInv showed much higher transfructosylating efficiency than Sc-wInv, reaching a maximum 6-kestose production of 98 g/L in medium with 30 % sucrose, after 48 h. Under these conditions, 6-kestose represented around 39 % of the total sugar content (Table 1). FOS synthesis was significantly less efficient in media with lower sucrose concentration, with 6-kestose yields of 2.5 + 0.1 and 13 + 2 g/L for Sc-wInv and Sc-mInv, respectively, in YP-sucrose 10 % and lower than 0.4 g/L in YP-2 % sucrose for both strains. This can be explained by low affinity of the enzyme for sucrose at the acceptor subsite. Therefore, a high substrate concentration would be required for efficient transglycosylation. On the other hand, high sucrose concentrations decrease water activity, which reduces the competition of hydrolysis against transglycosylation.
Optimization of FOS production
We have devised an experimental design aimed to maximize FOS production. The conditions used correspond to those described as “sucrose overload” treatment in Table 1 and are as follows: The yeast culture is grown 37 h at 30 °C (stage 1). Then, sucrose is added at high concentration (0.6 g/mL) and the temperature is set at 50 °C to increase the invertase activity (stage 2). The process takes place in the same vessel (one pot). After the temperature shift, glucose is not consumed by the yeast, and since glucose release is concomitant with both hydrolysis and transfructosylation, its production rate is a measurement of the enzyme global activity. The differences in the initial kinetics of glucose release (Fig. 4a) were in agreement with the different invertase expression levels reached after 37 h of growth (Fig. 2). A linear glucose release was observed in all cases up to 9–12 days of incubation, when activity slowed down reaching a glucose plateau, indicative of enzyme inactivation (Fig. 4a).
Glucose and 6-kestose content in aliquots taken from the recombinant cultures subjected to “sucrose overload.” The strains were grown in YP-2 % sucrose or YP-30 % sucrose, for 37 h at 30 °C. Extra sucrose (0.6 g/mL) was added to the cultures and the temperature was set at 50 °C. Counting from this moment, aliquots were taken at different time intervals for glucose and 6-kestose determination (a and b, respectively). Symbols code: YP-2 % sucrose (open symbols); YP-30 % sucrose (closed symbols); Sc-wInv (triangles); Sc-mInv (circles). Error bars represent standard deviation of triplicates
Mutant invertase N21S produced by Sc-mInv had much higher transfructosylating activity than the wild-type version (Fig. 4b). Cultures of Sc-mInv grown in YP-30 % sucrose in the first stage, reached 6-kestose yields of ca. 200 g/L, 6 days after the addition of the sucrose overload and temperature shift (Fig. 4b). At this point, 6-kestose was around 28 % of the total sugar content (Table 1). When the culture (stage 1) was carried out in YP-2 % sucrose, since N21S invertase is synthesized in lesser amount (see Fig. 2), 6-kestose production at stage 2 required a longer incubation time (Fig. 4b). In these conditions, FOS yield was slightly lower, probably due to enzyme inactivation.
Compared to the production in standard cultures, the sucrose overload scheme yields 6-kestose content significantly higher (twofold), although it requires a longer processing time. This disadvantage may be overcome by regulating the synthesis of invertase.
Discussion
Expression in S. cerevisiae of an allele of the SUC2 gene, which encodes a mutant version of invertase with enhanced transglycosylating activity, results in efficient synthesis of 6-kestose. The yield obtained by simply growing the yeast cultures in a medium with 30 % sucrose (98 g/L) was comparable to other one-stage processes designed for the synthesis of inulin-type FOS (Dominguez et al. 2012; Mussatto et al. 2009, 2012; Prata et al. 2010) and higher than those reported for other 6-kestose production systems (Katapodis et al. 2004; de Abreu et al. 2011; Fialho et al. 2013). Production of 6-kestose was significantly increased (twofold) by the addition of a sucrose supplement (0.6 g/mL). On the basis of these observations, we designed a simple one-pot process by which a yeast transformant strain expressing the engineered invertase produced 6-kestose with high yield (ca. 200 g/L), specificity (>95 % of total FOS), and purity (ca. 30 % of total sugar content). Prebiotic properties of 6-kestose have been reported. This type of FOS is resistant to the enzymes acting in the upper intestinal tract and supports the growth of different Bifidobacterium species (Marx et al. 2000). Moreover, compared to inulin-type FOS, levan-type FOS promote higher growth of Bifidobacterium lactis and induce higher synthesis of short-chain fatty acids (SCFAs) as acetate and lactate (Semjonovs et al. 2004). Human colon bacteria convert lactate into butyrate, the main energy source for colonocytes (Duncan et al. 2004). SCFAs may reduce the risk of developing gastrointestinal disorders, cancer, and cardiovascular diseases (Wong et al. 2006). In particular, butyrate has been associated to health benefits as nourishing the colonic mucosa and colon cancer prevention. Therefore, the development of an efficient system for 6-kestose synthesis may have relevant biotechnological impact in functional food and nutraceutical formulas.
The engineered version of Saccharomyces invertase used in this work carries the mutation N21S whose effect was discovered as a result of rational approach of targeted mutations directed to enhance the FOS synthetic capability of the enzyme (Lafraya et al. 2011). The recent resolution of the invertase molecular structure (Sainz-Polo et al. 2013) provides an accurate picture, at the atomic level, that explains enzyme function. As a retaining glycoside hydrolase, invertase proceeds through a two-step reaction. In the first step, the residue acting as nucleophile (D22) forms a covalent bond with the fructosyl unit of the sucrose, and glucose is released assisted by the acid/base catalyst (E203). In the second step, the fructose molecule may be donated to a water molecule (hydrolysis) or to another sucrose molecule (transfructosylation), to generate a trisaccharide. Figure 5 shows the invertase catalytic site in complex with a 6-kestose molecule. The docking analysis suggests that N21 forms a hydrogen bond with the fructosyl unit that binds covalently to the nucleophile in the reaction intermediate. The substitution N21S disrupts this hydrogen bond since the estimated distance between the Oγ from S21 and the O4 from the fructosyl unit (4.3 Å) is longer than the hydrogen bond distance in proteins (2.5–3.2 Å). This would result in a reaction intermediate where the fructosyl moiety may have a higher rotational freedom to be coupled to the acceptor sucrose, increasing the transfructosylation efficiency. Moreover, the resolution of the quaternary structure of this enzyme points to the involvement of two residues (K385 and S412) from a neighboring subunit, in binding the acceptor sucrose (Fig. 5). Site-directed mutagenesis of these residues may render mutants with higher transfructosylating capabilities or a different chemical profile of FOS.
Structure of the wild-type (a) and N21S mutant (b) invertase catalytic pocket complexed with a 6-kestose molecule. The 6-kestose molecule is depicted in orange (fructosyl moiety) and brown (sucrose moiety). Residues potentially forming hydrogen bonds with 6-kestose are labeled in green (A subunit) or blue (B subunit). The three residues of the catalytic triad are highlighted in red
The procedure described in this communication is well suited for the food industry since it makes use of S. cerevisiae, an ideal organism for food applications. The same scheme could be adapted to the production of different types of FOS by transforming S. cerevisiae with genes encoding new versions of engineered invertase.
References
Alvaro-Benito M de AM, Fernandez-Arrojo L, Plou FJ, Jimenez-Barbero J, Ballesteros A, Polaina J, Fernandez-Lobato M (2007) Characterization of a beta-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J Biotechnol 132:75–81
de Abreu MA, Alvaro-Benito M, Plou FJ, Fernández-Lobato M, Alcalde M (2011) Screening β-fructofuranosidases mutant libraries to enhance the transglycosylation rates of β-(2→6) fructooligosaccharides. Comb Chem High Throughput Screen 14:730–738
del Castillo-Agudo L, Nieto Soria A, Sentandreu R (1992) Differential expression of the invertase-encoding SUC genes in Saccharomyces cerevisiae. Gene 120:59–65
Dominguez A, Nobre C, Rodrigues LR, Peres AM, Torres D, Rocha I, Lima N, Teixeira J (2012) New improved method for fructooligosaccharides production by Aureobasidium pullulans. Carbohydr Polym 89:1174–1179
Dominguez AL, Rodrigues AR, Lima NM, Teixeira JA (2014) An overview of the recent developments on fructooligosaccharide production and applications. Food Bioprocess Tech 7:324–337
Duncan SH, Louis P, Flint HJ (2004) Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol 70:5810–5817
Falony G, Verschaeren A, De Bruycker F, De Preter V, Verbeke K, Leroy F, De Vuyst L (2009) In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl Environ Microbiol 75:454–461
Fialho MB, Simoes K, Barros CD, Pessoni RAB, Braga MR, Figueiredo-Ribeiro RDL (2013) Production of 6-kestose by the filamentous fungus Gliocladium virens as affected by sucrose concentration. Mycoscience 54:198–205
Gietz RD, Sugino A (1988) New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534
Gietz RD, Woods RA (2002) Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350:87–96
Hohmann S (2002) Osmotic adaptation in yeast-control of the yeast osmolyte system. Int Rev Cytol 215:149–187
Jang KH, Kang SA, Cho Y, Kim YY, Lee YJ, Hong K, Seong KH, Kim SH, Kim CH, Rhee SK (2003) Prebiotic properties of levan in rats. J Microbiol Biotechnol 13:348–353
Jeurink PV, van Esch BC, Rijnierse A, Garssen J, Knippels LM (2013) Mechanisms underlying immune effects of dietary oligosaccharides. Am J Clin Nutr 98:572S–577S
Katapodis P, Kalogeris E, Kekos D, Macris BJ, Christakopoulos P (2004) Biosynthesis of fructo-oligosaccharides by Sporotrichum thermophile during submerged batch cultivation in high sucrose media. Appl Microbiol Biotechnol 63:378–382
Kilian SG, Kritzinger SM, Rycroft C, du Gibson GR, du Preez JC (2002) The effects of the novel bifidogenic trisaccharide, neokestose, on the human colonic microbiota. World J Microbiol Biotechnol 18:637–644
Lafraya A, Sanz-Aparicio J, Polaina J, Marín-Navarro J (2011) Fructo-oligosaccharide synthesis by mutant versions of Saccharomyces cerevisiae invertase. Appl Environ Microbiol 77:6148–6157
Lim JS, Lee JH, Kang SW, Park SW, Kim SW (2007) Studies on production and physical properties of neo-FOS produced by co-immobilized Penicillium citrinum and neo-fructosyltransferase. Eur Food Res Technol 225:457–462
Marx SP, Winkler S, Hartmeier W (2000) Metabolization of β-(2,6)-linked fructose-oligosaccharides by different bifidobacteria. FEMS Microbiol Lett 182:163–169
Mussatto SI, Aguilar CN, Rodrigues LR, Teixeira JA (2009) Colonization of Aspergillus japonicus on synthetic materials and application to the production of fructooligosaccharides. Carbohydr Res 344:795–800
Mussatto SI, Prata MB, Rodrigues LR, Teixeira JA (2012) Production of fructooligosaccharides and β-fructofuranosidase by batch and repeated batch fermentation with immobilized cells of Penicillium expansum. Eur Food Res Technol 235:13–22
Oozeer R, van Limpt K, Ludwig T, Ben Amor K, Martin R, Wind RD, Boehm G, Knol J (2013) Intestinal microbiology in early life: specific prebiotics can have similar functionalities as human-milk oligosaccharides. Am J Clin Nutr 98:561S–571S
Prata MB, Mussatto SI, Rodrigues LR, Teixeira JA (2010) Fructooligosaccharide production by Penicillium expansum. Biotechnol Lett 32:837–840
Roberfroid MB (2007) Inulin-type fructans: functional food ingredients. J Nutr 137:S2493–S2502
Roberfroid M, Slavin J (2000) Non-digestible oligosaccharides. Crit Rev Food Sci Nutr 40:461–480
Sainz-Polo MA, Ramírez-Escudero M, Lafraya A, González B, Marín-Navarro J, Polaina J, Sanz-Aparicio J (2013) Three-dimensional structure of Saccharomyces invertase: role of a non-catalytic domain in oligomerization and substrate specificity. J Biol Chem 288:9755–9766
Sangeetha PT, Ramesh MN, Prapulla SG (2005) Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends Food Sci Tech 16:442–457
Semjonovs SP, Marauska M, Linde R, Grube M, Zikmanis P, Bekers M (2004) Development of Bifidobacterium lactis Bb 12 on β-(2,6)-linked fructan-containing substrate. Eng Life Sci 4:433–437
Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiol 120:351–360
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ (2006) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243
Acknowledgments
Lucas del Castillo is thanked for plasmid pLC6 used for the amplification of the SUC2 gene. This work was funded by grant BIO2010-20508-C04-02 from Spain’s ‘Secretaría de Estado de Investigación, Desarrollo e Innovación.’ D T-P was supported by a FPU fellowship from ‘Ministerio de Economía y Competitividad.’
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marín-Navarro, J., Talens-Perales, D. & Polaina, J. One-pot production of fructooligosaccharides by a Saccharomyces cerevisiae strain expressing an engineered invertase. Appl Microbiol Biotechnol 99, 2549–2555 (2015). https://doi.org/10.1007/s00253-014-6312-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6312-4