Abstract
Since the anatomic description of the anterolateral ligament (ALL) by Claes et al. in [9], there has been a vigorous debate in literature on the existence and the function of this structure first described in 1879 by Dr. Paul Segond. The culmination of this debate was July 2018 and the publication of a consensus paper co-authored by a panel of influential international researchers and clinicians confirming the existence of this ligament. Its origin is posterior and proximal to the lateral epicondyle of the femur and its insertion is on tibia plateau midway between Gerdy’s tubercle and the fibular head. Biomechanically, the ALL acts as a rotational stabilizer of the knee and the combined reconstruction of anterior cruciate ligament (ACL) and ALL demonstrated an improvement in knee stability compared with isolated ACL reconstruction. This improvement in knee kinematics has an important clinical impact reducing the rate of ACL graft ruptures and failure of medial meniscus repairs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Anterior cruciate ligament (ACL) tears are among the most common knee injuries and the number of ACL reconstructions (ACLR) performed every year is increasing [1]. Isolated single-bundle ACLR is still the gold standard surgical procedure for patients presenting with an ACL tear. However, graft failure rate and persistent rotational instability reflected by a positive pivot shift remains a major concern after the surgery [2]. This residual pivot shift after ACLR showed a negative correlation with functional outcomes and a higher risk of developing osteoarthritis [3, 4]. The influence of different intraarticular surgical procedures or ACL graft choice has been evaluated but didn’t show any significant improvement on post-operative outcomes [5,6,7,8]. It is for this reason that since the new description of the anterolateral ligament (ALL) by Claes et al. in [9], orthopaedic surgeons have demonstrated a renewed interest in the role of the anterolateral structures of the knee in controlling rotatory laxity and their ability to share loads with the ACL graft [9,10,11,12]. While some authors demonstrated the ALL anatomy and its important contribution in knee stability others have questioned its role as knee stabilizer and even its existence [13,14,15,16,17]. However, in a consensus meeting in 2017, the ALL was identified as a clear anatomical structure within the anterolateral complex involved in the control of internal rotation of the knee [18]. Additionally biomechanical studies have shown that knee stability was better after combined ACLR + ALLR than after isolated ACLR in the setting of an ALL injury. Finally, this improvement in knee stability could explain the promising clinical results observed in patients who underwent combined ACLR + ALLR [19,20,21,22].
History
The ALL was first described in 1879 by Dr. Paul Segond as a “pearly, resistant, fibrous band” that could result in an avulsion fracture of the tibial plateau when the knee was forcefully internally rotated: the Segond Fracture [23]. However, Segond did not describe its precise anatomy and did not name it [24]. In 1914, a french anatomist, Vallois, described the lateral epicondyle meniscal ligament (LEML) whose femoral insertion was on the top of the femoral epicondyle, above the attachment of the lateral collateral ligament and its tibial insertion was on the superior edge of the meniscus [24, 25]. In 1921 in Strasbourg, Jost evaluated Vallois’ works in depth and reported that LEML not only had an insertion on lateral meniscus, but also on the tibia. Additionally he mentioned that this ligament was particularly well developed in animals requiring control over rotational stability of their knee [24, 26].
Hughston et al. in 1976 and Prof. W. Müller in 1982 described “a middle third of the lateral capsular ligament” and an “anterolateral femoro-tibial ligament”, respectively, providing rotation stabilization of the knee [27, 28].
The term “anterolateral ligament” was first used in literature in 1986 by Terry et al., but its existence was popularized beyond medical journals by Claes et al. in [9] even though many other authors have contributed to the identification of the ALL and the determination of its function [9, 29,30,31,32,33,34].
Anatomy and Histology
The anatomical characteristics of the ALL have been a source of an intensive debate that ended in 2018 with the publication of the results from the ALC consensus group meeting in London [18]. They confirmed that ALL is a structure within the anterolateral complex (ALC) that included from superficial to deep:
-
Superficial iliotibial (IT) band and iliopatellar band
-
Deep IT band and Kaplan fiber system
-
ALL
-
Capsule.
Its origin is posterior and proximal to the lateral epicondyle of the femur [18, 35]. It runs superficially to the lateral collateral ligament (LCL) and then crosses the joint line giving some branching attachment to the lateral meniscus [34, 36,37,38]. Finally it inserts on the tibia, 413 mm distal to the joint line, halfway between anterior border of the fibular head and the posterior border of Gerdy’s Tubercle [9, 18, 36, 37, 39]. According to a reward-winning study published by Claes et al. in [40], this location corresponds to the same location of Segond avulsion fractures [40]. However due to the presence of other structures that also attach on this region, a consensus could not be reached about which of these structures is strictly responsible for this lesion [18].
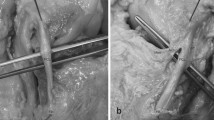
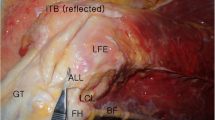
Following dissection protocols, the ALL could be identified in 83–100% of specimens [9, 36, 37, 39, 41, 42]. According to Daggett et al. a key to successful identification of the ALL is a careful reflection of the ITB from proximal to distal because toward the lateral epicondyle the ITB becomes thin and could closely adhere to the ALL (Fig. 1) [35].

Copyright: Fig. 2 + 5. Daggett M et al. Sugical dissection of the anterolateral ligament. Arthroscopy techniques, vol 5, no1 2016; e185–188
a Careful reflection of the iliotibial band to the Gerdy tubercle is required for visualization of the anterolateral ligament (right knee specimen in supine position). The fibers of the anterolateral ligament are often in close proximity to the deep fibers of the iliotibial band, and meticulous dissection is required to isolate these two structures. b After careful dissection, the entirety of the anterolateral ligament (ALL) can be identified as it overlaps the lateral collateral ligament (LCL) (right knee specimen in supine position). The ALL originates near the lateral epicondyle (LE) and inserts onto the tibia between the Gerdy tubercle and the fibular head.
On average, ALL measures 35 to 40 mm in length, 7 mm in width and 1–3 mm in thickness [15, 17, 42]. Histologically, it is composed of well organized collagenous fibers, fibroblasts, and nerves, indicating a potential proprioceptive role (Fig. 2) [36, 43,44,45].

Copyright: Fig. 4. Helito C. et al. Anatomy and Histology of the knee anterolateral ligament, OJSM 2013
Sections of the anterolateral ligament (L) showing its well-defined femoral bone attachment (B) in the left and its meniscal attachment (M) in the right. The bottom right image shows the histological structure, with dense connective tissue, arranged fibers, and little cellular material.
Biomechanics and Function
ALL is a stabilizer of the knee whose maximal load to failure and stiffness reported in literature varied from 175 to 205 N and 20 N/mm to 42 N/mm, respectively [39, 46, 47]. These results confirm that a semitendinosus graft (1216 N) or a gracilis graft (838 N) are both appropriate for ALL reconstruction [39].
While results about its contribution in an ACL intact knee remains controversial in literature, it is well documented that the ALL is an important restraint for internal rotation and anterior translation and plays a role in preventing pivot shift in ACL deficient knees [46, 48,49,50]. Two other structures were reported in literature as actively participating in this knee stabilization: The ITB and the lateral meniscus [46, 51,52,53]. Indeed Lording et al. and Shybut et al. reported an increased anterior translation and internal rotation of the knee after tears of the posterior root of the lateral meniscus [51, 52].
All authors agreed that the ALL is an anisometric structure. However, while some authors reported that the length of the ligament increased with knee flexion, others demonstrated that it decreased [11, 37, 39, 43, 54]. A possible explanation for this disagreement could be related to the previously misidentified origin of the ALL on the femur. With a femoral origin close to or anterior and distal to the lateral epicondyle center, Helito et al. and Zens et al. reported an increase in the ALL length with knee flexion [43, 54]. On the other hand, Dodds et al. demonstrated that the ALL slackened with knee flexion if it originated proximal and posterior to the lateral femoral epicondyle (Fig. 3).
This favorable anisometry would be a condition inherently necessary to allow physiological internal rotation of the tibia during knee flexion and to avoid risk of over-constraint of the lateral compartment of the knee [37, 55].
The problem of length change of the ALL during knee mobilization according to its femoral insertion has been solved by Imbert et al. who demonstrated an identical behavior of the ALL contingent on these two different femoral insertions [56].
Injury
Injuries to the anterolateral structures of the knee can occur at the time of an ACL tear or can be a result of overloading or subsequent giving-way episodes in chronic cases [57]. The traumatic mechanism for a combined ACL and ALL lesion is similar to one for isolated ACL injury: early flexion, dynamic valgus, and internal rotation [13].
Incidence of this injury reported in literature ranged from 80 to 100% of cases [27, 28, 30, 57, 58]. In a recent study, Ferretti et al. systematically explored the lateral compartment in 76 patients who underwent an ACL reconstruction [57]. Macroscopic tears were identified in 90% of patients and were divided as follows (Fig. 4a–d):

Copyright: Figs. 2 and 5 Ferretti A et al. Prevalence and Classification of Injuries of Anterolateral Complex in Acute Anterior Cruciate Ligament Tears, Arthroscopy 2017, vol 33, 2017:147–154)
Classification of injuries of anterolateral complex. a Type I lesion: multilevel rupture in which individual layers are torn at different levels with macroscopic hemorrhage involving the area of the ALL and extended to the anterolateral capsule only. b Type II lesion: multilevel rupture in which individual layers are torn at different levels with macroscopic hemorrhage extended from the area of the ALL and capsule to the posterolateral capsule. c Type III lesion: complete transverse tear involving the area of ALL near its insertion to the lateral tibial plateau, always distal to lateral meniscus. d Type IV lesion: bony avulsion. ALL, anterolateral ligament; GT, Gerdy tubercle; LCL, lateral collateral ligament; SF, Segond Fracture.
-
Type I (31.6%): multilevel rupture in which individual layers are torn at different levels with macroscopic hemorrhage involving the area of the ALL and extended to the anterolateral capsule only.
-
Type II (26.7%): multilevel rupture in which individual layers are torn at different levels with macroscopic hemorrhage extended from the area of the ALL and capsule to the posterolateral capsule.
-
Type III (21.7%): complete transverse tear involving the area of the ALL near its insertion to the lateral tibial plateau, always distal to the lateral meniscus.
-
Type IV (10%): bony avulsion of ALL (Segond fracture).
This study shows that injuries of the anterolateral secondary restraints often occur in cases of apparently isolated ACL tears. This confirms that rotational instability of the knee is not only the result of an ACL tear, but also involves anterolateral structures.
Diagnosis
Clinical diagnosis of an ALL tear remains a challenge for orthopaedic surgeons [13]. The pivot shift test remains the most reliable test to evaluate its integrity. Monaco et al. demonstrated that a grade III pivot shift could be seen only in the absence of both ALL and ACL in vitro [59]. This finding was not confirmed in literature though, as other authors showed that a high-grade pivot shift could be caused by injuries to the lateral meniscus, the iliotibial band, an increased tibial slope, or a general hyperlaxity [13, 60].
With regards to radiology, two modalities are commonly reported on for evaluation of the ALL: ultrasound (US) and magnetic resonance imaging (MRI).
On MRI, although a part of the ALL could be identified in most cases, the entire ligament remains difficult to analyze because of its small thickness and the presence of adjacent structures which cause a partial volume effect in the region [60, 61]. The ligament was entirely visualized in 20.6 to 100% of cases [61,62,63,64,65].
ALL tears also remain difficult to diagnose. In 206 patients with ACL injury, Claes et al. reported that the ALL was abnormal on 162 MRI (78.8%). On the other hand, Helito et al. and Cavaignac et al. identified ALL lesions in 32.6 and 53% of patients with ACL injury, respectively [61, 62]. These rates are far below those reported by Ferretti et al. (90%), which suggests that the false negative rate of MRI in diagnosing ALL injury remains high [57]. However, using a three-dimensional (3D) MRI, Muramatsu et al. identified a higher rate of ALL injury in patients with acute ACL tears (87.5%) as compared to previous authors using standard MRI (Fig. 5) [66].

Copyright: Fig. 2 Muramatsu K et al. Three-dimensional Magnetic Resonance Imaging of the Anterolateral Ligament of the Knee: An Evaluation of Intact and Anterior Cruciate Ligament Deficient Knees From the Scientific Anterior Cruciate Ligament Network International (SANTI) Study Group. Arthroscopy 2018; 34: 2207–17
Injury classification of anterolateral ligament (ALL, arrows) in anterior cruciate ligament deficient knees shown on coronal cross-sectional images: type A, normal ALL, visualized as a continuous, clearly defined low-signal band; type B, abnormal ALL showing warping, thinning, or iso-signal changes; and type C, abnormal ALL showing no clear continuity.
With regard to ultrasound, Cavaignac et al. demonstrated in a cadaveric study that ALL could be identified with US in all specimens and the findings corresponded precisely to the anatomical dissection [67]. In a comparative study including 30 patients with an acute ACL injury (<3 months old), they also showed that US and MRI could identify ALL tear in 53% and 63% of cases, respectively [62]. Additionally, Segond fracture was identified in 3% of patients on radiographs, 13% of patients on MRI, and 50% of patients on US (Fig. 6).

Copyright: Fig. 2. Faruch Bilfeld M et al. Anterolateral ligament injuries in knees with an anterior cruciate ligament tear: Contribution of ultrasonography and MRI. Eur Radiol 2018;28:58–65
Appearance of anterolateral ligament (ALL) on ultrasonography. Major axis of the anterolateral ligament of the knee; coronal plane image showing the ligament in the major axis. a Ultrasonography of normal ALL (arrows): hypoechogenic, fibrillar, thin structure crossing superficially the inferior genicular artery (arrow-head) and popliteal tendon (star). b Ultrasonography of injured ALL: the tibial insertion is hypoechogenic and thickened (arrow) with fluid accumulation in the soft tissues around the ligament. c Ultrasonography of injured ALL: the tibial insertion is hypoechogenic and thickened (arrow) and there is a bone avulsion at the tibial enthesis (arrow-head), i.e., Segond fracture. FC femoral condyle, LM lateral meniscus, TP tibial plateau.
This higher rate of Segond fracture diagnosed with US is explained by the fact that it has the highest spatial resolution [62]. Time between ACL injury and sonographic evaluation could be an important parameter to consider when analyzing the diagnostic performance. Indeed, Yoshida et al. reported that 33% of ACL-injured knees had abnormalities in the anterolateral structures of the knee when mean time to sonographic evaluation was 4 months (range: 2 days–1 year) [68]. Technically, to identify the ALL on US, the leg has to be flexed and internally rotated placing tension on the ligament. The tibial insertion has to be identified first and then the ALL is followed proximally to its femoral insertion [67].
ALL tears have to be searched for near its tibial insertion. Cavaignac et al. [62] reported that all ALL injuries were at its tibial insertion, which was consistent with results of Van Dyck et al. and Claes et al. who found that tibial enthesis was involved in 71.8 and 77.8% of cases, respectively [69, 70]. The predominance of tears in this region could be explained by the biomechanical study of Wang et al. that demonstrated a significantly higher strain in the distal portion of the ALL when internal rotation was applied on the knee [71].
Finally, in a recent systematic review, Puzzitiello et al. have shown that an injury of the ALL, as seen on MRI or US, had a significant correlation with a high-grade pivot shift in most studies [60]. Additionally, although both exams could be useful to diagnose an ALL tear, their actual performance does not allow us to definitively rule out an ALL injury if the imaging findings are negatives.
Surgical Indication
Indications for a combined ACLR + ALLR are questioned in literature due to current lack of clinical evidence [72]. However, based on promising clinical results and evidence that the addition of an extra-articular reconstruction to the ACLR improves rotational laxity, an expert group proposed criteria to identify patients eligible for such surgical procedure (Table 1) [13].
Copyright: Table 3. Delaloye JR et al. Clinical outcomes after combined anterior cruciate ligament and anterolateral ligament reconstruction. Tech Orthop. 2018 Dec;33(4):225–231.
Among decisive criteria, members of the international ALC consensus groups agreed that revision ACLR, high-grade pivot shift, hyperlaxity, and young patients returning to pivoting activities represented appropriate indications for an ALLR [18].
Surgical Techniques
Based on anatomical and biomechanical studies different surgical techniques have been proposed for ALL reconstruction using a single or a double gracilis graft [73]. The technique presented below is the one developed by Sonnery-Cottet et al. [74] (Fig. 7).

Copyright: Fig. 1 A Delaloye JR et al. Clinical Outcomes After Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Tech orthop 2018
Anterolateral ligament reconstruction.
This minimally invasive ALL reconstruction has demonstrated excellent clinical and biomechanical results [19, 20, 22, 75].
Step 1—Three bony landmarks are marked at the start of the operation (knee 90° of flexion): Lateral epicondyle, fibula head, and Gerdy’s tubercle (Fig. 8).

Copyright: Fig. 1 Sonnery-Cottet et al. Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Arthroscop Tech vol 5 No 6 2016 e 1253–e1259
As shown in a right knee (lateral view), 3 stab incisions (blue ovals) are positioned in relation to the 3 bony landmarks for combined anterior cruciate ligament and anterolateral ligament reconstruction. One is placed on the femoral side, slightly proximal and posterior to the lateral epicondyle (LE). Two tibial stab incisions are subsequently positioned 8 mm below the joint line between the Gerdy tubercle (GT) and fibular head (FH).
Step 2—One femoral stab incision: slightly proximal and posterior to the epicondyle.
Two tibial stab incisions: 1 cm under the femoro-tibial articulation.
One is just above the superolateral margin of the Gerdy tubercle the other is midway between the previously marked fibular head and the Gerdy tubercle
Step 3—Three 2.4 mm K-wires are drilled into the bone through the skin incision at the selected points. A control of the adequate non-isometry can be performed using a suture passed around the guidewires (Fig. 3). The suture has to be tight in extension, and slightly slack in flexion. If it tightens in flexion, then the femoral socket position is too distal and anterior.
Step 4—A 4.5 mm cannulated drill bit is used to overdrill the k-wires and prepare three 20 mm deep sockets. Connect the 2 tibial bony sockets using a right-angled clamp to create a bony bridge. A suture is then passed in a retroverted fashion to create a loop and ease graft passage (Fig. 9B).

Copyright: Fig. 2. Delaloye JR et al. Combined Anterior Cruciate Ligament Repair and Anterolateral Ligament Reconstruction, Arthrosc Tech vol 8, No1 (2019); e23-e29
Right knee. a Femoral fixation of one end of the gracilis with the SwiveLock anchor device. b a loop of suture relay is placed through the 2 convergent transosseous tunnels. c The free end of the gracilis is routed from the femur to the tibia deep to the iliotibial band, d through the tibial transosseous tunnel using the suture relay, and e back to the femoral incision deep to the iliotibial band. FH, fibular head; GT, Gerdy’s tubercle; LE, lateral epicondyle.
Step 5—Harvest the gracilis tendon. Both ends are whipstitched with a number 2 suture.
Step 6—Femoral fixation of the graft. The gracilis graft is passed into an 4.75 mm anchor and then placed into socket (Fig. 9a).
Step 7—Graft passage deep to the iliotibial band using an arthroscopic grasper introduced through the stab incision next to the fibula head. Shuttle of the graft through the anterior tibial bone tunnel using the previously passed suture. Introduction of the arthroscopic gasper through the femoral incision and deep to the iliotibial band. Then pull back of the gracilis graft through the femoral incision resulting in a triangle configuration of the graft through the tibial bone tunnel (Fig. 9c–e).
Step 8—Final tensioning of the graft with the knee in full extension and neutral rotation. Fixation of the graft on the femoral side using the sutures outgoing from the anchor.
Post-operative Rehabilitation
After an ALL reconstruction, particularly if performed in conjunction with an ACL reconstruction, the rehabilitation should be carried out in a similar way to conventional ACL rehabilitation [13]:
-
Full weight bearing without brace.
-
Progressive range of motion exercises. Control of the absence of extension deficit 3 weeks post-operative.
-
Gradual return to sports activities is allowed starting at 4 months for non-pivoting sports, at 6 months for pivoting noncontact sports, and at 8–9 months for pivoting contact sports.
Biomechanics of ALL Reconstructions
Several cadaveric studies have examined the kinematics of the knee after ACLR with or without ALLR [75,76,77,78,79,80,81].
In the absence of an ALL injury, Noyes et al. and Herbst and al. demonstrated that an isolated ACLR was able to restore the stability of the knee [79, 80]. However, their results also showed that in ALL deficient knee this isolated ACL reconstruction was not sufficient and internal rotation stability of the knee was improved when a lateral extra-articular procedure was added. These results are in accordance with most studies that demonstrated that combined ACLR + ALLR could significantly improve knee kinematics in comparison with isolated ACLR [75,76,77,78]. Inderhaugh et al. reported that anatomic ALLR tensioned in full extension, added to ACLR could restore the intact knee laxity in an ACL and ALL injured knee unlike isolated ACLR [75]. This higher knee stability was seen for isolated anterior translation, internal rotation of the knee, as well as stimulated pivot shift. Indeed, except for Noyes et al. who failed to demonstrate an improvement of knee stability when performing a pivot shift test after combined ACLR + ALLR in comparison with isolated ACLR, most other authors demonstrated a higher knee stability during the test when both ligaments were reconstructed [75, 77,78,79,80].
A main concern after ALLR is the risk of over-constraint of the knee [76, 78, 80]. Herbst et al. reported a decrease in internal rotation after ACLR and lateral extra-articular tenodesis (LET) in comparison with an intact knee. The largest difference was observed when a combined ACLR and LET were performed in an isolated ACL deficient knee. Interestingly, even in this situation the difference of internal rotation never reached significance. Schon et al. also reported on over-constraint in internal rotation of the knee when ALLR was performed using a semitendinosus graft tensioned at 88 N [76]. This high tension has been highly questioned and may explain the over-constraint observed [82]. Indeed, Inderhaug et al. demonstrated the absence of any over-constraint of the knee when a 20 N tension was applied on the graft [75].
Clinical Results after ALLR
Clinical Outcomes
In 2015, Sonnery-Cottet et al. published the first clinical series of 92 patients who underwent a combined ACLR + ALLR [21]. At a mean follow-up of 32.4 months (range: 24–39 months), Tegner score was 7.1 ± 1.8 and side-to-side laxity was 0.7 ± 0.8 mm. Lysholm, subjective and objective International Knee Documentation Committee (IKDC) scores were significantly improved after surgery (p < 0.0001). At final follow-up, 91.6% of patients graded A IKDC subjective score while Lysholm and IKDC subjective scores were 92 ± 9.8 and 86.7 ± 12.3, respectively.
In several comparative studies, clinical outcomes of patients after combined ACLR + ALLR were similar or significantly better than those after isolated ACLR. These observations were obtained regardless of the studied subpopulation (high-risk patient, chronic ACL injury, Hyperlaxity) (Table 2).
Graft Rupture
Although ACL reconstruction is associated with superior quality of life, sports function, and knee symptoms when compared to non-operative treatment, the graft failure rate is up to 18% in high-risk population [83, 84]. Combined ACLR + ALLR have been proposed to reduce the stress applied on the graft during its ligamentization with the expectation that it will reduce the risk of raft rupture [46, 85].
In a comparative study, Sonnery-Cottet et al. demonstrated that combined ACLR + ALLR in a high-risk population was associated with significantly decreased graft rupture rates when compared to isolated ACLR [20]. These graft rupture rates were found to be 10.77% (range, 6.60–17.32%) for quadrupled hamstring tendon (4HT) grafts, 16.77% (9.99–27.40%) for bone-patellar tendon-bone (B-PT-B) grafts, and 4.13% (2.17–7.80%) for hamstring tendon graft combined with ALLR (HT + ALL) at a mean follow-up of 38.4 months (Fig. 10).

Copyright: Fig. 3, Sonnery-Cottet et al. Anterolateral Ligament Reconstruction Is Associated With Significantly Reduced ACL Graft Rupture Rates at a Minimum Follow-up of 2 Years A Prospective Comparative Study of 502Patients From the SANTI Study Group. Am J Sports Med 2017 45(7):1547–1557
Survivorship data from Kaplan–Meier analysis stratified by anterior cruciate ligament reconstruction technique. ALL, anterolateral ligament; B-PT-B, bone-patellar tendon-bone; HT, hamstring tendon. Reprinted with permission from American Journal of Sports Medicine.
In patients with hypermobility and knee hyperextension, Helito et al. also demonstrated a significantly lower graft failure in patients after combined ACLR + ALLR (3.3%) than after isolated ACLR (21.7%) (p = 0.03) [86].
In patients with chronic ACL injuries or those with revision ACLR, graft rupture rates at a minimum 2 year follow-up were also lower in patients with ALLR but this difference was not statistically significant [87, 88].
Finally, In a series of 70 professional athletes with a mean follow-up of 3.9 years, Rosenstiel et al. reported that graft failure after combined ACLR + ALLR was 5.7% [89].
Protective Effect on Medial Meniscal Repairs
Biomechanical studies previously cited have demonstrated that combined ACLR + ALLR improved the rotational stability of the knee in comparison to isolated ACLR [75, 81]. This higher stability could explain the protective effect of the ALLR on medial meniscus repair performed in patients with ACLR [19]. Sonnery-Cottet et al. showed that the survival rate of a meniscal repair at 36-month follow-up was 91.2% (95% IC, 85.4%–94.8) after combined ACLR + ALLR compared to 83.8% (95% CI, 77.1–88.7%) (p = 0.033) after isolated ACLR. The probability of failure of a medial meniscal repair was more than two times lower if ALLR was performed in patients with ACLR (hazard ratio, 0.443; 95% CI, 0.218–0.866) (Fig. 11).

Copyright: Fig. 2 Sonnery-Cottet et al. Anterolateral Ligament Reconstruction Protects the Repaired Medial Meniscus: A Comparative Study of 383 Anterior Cruciate Ligament Reconstructions From the SANTI Study Group With a Minimum Follow-up of 2 Years. Am J Sports med 2018 Jul;46(8):1819–1826
Kaplan–Meier survivorship with reoperation for medial meniscal injury as an endpoint. ACLR, anterior cruciate ligament anterolateral ligament reconstruction; ALLR, reconstruction. Reprinted with permission from American Journal of Sports Medicine.
This protective effect on the medial meniscal repair could play an important role in long-term preservation of the knee articulation in patients after ACLR. Indeed, Claes et al. and Shelbourne et al. reported a three times higher risk to develop OA in patients with meniscectomy compared to those without meniscectomy at a mean post-operative follow-up of 10 years (Odds ratio 3.54, 95% CI 2.56–4.91) and 22.5 years (Odds ratio 2.98, 95% CI 1.91–4.66), respectively [90, 91].
Return to Sport
Low rates of return to sport are a major concern after ACLR, particularly in a high-risk population. One systematic review has demonstrated that on average, only 65% of patients return to their pre-injury level of sport and only 55% to competitive sport [92].
Sonnery-Cottet et al. reported a higher rate of return to sport for patients who underwent a combined ACLR + ALLR (68.8%) in comparison with those who underwent an isolated ACLR using B-PT-B (63.5%) or 4HT grafts (59.9%). However the difference did not reach statistical significance (p = 0.231) [20]. Regardless of the type of graft, factors that significantly increased the return to pre-injury level of sport were male sex and absence of meniscal tear.
After revision ACLR, Lee et al. reported that patients with combined ACLR + ALLR had a significantly higher rate of return to the same level of sports activity than those with isolated ACLR (57.1 vs. 25.6%, p = 0.008) [88].
Finally, according to Rosenstiel et al. professional athletes who underwent combined ACLR + ALLR were able to return to the same competitive level of sport in 85.7% of cases with a mean delay from the surgery of 7.9 months (range, 5–12 months) [89].
Post-operative Complications
The rates of reoperation after ACLR reported in literature remain higher than desired varying from 18.9 to 26.7% [93, 94]. Based on historical series of non-anatomic LET that reported high rates of knee stiffness and poor clinical results, concerns existed about the addition of an anatomic ALLR in patients with ACLR [95, 96]. However, more recent studies with a minimum 2-year follow-up demonstrated that this procedure did not appear to be associated with increased risk of reoperation or post-operative stiffness [21, 22, 88, 97]. Indeed, the first clinical series reported that 8 of 92 patients required a reoperation of the ipsilateral knee (8.7%) while 7 patients sustained a contralateral ACL rupture (7.6%) [21]. Thaunat et al. also reported excellent results in a large study of 548 patients, where 77 (14.1%) required an ipsilateral knee reoperation, while 47 suffered a contralateral ACL tear (8.6%) at a mean of 20.4 ± 8.0 months after the index procedure [22]. The only complications specifically related to the ALL procedure (3 patients) were all related to femoral hardware that required removal. Lee et al. also reported one complication in his 42 patients after revision ACLR and ALLR, which was a femoral interference screw protrusion that required removal [88]. Ibrahim et al. reported no patients that needed a reoperation and the only post-operative complication reported in their series of 53 patients with combined ACLR + ALLR was a superficial infection treated with antibiotics [97].
Based on biomechanical results, authors warned of a risk of over-constraint of the knee and early development of arthrosis after ALLR [76, 78]. However, no substantial clinical data is available to confirm or disprove this concern with regards to anatomic reconstruction of the ALL. The only study so far was by Ferretti et al. who showed no increased risk of OA at a minimum of 10 years follow-up in patients who underwent a combined ACLR and LET [98].
Conclusion
The ALL is an important stabilizing structure of the knee whose origin is posterior and proximal to the lateral epicondyle of the femur and its insertion is on the tibia plateau midway between Gerdy’s tubercle and the fibular head. In ACL and ALL deficient knees, biomechanical studies have demonstrated that combined ACLR + ALLR restores a higher stability to the knee compared to isolated ACLR. This improvement could explain the excellent clinical outcomes and the reduced rates of graft failure and secondary meniscectomy reported in patients after combined ACLR + ALLR. Furthermore, the addition of an ALLR is a safe and reproducible procedure with no evidence of the adverse events that led to the historical widespread abandonment of other types of LET. As recently reported by Rossi, the question to be considered is not “if” augmentation should be considered, but rather “when” should it be considered, and maybe more importantly, “how” to augment [99]. On going randomized controlled trials (RCT) comparing isolated ACLR and ACLR + ALLR could soon shed light on essential points [100, 101]. Preliminary results of the RCT performing by Sonnery-Cottet et al. will be published later in 2019 [101]. Until then, current clinical data from multiple centers gives confidence in the strength of evidence supporting an important role for ALLR in the ACL-injured knee.
References
Mall NA, Chalmers PN, Moric M, Tanaka MJ, Cole BJ, Bach BR Jr, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–70. https://doi.org/10.1177/0363546514542796.
Chambat P, Guier C, Sonnery-Cottet B, Fayard JM, Thaunat M. The evolution of ACL reconstruction over the last fifty years. Int Orthop. 2013;37(2):181–6. https://doi.org/10.1007/s00264-012-1759-3.
Ayeni OR, Chahal M, Tran MN, Sprague S. Pivot shift as an outcome measure for ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):767–77. https://doi.org/10.1007/s00167-011-1860-y.
Jonsson H, Riklund-Ahlstrom K, Lind J. Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5–9 years after surgery. Acta Orthop Scand. 2004;75(5):594–9. https://doi.org/10.1080/00016470410001484.
Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985–92. https://doi.org/10.1177/0363546512454414.
Gifstad T, Sole A, Strand T, Uppheim G, Grontvedt T, Drogset JO. Long-term follow-up of patellar tendon grafts or hamstring tendon grafts in endoscopic ACL reconstructions. Knee Surg Sports Traumatol Arthrosc. 2013;21(3):576–83. https://doi.org/10.1007/s00167-012-1947-0.
Mascarenhas R, Cvetanovich GL, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, et al. Does double-bundle anterior cruciate ligament reconstruction improve postoperative knee stability compared with single-bundle techniques? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31(6):1185–96. https://doi.org/10.1016/j.arthro.2014.11.014.
Forster MC, Forster IW. Patellar tendon or four-strand hamstring? A systematic review of autografts for anterior cruciate ligament reconstruction. Knee. 2005;12(3):225–30. https://doi.org/10.1016/j.knee.2004.06.008.
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223(4):321–8. https://doi.org/10.1111/joa.12087.
Delaloye JR, Murar J, Gonzalez M, Amaral T, Kakatkar V, Sonnery-Cottet B. Clinical outcomes after combined anterior cruciate ligament and anterolateral ligament reconstruction. Tech Orthop. 2018. https://doi.org/10.1097/BTO.0000000000000326.
Weber AE, Zuke W, Mayer EN, Forsythe B, Getgood A, Verma NN et al. Lateral augmentation procedures in anterior cruciate ligament reconstruction: anatomic, biomechanical, imaging, and clinical evidence. Am J Sports Med. 2018;363546517751140. https://doi.org/10.1177/0363546517751140.
Musahl V, Herbst E, Burnham JM, Fu FH. The anterolateral complex and anterolateral ligament of the knee. J Am Acad Orthop Surg. 2018;26(8):261–7. https://doi.org/10.5435/JAAOS-D-16-00758.
Sonnery-Cottet B, Daggett M, Fayard JM, Ferretti A, Helito CP, Lind M, et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament—deficient knee. J Orthop Traumatol. 2017;18(2):91–106. https://doi.org/10.1007/s10195-017-0449-8.
Ingham SJM, de Carvalho RT, Martins CAQ, Lertwanich P, Abdalla RJ, Smolinski P, et al. Anterolateral ligament anatomy: a comparative anatomical study. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1048–54. https://doi.org/10.1007/s00167-015-3956-2.
Kraeutler MJ, Welton KL, Chahla J, LaPrade RF, McCarty EC. Current concepts of the anterolateral ligament of the knee: anatomy, biomechanics, and reconstruction. Am J Sports Med. 2018;46(5):1235–42. https://doi.org/10.1177/0363546517701920.
Williams A. Editorial commentary: the anterolateral ligament: the emperor’s new clothes? Arthroscopy. 2018;34(4):1015–21. https://doi.org/10.1016/j.arthro.2017.12.026.
Patel RM, Brophy RH. Anterolateral ligament of the knee: anatomy, function, imaging, and treatment. Am J Sports Med. 2018;46(1):217–23. https://doi.org/10.1177/0363546517695802.
Getgood A, Brown C, Lording T, Amis A, Claes S, Geeslin A, et al. The anterolateral complex of the knee: results from the International ALC Consensus Group Meeting. Knee Surg Sports Traumatol Arthrosc. 2018. https://doi.org/10.1007/s00167-018-5072-6.
Sonnery-Cottet B, Saithna A, Blakeney WG, Ouanezar H, Borade A, Daggett M, et al. Anterolateral ligament reconstruction protects the repaired medial meniscus: a comparative study of 383 anterior cruciate ligament reconstructions from the SANTI study group with a minimum follow-up of 2 years. Am J Sports Med. 2018;46(8):1819–26. https://doi.org/10.1177/0363546518767659.
Sonnery-Cottet B, Saithna A, Cavalier M, Kajetanek C, Temponi EF, Daggett M, et al. Anterolateral ligament reconstruction is associated with significantly reduced ACL graft rupture rates at a minimum follow-up of 2 years: a prospective comparative study of 502 patients from the SANTI study group. Am J Sports Med. 2017;45(7):1547–57. https://doi.org/10.1177/0363546516686057.
Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BH, Murphy CG, Claes S. Outcome of a combined anterior cruciate ligament and anterolateral ligament reconstruction technique with a minimum 2-year follow-up. Am J Sports Med. 2015;43(7):1598–605. https://doi.org/10.1177/0363546515571571.
Thaunat M, Clowez G, Saithna A, Cavalier M, Choudja E, Vieira TD, et al. Reoperation rates after combined anterior cruciate ligament and anterolateral ligament reconstruction: a series of 548 patients from the SANTI study group with a minimum follow-up of 2 years. Am J Sports Med. 2017;45(11):2569–77. https://doi.org/10.1177/0363546517708982.
Segond P. Recherches cliniques et experimentales sur les epanchements sanguins du genou par entorse. Progres Med. 1879(7):297–9, 319–21, 40–41.
Cavaignac E, Ancelin D, Chiron P, Tricoire JL, Wytrykowski K, Faruch M, et al. Historical perspective on the “discovery” of the anterolateral ligament of the knee. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):991–6. https://doi.org/10.1007/s00167-016-4349-x.
Hv V. Etude anatomique de l’articulation du genou chez les primates. Montpellier: Abeille; 1914.
Jost A. Sur la morphogenèse et le rôle fonctionnel des ligaments épicondylo-méniscaux du genou. Compte rendu de la société de biologie. 1921;LXXXIV.
Hughston JC, Andrews JR, Cross MJ, Moschi A. Classification of knee ligament instabilities. Part II. The lateral compartment. J Bone Joint Surg Am. 1976;58(2):173–9.
Müeller W. The knee: form, function and ligamentous reconstruction surgery. 1982.
Terry GC, Hughston JC, Norwood LA. The anatomy of the iliopatellar band and iliotibial tract. Am J Sports Med. 1986;14(1):39–45. https://doi.org/10.1177/036354658601400108.
Puddu GFMP, Conteduca F. Lesioni Combinateanteriori Acute. Il Ginocchio. 1987;6:303–6.
Irvine GB, Dias JJ, Finlay DB. Segond fractures of the lateral tibial condyle: brief report. J Bone Joint Surg Br. 1987;69(4):613–4.
Campos JC, Chung CB, Lektrakul N, Pedowitz R, Trudell D, Yu J, et al. Pathogenesis of the Segond fracture: anatomic and MR imaging evidence of an iliotibial tract or anterior oblique band avulsion. Radiology. 2001;219(2):381–6. https://doi.org/10.1148/radiology.219.2.r01ma23381.
Vieira EL, Vieira EA, da Silva RT, Berlfein PA, Abdalla RJ, Cohen M. An anatomic study of the iliotibial tract. Arthroscopy. 2007;23(3):269–74. https://doi.org/10.1016/j.arthro.2006.11.019.
Vincent JP, Magnussen RA, Gezmez F, Uguen A, Jacobi M, Weppe F, et al. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):147–52. https://doi.org/10.1007/s00167-011-1580-3.
Daggett M, Busch K, Sonnery-Cottet B. Surgical dissection of the anterolateral ligament. Arthrosc Tech. 2016;5(1):e185–8. https://doi.org/10.1016/j.eats.2015.10.019.
Helito CP, Demange MK, Bonadio MB, Tirico LE, Gobbi RG, Pecora JR, et al. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1(7):2325967113513546. https://doi.org/10.1177/2325967113513546.
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Joint J. 2014;96-B(3):325–31. https://doi.org/10.1302/0301-620X.96B3.33033.
Helito CP, Bonadio MB, Soares TQ, da Mota e Albuquerque RF, Natalino RJ, Pecora JR et al. The meniscal insertion of the knee anterolateral ligament. Surg Radiol Anat. 2016;38(2):223–8. https://doi.org/10.1007/s00276-015-1533-5.
Kennedy MI, Claes S, Fuso FA, Williams BT, Goldsmith MT, Turnbull TL, et al. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43(7):1606–15. https://doi.org/10.1177/0363546515578253.
Claes S, Luyckx T, Vereecke E, Bellemans J. The Segond fracture: a bony injury of the anterolateral ligament of the knee. Arthroscopy. 2014;30(11):1475–82. https://doi.org/10.1016/j.arthro.2014.05.039.
Daggett M, Ockuly AC, Cullen M, Busch K, Lutz C, Imbert P, et al. Femoral origin of the anterolateral ligament: an anatomic analysis. Arthroscopy. 2016;32(5):835–41. https://doi.org/10.1016/j.arthro.2015.10.006.
Van der Watt L, Khan M, Rothrauff BB, Ayeni OR, Musahl V, Getgood A et al. The structure and function of the anterolateral ligament of the knee: a systematic review. Arthroscopy. 2015;31(3):569–82 e3. https://doi.org/10.1016/j.arthro.2014.12.015.
Helito CP, do Prado Torres JA, Bonadio MB, Aragao JA, de Oliveira LN, Natalino RJ et al. Anterolateral ligament of the fetal knee: an anatomic and histological study. Am J Sports Med. 2017;45(1):91–6. https://doi.org/10.1177/0363546516664888.
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3186–95. https://doi.org/10.1007/s00167-014-3117-z.
Helito CP, do Amaral C, Jr., Nakamichi YD, Gobbi RG, Bonadio MB, Natalino RJ et al. Why do authors differ with regard to the femoral and meniscal anatomic parameters of the knee anterolateral ligament?: Dissection by layers and a description of its superficial and deep layers. Orthop J Sports Med. 2016;4(12):2325967116675604. https://doi.org/10.1177/2325967116675604.
Sonnery-Cottet B, Lutz C, Daggett M, Dalmay F, Freychet B, Niglis L, et al. The involvement of the anterolateral ligament in rotational control of the knee. Am J Sports Med. 2016;44(5):1209–14. https://doi.org/10.1177/0363546515625282.
Helito CP, Bonadio MB, Rozas JS, Wey JM, Pereira CA, Cardoso TP, et al. Biomechanical study of strength and stiffness of the knee anterolateral ligament. BMC Musculoskelet Disord. 2016;17:193. https://doi.org/10.1186/s12891-016-1052-5.
Huser LE, Noyes FR, Jurgensmeier D, Levy MS. Anterolateral ligament and iliotibial band control of rotational stability in the anterior cruciate ligament-intact knee: defined by tibiofemoral compartment translations and rotations. Arthroscopy. 2017;33(3):595–604. https://doi.org/10.1016/j.arthro.2016.08.034.
Monaco E, Fabbri M, Mazza D, Daggett M, Redler A, Lanzetti RM, et al. The effect of sequential tearing of the anterior cruciate and anterolateral ligament on anterior translation and the pivot-shift phenomenon: a cadaveric study using navigation. Arthroscopy. 2018;34(4):1009–14. https://doi.org/10.1016/j.arthro.2017.09.042.
Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, et al. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44(3):585–92. https://doi.org/10.1177/0363546515618387.
Shybut TB, Vega CE, Haddad J, Alexander JW, Gold JE, Noble PC, et al. Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43(4):905–11. https://doi.org/10.1177/0363546514563910.
Lording T, Corbo G, Bryant D, Burkhart TA, Getgood A. Rotational laxity control by the anterolateral ligament and the lateral meniscus is dependent on knee flexion angle: a cadaveric biomechanical study. Clin Orthop Relat Res. 2017;475(10):2401–8. https://doi.org/10.1007/s11999-017-5364-z.
Kittl C, El-Daou H, Athwal KK, Gupte CM, Weiler A, Williams A, et al. The role of the anterolateral structures and the acl in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44(2):345–54. https://doi.org/10.1177/0363546515614312.
Zens M, Niemeyer P, Ruhhammer J, Bernstein A, Woias P, Mayr HO, et al. Length changes of the anterolateral ligament during passive knee motion: a human cadaveric study. Am J Sports Med. 2015;43(10):2545–52. https://doi.org/10.1177/0363546515594373.
Qi W, Hosseini A, Tsai TY, Li JS, Rubash HE, Li G. In vivo kinematics of the knee during weight bearing high flexion. J Biomech. 2013;46(9):1576–82. https://doi.org/10.1016/j.jbiomech.2013.03.014.
Imbert P, Lutz C, Daggett M, Niglis L, Freychet B, Dalmay F, et al. Isometric characteristics of the anterolateral ligament of the knee: a cadaveric navigation study. Arthroscopy. 2016;32(10):2017–24. https://doi.org/10.1016/j.arthro.2016.02.007.
Ferretti A, Monaco E, Fabbri M, Maestri B, De Carli A. Prevalence and classification of injuries of anterolateral complex in acute anterior cruciate ligament tears. Arthroscopy. 2017;33(1):147–54. https://doi.org/10.1016/j.arthro.2016.05.010.
Terry GC, Norwood LA, Hughston JC, Caldwell KM. How iliotibial tract injuries of the knee combine with acute anterior cruciate ligament tears to influence abnormal anterior tibial displacement. Am J Sports Med. 1993;21(1):55–60. https://doi.org/10.1177/036354659302100110.
Monaco E, Ferretti A, Labianca L, Maestri B, Speranza A, Kelly MJ, et al. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):870–7. https://doi.org/10.1007/s00167-011-1640-8.
Puzzitiello RN, Agarwalla A, Zuke WA, Garcia GH, Forsythe B. Imaging diagnosis of injury to the anterolateral ligament in patients with anterior cruciate ligaments: association of anterolateral ligament injury with other types of knee pathology and grade of pivot-shift examination: a systematic review. Arthroscopy. 2018. https://doi.org/10.1016/j.arthro.2018.04.025.
Helito CP, Helito PVP, Costa HP, Demange MK, Bordalo-Rodrigues M. Assessment of the anterolateral ligament of the knee by magnetic resonance imaging in acute injuries of the anterior cruciate ligament. Arthroscopy. 2017;33(1):140–6. https://doi.org/10.1016/j.arthro.2016.05.009.
Cavaignac E, Faruch M, Wytrykowski K, Constant O, Murgier J, Berard E, et al. Ultrasonographic evaluation of anterolateral ligament injuries: correlation with magnetic resonance imaging and pivot-shift testing. Arthroscopy. 2017;33(7):1384–90. https://doi.org/10.1016/j.arthro.2017.01.040.
Helito CP, Helito PV, Costa HP, Bordalo-Rodrigues M, Pecora JR, Camanho GL et al. MRI evaluation of the anterolateral ligament of the knee: assessment in routine 1.5-T scans. Skeletal Radiol. 2014;43(10):1421–7. https://doi.org/10.1007/s00256-014-1966-7.
Devitt BM, O’Sullivan R, Feller JA, Lash N, Porter TJ, Webster KE, et al. MRI is not reliable in diagnosing of concomitant anterolateral ligament and anterior cruciate ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1345–51. https://doi.org/10.1007/s00167-017-4538-2.
Porrino J Jr, Maloney E, Richardson M, Mulcahy H, Ha A, Chew FS. The anterolateral ligament of the knee: MRI appearance, association with the Segond fracture, and historical perspective. AJR Am J Roentgenol. 2015;204(2):367–73. https://doi.org/10.2214/AJR.14.12693.
Muramatsu K, Saithna A, Watanabe H, Sasaki K, Yokosawa K, Hachiya Y, et al. Three-dimensional magnetic resonance imaging of the anterolateral ligament of the knee: an evaluation of intact and anterior cruciate ligament-deficient knees from the scientific anterior cruciate ligament network international (SANTI) study group. Arthroscopy. 2018;34(7):2207–17. https://doi.org/10.1016/j.arthro.2018.02.014.
Cavaignac E, Wytrykowski K, Reina N, Pailhe R, Murgier J, Faruch M, et al. Ultrasonographic identification of the anterolateral ligament of the knee. Arthroscopy. 2016;32(1):120–6. https://doi.org/10.1016/j.arthro.2015.07.015.
Yoshida M, Herbst E, Albers M, Musahl V, Fu FH, Onishi K. The anterolateral complex in anterior cruciate ligament deficient knees demonstrate sonographic abnormalities on high-resolution sonography. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1024–9. https://doi.org/10.1007/s00167-017-4512-z.
Van Dyck P, Clockaerts S, Vanhoenacker FM, Lambrecht V, Wouters K, De Smet E, et al. Anterolateral ligament abnormalities in patients with acute anterior cruciate ligament rupture are associated with lateral meniscal and osseous injuries. Eur Radiol. 2016;26(10):3383–91. https://doi.org/10.1007/s00330-015-4171-8.
Claes S, Bartholomeeusen S, Bellemans J. High prevalence of anterolateral ligament abnormalities in magnetic resonance images of anterior cruciate ligament-injured knees. Acta Orthop Belg. 2014;80(1):45–9.
Wang Y, Li S, Xu D, Qian L, Jiang C, Fu M, et al. Strain distribution of the anterolateral ligament during internal rotation at different knee flexion angles: a biomechanical study on human cadavers. Knee. 2019. https://doi.org/10.1016/j.knee.2019.01.001.
Pula DA. Editorial commentary: the anterolateral ligament really exists, now show me how to find it. Arthroscopy. 2019;35(2):528–9. https://doi.org/10.1016/j.arthro.2018.11.004.
DePhillipo NN, Cinque ME, Chahla J, Geeslin AG, LaPrade RF. Anterolateral ligament reconstruction techniques, biomechanics, and clinical outcomes: a systematic review. Arthroscopy. 2017;33(8):1575–83. https://doi.org/10.1016/j.arthro.2017.03.009.
Sonnery-Cottet B, Barbosa NC, Tuteja S, Daggett M, Kajetanek C, Thaunat M. Minimally invasive anterolateral ligament reconstruction in the setting of anterior cruciate ligament injury. Arthrosc Tech. 2016;5(1):e211–5. https://doi.org/10.1016/j.eats.2015.11.005.
Inderhaug E, Stephen JM, Williams A, Amis AA. Anterolateral tenodesis or anterolateral ligament complex reconstruction: effect of flexion angle at graft fixation when combined with ACL reconstruction. Am J Sports Med. 2017;45(13):3089–97. https://doi.org/10.1177/0363546517724422.
Schon JM, Moatshe G, Brady AW, Serra Cruz R, Chahla J, Dornan GJ, et al. Anatomic anterolateral ligament reconstruction of the knee leads to overconstraint at any fixation angle. Am J Sports Med. 2016;44(10):2546–56. https://doi.org/10.1177/0363546516652607.
Nielsen ET, Stentz-Olesen K, de Raedt S, Jorgensen PB, Sorensen OG, Kaptein B, et al. Influence of the anterolateral ligament on knee laxity: a biomechanical cadaveric study measuring knee kinematics in 6 degrees of freedom using dynamic radiostereometric analysis. Orthop J Sports Med. 2018;6(8):2325967118789699. https://doi.org/10.1177/2325967118789699.
Geeslin AG, Moatshe G, Chahla J, Kruckeberg BM, Muckenhirn KJ, Dornan GJ, et al. Anterolateral knee extra-articular stabilizers: a robotic study comparing anterolateral ligament reconstruction and modified lemaire lateral extra-articular tenodesis. Am J Sports Med. 2018;46(3):607–16. https://doi.org/10.1177/0363546517745268.
Noyes FR, Huser LE, Jurgensmeier D, Walsh J, Levy MS. Is an anterolateral ligament reconstruction required in ACL-reconstructed knees with associated injury to the anterolateral structures? A robotic analysis of rotational knee stability. Am J Sports Med. 2017;45(5):1018–27. https://doi.org/10.1177/0363546516682233.
Herbst E, Arilla FV, Guenther D, Yacuzzi C, Rahnemai-Azar AA, Fu FH, et al. Lateral extra-articular tenodesis has no effect in knees with isolated anterior cruciate ligament injury. Arthroscopy. 2018;34(1):251–60. https://doi.org/10.1016/j.arthro.2017.08.258.
Spencer L, Burkhart TA, Tran MN, Rezansoff AJ, Deo S, Caterine S, et al. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43(9):2189–97. https://doi.org/10.1177/0363546515589166.
Sonnery-Cottet B, Daggett M, Helito CP, Cavalier M, Choudja E, Vieira TD et al. Anatomic anterolateral ligament reconstruction leads to overconstraint at any fixation angle: letter to the editor. Am J Sports Med. 2016;44(10):NP57-NP8. https://doi.org/10.1177/0363546516669313.
Ardern CL, Sonesson S, Forssblad M, Kvist J. Comparison of patient-reported outcomes among those who chose ACL reconstruction or non-surgical treatment. Scand J Med Sci Sports. 2017;27(5):535–44. https://doi.org/10.1111/sms.12707.
Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(11):2827–32. https://doi.org/10.1177/0363546516651845.
Roessler PP, Schuttler KF, Heyse TJ, Wirtz DC, Efe T. The anterolateral ligament (ALL) and its role in rotational extra-articular stability of the knee joint: a review of anatomy and surgical concepts. Arch Orthop Trauma Surg. 2016;136(3):305–13. https://doi.org/10.1007/s00402-015-2395-3.
Camilo P. Helito MFS, Pedro N. Giglio, Marcelo B. Bonadio, José R. Pécora, Marco K. Demange, Gilberto L. Camanho, Riccardo G. Gobbi. Combined reconstruction of the anterolateral ligament in patients with hypermobility and knee hyperextension with ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. ISAKOS Biennial Congress ePoster. 2019.
Helito CP, Camargo DB, Sobrado MF, Bonadio MB, Giglio PN, Pecora JR, et al. Combined reconstruction of the anterolateral ligament in chronic ACL injuries leads to better clinical outcomes than isolated ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018. https://doi.org/10.1007/s00167-018-4934-2.
Lee DW, Kim JG, Cho SI, Kim DH. Clinical outcomes of isolated revision anterior cruciate ligament reconstruction or in combination with anatomic anterolateral ligament reconstruction. Am J Sports Med. 2019;47(2):324–33. https://doi.org/10.1177/0363546518815888.
Rosenstiel N, Praz C, Ouanezar H, Saithna A, Fournier Y, Hager JP, et al. Combined anterior cruciate and anterolateral ligament reconstruction in the professional athlete: clinical outcomes from the scientific anterior cruciate ligament network international study group in a series of 70 patients with a minimum follow-up of 2 years. Arthroscopy. 2019;35(3):885–92. https://doi.org/10.1016/j.arthro.2018.09.020.
Claes S, Hermie L, Verdonk R, Bellemans J, Verdonk P. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):1967–76. https://doi.org/10.1007/s00167-012-2251-8.
Shelbourne KD, Benner RW, Gray T. Results of anterior cruciate ligament reconstruction with patellar tendon autografts: objective factors associated with the development of osteoarthritis at 20 to 33 years after surgery. Am J Sports Med. 2017:363546517718827. https://doi.org/10.1177/0363546517718827.
Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014;48(21):1543–52. https://doi.org/10.1136/bjsports-2013-093398.
Kartus J, Magnusson L, Stener S, Brandsson S, Eriksson BI, Karlsson J. Complications following arthroscopic anterior cruciate ligament reconstruction. A 2–5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 1999;7(1):2–8. https://doi.org/10.1007/s001670050112.
Hettrich CM, Dunn WR, Reinke EK, Group M, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534-40. https://doi.org/10.1177/0363546513490277.
O’Brien SJ, Warren RF, Pavlov H, Panariello R, Wickiewicz TL. Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg Am. 1991;73(2):278–86.
Strum GM, Fox JM, Ferkel RD, Dorey FH, Del Pizzo W, Friedman MJ, et al. Intraarticular versus intraarticular and extraarticular reconstruction for chronic anterior cruciate ligament instability. Clin Orthop Relat Res. 1989;245:188–98.
Ibrahim SA, Shohdy EM, Marwan Y, Ramadan SA, Almisfer AK, Mohammad MW, et al. Anatomic reconstruction of the anterior cruciate ligament of the knee with or without reconstruction of the anterolateral ligament: a randomized clinical trial. Am J Sports Med. 2017;45(7):1558–66. https://doi.org/10.1177/0363546517691517.
Ferretti A, Monaco E, Ponzo A, Basiglini L, Iorio R, Caperna L, et al. Combined intra-articular and extra-articular reconstruction in anterior cruciate ligament-deficient knee: 25 years later. Arthroscopy. 2016;32(10):2039–47. https://doi.org/10.1016/j.arthro.2016.02.006.
Rossi MJ. Editorial commentary: anterolateral ligament augmentation for the anterior cruciate ligament-deficient knee debate-the proof is in the pudding. Arthroscopy. 2019;35(3):893–5. https://doi.org/10.1016/j.arthro.2018.12.018.
Getgood A BD, Litchfield B, Stability Study Group. Multicenter randomized clinical trial comparing anterior cruciate ligament reconstruction with and without lateral extra-articular tenodesis in individuals who are at high risk of graft failure. NCT02018354. https://clinicaltrials.gov/ct2/show/NCT02018354?term=NCT02018354&rank=1. Accessed 3 Aug 2018.
Sonnery-Cottet B, Pioger C, Vieira TD, Saithna A, Franck F, Fayard J-M, Kajetanek C, Thaunat M. A randomised controlled trial of ACL reconstruction with or without anterolateral ligament reconstruction: preliminary results from the SANTI study. NCT03740022. https://clinicaltrials.gov/ct2/show/NCT03740022. Accessed 14 Nov 2018.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Delaloye, JR., Murar, J., Pioger, C., Franck, F., Vieira, T.D., Sonnery-Cottet, B. (2021). The Role of Anterolateral Ligament Reconstruction in Anterior Instability. In: Kim, J.G. (eds) Knee Arthroscopy. Springer, Singapore. https://doi.org/10.1007/978-981-15-8191-5_10
Download citation
DOI: https://doi.org/10.1007/978-981-15-8191-5_10
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-8190-8
Online ISBN: 978-981-15-8191-5
eBook Packages: MedicineMedicine (R0)