Abstract
Background
Injury to the anterolateral ligament (ALL) has been reported to contribute to high-grade anterolateral laxity after anterior cruciate ligament (ACL) injury. Failure to address ALL injury has been suggested as a cause of persistent rotational laxity after ACL reconstruction. Lateral meniscus posterior root (LMPR) tears have also been shown to cause increased internal rotation of the knee.
Questions/purposes
The purpose of this study was to determine the functional relationship between the ALL and LMPR in the control of internal rotation of the ACL-deficient knee. Specifically: (1) We asked if there was a difference in internal rotation among: the intact knee; the ACL-deficient knee; the ACL/ALL-deficient knee; the ACL/LMPR-deficient knee; and the ACL/ALL/LMPR-deficient knee. (2) We also asked if there was a difference in anterior translation among these conditions.
Methods
Sixteen fresh frozen cadaveric knee specimens (eight men, mean age 79 years) were potted into a hip simulator (femur) and a 6 degree-of-freedom load cell (tibia). Rigid optical trackers were inserted into the proximal femur and distal tibia, allowing for the motion of the tibia with respect to the femur to be tracked during biomechanical tests. A series of points on the femur and tibia were digitized to create bone coordinate systems that were used to calculate internal rotation and anterior translation. Biomechanical testing involved applying a 5-Nm internal rotation moment to the tibia from full extension to 90° of flexion. Anterior translation was performed by applying a 90-N anterior load using a tensiometer. Both tests were performed in 15° increments tested sequentially in the following conditions: (1) intact; and (2) ACL injury (ACL−). The specimens were then randomized to either have the ALL sectioned (3) first (M+/ALL−); or (4) the LMPR sectioned first (M−/ALL+) followed by the other structure (M−/ALL−). A one-way analysis of variance was performed for each sectioning condition at each angle of knee flexion (α = 0.05).
Results
At 0° of flexion there was an effect of tissue sectioning such that internal rotation of the M−/ALL− condition was greater than ACL− by 1.24° (p = 0.03; 95% confidence interval [CI], 0.16–2.70) and the intact condition by 2.5° (p = 0.01; 95% CI, 0.69–3.91). In addition, the mean (SD) internal rotations for the M+/ALL− (9.99° [5.39°]) and M−/ALL+ (12.05° [5.34°]) were greater by 0.87° (p = 0.04; 95% CI, 0.13–3.83) and by 2.15°, respectively, compared with the intact knee. At 45° the internal rotation for the ACL− (19.15° [9.49°]), M+/ALL− (23.70° [7.00°]), and M−/ALL− (18.80° [8.27°]) conditions was different than the intact (12.78° [9.23°]) condition by 6.37° (p = 0.02; 95% CI, 1.37–11.41), 8.47° (p < 0.01; 95% CI, 3.94–13.00), and 6.02° (p = 0.01; 95% CI, 1.73–10.31), respectively. At 75° there was a 10.11° difference (p < 0.01; 95% CI, 5.20–15.01) in internal rotation between the intact (13.96° [5.34°]) and the M+/ALL− (23.22° [4.46°]) conditions. There was also a 4.08° difference (p = 0.01; 95% CI, 1.14–7.01) between the intact and M−/ALL− (18.05° [7.31°]) conditions. Internal rotation differences of 6.17° and 5.43° were observed between ACL− (16.28° [6.44°]) and M+/ALL− (p < 0.01; 95% CI, 2.45–9.89) as well as between M+/ALL− and M−/ALL− (p = 0.01; 95% CI, −8.17 to −1.63). Throughout the range of flexion, there was no difference in anterior translation with progressive section of the ACL, meniscus, or ALL.
Conclusions
The ALL and LMPR both play a role in aiding the ACL in controlling internal rotation laxity in vitro; however, these effects seem to be dependent on flexion angle. The ALL has a greater role in controlling internal rotation at flexion angles > 30o. The LMPR appears to have more of an effect on controlling rotation closer to extension.
Clinical Relevance
Injury to the ALL and/or LMPR may contribute to high-grade anterolateral laxity after ACL injury. The LMPR and the ALL, along with the iliotibial tract, appear to act in concert as secondary stabilizers of anterolateral rotation and could be considered as the “anterolateral corner” of the knee.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) injury results in both translational and rotational laxity. It is well understood that ACL reconstruction may fail to fully restore rotational stability to the knee [21, 35, 40] and that residual rotational laxity is associated with poor patient-reported outcome scores [20, 21]. Recent interest in the anterolateral ligament (ALL) has refocused attention on the secondary restraints to internal rotation and the potential contribution that injury to these structures may make to residual instability. In addition to the ACL, the ALL [31], iliotibial band [11, 17], lateral meniscus [27], and medial meniscotibial ligament [32] may all act as secondary restraints to internal rotation at the knee.
Debate continues regarding the anatomy and biomechanical function of the anterolateral structures of the knee [29]. Some authors have described the ALL as a distinct ligamentous structure [3, 4, 6, 18, 43], whereas others have reported only a capsular thickening [7]. Similarly, some cadaveric biomechanical studies demonstrate an increase in anterolateral rotation after sectioning of the ALL in the ACL-deficient knee [39], whereas others report little effect [36]. The clinical relevance of this structure has yet to be fully determined.
The lateral meniscus posterior root (LMPR) has also been shown to contribute to rotational laxity after an injury to the ACL [37]. Tears to the LMPR occur in approximately 7% to 12% of patients with ACL injury [9]. In a cadaveric study, Shybut et al. [37] demonstrated lateral compartment anterior translation of 8.1 mm during a simulated pivot shift after division of both the LMPR and the ACL compared with 6.0 mm after ACL sectioning alone. Although the role of the LMPR in aiding the control of anterolateral laxity seems evident after an ACL injury, less is known regarding the functional relationship between the LMPR and the ALL. Anatomic studies of the ALL have demonstrated a firm attachment of the ALL to the lateral meniscus [3, 4, 15, 18, 23, 43], the importance of which remains unknown, but which may indicate a functional interdependence of these two structures in the restraint of internal rotation.
The purpose of this study was to determine the functional relationship between the ALL and LMPR in the control of internal rotation throughout the range of flexion. Our hypothesis was that division of these structures would have a similar effect on increasing internal rotation in the ACL-deficient knee. Specifically: (1) We asked if there was a difference in internal rotation among: the intact knee; the ACL-deficient knee; the ACL/ALL-deficient knee; the ACL/LMPR-deficient knee; and the ACL/ALL/LMPR-deficient knee. (2) We also asked if there was a difference in anterior translation among these conditions.
Materials and Methods
A cadaveric study using an optical navigation system was undertaken. To answer our question regarding internal rotation, knees were tested under an internal rotational torque at various degrees of knee flexion with sequential sectioning of the structures under investigation. To answer our question regarding anterior translation, a simulated Lachman test was performed for each sectioning state.
Experimental Setup
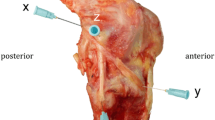
Sixteen fresh frozen intact (midfemur to midtibia) cadaveric specimens (eight men; mean [SD] age 79 [11] years) were thawed 24 hours before testing. Ethical review board approval was not necessary to conduct the investigation, because deidentified cadaveric specimens are exempt from review at our institution. The specimens had 5 cm of soft tissue removed from the proximal femur and distal tibia to allow potting of the specimens into sections of polyvinyl chloride through dental cement (Denstone® dental cement; Hereaus Holdings GmbH, Hanau, Germany). The femoral end was inserted into a custom-designed hip simulator rigidly mounted to a surgical table [39] while the tibial end was rigidly attached to a 6 degree-of-freedom load cell (MM3A-500; Advanced Mechanical Technology Inc, Watertown, MA, USA) with a measurement accuracy of 5 N and 0.14 Nm (Fig. 1).
Two rigid optical tracking smart marker clusters (Optotrak Certus; Northern Digital Inc, Waterloo, Ontario, Canada) were inserted into the proximal tibia and distal femur using orthopaedic bone pins (Fig. 1). These markers allowed the tracking of tibial motion with respect to the femur during testing with an accuracy of 0.1 mm. A series of anatomic landmarks on the femur and tibia were digitized after testing allowing the creation of bone-specific coordinate systems. Three-dimensional knee kinematics were calculated using the joint coordinate system method as described by Grood and Suntay [12, 39] (Fig. 1).
Sectioning Protocol
Once the specimens were attached to the simulator, they were all tested under the following conditions: (1) intact knee (intact)—all soft tissues surrounding the knee remained intact; and (2) complete sectioning of the ACL (ACL−)—the ACL was sectioned through an anteromedial arthrotomy. The specimens were then randomly assigned to have either the inframeniscal portion of the ALL sectioned first, leaving the LMPR intact (M+/ALL−), or the LMPR sectioned first leaving the ALL intact (M−/ALL+). The ALL was sectioned from the lateral collateral ligament to Gerdy’s tubercle through an oblique anterolateral incision with anterior retraction of the iliotibial band. This sectioning technique would divide the anterolateral capsule as well as all described versions of the ALL, consistent with all previously published sectioning studies investigating the function of the ALL. The LMPR was sectioned through the previously created arthrotomy. Finally, in all specimens, the remaining tissue (either the ALL or LMPR) was sectioned (M−/ALL−). For each condition, a 5-Nm internal rotation moment was applied manually through the load cell with each motion occurring three times. Testing was performed between 0° and 90° of knee flexion in 15° increments. Both internal rotation and secondary anterior translation under the internal rotational torque were measured.
Data Analysis and Statistics
The magnitude of each motion (degrees) that corresponded to the 5-Nm internal rotation load target was determined and the mean value across each of the three trials was used for all combinations of conditions and knee angles. To determine the effect of the intact, ACL−, and M−/ALL− conditions, a one-way analysis of variance (ANOVA) (three sections) was conducted. To determine the effect of M−/ALL+ and M+/ALL−, separate one-way ANOVAs were conducted within each randomized data set. Finally, an independent t-test was used to determine whether the internal rotations were significantly different between M−/ALCC+ and M+/ALL−. Post hoc testing was performed with a Bonferroni adjustment and effect sizes were calculated as partial eta squared (η2) and interpreted as small (0.01), medium (0.06), or large (0.14) [24]. All statistical analyses were performed separately at each knee angle and were performed with SPSS statistical software (Version 21; IBM Corp, Armonk, NY, USA) with α set at 0.05.
Results
Internal Rotation
At 0° of flexion (ie, full extension), there were differences in the internal rotation associated with the progressive sectioning of the ACL, LMPR, and the ALL (p = 0.02; η2 = 0.15; power = 0.75). Specifically, there was a difference in the mean (SD) internal rotation for M−/ALL− (11.42° [4.77°]) compared with ACL− (9.90° [4.67°]) and the intact conditions by 1.53° (p = 0.03; 95% confidence interval [CI], 0.16–2.70) and 2.30° (p = 0.01; 95% CI, 0.69–3.91), respectively (Fig. 2A). In addition, the mean (SD) internal rotations for the M+/ALL− (9.99° [5.39°]) and M−/ALL+ (12.05° [5.34°]) were greater by 0.87° (p = 0.04; 95% CI, 0.13–3.83) and 2.93° (p = 0.05; 95% CI, 0.24–4.61), respectively, compared with the intact knee (Fig. 2A).
The mean (SD) internal rotation is shown after each sectioning condition at (A) 0°, (B) 15°, (C) 30°, (D) 45°, (E) 60°, (F) 75°, and (G) 90° of flexion (*p < 0.05). ACL− = sectioned ACL; M−/ALL+ = sectioned ACL, sectioned meniscus, intact ALL; M+/ALL− = sectioned ACL, intact meniscus, sectioned ALL; M−/ALL− = sectioned ACL, sectioned meniscus, sectioned ALL.
At 15° and 30° of knee flexion, there were no differences in internal rotation when the ACL, LMPR, or ALL was sectioned (Fig. 2B–C).
At 45° the mean (SD) internal rotation for the ACL− (19.15° [9.49°]), M+/ALL− (23.70° [7.00°]), and M−/ALL− (18.80° [8.27°]) conditions was different than the intact (12.78° [9.23°]) condition by 6.37° (p = 0.02; 95% CI, 1.37–11.41), 10.92° (p < 0.01; 95% CI, 3.94–3.00), and 6.02° (p = 0.01; 95% CI, 1.73–10.31), respectively (Fig. 2D).
At 60° of knee flexion, there were no differences in internal rotation when the ACL, LMPR, or ALL was sectioned (Fig. 2E).
At 75° there was a 9.26° difference (p < 0.01; 95% CI, 5.20–15.01) in the mean (SD) internal rotation between the intact (13.96° [5.34°]) and the M+/ALL− (23.22° [4.46°]). There was also a 4.09° difference (p = 0.01; 95% CI, 1.14–7.01) between the intact and M−/ALL− (18.05° [7.31°]) conditions. Furthermore, an internal rotation difference of 6.94° (p < 0.01; 95% CI, 2.45–9.89) was observed between ACL− (16.28° [6.44°]) and M+/ALL− in addition to a 5.17° internal rotation difference between M+/ALL− and M−/ALL− (p = 0.01; 95% CI, 1.63–8.64) (Fig. 2F).
Finally, at 90° of knee flexion, there were no differences in internal rotation when the ACL, LMPR, or ALL was sectioned (Fig. 2G).
Anterior Translation
Throughout the range of flexion, there was no difference in anterior translation with progressive sectioning of the ACL, meniscus, or ALL (Fig. 3).
Discussion
Both the anterolateral capsular complex and the lateral meniscus are secondary stabilizers to internal rotation at the knee. Anatomic studies report an insertion of the anterolateral structures onto the lateral meniscus [3, 15], the functional implication of which is unknown. In our study, we confirmed that both the LMPR and ALL contributed as secondary stabilizers to internal rotation, but not to anterior translation, in the ACL-deficient knee. Their contribution to rotational control was dependent on knee flexion angle with the meniscal root of greater importance in extension and the ALL in deeper flexion (45° and 75°).
The limitations of the study include the use of elderly cadavers that may not be representative of the normal ACL-injured population. Despite this, all specimens achieved full extension and had an intact ACL at arthrotomy and were considered acceptable for this in vitro study. Although the distal femoral attachment of the iliotibial tract (Kaplan’s fibers) was intact, the proximal attachment of the tract was not and this may have impacted the results. Although a hip simulator that requires manually applied forces with optical tracking to assess knee kinematics was used in this study, this method has been previously published and the measurement variability was comparable to studies that have used a 6 degree-of-freedom robot [39].
Biomechanical studies of the role of ALL and anterolateral structures have produced conflicting results with some describing increased internal rotation with division of these structures [26, 34, 39], whereas others report no difference [19, 36]. This may reflect ongoing confusion regarding the definition of the structures being investigated as well as the role of other secondary stabilizers such as the iliotibial band. In particular, Parsons et al. [31], using a 6 degree-of-freedom robot and a force subtraction model, found the ALL to be the primary restraint to internal rotation at knee flexion angles > 35°, with the ACL providing the greatest restraint closer to extension. The iliotibial band was removed in this experiment. Zens et al. [46] found the ALL to increase in length with greater degrees of knee flexion, also suggesting a greater role in deeper flexion. These results would support our findings regarding the role of the anterolateral capsular structures and their importance in deeper flexion and question the role of anatomic ALL reconstruction in controlling the pivot shift phenomenon, which occurs at lower knee flexion angles.
Lateral meniscal root injuries have been reported in up to 12% of ACL-injured knees [44]. The importance of these injuries to knee stability has recently become apparent. Musahl et al. [27] reported an increase in lateral compartment translation during the pivot shift of 6 mm after lateral meniscectomy in the ACL-deficient knee. In a cadaveric biomechanical study specifically examining the role of the lateral meniscal posterior root during a robotic pivot shift, Shybut et al. [37] demonstrated an increase in lateral compartment translation of 2.1° in extension after division of the root compared with the ACL-deficient knee. This was in comparison to an increase of 3.4° for the ACL-deficient knee compared with the intact state. The difference between the root-deficient and ACL-deficient knee reduced with increasing knee flexion and was negligible after 35°.
It is not clear why the meniscus should be important only near extension; however, it may relate to the mobility of the meniscus [42], the changing shape of the femoral condyle, and rollback of the tibiofemoral contact point that occurs with knee flexion [16]. Furthermore, because the lateral meniscus has been shown to reduce the bony tibial slope toward the horizontal [8], loss of the meniscal root may increase the functional slope and create a rotational moment under compressive load [38].
Injuries to the meniscal roots have been shown to have biomechanical consequences similar to total meniscectomy [1, 25] and may lead to meniscal extrusion and progressive degenerative change [2, 14, 22]. Because this effect may be mitigated in the lateral compartment by the presence of intact meniscofemoral ligaments [2, 10, 33], the clinical benefit of root repair is less clear. Further clinical research is needed to determine whether meniscal root repair at the time of ACL reconstruction will be beneficial for both knee stability and chondroprotection.
Studies investigating the relationship between the ALL and the lateral meniscus are scarce. A case series of 90 knees with an ACL injury shown on MRI noted that 41% had an abnormality of the ALL with 61% of these having a concomitant lateral meniscus tear [41]. In contrast, of those knees with an intact ALL, only 31% were observed to have a lateral meniscus tear. A recent study by Musahl et al. [28] demonstrated that injury to the anterolateral structures, the lateral or medial meniscus, resulted in increased rotational laxity. These studies support the concept of a functional relationship between the lateral meniscus and the anterolateral structures. Furthermore, although sectioning studies generally show an additive effect of the sequential division of structures that act as restraints to the same movement [13, 45], subsequent sectioning of the second structure in the current investigation was not observed to have an additive effect (ie, sectioning the ALL after the LMPR, or the LMPR after the ALL, did not further increase internal rotation). This further supports a functional link between the two structures.
Sectioning the structures about the knee may alter the center of rotation with division of the ACL known to move the center of rotation medially [5]. This may impact rotational measurement, because a similar lateral compartment anterior translation will register less internal rotation but more anterior translation under an internal rotational torque as the center of rotation moves medially [30]. Whereas secondary anterior translation did not reach significance in our study, the increases measured may explain the paradoxic decrease in internal rotation seen in some sectioning conditions.
Injury to the secondary stabilizers of the knee likely contributes to the spectrum of instability observed after ACL injury and may be responsible for residual rotatory instability after routine intraarticular ACL reconstruction. The ALL and LMPR appear to act in concert, playing complimentary roles as secondary stabilizers to internal rotation at different stages of the knee flexion cycle. These structures, together with the iliotibial band, could be considered to constitute the “anterolateral corner” of the knee. Further research is required to determine under what circumstances, and using what techniques, surgical intervention is warranted for injury to these structures. In particular, the appropriate indications for extraarticular tenodesis or anatomic ALL reconstruction and their efficacy in controlling pathologic internal rotation require further delineation as does the in vivo impact of lateral meniscal root repair on clinical instability and chondroprotection.
Change history
02 August 2017
An erratum to this article has been published.
References
Allaire R, Muriuki M, Gilbertson L, Harner CD. Biomechanical consequences of a tear of the posterior root of the medial meniscus. Similar to total meniscectomy. J Bone Joint Surg Am. 2008;90:1922–1931.
Bao HRC, Zhu D, Gong H, Gu GS. The effect of complete radial lateral meniscus posterior root tear on the knee contact mechanics: a finite element analysis. J Orthop Sci. 2012;18:256–263.
Caterine S, Litchfield R, Johnson M, Chronik B, Getgood A. A cadaveric study of the anterolateral ligament: re-introducing the lateral capsular ligament. Knee Surg Sports Traumatol Arthrosc. 2015;23:3186–3195.
Claes S, Vereecke E, Maes M, Victor J, Verdonk P, Bellemans J. Anatomy of the anterolateral ligament of the knee. J Anat. 2013;223:321–328.
Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech. 2005;38:241–253.
Dodds AL, Halewood C, Gupte CM, Williams A, Amis AA. The anterolateral ligament: anatomy, length changes and association with the Segond fracture. Bone Joint J. 2014;96:325–331.
Dombrowski ME, Costello JM, Ohashi B, Murawski CD, Rothrauff BB, Arilla FV, Friel NA, Fu FH, Debski RE, Musahl V. Macroscopic anatomical, histological and magnetic resonance imaging correlation of the lateral capsule of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:2854–2860.
Elmansori A, Lording T, Dumas R, Elmajri K, Neyret P, Lustig S. Proximal tibial bony and meniscal slopes are higher in ACL injured subjects than controls: a comparative MRI study. Knee Surg Sports Traumatol Arthrosc. 2017 Feb 17. [Epub ahead of print]
Feucht MJ, Salzmann GM, Bode G, Pestka JM, Kühle J, Südkamp NP, Niemeyer P. Posterior root tears of the lateral meniscus. Knee Surg Sports Traumatol Arthrosc. 2014;23:119–125.
Forkel P, Herbort M, Schulze M, Rosenbaum D, Kirstein L, Raschke M, Petersen W. Biomechanical consequences of a posterior root tear of the lateral meniscus: stabilizing effect of the meniscofemoral ligament. Arch Orthop Trauma Surg. 2013;133:621–626.
Gadikota HR, Kikuta S, Qi W, Nolan D, Gill TJ, Li G. Effect of increased iliotibial band load on tibiofemoral kinematics and force distributions: a direct measurement in cadaveric knees. J Orthop Sports Phys Ther. 2013;43:478–485.
Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144.
Haimes JL, Wroble RR, Grood ES, Noyes FR. Role of the medial structures in the intact and anterior cruciate ligament-deficient knee. Limits of motion in the human knee. Am J Sports Med. 1994;22:402–409.
Hein CN, Deperio JG, Ehrensberger MT, Marzo JM. Effects of medial meniscal posterior horn avulsion and repair on meniscal displacement. Knee. 2011;18:189–192.
Helito CP, Demange MK, Bonadio MB. Anatomy and histology of the knee anterolateral ligament. Orthop J Sports Med. 2013;1:2325967113513546.
Iwaki H, Pinskerova V, Freeman MA. Tibiofemoral movement 1: the shapes and relative movements of the femur and tibia in the unloaded cadaver knee. J Bone Joint Surg Br. 2000;82:1189–1195.
Jakob RP, Hassler H, Staeubli HU. Observations on rotatory instability of the lateral compartment of the knee. Experimental studies on the functional anatomy and the pathomechanism of the true and the reversed pivot shift sign. Acta Orthop Scand Suppl. 1981;191:1–32.
Kennedy MI, Claes S, Fuso FAF, Williams BT, Goldsmith MT, Turnbull TL, Wijdicks CA, LaPrade RF. The anterolateral ligament: an anatomic, radiographic, and biomechanical analysis. Am J Sports Med. 2015;43:1606–1615.
Kittl C, Daou El H, Athwal KK, Gupte CM, Weiler A, Williams A, Amis AA. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee. Am J Sports Med. 2016;44:345–354.
Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:629–634.
Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84:1560–1572.
Lerer DB, Umans HR, Hu MX, Jones MH. The role of meniscal root pathology and radial meniscal tear in medial meniscal extrusion. Skeletal Radiol. 2004;33:569–574.
Macchi V, Porzionato A, Morra A, Stecco C, Tortorella C, Menegolo M, Grignon B, De Caro R. The anterolateral ligament of the knee: a radiologic and histotopographic study. Surg Radiol Anat. 2016;38:341–348.
Maher JM, Markey JC, Ebert-May D. The other half of the story: effect size analysis in quantitative research. Cell Biol Educ. 2013;12:345–351.
Marzo JM, Gurske-DePerio J. Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med. 2008;37:124–129.
Monaco E, Ferretti A, Labianca L, Maestri B, Speranza A, Kelly MJ, D’Arrigo C. Navigated knee kinematics after cutting of the ACL and its secondary restraint. Knee Surg Sports Traumatol Arthrosc. 2011;20:870–877.
Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38:1591–1597.
Musahl V, Rahnemai-Azar AA, Costello J, Arner JW, Fu FH, Hoshino Y, Lopomo N, Samuelsson K, Irrgang JJ. The influence of meniscal and anterolateral capsular injury on knee laxity in patients with anterior cruciate ligament injuries. Am J Sports Med. 2016;44:3126–3131.
Musahl V, Rahnemai-Azar AA, van Eck CF, Guenther D, Fu FH. Anterolateral ligament of the knee, fact or fiction? Knee Surg Sports Traumatol Arthrosc. 2015;24:2–3.
Noyes FR, Jetter AW, Grood ES, Harms SP, Gardner EJ, Levy MS. Anterior cruciate ligament function in providing rotational stability assessed by medial and lateral tibiofemoral compartment translations and subluxations. Am J Sports Med. 2015;43:683–692.
Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43:669–674.
Peltier A, Lording T, Maubisson L, Ballis R, Neyret P, Lustig S. The role of the meniscotibial ligament in posteromedial rotational knee stability. Knee Surg Sports Traumatol Arthrosc. 2015;23:2967–2973.
Perez-Blanca A, Espejo-Baena A, Amat Trujillo D, Prado Nóvoa M, Espejo-Reina A, Quintero López C, Ezquerro Juanco F. Comparative biomechanical study on contact alterations after lateral meniscus posterior root avulsion, transosseous reinsertion, and total meniscectomy. Arthroscopy. 2016;32:624–633.
Rasmussen MT, Nitri M, Williams BT, Moulton SG, Cruz RS, Dornan GJ, Goldsmith MT, LaPrade RF. An in vitro robotic assessment of the anterolateral ligament, part 1: secondary role of the anterolateral ligament in the setting of an anterior cruciate ligament injury. Am J Sports Med. 2016;44:585–592.
Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:1323–1329.
Saiegh YA, Suero EM, Guenther D, Hawi N, Decker S, Krettek C, Citak M, Omar M. Sectioning the anterolateral ligament did not increase tibiofemoral translation or rotation in an ACL-deficient cadaveric model. Knee Surg Sports Traumatol Arthrosc. 2015;21:257.
Shybut TB, Vega CE, Haddad J, Alexander JW, Gold JE, Noble PC, Lowe WR. Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43:905–911.
Simon RA, Everhart JS, Nagaraja HN, Chaudhari AM. A case-control study of anterior cruciate ligament volume, tibial plateau slopes and intercondylar notch dimensions in ACL-injured knees. J Biomech. 2010;43:1702–1707.
Spencer L, Burkhart TA, Tran MN, Rezansoff AJ, Deo S, Caterine S, Getgood AM. Biomechanical analysis of simulated clinical testing and reconstruction of the anterolateral ligament of the knee. Am J Sports Med. 2015;43:2189–2197.
Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975–983.
Van Dyck P, Clockaerts S, Vanhoenacker FM, Lambrecht V, Wouters K, De Smet E, Gielen JL, Parizel PM. Anterolateral ligament abnormalities in patients with acute anterior cruciate ligament rupture are associated with lateral meniscal and osseous injuries. Eur Radiol. 2016;26:3383–3391.
Vedi V, Williams A, Tennant SJ, Spouse E, Hunt DM, Gedroyc WM. Meniscal movement. An in-vivo study using dynamic MRI. J Bone Joint Surg Br. 1999;81:37–41.
Vincent J-P, Magnussen RA, Gezmez F, Uguen A, Jacobi M, Weppe F, Al-Saati MF, Lustig S, Demey G, Servien E, Neyret P. The anterolateral ligament of the human knee: an anatomic and histologic study. Knee Surg Sports Traumatol Arthrosc. 2011;20:147–152.
West RV, Kim JG, Armfield D, Harner CD. Lateral meniscal root tears associated with anterior cruciate ligament injury: classification and management (SS-70). Arthroscopy. 2004;20:e32–e33.
Wroble RR, Grood ES, Cummings JS, Henderson JM, Noyes FR. The role of the lateral extraarticular restraints in the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21:257–263.
Zens M, Niemeyer P, Ruhhammer J, Bernstein A, Woias P, Mayr HO, Sudkamp NP, Feucht MJ. Length changes of the anterolateral ligament during passive knee motion: a human cadaveric study. Am J Sports Med. 2015;43:2545–2552.
Acknowledgments
We thank Luke Spencer FRACS, Orson Lui and Chris Brooker for their help with study design and data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Research funding support was received from an unrestricted internal research grant from Lawson Health Research (AG), the Department of Surgery at Western University (AG), and the Canadian Health Research Institute (TAB).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Departments of Surgery and Mechanical Engineering, Western University, London, Ontario, Canada.
An erratum to this article is available at https://doi.org/10.1007/s11999-017-5459-6.
About this article
Cite this article
Lording, T., Corbo, G., Bryant, D. et al. Rotational Laxity Control by the Anterolateral Ligament and the Lateral Meniscus Is Dependent on Knee Flexion Angle: A Cadaveric Biomechanical Study. Clin Orthop Relat Res 475, 2401–2408 (2017). https://doi.org/10.1007/s11999-017-5364-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-017-5364-z