Abstract
Currently, the main microalgae-based product available in the market is whole dried biomass (single-cell protein), where Chlorella and Spirulina are the dominant genera. Some speciality chemicals that include beta-carotene, astaxanthin, phycocyanin, eicosapentaenoic acid, and docosahexaenoic acid have already your market share consolidated. All of these bioactive products have applications as a natural colorant, additive, and food supplement. Independent of this, several emerging bioproducts such as lutein, fucoxanthin, phycoerythrin, beta-glucans, exopolysaccharides, arachidonic acid, recombinant proteins, and single-cell protein are in the advanced status of technological development, able to achieve commercial exploitation in the coming years. In this sense, this chapter aims to present status and perspectives on the next-generation of microalgae-based products and their technological advancements.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Microalgae, including cyanobacteria, constitute a diversified group of organisms that are mainly unicellular, aquatic, and photosynthetic eukaryotes (Maroneze et al. 2016). They can live in adverse environmental conditions using only light, water, and simple substances. The great diversity of microalgae, still not fully exploited, offers a series of biologically active metabolites which are of commercial interest. These bioactive metabolites present antioxidant, antimicrobial, antifungal, antiviral, anti-inflammatory, and anticancer activities (Kothari et al. 2017; Bule et al. 2018; Martínez-Francés and Escudero-Oñate 2018).
The interest in microalgae as promising sources of valuable metabolites recently recovered great importance, driven in part by the focus of microalgae biotechnology to produce renewable and commercially viable biofuels, which can only be possible if higher value products are purposely explored (Dias et al. 2019). Thus, the researchers have been concentrated in the diversity of bioactive molecules that can be obtained from microalgae, and that can be profitable and counterbalance production costs (Deprá et al. 2018; Srivastava et al. 2019; Dixit et al. 2019; Jagadevan et al. 2018; Anand et al. 2017).
Today, the most important products obtained of microalgae are the whole dried biomass (single-cell protein), eicosapentaenoic acid (EPA),docosahexaenoic acid (DHA), β-carotene, astaxanthin, and phycocyanin, established in the market of bioactive compounds for use as natural colorant, supplement, and food additive (Jacob-Lopes et al. 2018). In general, the cost of producing these products is generally higher than of traditional sources. However, some metabolites obtained from microalgae have advantages over their conventional sources. Synthetic molecules are less effective than natural sources, which make their use less competitive industrially (Enzing et al. 2014). Moreover, not always, there is a similar alternative available. Phycocyanin, for example, is the unique natural blue colorant available for use.
Beyond the microalgae products already established, several others are on the way to successful commercialization, utilizing the experience of the products that already reached the food and feed market (Borowitzka 2013; Sathasivam et al. 2017). Therefore, the primary objective of this chapter is to compile information and present status and perspectives of the microalgae-based products will reach to commercial exploitation shortly. These represented the next-generation of microalgae-based products.
2.2 A Brief Overview of the Current Marketed Microalgae Based-Products
In the early 1960s, large-scale commercial Chlorella cultures were started, followed by Spirulina in the early 1970s. In this period, the main microalgae product that the industry targeted was the single-cell protein, with applications directed to food and prophylactic use. In the 1980s, emerged the production of β-carotene and astaxanthin from Dunaliella salina and Haematococcus pluvialis, approved as a food coloring and antioxidant. Later, started the production of polyunsaturated fatty acids, namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Table 2.1 summarizes the current market and sales prices of the main commercial products microalgae-based. These bioproducts reached a consolidated food and feed market.
Most of the microalgae-based products on the market are intracellular. Therefore, biomass production represents the primary criterion for technical-economic viability. Moreover, they must comply with many regulations and standards, once are generally destined for human and animal consumption. High value-added products, such as pigments and polyunsaturated fatty acids, have a higher production cost and, consequently, sale higher than whole dried biomass. This can be, partly, explained by the downstream processing of these products. The downstream processing for whole dried biomass production is relatively simple, seeing that only biomass drying is necessary. On the other hand, intracellular products such as pigments and polyunsaturated fatty acid require costly extraction and purification steps. In general, the expenditure involved in downstream processes for whole dried biomass production is about 2%, while for polyunsaturated fatty acids and pigments are about 70% and 85%, respectively (Jacob-Lopes et al. 2018).
Microalgae products, especially high value-added products, using innovative technologies, have attracted commercial attention, seeing that can be obtained at a reasonable cost. Noteworthy, the market value of these products depends not only on production costs, but also on market conditions, including competition from similar chemicals, supply–demand ratio, and suitability for specific applications (Budzianowski 2017).
Today, in addition to the products mentioned in Table 2.1, there are several other products in different stages of development. Therefore, in this chapter, the status and perspectives of microalgae-based products in advanced development have been reviewed.
2.3 Technological Processes for Manufacturing Microalgae-Based Products
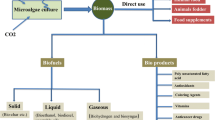
The diversity of microalgae and the capacity of these microorganisms to produce chemical specialities made them a target of research and development. They thrive in adverse environments, and their chemical composition under specific conditions can form and accumulate bioproducts of interest (da Silva Vaz et al. 2016). These products can be allocated in the energy, chemicals and materials, food and feed, and pharmaceuticals and personal cares markets (Barsanti and Gualtieri 2018). Figure 2.1 portrays the schematic of the main steps involved in the industrial process related to the production of microalgae-based products.
The industrialization of microalgae-based products requires large-scale culture systems, which are typically raceway ponds, tubular photobioreactors, or heterotrophic bioreactors (Maroneze and Queiroz 2018). Among these, stands out the raceway ponds systems and tubular photobioreactors. The purchase cost for raceway ponds and tubular photobioreactors is estimated at 4–6 kUSD/m3 and 40–50 kUSD/m3, respectively (Ramírez-Mérida et al. 2017; Deprá et al. 2019). The cost of each configuration is related to the quality of the materials required. The magnification of these reaction vessels enables reducing the cost of the process and product. For example, the cost of microalgae biomass (USD/kg dry matter) for raceway ponds and tubular photobioreactors in a scale 1000 m2 is estimated at US $40.36 and US $21.43 and for a scale of 100 ha is of US $7.04 and US $5.13, respectively (Spruijt et al. 2015).
After the upstream processing, the choice of the downstream process is crucial, once influences the economic viability of microalgae-based products. The downstream processing involves harvesting, pre-treatment, extraction, and purification. Current harvesting strategies include physical and chemical methods (Ummalyma et al. 2017; Zhu et al. 2017; Mathimani and Mallick 2018). Despite the importance of harvesting to the economics and energy balance, there is no universal harvesting technique for microalgae. However, centrifugation is the commonly employed technique (Singh and Patidar 2018).
After harvest, the drying and cell disruption are most utilized pre-treatment methods and are selected based on extraction procedure and in the desired product (Khanra et al. 2018). The most used drying methods are spray-drying and freeze-drying and cellular rupture can be broadly categorized into two methods, non-mechanical and mechanical. Each method has its advantages and disadvantages (Lee et al. 2017; Dias et al. 2019). In general, the pre-treatment is the key to increase the extraction efficiency of microalgae biocompounds. The extraction follows the process of pre-treatment.
The extraction by supercritical fluids and solvents are widely used to extract biocompounds such as pigments and polyunsaturated fatty acids. Finally, purification is given by several chromatographic methods as supercritical fluids chromatography and ion-exchange chromatography. The purification is done to compounds of microalgae applied to products of high value, to improve its bioavailability, and the method is selected based on the intended product (Voort and VulstekeSaut 2015; Ventura et al. 2018).
2.4 Microalgae-Based Products in Advanced Development
Microalgae offer a chemically diverse and unexplored reservoir of biomolecules that can be harnessed for commercial use. Although many of these compounds have been identified, the metabolic pathways for synthesis are poorly understood for most of them, and extraction and purification methods necessitated to be perfected (Kumar et al. 2019). In this context, independent of the commercially established microalgae products, there are several others at different stages of research and development. The microalgae-based products in advanced development are discussed below.
2.4.1 Single-Cell Protein
The single-cell protein (whole-cell powder) traditionally have been produced as the main microalgae-based product worldwide. This market share is majoritarily represented by the genera Chlorella and Spirulina as reported early. In consolidated technological routes, the product is sold without any kind of processing except drying. Their application is mainly as dietary supplements for human use (Ramírez-Mérida et al. 2017).
Furthermore, based on diversity of the chemical composition of these microorganisms, other species besides Spirulina/Chlorella have been considered for production as whole biomass, including mainly Nannochloropsis, Isochrysis, Pavlova, Phaeodactylum, Chaetoceros, Skeletonema, Thalassiosira, and Tetraselmis. These products are presented as live cells, fresh cells, frozen cells, and freeze cells. The applications include starter cultures, concentrates for aquaculture and formulated feeds for aquaculture (Enzing et al. 2014). Besides the unit operations of dewatering, freezing, and drying, some products can include the addition of food-grade preservatives aiming to extend the shelf-life of the product. In terms of safety aspects of these new species, all are classified as “no toxins known (NT)” (Matos 2017).
2.4.2 Recombinant Proteins
Technological advances in the field of recombinant proteins and vaccine delivery systems have driven the use of alternative sources against animal-produced vaccines. Currently, it is estimated that the global market should reach about US $2850.5 million by 2022 (Market and Markets 2017).
In this context, genetically accessible photosynthetic organisms, such as microalgae, the present potential for the generation of high-quality recombinant proteins (Surzycki et al. 2009). Its cellular machinery, mainly involving the presence of chloroplasts, makes these microorganisms excellent sites specific for the double production of complex recombinant proteins. This is because chloroplasts are now known to have peculiar properties, being they the ability to accumulate higher levels of transgenic proteins, since they do not have gene silencing mechanisms. Besides, they can be supported by multiple genes in a single event due to the availability of numerous insertion sites (Specht et al. 2010).
The mechanism of action for the secretion of proteins is related to the cellular structure of microalgae. The constructs of the genes and the transformation methods depend on the cellular locations of the accumulation of recombinant proteins. It is known that modification of plastid causes accumulation of the transgene material in the chloroplast. However, nuclear genetic materials are accumulated in the cytosol. Thus, appropriate nuclear transformation allows targeting to the endoplasmic and Golgi reticulum for packaging and export to the extracellular medium (Akbari et al. 2014). At the exemplification level, the microalgae Chlamydomonas reinhardtii presents the ability to allow adequate folding of proteins strongly bound by disulfide, which is not naturally achieved in other bacterial production platforms.
In addition, among the structural advantages, microalgae are attributed to the potential for rapid cell development, large-scale production and also the non-transmission of animal pathogens (Yan et al. 2016).
The initial studies were supported by hepatitis B antigens of the microalgae Dunaliella salina (Siripornadulsil et al. 2007). However, several studies report the use of the microalgae Chlamydomonas reinhardtii as the primary potential source in the production of pharmaceutical proteins such as erythropoietin, interferon β insulin, and immunoglobulin A. Recently, this microalga has been used in experimental formulations of vaccines against Staphylococcus aureus, while the microalgae Schizochytrium sp. has been used to develop novel zika virus vaccines and in both cases the performance of these vaccines presented more satisfactory results in triggering both the humoral mucosal and systemic immune response (Márquez-Escobar et al. 2018; Miquel-Clopés et al. 2019).
Additionally, among the main applications of recombinant proteins, it is assumed that this industrial follow-up may be more economical for vaccines such as HPV, as well as the fundamental element for the development of new vaccines for which there is still no alternative (Specht and Mayfield 2014). This is because, at the comparison level, costs for the production of functional antibodies are estimated at US $150/g in mammalian cells, while in plants the average values estimated are of US $0.05/g. In addition, microalgae are a promising system, and their costs are estimated at US $0.002/g (Potvin and Zhang 2010; Barrera and Mayfield 2013). Therefore, the use of microalgae becomes particularly significant because of the cost-effectiveness of such recombinant proteins in the field of industrial vaccines.
2.4.3 Phycoerythrin
The phycoerythrin (PE) is a photosynthetic pigment belonging to phycobiliproteins, which also include phycocyanin, allophycocyanin, and phycoerythrocyanin. This pigment is considered one of the brightest natural fluorophores identified. The chemical structure of phycoerythrin is shown in Fig. 2.2. According to the light absorption properties, the PE can be classified into two main classes: R-PE (λmax = 565 nm, 499 nm and one shoulder at 545 nm) and B-PE (λmax = 565 nm, 546 nm and one shoulder at 499 nm). The spectral difference between phycoerythrins is attributed to the presence of prosthetic groups, denominated bilins that covalently bind to cysteine-specific residues on protein subunits. R-PE is commonly extracted and purified from Porphyra, Gastroclonium, and Polysiphonia macroalgae. R-PE is generally used for flow cytometry and other applications that require high sensitivity but not photostability (Gargouch et al. 2018; Leney et al. 2018).
Chemical structure of phycoerythrin. Source: Adapted from Hsieh-Lo et al. (2019)
On the other hand, B-PE is the main phycobiliprotein extracted from red microalgae. The Porphyridium cruentum microalgae have been produced for commercialization this pigment. The B-phycoerythrin of Porphyridium cruentum is used mainly as an indicator in the quantitative assay for the oxygen-radical absorbing capacity of antioxidants in serum or plasma. B-PE is commonly marketed as a lyophilized powder containing preservatives. The commercial value of B-PE of Porphyridium cruentum is about 681.62 USD/mg (Sigma-Aldrich 2019). This high value has encouraged the development of efficient extraction and purification procedures to maximize the purity and commercial yield of B-PE. The complexity of downstream procedures and low yields limit the potential for practical implementation to the commercial level. Noteworthy, under specific conditions, dependent on abiotic and biotic factors, the microalgae may potentiate B-PE accumulation. The B-PE accumulation is desired to optimize its large-scale production (Tang et al. 2016).
In addition, the B-PE, as a natural pigment, exhibits beneficial biological activities for human health. Recent studies have shown that the B-phycoerythrin from Porphyridium microalgae species can reduce myeloid tumor cell proliferation in vitro. Treatment with B-phycoerythrin induces apoptosis and can increase glutathione reductase and superoxide dismutase activity. These studies indicate the potential of B-PE as a source of bioactive protein (Minkova et al. 2011; Gargouch et al. 2018).
2.4.4 Fucoxanthin
The fucoxanthin belongs to the class of non-provitamin A carotenoids, which gives brown microalgae their characteristic color. When ingested, the fucoxanthin is metabolized by digestive enzymes in the gastrointestinal tract into fucoxanthinol and then absorbed into intestinal cells. In the liver, the fucoxanthinol is converted to amaruciaxanthin A (Martin 2015). Both structures are shown in Fig. 2.3. Experimental evidence reveals that fucoxanthin and its metabolites have present remarkable biological properties, without any evident toxicity (Rokkaku et al. 2013; Asai et al. 2004; Rwigemera et al. 2015, 2014). These metabolites exhibit antioxidant, anti-inflammatory, anticancer, anti-obesity, antidiabetic, hepatoprotective, antimalarial activities (Crupi et al. 2013). In particular, fucoxanthin is a promising nutritional supplement for the treatment of obesity and related pathologies. Nutrigenomic studies reveal that fucoxanthin induces uncoupling protein-1 in abdominal white adipose tissue, conducting to oxidation of fatty acids and heat production in brown adipose tissue(Miyashita et al. 2011; Gammone and D'Orazio 2015; Maeda 2015). The fucoxanthin and its metabolite fucoxanthinol suppress triglyceride absorption. The suppressive effect of fucoxanthinol, however, is more pronounced than that of fucoxanthin (Matsumoto et al. 2010). In contrast, the suppressive effect of amaruciaxanthin A on the differentiation of 3T3-L1 is more pronounced than that of fucoxanthinol (Yim et al. 2011). The fucoxanthin metabolites are considered the active forms that play physiological functions in the human organism. However, both the fucoxanthin as its metabolites has an anti-obesity effect (Hu et al. 2016).
Chemical structure of fucoxanthin, fucoxanthinol, and amaruciaxanthin A. Source: Adapted from Hashimoto et al. (2009)
The dietary fucoxanthin preferentially accumulates in the heart and liver as fucoxanthinol and in white adipose tissue as amaruciaxanthin A (Hashimoto et al. 2009; Miyashita and Hosokawa 2018). As amaruciaxanthin A is the dominant metabolite of fucoxanthin that accumulates in white adipose tissue, studies suggest that amaruciaxanthin A may be the molecule responsible for the anti-obesity effect of fucoxanthin in vivo (Yim et al. 2011).
The fucoxanthin has a unique chemical structure, similar to neoxanthin and peridinine, which is different from that of other carotenoids, such as β-carotene and lutein. The alenic linkage, 5,6-mono-epoxide, and nine double conjugated plays an important role within fucoxanthin structure. In part, the biological properties of fucoxanthin and its metabolites are assumed due to the presence of its allenic bond (Fig. 2.3) (Sachindra et al. 2007).
Currently, the commercialization of fucoxanthin is mainly obtained from macroalgae as Macrocystis, Laminaria, and Undaria. Phaeodactylum tricornutum is the unique microalgae produced to obtaining fucoxanthin. Your application is as a dietary supplement for humans, mainly as concentrates or microalgae extracts (Algaech 2019). Beyond the microalgae Phaeodactylum tricornutum, there is the opportunity for fucoxanthin production from other microalgae species such as Odontella aurita, Cyclotella cryptica, Chaetoceros calcitrans, and Isochrysis galbana (Foo et al. 2015; Kim et al. 2012a, b). Typical concentrations found in microalgae are at least an order of magnitude greater than those found in macroalgae (McClure et al. 2018). The microalgae Isochrysis galbana, for example, is capable of producing 18.2 mg of fucoxanthin per g DW, while species of macroalgae (E. bicyclis, K. crassifolia, A. crassifolia, S. horneri, and C. hakodatensis) produce only 0.04–1.52 mg of fucoxanthin per g DW (Airanthi et al. 2011; Gong and Bassi 2016). In addition, the microalgae Odontella aurita presents concentrations up to 18.47 mg/g DW, can accumulate, under specific growth conditions, concentrations superiors than 20 mg/g DW (Xia et al. 2013). Considering the high content of fucoxanthin in Isochrysis sp. and Odontella aurita, these microalgae could be proposed as a source of this compound for nutraceutical and pharmaceutical applications. Noteworthy, although the content of fucoxanthin in microalgae is more significant than the found in macroalgae, this pigment continues to be produced mainly by macroalgae, because its cultivation is cheaper. In contrast, an advantage of cultivating microalgae in closed systems and controlled is the availability of a continuous supply of biomass throughout the year (Crupi et al. 2013).
2.4.5 Lutein
The lutein is a carotenoid of lemon-yellow tint considered essential for human health. This compound is not produced by humans and should be ingested through diet. Lutein contains a characteristic structure composed of 10 conjugated double bonds (Fig. 2.4). Its primary biological functions are determined by the extended system of conjugated double bonds, which is also responsible for its color (Stringheta et al. 2009). In the diet, free or esterified lutein is absorbed from the gastrointestinal tract via uptake by enterocytes. This carotenoid is predominantly found in the esterified form. However, for commercialization, it is normally saponified for esters removal (Kijlstra et al. 2012). Scientific information about its bioavailability demonstrates that free lutein is more bioavailable than lutein in the esterified form (Norkus et al. 2010).
Chemical structure of the lutein. Source: Adapted from Bernstein et al. (2016)
The lutein exhibits antioxidant and anti-inflammatory properties and has demonstrated, from several studies, to be useful in protecting against atherosclerosis and in preventing macular degeneration and cardiovascular diseases (Chung et al. 2017; Gong and Bassi 2016). In particular, numerous studies emphasize a relationship between lutein consumption and a reduction in the incidence of eye diseases (Johnson 2014; Bernstein et al. 2016). Studies in patients with age-related macular degeneration (AMD) show that lutein-containing supplementation may impede the progression of AMD and improve visual function (Weigert et al. 2011; Ma et al. 2012a, b). Moreover, studies have associated lutein supplementation with the prevention of cataract development and progression (Arnal et al. 2009; Karppi et al. 2012). Despite the scientific evidence presented between lutein consumption and the reduction in the incidence of eye diseases, requests for health claims have not yet obtained ubiquitously successful (Nwachukwu et al. 2016).
The world market for lutein as from different sources was estimated at USD 308 million in 2018 and is expected to reach US $396.4 million by 2024. This increase is a projection based on demand for lutein for various applications. The lutein is especially used as dye, food additive, and as an eye health supplement (Lin et al. 2015; Global 2019).
Currently, most of the lutein is produced from marigold flowers. The disadvantage of calendula cultivation as a source of lutein is the demand for land and manpower intensive. In this context, the microalgae represent an alternative to traditional sources, with a sufficiently high content of lutein. The market estimate for lutein obtained from microalgae is US $3.14 million (Hu 2019). When compared to calendula cultivation, microalgae present advantages such as higher growth rate, a predominance of lutein in free form, annual productivity regardless of seasons, and coproduction of value-added compounds (Fernández-Sevilla et al. 2010; Araya et al. 2014).
Some strains of microalgae such as Chlorella vulgaris, Muriellopsis sp., Scenedesmus sp., Desmodesmus sp., C. zofingensis, and C. protothecoides are recognized as lutein producers (Chen et al. 2017; Xie et al. 2017). The quantity of lutein generated by microalgae depends on some environmental conditions, such as light intensity and temperature (Bhalamurugan et al. 2018). Based on studies, a lutein content in the microalgae biomass of 5 g/kg is estimated. Although they have a relatively high content of lutein and advantages when compared to marigold flowers, there are still no lutein products obtained from commercially established microalgae. There is only a small market participation represented by the genera Chlorella and Scenedesmus, turned for application as food supplement and supplement feed (Camacho et al. 2019; A4F 2019).
Some technical inconvenient, such as elevated harvest costs and high energy demand for the pre-treatment and lutein extraction, persist. Therefore, strategies to reduce the cost of production and the search for strains with higher lutein content are being evaluated to make microalgae economically viable sources of lutein (Acién et al. 2012).
2.4.6 Beta-Glucans
The β-glucans are heterogeneous polysaccharides united by β-(1,3); (1,4) or (1,6) glycosidic bonds, found in plants, fungi, bacteria, and microalgae (Fig. 2.5). The β-(1,3)-glucan is the simple of all. A well-known example of this structure is paramylon found in microalgae such as Euglena sp. and Astasia longa. The β-(1,3;1,4)-glucan can be found in the cell wall of microalgae as Micrasterias sp. and Monodus subterraneus, and the branched β-(1,3;1,6)-glucan is found in diatoms and chrysophytes (Barsanti et al. 2011). These polysaccharides have been extensively studied and are considered safe for use. Based on clinical evidence, the β-glucans have been established as beneficial natural polysaccharides in the treatment of multiple infirmities. The β-glucans exhibit antitumor, anti-inflammatory, anti-osteoporotic, and immunomodulating activities (Jayachandran et al. 2018).
β-glucan structures identified in microalgae. Source: Adapted from Barsanti et al. (2011)
The β-glucan structures vary according to the different sources, and their physiological functions and biological activity vary each other. The mechanism of action of β-glucans in the human biological system depends on its molecular weight and solubility in water, which depends on its molecular structure. The higher the molecular weight, the higher the viscosity and the greater the health benefits (Maheshwari et al. 2019). With the remarkable range of benefits offered by these polysaccharides, it is expected, stimulation scientific efforts to understand better the structure/function relationships and the mechanisms involved behind their biological activity (Izydorczyk 2016).
β-glucans can be ingested as a dietary supplement or as part of a diet. Currently, the most commercially available β-glucans are in the great part native of fungi; however, there are clear opportunities for successful commercialization of β-glucans derived from microalgae (Kim et al. 2019). The microalgae cultivation is more straightforward, the costs are lower, and the productivity of β-glucan is higher than, for example, in the cultivation of mushrooms and yeasts. Several species of mushrooms cannot be cultivated and are collected from nature. The microalgae, on the other hand, are easily cultivable and represent a more interesting alternative (Schulze et al. 2016). Moreover, the microalgae-based processes do not involve costly extraction steps and, therefore, their cost is substantially smaller. Another advantage of microalgae compared to traditional sources of β-glucan is its bioavailability. In microalgae, the β-glucan is mainly β-(1,3)-glucan, while yeast-derived products are mostly a combination with β-(1,6)-glucan, which affects their bioavailability (Stephen 2016).
The microalgae Euglena gracilis is a rich source of β-(1,3)-glucan and has been exploited for the production of this polysaccharide (Russo et al. 2017). Their application is as immune-support ingredient. The products are presented mainly as concentrates or whole-cell of Euglena gracilis (Gissibl et al. 2019). Other microalgae species source of β-glucan include Scenedesmus obtusiusculus, Porphyridium purpureum, Pavlova mesolychnon, Phaeodactylum tricornutum, Odontella aurita, and Chaetoceros muelleri (Bashir and Choi 2017). It is expected that the increasing use of this ingredient mainly in the pharmaceutical and nutraceutical industry boost its development and production from microalgae (Barsanti and Gualtieri 2018).
2.4.7 Exopolysaccharides
Among the polysaccharides and their derivatives, the sulphated exopolysaccharides (sPS) of microalgae are in focus. The sPS of microalgae are complex polymers composed of numerous monosaccharides, the most abundant being xylose, glucose, and galactose (Marcati et al. 2014). The structure of the sPS and its complexity varies according to the microalgae species. Therefore, each new purified sPS is a new compound with unique structures and, consequently, with unclear biological activities. Thereby, the precise knowledge of the structural relationship with its biological activities remains under-revealed for most sPS structures (Pandeirada et al. 2019). However, it is reported that the bioactivity of the sPS is related to its molecular weight, monosaccharide composition, sulfate content and the position of the sulfate ester group (Hahn et al. 2012).
Several studies have already highlighted the pharmacological activities of the sPS of microalgae (de Jesus Raposo et al. 2014; Sun et al. 2012; Challouf et al. 2011; Liu et al. 2016). They perform, at the cellular level, different functions. The bioactive properties of these biomolecules have been associated with anti-inflammatory, immunomodulatory, anti-tumor, antiviral, antioxidant, antibacterial, anti-lipidemic, and anti-glycemic activities (de Jesus Raposo et al. 2013; Sanjeewa et al. 2018).
Microalgae with potential for production of sPS include Chlorella stigmatophora, Chlorella capsulata, Tetraselmis sp., Nannochloropsis oculata, Botryococcus sudeticus, Neochloris oleoabundans, Gyrodinium impudicum, Dunaliella tertiolecta, Isochrysis sp., Phaeodactylum tricornutum, Porphyridium marinum, P. cruentum, P. purpureum, Cylindrotheca closterium, and Spirulina platensis (Pletikapić et al. 2011; Guzman et al. 2003; Guzman-Murillo and Ascencio 2000; Soanen et al. 2016; Balti et al. 2018; Goo et al. 2013). Unlike macroalgae, the microalgae do not have proper names for their sPS, except spirulan, produced by Spirulina platensis (Raposo and Morais 2015). Data demonstrated that spirulan isolated from Spirulina platensis is a potent antiviral agent (Lee et al. 2000).
In sPS it was verified that higher sulfate content induces an increased in the antiviral activity (de Jesus Raposo et al. 2014). In general, the content and position of the sulfated groups may differ in the various sPS, which is dependent on the strain, culture conditions, and extraction procedures (de Jesus Raposo et al. 2015).
Sulphated exopolysaccharides can find applications in functional foods, cosmetics, and pharmaceuticals. The commercial exploitation of these polymers from microalgae is limited basically by their low concentrations (Patel et al. 2013). Information regarding the estimated price range and market of these biomolecules remains unclear.
2.4.8 Arachidonic Acid
The arachidonic acid (AA) is considered a constituent of biomembranes, essential for cell function, especially in the nervous system, skeletal muscle, and the immune system. Besides that, it is a precursor of prostaglandins and eicosanoids. Due to its importance in the development and function of the central nervous system and retina, AA has been recommended for infant formula supplementation (Tallima and El Ridi 2018).
The AA is obtained from the diet or through converting linoleic acid by desaturation and elongation processes. The biosynthesis of AA from linoleic acid is shown in Fig. 2.6 (Hanna and Hafez 2018). It is noteworthy that only a small portion of linoleic acid is converted to AA in the body. The production of AA on an industrial scale originates mainly from fungal strains belonging to the genus Mortierella sp., of which the M. alpina species is considered the predominant source with a content of up to 70% of the total lipids (Dediukhina et al. 2011). The Lobosphaera incisa microalgae are also capable of producing a high AA content, capable of reaching up to 77% of total fatty acids. These have been exploited for AA production as a food supplement for infants (Camacho et al. 2019). The laboratory-scale production has already been enlarged and is found currently on pilot-scale. Open culture systems, such as cascade raceways and raceway ponds, and closed systems, such as tubular and flat-plate photobioreactors, have been used in the cultivation of Lobosphaera incisa. Typically, an induction phase is required for AA accumulation. Other microalgae species, such as Porphyridium purpureum, P. cruentum, Myrmecia incisa, Gracilaria sp., and Rodomella subfusca, have an AA content of 40–60% of total fatty acids. These represent a promising alternative source for AA production (Shanab et al. 2018).
Biosynthesis of AA from linoleic acid. Source: Adapted from Shanab et al. (2018)
2.5 Microalgae-Based Products in Early Development
Regardless of commercially established microalgae-based products and those in advanced development, there are others in early development with potential for commercial exploitation that includes biofuels, polyhydroxyalkanoates, enzymes, violaxanthin, zeaxanthin, prebiotics, phenolics, glutathione, and sterols.
Among biofuels, it is possible to mainly produce biodiesel and biohydrogen from microalgae (Shuba and Kifle 2018). However, economic aspects are the barrier to the final commercialization of these biofuels (Su et al. 2017). A critical analysis of the current status of microalgae biofuels indicates the unfeasibility of their production, which is amplified by the low price of fossil resources (Deprá et al. 2018). Despite this, there is optimism based on the sustainability of microalgae as feedstock and the technological advance already achieved to make them competitive with petroleum (Adeniyi et al. 2018).
Recently, microalgae have been proposed for the production of polyhydroxyalkanoates (PHA). This polymer is produced from microorganisms and considered as a substitute for petroleum-based plastics (Cassuriaga et al. 2018). Currently, the production of PHAs by bacteria is limited by the cost of production (Rahman and Miller 2017). Given this scenario, microalgae were suggested for the production of PHAs at a relatively minor cost. Attributed to the minimum nutrient requirements needed for cultivation. Numerous studies, under specific growth conditions, evaluated PHA production during the cultivation of different strains of microalgae. Concentrations of 5–55% of PHA (dry cell weight) were reported (Nishioka et al. 2001; Kavitha et al. 2016; Kovalcik et al. 2017; Toh et al. 2008; Coelho et al. 2015).
Commercially important enzymes can be produced from microalgae and can be used as biocatalysts in a range of industrial applications. Previously neglected, the microalgae have now been proposed as enzymatic factories. Enzymes derived from microalgae include cellulases, galactosidases, proteases, lipases, phytases, laccases, and amylases (Brasil et al. 2017). To date, there have been no reports of commercial production of microalgae-based enzymes, but laboratory studies have demonstrated the potential of these microorganisms to synthesize these substances (Yong et al. 2016). In this context, strategies to optimize enzyme concentration and reduce operating costs are underway. The concept of biorefinery has been approached as an efficient strategy to overcome the existing bottlenecks and make possible the production of enzymes in the future.
Microalgae compounds with promising biological activities are being evaluated with a focus on human health and disease prevention (Dewi et al. 2018). In vitro and animal studies support the potential of microalgae and their isolated bioproducts as an innovative strategy for the treatment of various diseases (Talero et al. 2015; El-Hack et al. 2019). The carotenoid violaxanthin isolated from microalgae strains, for example, demonstrated in vitro to have antiproliferative effects against the growth of cancer cells (Cha et al. 2008; Pasquet et al. 2011; Soontornchaiboon and Kim 2011; Amaro et al. 2013). Microalgae such as Chlorella protothecoides, Chlorella vulgaris, and Scenedesmus obliquus produce this carotenoid (Grudzinski et al. 2016; Patias et al. 2017).
The zeaxanthin commonly referred to as macular pigment such as lutein can be synthesized by microalgae as Dunaliella, Spirulina, Synechococcus, Chlorella, Prochlorococcus, and Prochlorothrix. Zeaxanthin currently available in synthetic form has a complex production process and low biological activity. On the other hand, the production from vegetables and fruits presents low extraction and production rate. In this context, the production of zeaxanthin from microalgae can be seen as a promising alternative for applications as dye and food supplement. From genetic engineering and the use of new technologies, it is possible to increase the accumulation of zeaxanthin in microalgae, which can make them commercially viable sources (Sajilata et al. 2008; Zhang et al. 2018).
Active compounds such as prebiotics found in microalgae include galacto-oligosaccharides, xylooligosaccharides, oligosaccharides derived from agarose and alginate, galactans and arabinoxylans, although the criteria for classification as prebiotics have not been validated for some of them (de Jesus Raposo et al. 2016). This is a promising area of research, capable of providing biocompounds that are essential for the prevention of various human diseases.
Microalgae can also produce phenolics and glutathione (GSH) with antioxidant properties applicable in the food, pharmaceutical, and cosmetic industries (Maadane et al. 2015; Goiris et al. 2015; do Nascimento et al. 2019; Sathasivam et al. 2017; Choochote et al. 2014). Currently, synthetic antioxidants are the most utilized in industrial applications. However, concerns regarding its safety and toxicity led to the search for natural antioxidants (Morowvat and Ghasemi 2016).
Due to the content of sterols of some microalgae, these are commonly used to promote the growth of juveniles. Beyond cholesterol, unusual sterols like brassicasterol, campesterol, stigmasterol, and sitosterol are reported. Noteworthy, microalgae sterols seem to present hypocholesterolemic properties (de Jesus Raposo et al. 2013; Fagundes et al. 2019).
2.6 Conclusion and Way Forward
With the emergence of new techniques and improving them for the study of microalgae-based products, especially those with bioactive potential, this group of microorganisms gains a closer look as a source of natural products. Given their extraordinary biological availability and structural variety, the screening of microalgae bioactive compounds will be certainly successful in the global market. However, there are many obstacles related to this that need to be overcome to make microalgae-based products an industrial reality. Some recommendations of the way forward are: (1) select microalgae strains suitable to be controlled at the industrial level. The use of biotechnology techniques, including the genetic engineering tools, could be a major leap toward the search for compounds with unique and multifunctional activities; (2) culture conditions should be improved to raise the amount of the microalgae bioactive compounds of particular interest inside their chemical composition; (3) bioreactors engineering must be enhanced to achieve higher workloads and then scale-up. These advances will impact the process design; (4) implement approaches that maximize the technical, economic, and environmental performance of microalgae-based processes and products. We can cite three main strategies for this purpose: the process integration, the process intensification, and the integrated biorefinery model; (5) perform assays that allow determining the biological effects, such as antiviral, anticancer, antibacterial, and antioxidant, associated with robust extraction methods; and (6) establish duly safety and regulatory issues for the microalgae bioproducts, since many of these bioproducts are intended for human or animal consumption. Finally, since all these concepts are demonstrated, the scientific communities must carefully elucidate the aspects related to the scaling up.
References
A4f (2019) Industrial production: microalgae track record. Available https://www.a4f.pt. Accessed 17 July 19
Acién FG, Fernández JM, Magán JJ, Molina E (2012) Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol Adv 30(6):1344–1353. https://doi.org/10.1016/j.biotechadv.2012.02.005
Adeniyi OM, Azimov U, Burluka A (2018) Algae biofuel: current status and future applications. Renew Sust Energ Rev 90:316–335. https://doi.org/10.1016/j.rser.2018.03.067
Airanthi MWA, Hosokawa M, Miyashita K (2011) Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci 76(1):C104–C111. https://doi.org/10.1111/j.1750-3841.2010.01915.x
Akbari F, Eskandani M, Khosroushahi AY (2014) The potential of transgenic green microalgae; a robust photobioreactor to produce recombinant therapeutic proteins. World J Microbiol Biotechnol 30(11):2783–2796. https://doi.org/10.1007/s11274-014-1714-0
Algaech (2019) FucoVital fucoxanthin. Available https://www.algatech.com. Accessed 29 July 19
Amaro HM, Barros R, Guedes AC, Sousa-Pinto I, Malcata FX (2013) Microalgal compounds modulate carcinogenesis in the gastrointestinal tract. Trends Biotechnol 31(2):92–98. https://doi.org/10.1016/j.tibtech.2012.11.004
Anand V, Singh PK, Banerjee C, Shukla P (2017) Proteomic approaches in microalgae: perspectives and applications. Biotech 7(3):197
Araya B, Gouveia L, Nobre B, Reis A, Chamy R, Poirrier P (2014) Evaluation of the simultaneous production of lutein and lipids using a vertical alveolar panel bioreactor for three Chlorella species. Algal Res 6:218–222. https://doi.org/10.1016/j.algal.2014.06.003
Arnal E, Miranda M, Almansa I, Muriach M, Barcia JM, Romero FJ, Bosch-Morell F (2009) Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol 247(1):115–120
Asai A, Sugawara T, Ono H, Nagao A (2004) Biotransformação de fucoxantinol em amaruciaxantina A em ratinhos e em células HepG2: Formação e citotoxicidade de metabolitos de fucoxantina. Drug Metab Dispos 32:205–211. https://doi.org/10.1124/dmd.32.2.205
Balti R, Le Balc’h R, Brodu N, Gilbert M, Le Gouic B, Le Gall S, Massé A (2018) Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem 74:175–184. https://doi.org/10.1016/j.procbio.2018.06.021
Barrera DJ, Mayfield SP (2013) High-value recombinant protein production in microalgae. Handb Microalgal Cult 2:532–544
Barsanti L, Gualtieri P (2018) Is exploitation of microalgae economically and energetically sustainable? Algal Res 31:107–115. https://doi.org/10.1016/j.algal.2018.02.001
Barsanti L, Passarelli V, Evangelista V, Frassanito AM, Gualtieri P (2011) Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat Prod Rep 28(3):457–466. https://doi.org/10.1039/C0NP00018C
Bashir KM, Choi JS (2017) Clinical and physiological perspectives of β-glucans: the past, present, and future. Int J Mol Sci 18(9):1906. https://doi.org/10.3390/ijms18091906
Bernstein PS, Li B, Vachali PP, Gorusupudi A, Shyam R, Henriksen BS, Nolan JM (2016) Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res 50:34–66. https://doi.org/10.1016/j.preteyeres.2015.10.003
Bhalamurugan GL, Valerie O, Mark L (2018) Valuable bioproducts obtained from microalgal biomass and their commercial applications: a review. Environ Eng Res 23(3):229–241. https://doi.org/10.4491/eer.2017.220
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25(3):743–756
Brasil BDSAF, de Siqueira FG, Salum TFC, Zanette CM, Spier MR (2017) Microalgae and cyanobacteria as enzyme biofactories. Algal Res 25:76–89. https://doi.org/10.1016/j.algal.2017.04.035
Budzianowski WM (2017) High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew Sust Energ Rev 70:793–804. https://doi.org/10.1016/j.rser.2016.11.260
Bule MH, Ahmed I, Maqbool F, Bilal M, Iqbal HM (2018) Microalgae as a source of high-value bioactive compounds. Front Biosci 10:197–216
Camacho F, Macedo A, Malcata F (2019) Potential industrial applications and commercialization of microalgae in the functional food and feed industries: a short review. Mar Drugs 17:312. https://doi.org/10.3390/md17060312
Cassuriaga APA, Freitas BCB, Morais MG, Costa JAV (2018) Innovative polyhydroxybutyrate production by Chlorella fusca grown with pentoses. Bioresour Technol 265:456–463. https://doi.org/10.1016/j.biortech.2018.06.026
Cha KH, Koo SY, Lee DU (2008) Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem 56(22):10521–10526. https://doi.org/10.1021/jf802111x
Challouf R, Trabelsi L, Ben Dhieb R, El Abed O, Yahia A, Ghozzi K, Ben Ouada H (2011) Evaluation of cytotoxicity and biological activities in extracellular polysaccharides released by cyanobacterium Arthrospira platensis. Braz Arch Biol Technol 54(4):831–838. https://doi.org/10.1590/S1516-89132011000400024
Chen JH, Chen CY, Chang JS (2017) Lutein production with wild-type and mutant strains of Chlorella sorokiniana MB-1 under mixotrophic growth. J Taiwan Inst Chem Eng 79:66–73. https://doi.org/10.1016/j.jtice.2017.04.022
Choochote W, Suklampoo L, Ochaikul D (2014) Evaluation of antioxidant capacities of green microalgae. J Appl Phycol 26(1):43–48
Chung RW, Leanderson P, Lundberg AK, Jonasson L (2017) Lutein exerts anti-inflammatory effects in patients with coronary artery disease. Atherosclerosis 262:87–93. https://doi.org/10.1016/j.atherosclerosis.2017.05.008
Coelho VC, da Silva CK, Terra AL, Costa JAV, de Morais MG (2015) Polyhydroxybutyrate production by Spirulina sp. LEB 18 grown under different nutrient concentrations. Afr J Microbiol Res 9(24):1586–1594. https://doi.org/10.5897/AJMR2015.7530
Crupi P, Toci AT, Mangini S, Wrubl F, Rodolfi L, Tredici MR, Antonacci D (2013) Determination of fucoxanthin isomers in microalgae (Isochrysis sp.) by high-performance liquid chromatography coupled with diode-array detector multistage mass spectrometry coupled with positive electrospray ionization. Rapid Commun Mass Spectrom 27(9):1027–1035. https://doi.org/10.1002/rcm.6531
da Silva Vaz B, Moreira JB, de Morais MG, Costa JAV (2016) Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci 7:73–77. https://doi.org/10.1016/j.cofs.2015.12.006
de Jesus Raposo MF, de Morais RMSC, de Morais AMMB (2013) Health applications of bioactive compounds from marine microalgae. Life Sci 93(15):479–486. https://doi.org/10.1016/j.lfs.2013.08.002
de Jesus Raposo MF, de Morais AMMB, de Morais RMSC (2014) Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci 101(1-2):56–63. https://doi.org/10.1016/j.lfs.2014.02.013
de Jesus Raposo M, de Morais A, de Morais R (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13(5):2967–3028. https://doi.org/10.3390/md13052967
de Jesus Raposo M, de Morais A, de Morais R (2016) Emergent sources of prebiotics: seaweeds and microalgae. Mar Drugs 14(2):27. https://doi.org/10.3390/md14020027
Dediukhina ÉG, Chistiakova TI, Vaĭnshteĭn MB (2011) Biosynthesis of arachidonic acid by micromycetes (review). Prikl Biokhim Mikrobiol 47(2):125–134. https://doi.org/10.1134/S0003683811020037
Deprá MC, dos Santos AM, Severo IA, Santos AB, Zepka LQ, Jacob-Lopes E (2018) Microalgal biorefineries for bioenergy production: can we move from concept to industrial reality? Bioenergy Res 11(4):727–747
Deprá MC, Mérida LG, de Menezes CR, Zepka LQ, Jacob-Lopes E (2019) A new hybrid photobioreactor design for microalgae culture. Chem Eng Res Des 144:1–10. https://doi.org/10.1016/j.cherd.2019.01.023
Dewi IC, Falaise C, Hellio C, Bourgougnon N, Mouget JL (2018) Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. In: Microalgae in health and disease prevention. Academic, Cambridge, pp 235–261. https://doi.org/10.1016/B978-0-12-811405-6.00012-8
Dias RR, Vieira KR, Pinheiro PN, Zepka LQ, Jacob-Lopes E (2019) Biodiesel from microalgae. In: A closer look at biodiesel production. Nova Science Publishers, Hauppauge
Dixit M, Gupta GK, Shukla P (2019) Insights into the resources generation from pulp and paper industry wastes: challenges, perspectives and innovations. Bioresour Technol 297:122496
do Nascimento TC, Cazarin CB, Maróstica MR Jr, Risso ÉM, Amaya-Farfan J, Grimaldi R, Zepka LQ (2019) Microalgae biomass intake positively modulates serum lipid profile and antioxidant status. J Funct Foods 58:11–20. https://doi.org/10.1016/j.jff.2019.04.047
El-Hack MEA, Abdelnour S, Alagawany M, Abdo M, Sakr MA, Khafaga AF, Gebriel MG (2019) Microalgae in modern cancer therapy: current knowledge. Biomed Pharmacother 111:42–50. https://doi.org/10.1016/j.biopha.2018.12.069
Enzing C, Ploeg M, Barbosa M, Sijtsma L (2014) Microalgae-based products for the food and feed sector: an outlook for Europe. JRC Sci Policy Rep 2791:19–37. https://doi.org/10.2791/3339
Fagundes MB, Falk RB, Facchi MMX, Vendruscolo RG, Maroneze MM, Zepka LQ, Wagner R (2019) Insights in cyanobacteria lipidomics: a sterols characterization from Phormidium autumnale biomass in heterotrophic cultivation. Food Res Int 119:777–784. https://doi.org/10.1016/j.foodres.2018.10.060
Fernández-Sevilla JM, Fernández FA, Grima EM (2010) Biotechnological production of lutein and its applications. Appl Microbiol Biotechnol 86(1):27–40
Foo SC, Yusoff FM, Ismail M, Basri M, Chan KW, Khong NM, Yau SK (2015) Production of fucoxanthin-rich fraction (FxRF) from a diatom, Chaetoceros calcitrans (Paulsen) Takano 1968. Algal Res 12:26–32. https://doi.org/10.1016/j.algal.2015.08.004
Gammone M, D'Orazio N (2015) Anti-obesity activity of the marine carotenoid fucoxanthin. Mar Drugs 13(4):2196–2214. https://doi.org/10.3390/md13042196
Gargouch N, Karkouch I, Elleuch J, Elkahoui S, Michaud P, Abdelkafi S, Fendri I (2018) Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: a powerful agent in industrial applications. Int J Biol Macromol 120:2106–2114. https://doi.org/10.1016/j.ijbiomac.2018.09.037
Gissibl A, Sun A, Care A, Nevalainen H, Sunna A (2019) Bioproducts from Euglena gracilis: synthesis and applications. Front Bioeng Biotechnol 7:108. https://doi.org/10.3389/fbioe.2019.00108
Global (2019) Global lutein market to witness a CAGR of 5.2% during 2018–2024. Available in https://www.globenewswire.com. Accessed 30 May 19
Goiris K, Muylaert K, De Cooman L (2015) Microalgae as a novel source of antioxidants for nutritional applications. In: Handbook of marine microalgae. Academic, Cambridge, pp 269–280. https://doi.org/10.1016/B978-0-12-800776-1.00017-0
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34(8):1396–1412. https://doi.org/10.1016/j.biotechadv.2016.10.005
Goo BG, Baek G, Choi DJ, Park YI, Synytsya A, Bleha R, Park JK (2013) Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta. Bioresour Technol 129:343–350. https://doi.org/10.1016/j.biortech.2012.11.077
Grudzinski W, Krzeminska I, Luchowski R, Nosalewicz A, Gruszecki WI (2016) Strong-light-induced yellowing of green microalgae Chlorella: a study on molecular mechanisms of the acclimation response. Algal Res 16:245–254. https://doi.org/10.1016/j.algal.2016.03.021
Guzman S, Gato A, Lamela M, Freire-Garabal M, Calleja JM (2003) Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 17(6):665–670. https://doi.org/10.1002/ptr.1227
Guzman-Murillo MA, Ascencio F (2000) Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett Appl Microbiol 30(6):473–478. https://doi.org/10.1046/j.1472-765x.2000.00751.x
Hahn T, Lang S, Ulber R, Muffler K (2012) Novel procedures for the extraction of fucoidan from brown algae. Process Biochem 47(12):1691–1698. https://doi.org/10.1016/j.procbio.2012.06.016
Hanna VS, Hafez EAA (2018) Synopsis of arachidonic acid metabolism: a review. J Adv Res 11:23–32. https://doi.org/10.1016/j.jare.2018.03.005
Hashimoto T, Ozaki Y, Taminato M, Das SK, Mizuno M, Yoshimura K, Kanazawa K (2009) The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr 102(2):242–248. https://doi.org/10.1017/S0007114508199007
Hsieh-Lo M, Castillo G, Ochoa-Becerra MA, Mojica L (2019) Phycocyanin and phycoerythrin: strategies to improve production yield and chemical stability. Algal Res 42:101600. https://doi.org/10.1016/j.jbiotec.2018.07.025
Hu IC (2019) Production of potential coproducts from microalgae. In: Biofuels from algae. Elsevier, Amsterdam, pp 345–358. https://doi.org/10.1016/B978-0-444-64192-2.00014-7
Hu X, Tao N, Wang X, Xiao J, Wang M (2016) Marine-derived bioactive compounds with anti-obesity effect: a review. J Funct Foods 21:372–387. https://doi.org/10.1016/j.jff.2015.12.006
Izydorczyk MS (2016) β-Glucans: measurement and processing. Module Food Sci. https://doi.org/10.1016/B978-0-08-100596-5.00148-7
Jacob-Lopes E, Maroneze MM, Deprá MC, Sartori RB, Dias RR, Zepka LQ (2018) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci. https://doi.org/10.1016/j.cofs.2018.12.003
Jagadevan S, Banerjee A, Banerjee C, Guria C, Tiwari R, Baweja M, Shukla P (2018) Recent developments in synthetic biology and metabolic engineering in microalgae towards biofuel production. Biotechnol Biofuels 11(1):185
Jayachandran M, Chen J, Chung SSM, Xu B (2018) A critical review on the impacts of β-glucans on gut microbiota and human health. J Nutr Biochem. https://doi.org/10.1016/j.jnutbio.2018.06.010
Johnson EJ (2014) Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr Rev 72(9):605–612. https://doi.org/10.1111/nure.12133
Karppi J, Laukkanen JA, Kurl S (2012) Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br J Nutr 108(1):148–154. https://doi.org/10.1017/S0007114511005332
Kavitha G, Kurinjimalar C, Sivakumar K, Kaarthik M, Aravind R, Palani P, Rengasamy R (2016) Optimization of polyhydroxybutyrate production utilizing waste water as nutrient source by Botryococcus braunii Kütz using response surface methodology. Int J Biol Macromol 93:534–542. https://doi.org/10.1016/j.ijbiomac.2016.09.019
Khanra S, Mondal M, Halder G, Tiwari ON, Gayen K, Bhowmick TK (2018) Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: a review. Food Bioprod Process 110:60–84. https://doi.org/10.1016/j.fbp.2018.02.002
Kijlstra A, Tian Y, Kelly ER, Berendschot TT (2012) Lutein: more than just a filter for blue light. Prog Retin Eye Res 31(4):303–315. https://doi.org/10.1016/j.preteyeres.2012.03.002
Kim SM, Jung YJ, Kwon ON, Cha KH, Um BH, Chung D, Pan CH (2012a) A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl Biochem Biotechnol 166(7):1843–1855
Kim SM, Kang SW, Kwon ON, Chung D, Pan CH (2012b) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: characterization of extraction for commercial application. J Korean Soc Appl Biol Chem 55(4):477–483. https://doi.org/10.1007/s13765-012-2108-3
Kim K, Ehrlich A, Perng V, Chase JA, Raybould H, Li X, Liu Y (2019) Algae-derived β-glucan enhanced gut health and immune responses of weaned pigs experimentally infected with a pathogenic E. coli. Anim Feed Sci Technol 248:114–125. https://doi.org/10.1016/j.anifeedsci.2018.12.004
Kothari R, Pandey A, Ahmad S, Kumar A, Pathak VV, Tyagi VV (2017) Microalgal cultivation for value-added products: a critical enviro-economical assessment. Biotech 7(4):243
Kovalcik A, Meixner K, Mihalic M, Zeilinger W, Fritz I, Fuchs W, Drosg B (2017) Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int J Biol Macromol 102:497–504. https://doi.org/10.1016/j.ijbiomac.2017.04.054
Kumar BR, Deviram G, Mathimani T, Duc PA, Pugazhendhi A (2019) Microalgae as rich source of polyunsaturated fatty acids. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2019.01.017
Lee JB, Hayashi T, Hayashi K, Sankawa U (2000) Structural analysis of calcium spirulan (Ca− SP)-derived oligosaccharides using electrospray ionization mass spectrometry. J Nat Prod 63(1):136–138. https://doi.org/10.1021/np990348b
Lee SY, Cho JM, Chang YK, Oh YK (2017) Cell disruption and lipid extraction for microalgal biorefineries: a review. Bioresour Technol 244(2):1317–1328. https://doi.org/10.1016/j.biortech.2017.06.038
Leney AC, Tschanz A, Heck AJ (2018) Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. FEBS J 285(1):178–187. https://doi.org/10.1111/febs.14331
Lin JH, Lee DJ, Chang JS (2015) Lutein in specific marigold flowers and microalgae. J Taiwan Inst Chem Eng 49:90–94. https://doi.org/10.1016/j.jtice.2014.11.031
Liu L, Pohnert G, Wei D (2016) Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar Drugs 14(10):191. https://doi.org/10.3390/md14100191
Ma L, Yan SF, Huang YM, Lu XR, Qian F, Pang HL, Wang X (2012a) Effect of lutein and zeaxanthin on macular pigment and visual function in patients with early age-related macular degeneration. Ophthalmology 119(11):2290–2297. https://doi.org/10.1016/j.ophtha.2012.06.014
Ma L, Dou HL, Huang YM, Lu XR, Xu XR, Qian F, Wang X (2012b) Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol 154(4):625–634. https://doi.org/10.1016/j.ajo.2012.04.014
Maadane A, Merghoub N, Ainane T, El Arroussi H, Benhima R, Amzazi S, Wahby I (2015) Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J Biotechnol 215:13–19. https://doi.org/10.1016/j.jbiotec.2015.06.400
Maeda H (2015) Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review. J Oleo Sci 56:14226. https://doi.org/10.5650/jos.ess14226
Maheshwari G, Sowrirajan S, Joseph B (2019) β-Glucan, a dietary fiber in effective prevention of lifestyle diseases–an insight. Bioact Carbohydr Diet Fibre 19:100187. https://doi.org/10.1016/j.bcdf.2019.100187
Marcati A, Ursu AV, Laroche C, Soanen N, Marchal L, Jubeau S, Michaud P (2014) Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Res 5:258–263. https://doi.org/10.1016/j.algal.2014.03.006
Market and Markets (2017) Protein expression market by type (E. Coli, mammalian, yeast, pichia, insect, baculovirus, Cell-free), products (competent cells, reagents, instruments, services), application (therapeutic, research, industrial) & end user - global forecast to 2022. Available https://www.marketsandmarkets.com/Market-Reports/protein-expression-market-180323924.html. Accessed 11 June 19
Maroneze MM, Queiroz MI (2018) Microalgal production systems with highlights of bioenergy production. In: Energy from microalgae. Springer, Cham, pp 5–34. https://doi.org/10.1007/978-3-319-69093-3_2
Maroneze MM, Siqueira SF, Vendruscolo RG, Wagner R, de Menezes CR, Zepka LQ, Jacob-Lopes E (2016) The role of photoperiods on photobioreactors–A potential strategy to reduce costs. Bioresour Technol 219:493–499. https://doi.org/10.1016/j.biortech.2016.08.003
Márquez-Escobar VA, Bañuelos-Hernández B, Rosales-Mendoza S (2018) Expression of a Zika virus antigen in microalgae: towards mucosal vaccine development. J Biotechnol 282:86–91. https://doi.org/10.1016/j.jbiotec.2018.07.025
Martin L (2015) Fucoxanthin and its metabolite fucoxanthinol in cancer prevention and treatment. Mar Drugs 13(8):4784–4798. https://doi.org/10.3390/md13084784
Martínez-Francés E, Escudero-Oñate C (2018) Cyanobacteria and microalgae in the production of valuable bioactive compounds. In: Microalgal biotechnology. IntechOpen, London. https://doi.org/10.5772/intechopen.74043
Mathimani T, Mallick N (2018) A comprehensive review on harvesting of microalgae for biodiesel–key challenges and future directions. Renew Sust Energ Rev 91:1103–1120. https://doi.org/10.1016/j.rser.2018.04.083
Matos ÂP (2017) The impact of microalgae in food science and technology. J Am Oil Chem Soc 94(11):1333–1350. https://doi.org/10.1007/s11746-017-3050-7
Matsumoto M, Hosokawa M, Matsukawa N, Hagio M, Shinoki A, Nishimukai M, Hara H (2010) Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur J Nutr 49(4):243–249
McClure DD, Luiz A, Gerber B, Barton GW, Kavanagh JM (2018) An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res 29:41–48. https://doi.org/10.1016/j.algal.2017.11.015
Minkova KM, Toshkova RA, Gardeva EG, Tchorbadjieva MI, Ivanova NJ, Yossifova LS, Gigova LG (2011) Antitumor activity of B-phycoerythrin from Porphyridium cruentum. J Pharm Res 4(5):1480–1482
Miquel-Clopés A, Bentley EG, Stewart JP, Carding SR (2019) Mucosal vaccines and technology. Clin Exp Immunol 196(2):205–214. https://doi.org/10.1111/cei.13285
Miyashita K, Hosokawa M (2018) Therapeutic effect of fucoxanthin on metabolic syndrome and type 2 diabetes. In: Nutritional and therapeutic interventions for diabetes and metabolic syndrome. Academic, Cambridge, pp 343–355. https://doi.org/10.1016/B978-0-12-812019-4.00027-1
Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M (2011) The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric 91(7):1166–1174. https://doi.org/10.1002/jsfa.4353
Morowvat MH, Ghasemi Y (2016) Evaluation of antioxidant properties of some naturally isolated microalgae: Identification and characterization of the most efficient strain. Biocatal Agric Biotechnol 8:263–269. https://doi.org/10.1016/j.bcab.2016.09.010
Nishioka M, Nakai K, Miyake M, Asada Y, Taya M (2001) Production of poly-β-hydroxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate-limited conditions. Biotechnol Lett 23(14):1095–1099
Norkus EP, Norkus KL, Dharmarajan TS, Schierle J, Schalch W (2010) Serum lutein response is greater from free lutein than from esterified lutein during 4 weeks of supplementation in healthy adults. J Am Coll Nutr 29(6):575–585. https://doi.org/10.1080/07315724.2010.10719896
Nwachukwu ID, Udenigwe CC, Aluko RE (2016) Lutein and zeaxanthin: production technology, bioavailability, mechanisms of action, visual function, and health claim status. Trends Food Sci Technol 49:74–84. https://doi.org/10.1016/j.tifs.2015.12.005
Pandeirada CO, Maricato É, Ferreira SS, Correia VG, Pinheiro BA, Evtuguin DV, Nunes C (2019) Structural analysis and potential immunostimulatory activity of nannochloropsis oculata polysaccharides. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.06.001
Pasquet V, Morisset P, Ihammouine S, Chepied A, Aumailley L, Berard JB, Lafferriere M (2011) Antiproliferative activity of violaxanthin isolated from bioguided fractionation of Dunaliella tertiolecta extracts. Mar Drugs 9(5):819–831. https://doi.org/10.3390/md9050819
Patel AK, Laroche C, Marcati A, Ursu AV, Jubeau S, Marchal L, Michaud P (2013) Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour Technol 145:345–350. https://doi.org/10.1016/j.biortech.2012.12.038
Patias LD, Fernandes AS, Petry FC, Mercadante AZ, Jacob-Lopes E, Zepka LQ (2017) Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res Int 100:260–266. https://doi.org/10.1016/j.foodres.2017.06.069
Pletikapić G, Radić TM, Zimmermann AH, Svetličić V, Pfannkuchen M, Marić D, Žutić V (2011) AFM imaging of extracellular polymer release by marine diatom Cylindrotheca closterium (Ehrenberg) Reiman & JC Lewin. J Mol Recognit 24(3):436–445. https://doi.org/10.1002/jmr.1114
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28(6):910–918. https://doi.org/10.1016/j.biotechadv.2010.08.006
Rahman A, Miller CD (2017) Microalgae as a source of bioplastics. In: Algal green chemistry. Elsevier, Amsterdam, pp 121–138. https://doi.org/10.1016/B978-0-444-63784-0.00006-0
Ramírez-Mérida LGR, Zepka LQ, Jacob-Lopes E (2017) Current production of microalgae at industrial scale. In: Recent advances in renewable energy. Bentham Science, Sharjah, pp 242–260
Raposo MFJ, Morais AMMB (2015) Microalgae for the prevention of cardiovascular disease and stroke. Life Sci 125:32–41. https://doi.org/10.1016/j.lfs.2014.09.018
Rokkaku T, Kimura R, Ishikawa C, Yasumoto T, Senba M, Kanaya F, Mori N (2013) Anticancer effects of marine carotenoids, fucoxanthin and its deacetylated product, fucoxanthinol, on osteosarcoma. Int J Oncol 43(4):1176–1186. https://doi.org/10.3892/ijo.2013.2019
Russo R, Barsanti L, Evangelista V, Frassanito AM, Longo V, Pucci L, Gualtieri P (2017) Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors. Food Sci Nutr 5(2):205–214. https://doi.org/10.1002/fsn3.383
Rwigemera A, Mamelona J, Martin LJ (2014) Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell Biol Toxicol 30(3):157–167
Rwigemera A, Mamelona J, Martin LJ (2015) Comparative effects between fucoxanthinol and its precursor fucoxanthin on viability and apoptosis of breast cancer cell lines MCF-7 and MDA-MB-231. Anticancer Res 35(1):207–219
Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K (2007) Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem 55(21):8516–8522. https://doi.org/10.1021/jf071848a
Sajilata MG, Singhal RS, Kamat MY (2008) The carotenoid pigment zeaxanthin—a review. Compr Rev Food Sci Food Saf 7(1):29–49. https://doi.org/10.1111/j.1541-4337.2007.00028.x
Sanjeewa KA, Kang N, Ahn G, Jee Y, Kim YT, Jeon YJ (2018) Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: a review. Food Hydrocoll 81:200–208. https://doi.org/10.1016/j.foodhyd.2018.02.040
Sathasivam R, Radhakrishnan R, Hashem A, Abdallah EF (2017) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2017.11.003
Schulze C, Wetzel M, Reinhardt J, Schmidt M, Felten L, Mundt S (2016) Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J Appl Phycol 28(5):2719–2725
Shanab SM, Hafez RM, Fouad AS (2018) A review on algae and plants as potential source of arachidonic acid. J Adv Res 11:3–13. https://doi.org/10.1016/j.jare.2018.03.004
Shuba ES, Kifle D (2018) Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew Sust Energ Rev 81:743–755. https://doi.org/10.1016/j.rser.2017.08.042
Sigma-Aldrich (2019) B-Phycoerythrin from Porphyridium cruentum. Available https://www.sigmaaldrich.com. Accessed 24 July 19
Singh G, Patidar SK (2018) Microalgae harvesting techniques: a review. J Environ Manag 217:499–508. https://doi.org/10.1016/j.jenvman.2018.04.010
Siripornadulsil S, Dabrowski K, Sayre R (2007) Microalgal vaccines. In: Transgenic microalgae as green cell factories. Springer, New York, pp 122–128
Soanen N, Da Silva E, Gardarin C, Michaud P, Laroche C (2016) Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour Technol 213:231–238. https://doi.org/10.1016/j.biortech.2016.02.075
Soontornchaiboon W, Kim SM (2011) Antiproliferative activities of violaxanthin extracted from microalga Chlorella ellipsoidea. In: 12th Asian Food Conference. p. 514
Specht EA, Mayfield SP (2014) Algae-based oral recombinant vaccines. Front Microbiol 5:60. https://doi.org/10.3389/fmicb.2014.00060
Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 32(10):1373–1383. https://doi.org/10.1007/s10529-010-0326-5
Spruijt J, Schipperus R, Kootstra AMJ, de Visser CLM (2015) AlgaeEconomics: bio-economic production models of micro-algae and downstream processing to produce bio energy carriers. EnAlgae Swansea University, Swansea
Srivastava N, Srivastava M, Malhotra BD, Gupta VK, Ramteke PW, Silva RN, Shukla P, Dubey KK, Mishra PK (2019) Nanoengineered cellulosic biohydrogen production via dark fermentation: a novel approach. Biotechnol Adv 37:107384
Stephen D (2016) Beta-glucan from algae: ‘it’s a simpler, more bioavailable, and better priced product’. Available https://www.foodnavigator-usa.com
Stringheta PC, Nachtigall AM, Oliveira TD, Ramos AM, Sant’ana HMP, Gonçalves MPJC (2009) Luteína: propriedades antioxidantes e benefícios à saúde. Aliment Nutr Araraquara 17(2):229–238
Su Y, Song K, Zhang P, Su Y, Cheng J, Chen X (2017) Progress of microalgae biofuel’s commercialization. Renew Sust Energ Rev 74:402–411. https://doi.org/10.1016/j.rser.2016.12.078
Sun L, Wang L, Zhou Y (2012) Immunomodulation and antitumor activities of different-molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr Polym 87(2):1206–1210. https://doi.org/10.1016/j.carbpol.2011.08.097
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37(3):133–138. https://doi.org/10.1016/j.biologicals.2009.02.005
Talero E, García-Mauriño S, Ávila-Román J, Rodríguez-Luna A, Alcaide A, Motilva V (2015) Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar Drugs 13(10):6152–6209. https://doi.org/10.3390/md13106152
Tallima H, El Ridi R (2018) Arachidonic acid: physiological roles and potential health benefits–a review. J Adv Res 11:33–41. https://doi.org/10.1016/j.jare.2017.11.004
Tang Z, Ju B, Li W, Wen S, Pu Y, Qin S (2016) One-step chromatographic procedure for purification of B-phycoerythrin from Porphyridium cruentum. Protein Expr Purif 123:70–74. https://doi.org/10.1016/j.pep.2016.01.018
Toh PS, Jau MH, Yew SP, Abed RM, Sudesh K (2008) Comparison of polyhydroxyalkanoates biosynthesis, mobilization and the effects on cellular morphology in spirulina platensis and Synechocystis sp. Uniwg J Biosci 19(2):21–38
Ummalyma SB, Gnansounou E, Sukumaran RK, Sindhu R, Pandey A, Sahoo D (2017) Bioflocculation: an alternative strategy for harvesting of microalgae–an overview. Bioresour Technol 242:227–235. https://doi.org/10.1016/j.biortech.2017.02.097
Ventura SP M, Nobre BP, Ertekin F, Hayes M, Garciá-Vaquero M, Vieira F, Palavra AMF (2018) Extraction of value-added compounds from microalgae. In: Microalgae-based biofuels and bioproducts. pp. 461–483. https://doi.org/10.1016/B978-0-08-101023-5.00019-4
Voort MPJ, VulstekeSaut E (2015) Macro-economics of algae products. Swansea University, Swansea
Weigert G, Kaya S, Pemp B, Sacu S, Lasta M, Werkmeister RM, Schmetterer L (2011) Effects of lutein supplementation on macular pigment optical density and visual acuity in patients with age-related macular degeneration. Invest Ophthalmol Vis Sci 52(11):8174–8178. https://doi.org/10.1167/iovs.11-7522
Xia S, Wang K, Wan L, Li A, Hu Q, Zhang C (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11(7):2667–2681. https://doi.org/10.3390/md11072667
Xie Y, Zhao X, Chen J, Yang X, Ho SH, Wang B, Shen Y (2017) Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour Technol 244:664–671. https://doi.org/10.1016/j.biortech.2017.08.022
Yan N, Fan C, Chen Y, Hu Z (2016) The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci 17(6):962. https://doi.org/10.3390/ijms17060962
Yim MJ, Hosokawa M, Mizushina Y, Yoshida H, Saito Y, Miyashita K (2011) Suppressive effects of amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPARγ and C/EBPα mRNA expression. J Agric Food Chem 59(5):1646–1652. https://doi.org/10.1021/jf103290f
Yong SK, Lim BH, Saleh S, Tey LH (2016) Optimisation, purification and characterisation of extracellular lipase from Botryococcus sudeticus (UTEX 2629). J Mol Catal B Enzym 126:99–105. https://doi.org/10.1016/j.molcatb.2016.02.004
Zhang Y, Liu Z, Sun J, Xue C, Mao X (2018) Biotechnological production of zeaxanthin by microorganisms. Trends Food Sci Technol 71:225–234. https://doi.org/10.1016/j.tifs.2017.11.006
Zhu L, Nugroho YK, Shakeel SR, Li Z, Martinkauppi B, Hiltunen E (2017) Using microalgae to produce liquid transportation biodiesel: what is next? Renew Sust Energ Rev 78:391–400. https://doi.org/10.1016/j.rser.2017.04.089
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dias, R.R., Severo, I.A., Deprá, M.C., Maroneze, M.M., Zepka, L.Q., Jacob-Lopes, E. (2020). The Next-Generation of Microalgae-Based Products. In: Shukla, P. (eds) Microbial Enzymes and Biotechniques. Springer, Singapore. https://doi.org/10.1007/978-981-15-6895-4_2
Download citation
DOI: https://doi.org/10.1007/978-981-15-6895-4_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-6894-7
Online ISBN: 978-981-15-6895-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)