Abstract
Βeta-glucans, widespread glucose polymers in mushrooms, yeasts, and bacteria, but rarely found in microalgae, have wide applications and high medicinal and economical potential. Some β-glucans like paramylon from algae-like Euglena gracilis are well investigated, but there is little information about the β-glucan content of microalgae. Therefore, more than 40 species of cultured microalgae have been investigated for composition of their biomass regarding to lipids, carbohydrates including β-glucans and proteins. Most of algae species showed a rather similar biomass composition of about 10 % lipids, 25 % carbohydrates, and 40 % proteins if they have been cultivated in a full medium, rather low light conditions of 50 μmol photons m−2 s−1, 12/12 h light/dark cycle under aeration and a temperature of 25 ± 2 °C. The content of β-glucans varied between 1.7 and 24.2 % of dry weight, respectively. Two microalgae, Scenedesmus ovalternus SAG 52.80 and Porphyridium purpureum SAG 1380-1d with a yield of more than 20 % of dry weight were identified as the best β-glucan producers under standard cultivation conditions. Culture optimization experiments revealed that enhanced irradiance increased the β-glucan content of Scenedesmus obtusiusculus A 189, a novel green algae isolate, from 6.4 to 19.5 %, but the β-glucan content of the green alga S. ovalternus SAG 52.80 remained unaffected (24.2 vs. 23.3 %). Nitrate starvation enhanced the β-glucan content of S. obtusiusculus A 189 from 16 to 23 % and of S. ovalternus SAG 52.80 from 23.3 to 36.7 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to their wide potential applications, algae have become more and more valuable for science, technology, and economy in the last decades. Algae can be used as food and food supplements, for extracting high value products such as astaxanthin or for the production of renewable energy. Furthermore, products of algae are on the market as health food (Rosello-Sastre 2012; Borowitzka 2013) and studies have shown that algae or algae extracts are also useable for medicinal applications, based on their anti-inflammatory (Hasegawa et al. 1999; Cavalcante-Silva et al. 2012) or immunomodulatory effects (Kralovec et al. 2005; Bitencourt et al. 2011). It is discussed that these effects could be caused by the presence of β-(1-3/1-6)-glucans (Bobadilla et al. 2013).
Paramylon, a β-(1–3)-linked glucan, common in bacterial cell walls, was also detected in Euglena sp. Sugiyama et al. (2009) described hepatoprotective effects of high-dosed paramylon against acute liver injury induced by carbon tetrachloride and showed that oral administered paramylon was able to inhibit the development of chemical-induced atopic dermatitis-like skin lesions (Sugiyama et al. 2010) in animal studies. According to Watanabe et al. (2013), paramylon is considered to have preventive effects against colon cancer in mice. Even in vitro HIV-activity of sulfated paramylon has been described (Koizumi et al. 1993).
Many other polysaccharides from algae are in use, too, for example, carragenan or alginate (Bixler and Porse 2011), but in the literature, there is not much information available about the content of β-glucans in microalgae, and only a few species have been investigated (Howard et al. 1976; Suárez et al. 2008). The economic importance of β-glucans is increasing continuously. There is a huge market for dietary products and health food and their potential as pharmaceuticals (Vo et al. 2012) and also as nutritional supplements is high. Lentinan (Zhang et al. 2011) for example is in use in anti-cancer therapy, even though β-glucans are not cytotoxic and have probably no direct anti-tumor activity. Chemical modification like introduction of sulfate- and carboxymethyl groups can lead to anti-tumor active derivatives (Wang et al. 2004). Sulfation of β-glucans with different chain length can increase the anti-tumor activity (Tao et al. 2006). Some studies have shown that β-glucans can influence the innate and adaptive immune system by targeting macrophages or T cells (Chan et al. 2009), but not all the details are understood so far. This immune activity could explain the benefit of β-glucans used in anti-cancer therapy. Other reported effects are the anti-carcinogenic activity found in some β-glucan-enriched mushroom extracts, the prevention of oncogenesis (Akramiene et al. 2007), anti-inflammatory, anti-hyperglycemia, and anti-viral activities (Zhang et al. 2007). Essential for the biological activity of β-glucans is the chain length and the higher order structure (Sletmoen and Stokke 2008), assuming that the highest immune activity can be found in mediate-long triple helical chains.
Concerning the broad potential applications of β-glucans, new biological sources should be explored. Algae cultivation is much easier, the costs are lower and the productivity is much higher than mushroom cultivation. Many mushroom species cannot be cultivated but have to be collected from nature. From this point of view, microalgae could be interesting new sources for β-glucans. Changing of carbon and nitrogen sources in the culture medium of Euglena gracilis, an important producer of paramylon, resulted in an improvement of β-glucan production (Rodríguez-Zavala et al. 2010), so that culture optimization of potential β-glucan producers seems to be a promising way to get sufficient amounts of microalgae biomass for economically effective use.
In this study, a screening of different microalgae was carried out to get an overview of composition of primary metabolites with the special focus on β-glucan content in microalgae biomass cultured under standard cultivation conditions. Changing cultivation conditions to optimize carbohydrate production as described by Markou et al. (2012) resulted in both, an increased total glucan content but also an enhanced amount of β-glucans in the biomass obtained from the green algae Scenedesmus obtusiusculus A189 and Scenedesmus ovalternus SAG 52.80 and indicates an opportunity for an upregulation of β-glucan biosynthesis.
Material and methods
Forty-seven microalgae strains, including green algae, red algae, and diatoms, were isolated from different environmental samples or were obtained from strain collections, respectively; see Table 1 for details. The unialgal strains were integrated into the culture collection of the Institute of Pharmacy, University of Greifswald.
Cultivation and harvesting
Pre-cultures of each algae species were cultivated in 200 mL BG11, SWES, Dun and f/2 media, respectively (Table 1). The media were prepared according to the instructions of the Culture Collection of Algae at Göttingen University SAG (www.epsag.uni-goettingen.de) without soil extract. SWES, Dun and f/2 were modified as follows: SWES contained NaNO3 0.5 g L−1, K2HPO4 0.05 g L−1, MgSO4 0.6 g L−1, CaCl2 0.4 g L−1, NaCl 27 g L−1 and was supplemented with 10 mL o2 medium contained additionally a trace element solution consisting of 3 g L−1 Na2EDTA, 0.6 g L−1 H3BO3, 0.2 g L−1 FeSO4·7H2O, 0.14 g L−1 MnCl2·4H2O, 0.033 g L−1 ZnSO4·7H2O, 0.0007 g L−1 Co(NO3)2·6H2O and 0.0002 g L−1 CuSO4·5H2O. Dun Medium was supplemented with 1.2 g L−1 MgSO4, 0.8 g L−1 CaCl2 and 10 mL of the trace element solution used for preparation of the modified SWES medium. Modified f/2 medium contained additionally 2.45 g L−1 MgSO4·7H2O and 1.2 g L−1 KCl. Cultures were grown in ventilated Schott Bottles (VWR, Germany) until an optical density at 750 nm (OD750) of at least 2 was reached. Afterwards the pre-culture was used to inoculate 10 parallel algae cultures in 500 mL Schott Bottles containing 200 mL medium. The starting OD750 was 0.1, irradiance was 50 μmol photons m−2 s−1 at 12/12 h light/dark cycle (Osram Lumilux Cool White 39W, Germany). Cultures were ventilated with fresh air at a flow of approx. 1.5 vvm at a temperature of 25 ± 2 °C over a cultivation time of 14 days. This standard cultivation procedure was used to grow algae in a full medium and without light-stress conditions. Every second day, 0.1 mL of the cultures was used to measure optical density for growth control. Evaporated water was refilled with sterile distilled water every second day. Cultures were harvested by centrifugation at 5700 × g for 5 min and biomass was dried by lyophilization (Vaco5, Zirbus Technology, Germany).
For the light experiments, S. obtusiusculus A 189 was cultivated under different irradiances (50, 100, 150, and 200 μmol photons m−2 s−1) with three replicates for each irradiance. Other cultivation conditions and harvesting were identical to the standard cultivation conditions as described above.
For the nitrate (N) starvation experiments, pre-cultures of S. obtusiusculus A 189 and S. ovalternus SAG 52.80 with an OD750 of at least 3 were centrifuged at 1900 × g for 2 min. The supernatant was discarded and the algae pellet resuspended in BG-11 medium with N-concentrations of 100, 75, 50, 40, 35, 30, 25, 20, 15, 10 and 5 % of the standard concentration (1.5 g L−1 NaNO3). The algae were cultivated in 500 mL Schott Bottles, each filled with 200 mL of BG-11 medium, containing different N concentrations. Three replicates were cultivated for each concentration; the whole experiment was repeated twice. The irradiance was 200 μmol photons m−2 s−1, other conditions and harvesting were identical to the screening experiments described above.
Lipid extraction
For lipid extraction, 50 mg of dried biomass was suspended in 5 mL n-hexane and treated with an ultrasonic probe (Sonopuls HD2070 and UW2070, Bandelin, Germany) for 2 min to break the cell walls. Extraction was carried out at 1000 rpm for 30 min on a magnetic stirrer (MR3001, Heidolph, Germany) utilizing stirring bars of a shape 10 × 6 mm (VWR, Germany). After this, samples were centrifuged and the supernatant was collected in tared snap cap vials (VWR, Germany). The whole procedure was repeated twice, the combined supernatants were dried overnight. Extract yield was calculated gravimetrically.
Total carbohydrates
The total carbohydrate yield was measured using a modified thymol-sulfuric acid method according to Gröger (1961). 20 mg of defatted biomass was hydrolyzed for 2 h at 100 °C with 2 N HCl. After cooling, the mixture was filled to 10.0 mL with distilled water and filtered through 0.22 μm PVDF filters (Rotilabo, Roth, Germany). 100 μL of the filtrate was diluted to 1.0 mL with distilled water. For the calibration, a glucose stock solution with a concentration of 1 mg mL−1 was diluted with distilled water to final concentrations of 0.1, 0.08, 0.06, 0.04, 0.02, and 0.01 mg mL−1. 100 μL of test or calibration solution was added to 300 μL thymol reagent (1 mg mL−1 thymol in concentrated sulfuric acid), vortexed thoroughly and heated at 110 °C for 30 min. Absorbance at 509 nm was measured in 96-well plates (Nuncleon Delta Surface, Thermo Scientific, USA) in a plate reader (Fluostar Omega, BMG Labtech, Germany). As a blank 100 μL distilled water added to 300 μL thymol reagent was used.
Total protein
Total protein yield was measured with a modified ninhydrin method according to Starcher (2001). 1.0 g of ninhydrin was dissolved in 75 mL of ethylene glycol and 12.5 mL 4 N sodium acetate buffer (272 g sodium acetate trihydrate and 100 mL glacial acetic acid filled to 500.0 mL with distilled water). Directly before use, 14 μL mL−1 of a tin chloride solution (100 mg mL−1 SnCl2 in ethylene glycol) was added and mixed well.
5.0 mg of algal biomass was hydrolyzed in 1 mL 6 N HCl for 2 h at 100 °C, diluted to 10.0 mL with distilled water and filtered through a 0.22 μm PVDF filter. 100 μL of the filtrate was diluted to 2.0 mL with distilled water. Bovine serum albumin (BSA) was used for calibration. A stock solution (10 mg mL−1 BSA) was diluted with distilled water to 100 µL to final concentrations of 1, 2, 4, 6, 8, and 10 mg mL−1. 100 μL 6 N HCl was added, heated to 100 °C for 2 h and diluted to 2.0 mL. 100 μL of each calibration level was filled to 2.0 mL with distilled water. 200 μL of every calibration and test solutions were mixed with 300 μL ninhydrin reagent and incubated at 110 °C for 40 min. 200 μL water mixed with 300 μL ninhydrin reagent was used as blank. The absorption at 575 nm was measured in 96-well-plates in a plate reader.
β-glucan yield
For determination of β-glucan content, the test kit K-YBGL 04/08 “Mushroom and Yeast Beta-Glucan” from Megazyme International Ireland, was used, following the manufacturer’s instructions.
Results and discussion
Biomass, lipids, carbohydrates, and proteins
Table 1 summarizes the yield of biomass and primary metabolites of the 47 investigated microalgae strains. Biomass yield was between 0.19 and 1.37 g L−1 after 14 days. Under standard conditions, the green algae Scenedesmus spp. and Chlorella spp. grew best. Lipid content in general was low (<10 %), only some species showed higher lipid contents e.g. Cylindrotheca fusiformis and Nannochloropsis salina with 39.1 and 27.4 %, respectively. The total carbohydrate yield varied in a wide range between N. salina (11.8 %) and Porphyridium purpureum (59.2 %), but biomass of most algae contained on average about 25 % carbohydrates. In contrast to lipid and carbohydrates, most investigated microalgae revealed more than 40 % protein in the biomass, and only the marine species such as P. purpureum, N. salina, and C. fusiformis showed distinctly lower values of <20 %.
β-glucans
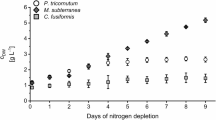
Under standard cultivation conditions, 31 of 47 algae species showed a β-glucan content between 5 and 10 % of dry weight. Phaeodactylum tricornutum and Mesotaenium caldariorum exhibited a very low value (1.7 %), but Scenedesmus ovalternus, Porphyridium purpureum, Cylindrotheca fusiformis, and Tetraselmis suecica revealed a very high β-glucan accumulation in the biomass with 24.2, 22.4, 17.9, and 16.1 %, respectively (Fig. 1a). Bobadilla et al. (2013) reported β-glucan contents between 5 and 33 % in different parts of brown alga Durvillaea antarctica, also determined with Megazyme® assay. Kozarski et al. (2011) and Synyttsya et al. (2008) investigated different fungi with this test kit and determined contents from 1 to 64 %.
α- and β-glucan content of microalgae. a Cultivation under standard conditions. b Influence of nitrate concentration on glucan content of Scenedesmus obtusiusculus A 189. c Influence of irradiance on glucan content of Scenedesmus obtusiusculus A 189. d Influence of nitrate concentration on glucan content of Scenedesmus ovalternus SAG 52.80. e Influence of irradiance on glucan content of Scenedesmus ovalternus SAG 52.80. f “β-glucan content” of cellulose, detectable with Megazyme® K-YBGL. Mean with range, n = 2 (except Fig. 1b, N5: n = 1). Statistical tests: 2way ANOVA Dunnett’s multiple comparison test; GraphPad Prism 6, California, US; *p < 0.05, **p < 0.005, ***p < 0.001 compared to control (b: 100 % N, c: 50 μmol photons m−2 s−1, d: 100 % N), only β-glucans tested
To get deeper insight into the mechanisms influencing synthesis or accumulation of primary metabolites with the special focus on β-glucans, two microalgae, Scenedesmus obtusiusculus A 189, a novel green algae isolate, and Scenedesmus ovalternus SAG 52.80 were selected for culture optimization experiments. Under standard cultivation conditions, biomass of Scenedesmus obtusiusculus A 189 contained 11.2 % total glucans of which 7.8 % were β-glucans. In cultivation of microalgae light is an important factor influencing growth and accumulation of primary metabolites in the biomass. It was reported in literature that higher irradiances led to lipid and carbohydrate accumulation in Scenedesmus obliquus (Ho et al. 2012). Gris et al. (2014) described for S. obliquus SAG 276-7 (currently Acutodesmus obliquus), a slight increase of total carbohydrate yield by increasing the irradiance from 50 to 200 μmol photons m−2 s−1. In this study, higher irradiances enhanced the β-glucan content of S. obtusiusculus A 189 significantly from 6 to 14 % under irradiation of 50 and 200 μmol photons m−2 s−1, respectively. A maximum of 19 % was reached at 150 μmol photons m−2 s−1 (Fig. 1c). Otherwise, the irradiance seems to have no influence on β-glucan yield of S. ovalternus SAG 52.80 (Fig. 1e). With 24.2 and 23.3 % (50 and 200 μmol photons m−2 s−1, respectively), the values did not distinguish significantly. Possibly SAG 52.80 has a wide light optimum, so that different irradiances do not influence the biosynthesis of β-glucans and the observed effect could be species dependent.
Under nitrate starvation, the content of total glucans of S. obtusiusculus A 189 increased from 22 % (control) to 34 % (30 % N). The β-glucan content rose from 16.0 % (100 % N, control) to 23.5 % (30 % N). At lower N-concentrations yield decreased again (Fig. 1b). For this alga, it could be shown that the combined use of higher irradiance and nitrate starvation enhanced the β-glucan content from approx. 6 to 23.5 %. Optimization experiments with S. ovalternus SAG 52.80, the alga with the highest β-glucan and the second highest total carbohydrate content under standard conditions, showed similar tendencies. The total glucan yield rose from 36 % (control) to 46 % (30 % N). The β-glucan content increased from 23.3 (control) to 36.7 % (20 % N). In contrast to A 189, the yield did not clearly decrease again under further nitrate limitation (Fig. 1d) and there was no influence of the irradiance. In both strains, SAG 52.80 and A 189 the α- and β-glucan content changed under N starvation conditions. The β-glucan content increased more than the α-glucan content, in A 189 from 6.5 to 10.3 % (α; + 3.8 %) and from 16.0 to 23.6 % (β; + 7.6 %), respectively. In SAG 52.80, the same tendency of the β-glucan content was observed (rise from 23.6 to 36.7 %), the α-glucans ranged between 6.2 and 12.8 %. These results could indicate that microalgae rather use β-glucans than α-glucans as storage carbohydrates. Recently, Li et al. (2013) described no influence of medium component starvation on accumulation of carbohydrates in cultures of Parachlorella kessleri, illuminated continuously with high irradiances of 780 μmol photons m−2 s−1. Even though growth of theses cultures was improved by aeration with 2 % CO2 in comparison to air, no differences in starch content were observed, so that CO2 limitation seems to have no influence on starch accumulation, but species differences should be considered.
According to the manufacturer’s instructions, the assay kit K-YBGL is not applicable in the presence of β-(1–4)-glucans like cellulose. Cellulose is a common cell wall component of green algae and as Ververis et al. (2007) reported, the cellulose content of mixed algal biomass was 7.1 %. Determination of pure cellulose with the assay kit showed that a content of 22.8 ± 4.3 % was detected (Fig. 1f), revealing that only approx. 1/5 to 1/4 of appearing cellulose can be hydrolyzed. Therefore, if cellulose content in algal biomass is <10 %, only 2.5 % or less of the determined β-glucans should be cellulose. In S. ovalternus SAG 52.80 for example 24.2 % β-glucans (standard conditions) were estimated with the test kit, so at least approx. 22 % should be β-(1-3/1-6)-glucans. In S. obtusiusculus A 189, at least approx. 5 % of the detected 7.8 % (standard conditions) can considered to be β-(1-3/1-6)-glucans. Taking into account these results, the test kit K-YBGL can be used also for determination of β-glucans in algal biomass but with a little inaccuracy that is reduced when the calculated β-glucan content is higher.
Forty-seven microalgae have been cultivated and analyzed for their composition of the three primary metabolites lipids, carbohydrates, and proteins. Although most algae showed very high protein yield, also species with high carbohydrate and lipid contents were identified, revealing the high diversity of biomass composition. When using the test kit K-YBGL 04/08 “Mushroom and Yeast Beta-Glucan” from Megazyme, the β-glucan content in algal biomass can be determined quick and easy. Since the presence of cellulose interfered only slightly, the kit also can be used for determination of β-glucans in microalgae. Four algae species with high β-glucan content from 16 to 24 % have been identified. Scenedesmus ovalternus SAG 52.80 was the best-growing alga and exhibited the highest β-glucan yield in the biomass. It could be shown that the β-glucan content can be influenced by nitrate starvation and irradiance, but factors such as the influence of temperature, starvation of other medium components and also CO2 should be considered. In further studies, β-glucans should be extracted, purified and the biological activity examined.
References
Akramiene D, Kondrotas A, Didziapetriene J, Kevelaitis E (2007) Effects of β-glucans on the immune system. Medicina (Kaunas) 43:597–606
Bitencourt MAO, Dantas GR, Lira DP, Barbosa-Filho JM, de Miranda GEC, de Oliveira Santos BV, Souto JT (2011) Aqueous and methanolic extracts of Caulerpa mexicana suppress cell migration and ear edema induced by inflammatory agents. Mar Drugs 10:1332–1345
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Bobadilla F, Rodriguez-Tirado C, Imarai M, Galotto MJ, Andersson R (2013) Soluble β-1,3/1,6-glucan in seaweed from the southern hemisphere and its immunomodulatory effect. Carbohydr Polym 92:241–248
Borowitzka MA (2013) High-value products from microalgae—their development and commercialisation. J Appl Phycol 25:743–756
Cavalcante-Silva LHA, da Matta CBB, de Araújo MV, Barbosa-Filho JM, de Lira DP, de Oliveira SBV, de Miranda GEC, Alexandre-Moreira MS (2012) Antinociceptive and anti-inflammatory activities of crude methanolic extract of red alga Bryothamnion triquetrum. Mar Drugs 9:1977–1992
Chan GC-F, Chan WK, Sze DM-Y (2009) The effects of β-glucan on human immune and cancer cells. J Hematol Oncol 25
Culture collection of Algae (SAG). Website of the Department EPSAG. http://www.uni-goettingen.de/en/list-of-media-and-recipes/186449.html. Accessed 1 December 2015
Gris B, Morosinotto T, Giacometti GM, Bertucco A, Sforza E (2014) Cultivation of Scenedesmus obliquus in photobioreactors: effects of light intensities and light–dark cycles on growth, productivity, and biochemical composition. Appl Biochem Biotechnol 172:2377–2389
Gröger WKL (1961) Determination of sugars in biological media with thymol in sulphuric acid. Clin Chim Acta 6:866–873
Hasegawa T, Ito K, Ueno S, Kumamoto S, Ando Y, Yamada A, Nomoto K, Yasunobu Y (1999) Oral administration of hot water extracts of Chlorella vulgaris reduces IgE production against milk casein in mice. Int J Immunopharmacol 21:311–323
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Howard RJ, Wright SW, Grant BR (1976) Structure and some properties of soluble 1,3-ß-glucan isolated from the green alga Caulerpa simpliciusculal. Plant Physiol 58:459–463
Koizumi N, Sakagami H, Utsumi A, Fujinaga S, Takeda M, Asano K, Sugawara I, Ichikawa S, Kondo H, Mori S, Miyatake K, Nakano Y, Nakashima H, Murakami T, Miyano N, Yamamoto N (1993) Anti-HIV (human immunodeficiency virus) activity of sulfated paramylon. Antivir Res 21:1–14
Kozarski M, Klaus A, Niksic M, Jakovljevic D, Helsper JPFG, Van Griensven LJLD (2011) Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem 129:1667–1675
Kralovec JA, Power MR, Liu F, Maydanski E, Ewart HS, Watson LV, Barrow CJ, Lin TJ (2005) An aqueous Chlorella extract inhibits IL-5 production by mast cells in vitro and reduces ovalbumin-induced eosinophil infiltration in the airway in mice in vivo. Int Immunopharmacol 5:689–698
Li X, Přibyl P, Bišová K, Kawano S, Cepák V, Zachleder V, Čížková M, Brányiková I, Vítová M (2013) The microalga Parachlorella kessleri—a novel highly efficient lipid producer. Biotechnol Bioeng 110:97–107
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biotechnol 96:631–645
Rodríguez-Zavala JS, Ortiz-Cruz MA, Mendoza-Hernández G, Moreno-Sánchez R (2010) Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J Appl Microbiol 109:2160–2172
Rosello-Sastre R (2012) Products from Microalgae—an overview. In: Posten, C; Walter, C: Microalgal Biotechnology: Integration and Economy. De Gruyter
Sletmoen M, Stokke BT (2008) Higher order structure of (1,3)-β-D-glucans and its influence on their biological activities and complexation abilities. Biopolymers 89:310–321
Starcher B (2001) A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem 292:125–129
Suárez ER, Bugden SM, Kai FB, Kralovec JA, Noseda MD, Barrow CJ, Grindley TB (2008) First isolation and structural determination of cyclic β-(1 → 2)—glucans from an alga, Chlorella pyrenoidosa. Carbohydr Res 343:2623–2633
Sugiyama A, Suzuki K, Mitra S, Arashida R, Yoshida E, Nakano R, Yabuta Y, Takeuchi T (2009) Hepatoprotective effects of paramylon, a β-1,3-D-glucan isolated from Euglena gracilis Z, on acute liver injury induced by carbon tetrachloride in rats. J Vet Med Sci 71:885–890. doi:10.1292/jvms.71.885
Sugiyama A, Hata S, Suzuki K, Yoshida E, Nakano R, Mitra S, Arashida R, Asayama Y, Yabuta Y, Takeuchi T (2010) Oral administration of paramylon, a β-1,3-D- glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. J Vet Med Sci 72:755–763
Synyttsya A, Mickova K, Jablonsky I, Slukova M, Copikova J (2008) Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J Food Sci 26:441–446
Tao Y, Zhang L, Cheung PCK (2006) Physicochemical properties and antitumor activities of water-soluble native and sulfated hyperbranched mushroom polysaccharides. Carbohydr Res 341:2261–2269
Ververis C, Georghiou K, Danielidis D, Hatzinikolaou DG, Santas P, Santas R, Corleti V (2007) Cellulose, hemicelluloses, lignin and ash content of some organic materials and their suitability for use as paper pulp supplements. Bioresour Technol 98:296–301
Vo T-S, Ngo D-H, Kim S-K (2012) Marine algae as a potential pharmaceutical source for anti-allergic therapeutics. Process Biochem 47:386–394
Wang Y, Zhang L, Li Y, Hou X, Zeng F (2004) Correlation of structure to antitumor activities of five derivatives of a β-glucan from Poria cocos sclerotium. Carbohydr Res 339:2567–2574
Watanabe T, Shimada R, Matsuyama A, Yuasa M, Sawamura H, Yoshida E, Suzuki K (2013) Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt foci in mice. Food Function 4:1685–1690
Zhang M, Cui SW, Cheung PCK, Wang Q (2007) Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol 18:4–19
Zhang Y, Li S, Wang X, Zhang L, Cheung PCK (2011) Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll 25:196–206
Acknowledgments
We thank Prof. Thomas Brück und Johannes Schmidt, Technical University of Munich, Department of Industrial Biocatalysis for providing of algae strains and optimizing the media for cultivation of Cylindrotheca fusiformis, Nannochloropsis salina, Phaeodactylum tricornutum, Picochlorum sp., and Porphyridium purpureum. The Federal Ministry of Education and Research is thanked for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schulze, C., Wetzel, M., Reinhardt, J. et al. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J Appl Phycol 28, 2719–2725 (2016). https://doi.org/10.1007/s10811-016-0812-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0812-9