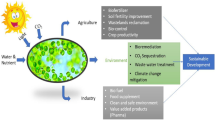
Abstract
Microalgae feature the ability to develop in different ecosystems, consequently because they are photosynthetic microorganisms with a simple structure. Recently, the interest production of microalgae-based products has increased, due to the integrity of these natural microorganisms in the production of fatty acids, lipids, carbohydrates, pigments, proteins, vitamins, antioxidants, enzymes, and bioactive molecules. It is crucial to study cultivation systems, species, and environmental factors, as they may have strong mastery over the cultivation of microalgae. Microalgae require cheap substrates, such as sunlight, temperature, and carbon dioxide, being used as affordable and effective biocatalysts to obtain products with high added value and commercial applicability (nutraceuticals, pharmaceuticals, biofuels, cosmetics, and functional foods, among others). Therefore, this chapter reports on the mechanisms of formation, production, and application of these components from microalgae (chemicals, enzymes, and bioactive molecules), in addition to providing a description of microalgae-based products, improving the application of microalgal biomass in several segments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Microalgae are considered photosynthetic microorganisms being able to grow in marine or freshwater systems with applications in industrial units (Pignolet et al. 2013). The classification of microalgae includes prokaryotic and eukaryotic microalgae (Borowitzka 2013). According to Gimpel et al. (2015), there are 40,000–70,000 species of microalgae referring to 9 classes, with species not yet discovered or classified.
As photosynthetic microorganisms, microalgae are considered valuable sources for many applications, through biomass, production of various compounds, and environmental applications. Commercial exploitation by these microorganisms has increased due to the need for reliable, efficient, and economical processes (Fernandes et al. 2015).
Microalgae are used to obtain compounds with high added value, requiring only sunlight, temperature, and carbon dioxide (CO2), for their superior growth (Vilchez et al. 1997). In addition, numerous strains of microalgae produce compounds such as lipids being possibly converted into biodiesel, and microalgae biomass is characterized by having valuable compounds, such as carbohydrates, fatty acids, pigments, proteins, vitamins, and antioxidants, favoring the transformation of these compounds into refined products for various segments (Nur and Buma 2019; Koller et al. 2014).
However, some factors influence the behavior of microalgae, such as high cost of installation and operation, difficulty in controlling culture conditions, contaminating microorganisms, unstable light supply, and local climate (Yen et al. 2013). Therefore, the classes of microalgae and their adaptation changes in climatic factors, in particular light and temperature, must be studied to obtain a successful, economical, and sustainable process (Bhalamurugan et al. 2018).
The industry is focused on expanding products for human nutrition, animal feed, aquaculture food, cosmetic products, pigments, biofertilizers, medicines, and biofuels. Notably, microalgae are producers of many important biochemicals that have not yet been discovered (Rizwan et al. 2018).
Therefore, this chapter addresses an overview of the mechanisms of formation, production, and applications of these components based on microalgae (chemicals, enzymes, and bioactive molecules). In addition, it provides a description of the microalgae-based products generated and their application in various commercial segments.
14.2 Microalgae-Based Products
14.2.1 Chemical Products
Several species of microalgae are considered promising candidates for obtaining useful materials, such as biofuels and chemicals; from this perspective, there is a great demand for more natural and sustainable products (Maeda et al. 2018).
Microalgae are microorganisms capable of accumulating macromolecules, such as proteins, lipids, and carbohydrates, through the capture of solar energy, CO2, and nutrients. Besides, they are widely used in contemporary nutraceutical foods, through their ability to synthesize aggregate products such as pigments (carotenoids), essential and non-essential amino acids, sugar, enzymes, fatty acids, essential vitamins, and minerals for human consumption (Matos 2017).
These chemical compounds of high added value can be extracted from different microalgae species, being used as bulk commodities in several industrial sectors. In order to obtain chemical products and bioactive compounds, it is essential to cultivate suitable species, together with cultivation systems and ideal conditions, to acquire the desired final product (Mata et al. 2010).
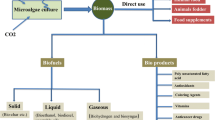
As shown in Fig. 14.1, the productivity of microalgae biomass can be directed to various industrial segments as a source of healthy food, a source of protein for fish farming, a source of animal feed (cattle, swine and poultry), production of cosmetics, medicines and biodiesel (Koller et al. 2012).
Cultivation of microalgae to generate products with high added value with different industrial segments. (Adapted from Bellou et al. (2014))
The processing of microalgae occurs in three stages: cultivation, harvesting, and extraction. However, the cultivation mode and the choice of species are of paramount importance to define the desired final product (Rizwan et al. 2018). Today, outdoor cultivation is the most economical and viable system in terms of energy and operating costs (Maeda et al. 2018).
The environmental conditions are determining factors for the development of microalgae cultures. In systems exposed to the outdoors, it is imperative to control the parameters, mainly for the generation of biomass (Eriksen 2008). Climatic factors such as carbon dioxide, sunlight, water, temperature, and nutrients are indispensable for the development of microalgae (Chisti 2007). These factors present daily and seasonal variations according to the climatic and geographic location; however, many species behave differently in the face of limiting factors (Bellou et al. 2014).
However, in systems exposed to the outdoors, it is not possible to control the temperature and light intensity, which vary during the day and throughout the year. Therefore, integration technologies and systems engineering are presented, which can be used to optimize the microalgae growth control system and, thus, thrive under ideal conditions (Zhu and Hiltunen 2016).
Notably, when choosing the biomass harvesting method, it is necessary to analyze the profile of the microalgae and their cultivation conditions. So far, the harvesting modes found are flocculation, centrifugation, filtration, sedimentation, and flotation. The capacity of the methods depends on the microalgae strains, including the size, morphology, and composition of the medium used (Japar et al. 2017). After harvesting, the biomass is subjected to the extraction process, obtaining valuable products to produce compounds with high added value (Olguín 2012). In this sense, Fig. 14.2 illustrates several methods of extraction for different chemicals obtained by microalgae.
Different chemical products based on microalgae by various extraction processes. (Adapted from Enamala et al. (2018))
More specifically, microalgae lipids are divided into storage lipids (triglycerides) and structural lipids (sterols and phospholipids) (Levasseur et al. 2020). However, the increase in lipid production for the generation of biofuels contributes to the sustainability and competitiveness of the microalgae market (Bekirogullari et al. 2017). In this perspective, biodiesel has many benefits, being able to reduce emissions of carbon monoxide, carbon dioxide, and sulfur into the atmosphere. Notably, biodiesel is biodegradable, non-toxic, and own similarities to conventional diesel, such as energy content and chemical and physical properties (Pragya et al. 2013).
Microalgae are fatty acid producers with a high degree of unsaturation and unusual chain lengths, besides to not being found in natural quantities or elsewhere (Hess et al. 2018); examples of fatty acids obtained from microalgae are arachidonic acid (AA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and linolenic acid, being useful to treat diverse disease and as a food source. Several species of microalgae feature the capacity to produce significant amounts of oils and fats, such as omega-3 and omega-6. Currently, DHA is the only microalgae PUFA produced on a commercial scale (Rizwan et al. 2018).
Carbohydrates are divided into sugars (monosaccharides) and polymers (disaccharides, oligosaccharides, and polysaccharides) (Markou et al. 2012). Some strains of microalgae have a high content of carbohydrates (starch and cellulose), being excellent substrates for the generation of bioethanol; the use of carbohydrate to obtain bioethanol becomes advantageous because microalgae proliferate and fix CO2 at a higher rate compared to other terrestrial plants (Ho et al. 2013).
Microalgal proteins are similar to food proteins, consequently, due to the excellent profile and compatibility of amino acids, they are used in the pharmaceutical industry to treat some diseases. On the other hand, proteins are defined by their low index of stability and denaturation under acidic and alkaline conditions, making extraction and separation difficult (Markou and Nerantzis 2013; Chew et al. 2017).
Microalgae feature a great capacity to produce several essential vitamins, for example, A, B1, B2, B6, B12, C, E, nicotinate, biotin, folic acid, and pantothenic acid; therefore, the index of microalgal vitamins is of high interest for application in food (Graziani et al. 2013). The number of vitamins is more concentrated in microalgae than in conventional foods (Fabregas and Herrero 1990).
14.2.2 Bioactive Molecules
Bioactive molecules are biologically active substances presenting desirable features in human health. Currently, there is growing interest in the generation of bioactive molecules from natural products through the use of microalgae biomass, driven by a growing body of research demonstrating the beneficial approaches of bioactive molecules to health (Ejike et al. 2017).
The market for bioactive food compounds by microalgae is an opportunity in the segment of bioactive molecules, dominated by synthetic substances and sources of animals and plants (Jacob-Lopes et al. 2019). This composition of biomolecules can be designated as a bioproduct, rich in macro- and micronutrients. Thus, studies show microalgae are an innovation biotechnological applications in industrial sectors related to biofuel, chemical, pharmaceutical, cosmetics, and food (Rodrigues et al. 2015; de Morais et al. 2020). Table 14.1 demonstrates the main bioactive molecules extracted from microalgae (Table 14.1).
These photosynthetic microorganisms can accumulate significant natural bioactive compounds. Among these molecules, natural pigments are the most exciting components produced. Its main classes of phytonutrients are carotenoids, chlorophyll, and phycobiliproteins (Rodrigues et al. 2015). Derivatives of carotenoids can be isolated, and the main include is neoxanthin, violaxanthin, lutein, zeaxanthin, canthaxanthin, mixoxanthophyll, echinenone, (all-E)-α-carotene, (all-E)-β-carotene, and also its isomeric structures (Z). Derived pigments that are produced only by microalgae are echinenone, mixoxanthophyll, and canthaxanthin with antioxidant potential (Nascimento et al. 2020a).
The β-carotene is well known to have the highest provitamin A activity (Raposo et al. 2013a). Natural pigments have beneficial health-related properties. Their antioxidant activity balances the harmful effects of free radicals that have been associated with reduced risk of developing several degenerative diseases (da Silva Vaz et al. 2016).
The studies promising pharmacological action bioactivity of chlorophylls compounds is during the photodynamic therapy. There is also, evidences supporting that the role of chlorophyll derivatives can rebalance the gut microbiota (Zepka et al. 2019).
Phycobiliproteins are pigments hydrophilic protein complexes found in microalgae with highly sensitive fluorescent properties that are comprised of C-phycocyanin, phycoerythrin, and allophycocyanin and thus can be used as a detector for specific pharmacological analysis (Levasseur et al., 2020).
Microalgal proteins are sources of alternative nutrition and easy digestibility, acting as antioxidants and antimicrobials, thereby alternative to a healthy diet due to their bioactive peptide, amino acid, fatty acid, and phycobiliprotein content (Zepka et al. 2010). Therefore, when are inserted into a diet, compounds become bio-based decreasing body weight and preventing diet-induced obesity (Patias et al. 2018). The Spirulina species, helps in the treatment of many diseases as a result of its exceptional antioxidant, antibacterial, anti-tumor, immunoprotection, and anti-inflammatory properties and also reduces appetite and improves food absorption (Moradi et al. 2019).
The microalgae strain can have a wide range of sterols, from cholesterol to β-sitosterol. These compounds become important due to their antioxidant, anticarcinogenic and anti-inflammatory activity (Fagundes et al. 2020).
Microalgal polysaccharides produce original biopolymers with unique structures and composition to obtain sulfate esters, which are referred to as sulfated polysaccharides (carrageenan, ulvan, and fucoidan), and exhibit various bioactivities, such as antiviral, antioxidant, and anti-inflammatory activities. The production of macromolecules represents high-value products with applications in cosmetics, emulsifiers, food, fabrics, medicines, and stabilizers. Studies are being proposed to use microalgal polysaccharides as a promising prebiotic fiber source (Tang et al. 2020).
But also, according to Lafarga et al. (2020), the microalgae contains a wide spectrum of prophylactic and pharmaceutical phytonutrients including excellent sources of vitamins and minerals. Additionally, there is a lot of attractive biochemical profile that needs better exploited, being the enzymes. Among microorganisms, microalgae become a promising source for future research (Rocha et al. 2018).
14.3 Microalgae Enzymes
The potential application of microalgal biomass extends beyond the bioproducts established to date. There is still great untapped timeliness for utilizing this resource. Indeed, the synthesis of enzymes by microalgae has been recently proposed as a potential niche for the generation of amylases, proteases, lipases, peroxidases, laccases, phytases, and galactosidases (Brasil et al. 2017; Ellatif et al. 2020; Spier et al. 2020).
Amylases belong to a series of glycohydrolase enzymes acting the carbohydrate hydrolysis reaction (Azzopardi et al. 2016; Mohanan and Satyanarayana 2019). Amylases were the first enzymes employed for industrial processes, with large-scale production. Its global market value was estimated at US $ 1.6 billion in 2020, with the largest commercial share of 25%–30% (Mehta and Satyanarayana 2016; Cripwell et al. 2020). Thus, amylases are applied in numerous segments, including the food industry (e.g., in the cheese ripening, baking, chocolate, infant cereal, and brewing and as flavoring), the pharmaceutical industry (high-fructose syrups), the textile and paper industries, and the manufacture of detergents and bioethanol (Brasil et al. 2017; Cripwell et al. 2020; Spier et al. 2020).
Among the enzymes described in microalgae, amylases are the least reported; this is due to their autotrophic metabolism (Patil et al. 2001). However, the species Chlorella sorokiniana, Chlamydomonas reinhardtii, Dallina parva, Dunaliella tertiolecta, Dunaliella marina, Klebsormidium sp., Oedogonium sp., Rhizoclonium sp., Rhizoclonium hieroglyphicum, Scenedesmus obliquus, and Spirogyra sp. demonstrated amylase activity (Kombrink and Weober 1980; Levi and Gibbs 1984; Patil and Mahajan 2016; Manoj et al. 2018).
Proteases are enzymes that catalyze hydrolytic reactions, resulting in the cleavage of protein molecules into peptides and amino acids and representing the second largest group in market volume. Proteases are extensively exploited in the cleaning, food, and textile manufacturer (Aguilar and Sato 2018; Sharma et al. 2017).
Microalgae studies have shown that protease activity may be related to environmental factors, such as luminosity or nutrient restriction, nitrogen source, and cell apoptosis (Brasil et al. 2017; Spier et al. 2020). Niven (1995) determined the influence of different nutrient sources on the protease activity in Anabaena variabilis. In turn, Lockau et al. (1988) and Strohmeier et al. (1994) explored the same microorganisms and their dependence on calcium in the production of protease. Moreover, protease activity has also been observed in Chlorella vulgaris and Arthrospira platensis (Nanni et al. 2001; Yada et al. 2005; Silva et al. 2017).
Lipases are important biocatalysts due to their capability to hydrolyze triglyceride into fatty acids and glycerol. Accordingly, lipases have attracted commercial attention, falling only behind amylases and proteases in terms of global enzyme sales. The technical features of these enzymes have enabled its introduction in numerous applications in the food, animal feed, pharmaceutical, detergent, paper, cellulose, and bioremediation industries (Brasil et al. 2017; Almeida et al. 2020; Spier et al. 2020).
The lipases investigated in Botryococcus sudeticus and Isochrysis galbana have promising characteristics for industrial applications, such as substrate specificities, pH endurance (pH 5–11), and temperature resistance (40–70 °C). Furthermore, microalgae species Arthrospira platensis and Nannochloropsis oceanica also demonstrated the activity of this enzyme (Demir and Teukel 2010; Godet et al. 2012; Savvidou et al. 2016; Yong et al. 2016; Brasil et al. 2017; Hubert et al. 2017; Spier et al. 2020).
Peroxidases are antioxidant enzymes that catalyze the redox reaction for various substrates. Therefore, peroxidases are deliberated a valuable catalyst for several medicinal, industrial, and bioremediation applications (e.g., decolorization of synthetic textile effluents) (Medina et al. 2016). The peroxidases activity was observed in some microalgae strains, as Coelastrella sp., Dunaliella tertiolecta, Galdieria sulphuraria, Euglena gracilis, Phaeodactylum tricornutum, Rhizoclonium sp., Oedogonium sp., and Porphyridium purpureum (Overbaugh and Fall 1982; Overbaugh and Fall 1985; Murphy et al. 2000; Oesterhelt et al. 2008; Baldev et al. 2013).
The laccase enzyme, act on the oxidation of complex substrates (e.g., phenols and aliphatic or aromatic amines) with the concurrent reduction of a molecule of oxygen and releasing water molecules (Li et al. 2020; Spier et al. 2020). Laccases are widely involved in bioremediation processes of brewing effluents, paper, textile, and pulp (Brasil et al. 2017). Thus, the species T. aeria and C. moewusii are investigated for the biodegradation of phenolic pollutants in industrial wastewaters (Otto et al. 2015). Moreover, these enzymes have also been described in Phormidium valderianum, Arthrospira platensis, and Oscillatoria boryana (Otto et al. 2010; Afreen et al. 2017; Ellatif et al. 2020).
Phytase enzymes catalyze the hydrolysis of phytate through a series of myo-inositol phosphate intermediate compounds and inorganic phosphate. The phytase own several applications in the industries, mostly in the food manufacturer, where they are used in the elaboration of animal feed, aiming at cost reduction, minimizing the environmental impact, increasing the phosphorus bioavailability, and decreasing the anti-nutrition effect of phytate in monogastric animals (Handa et al. 2020; Sharma et al. 2020).
Due to the commercial appeal of this enzyme, the transgenic microalgae Chlamydomonas reinhardtii were studied for the exploration of phytase at a suitable pH and gastrointestinal temperature that can be applied as food supplements. However, other investigated species, such as C. thermalis Geitler, S. bigranulatus Skuja, and S. lividus, also demonstrated phytase activity (Klanbut et al. 2002; Erpel et al. 2016).
Galactosidases are a family of glycoside hydrolase enzymes that further the hydrolysis the glycosidic bonds (Naumoff 2011; Vidya et al. 2020). The enzymes α-galactosidase and β-galactosidase are important glycoside hydrolases with employment in the food, feed, and pharmaceutical industries (Husain 2010; Zhao et al. 2018). The enzyme α-galactosidase aims to hydrolyze the α-galactosyl (α 1–6 linkages) terminal moieties of glycolipids and glycoproteins, whereas, β-galactosidase clive the D-galactosyl (β 1–4 linkages) residues from oligosaccharides or polymers (Spier et al. 2020; Vidya et al. 2020).
In microalgae, α-galactosidase activity was observed in Poterioochromonas malhamensis as a metabolic result of external osmotic pressure (Dey and Kauss 1981). On the other hand, the microalgae C. minutissima, D. tertiolecta, N. oculata, S. obliquus, and T. obliquus demonstrated the formation of β-galactosidase (Davies et al. 1994; Girard et al. 2014; Bentahar et al. 2018; Suwal et al. 2019; Zanette et al. 2019).
Therefore, microalgae can metabolize a wide pool of enzymes, proving how these species are versatile. However, the microalgae are still little explored in comparison to other microorganisms, but numerous enzymes are being investigated and can be applied in various sectors of the industry (Brasil et al. 2017; Spier et al. 2020).
14.4 Industrial Applications of Microalgae
Due to innumerable scientific studies, microalgae have shown great potential as an alternative source for several operations through the bio-refining procedure. Today, microalgae are applied in various industrial sectors, due to their high survival skills in aggressive environments of temperature, pH, light intensity, salinity, and accelerated growth rate (Bhattacharya and Goswami 2020; Tang et al. 2020; Geada et al. 2017). Microalgae are promising for the generation of biodiesel and other products, including feed, nutraceuticals, and food (Giordano and Wang 2018; Rahman 2020).
The world trade in algae biomass is estimated at about US $ 3.8 to 5.4 billion, and approximately 7000 tons of dry algae biomass are manufactured globally (Brasil et al. 2017). In addition, the data indicate that the algae trade is becoming increasingly popular and has the potential to be applied to various branches of the industrial sector afterward (Tang et al. 2020). Today, the United States, Asia, and Oceania control the microalgae generation trade. Despite this, research indicates that Europe is likely to become a significant powerhouse in the field of microalgae bioproducts in the future (Rahman 2020).
Currently, the introduction of synthetic compounds in food, cosmetics, and pharmaceutical products is occurring excessively, becoming emerging issues. Thus, it can cause damage to health, including some allergic reactions and hyperactivity. Therefore, consumers are increasingly demanding and tend to use more natural products, developed from non-toxic resources, hence the emergence of microalgae, as an option for sustainable production and natural sources. Thus, in the market of various sectors of the industry, such as food, beverages, nutritional supplements, and pharmaceutical products, they are implementing bioproducts based on microalgae of the species Chlorella sp. and Spirulina sp. (Tang et al. 2020). Simultaneously, the species Dunaliella and Arthrospira (Spirulina sp.) also have great potential for numerous commercial uses, as a component for the preparation of various products, not only focusing on the finished product; therefore, the use of microalgae in different sectors of the industry is related to the biomass parameters and structure related to each microalgae (Junior et al. 2020). Thinking in this context, Table 14.2 shows the products and uses of microalgae biomass in different sectors of the industry (Table 14.2).
In fact, microalgae biomass is capable of being used in many industrial sectors. Thus, they are used as a food source, offering a high quality of protein, superior to vegetables. At the same time, microalgae also produce sterols that are used in pharmaceutical sectors as medicine for cardiovascular diseases and microalgae extracts used in cosmetics (Rizwan et al. 2018).
About 200 years ago, the Chinese began to implement microalgae as a food source, given the hunger crisis in their country (Geada et al. 2018). Currently, they are used as food in Asian countries, due to their high nutritional value (Chen et al. 2016; Hong et al. 2015; Um and Kim 2009). According to Tang et al. (2020), a commercial product that uses microalgae in its preparation is M&M chocolate, where Spirulina sp. biomass is used as a natural dye. In addition, some establishments produce cooking oil using the technique related to microalgae, generating healthier cooking oil. However, despite efforts to implement microalgae as human food, safety regulations and high manufacturing costs make implementation unfeasible. Consequently, it is in the animal feed trade that microalgae biomass is used, because of its nutritional content and health-related advantages. As a result, biomass is generally marketed in dry or wet mode (Geada et al. 2018; Raja et al. 2016).
In the cosmetics area, the company Daniel Jouvance applies microalgae in the production of its products, due to the potential of microalgae to generate compounds that offer essential benefits for the skin (Tang et al. 2020). In addition, extracts derived from Spirulina sp. and Chlorella sp. are used as compounds in sunscreens. Therefore, it helps to combat sunburn and ultraviolet radiation (Jha et al. 2017).
In the pharmacology sector, representatives who use algae to develop their products include Agri Life SOM, Phytopharma (India) Limited, Piramal Healthcare, Rincon Pharmaceuticals, and Novo Nordisk India Private Ltd, since microalgae synthesize treated substances for the administration of anticancer drugs. Therefore, microalgae use substances of great importance where it is possible to use them for different uses in medical treatments that can be introduced in the development of new drug technologies for the elimination of diseases, specifically in incurable pathologies (Tang et al. 2020).
Through research related to microalgae so far, they demonstrate their development potential in numerous environmental and industrial applications. However, tests are needed to solve some challenges still encountered for microalgae industrialization technologies, such as high installation and operating costs, microbial contamination of the environment, and light and climate conditions, reaching an imbalance. In that regard, researchers must focus on research related to the processing of microalgae, assessing its potential as a raw material with high promising capacity in biotechnological processes, as well as carrying out tests and technological studies on life cycle assessment, thus obtaining results to prove the economic and sustainable viability concerning microalgae-based processing models (Caporgno and Mathys 2018; Rizwan et al. 2018).
14.5 Conclusions and Future Perspectives
The diversity of microalgal products confirms the excellent performance of these microorganisms in the manufacture of various chemical products, enzymes, and bioactive molecules. The components present in microalgae are precious, with a wide range of applicability, such as human and animal nutrition, biofuels, pharmaceuticals, and cosmetics. In order to obtain these compounds, a more detailed study of cultivation conditions, species, and mainly climatic factors is necessary. Compared to other microorganisms, microalgae have benefits in terms of cost-effectiveness, efficiency, and sustainability.
Microalgae should be exploited among the best strains that produce compounds such as pigments, carbohydrates, lipids, fatty acids, proteins, vitamins, antioxidants, and enzymes. However, parameters that interfere with crop growth, such as climatic factors, must be better analyzed so that the number of desired compounds is produced.
Therefore, the commercial-scale generation of microalgae becomes an economical source, encouraging the manufacture of new products developed and commercialized in the next decade. Until the moment, genetic modifications are being studied to increase the production yield of these microorganisms.
In the near future, new research is expected to endeavor to reduce product losses and thus reduce equipment and energy costs. Also, large-scale processing should be further developed, making processes economically viable and environmentally friendly.
References
Afreen S, Shamsi TN, Baig MA et al (2017) Um romance multicopper oxidase (lacase) de cianobactérias: purificação, caracterização com potencial na descoloração de corante antraquinônico. PLoS One 12:1–20. https://doi.org/10.1371/journal.pone.0175144
Aguilar JGS, Sato HH (2018) Microbial proteases: production and application in obtaining protein hydrolysates. Food Res Int 103:253–262. https://doi.org/10.1016/j.foodres.2017.10.044
Almeida JM, Alnoch RC, Souza EM et al (2020) Metagenomics: is it a powerful tool to obtain lipases for application in biocatalysis? BBA-Proteins Proteom 1868(2):140320. https://doi.org/10.1016/j.bbapap.2019.140320
Apt KE, Behrens PW (1999) Commercial developments in microalgal biotechnology. J Phycol 35(2):215–226. https://doi.org/10.1046/j.1529-8817.1999.3520215.x
Azzopardi E, Lloyd C, Teixeira SR et al (2016) Clinical applications of amylase: novel perspectives. Surgery 160(1):26–37. https://doi.org/10.1016/j.surg.2016.01.005
Baldev E, MubarakAli D, Ilavarasi A et al (2013) Degradation of synthetic dye, rhodamine B to environmentally non-toxic products using microalgae. Colloids Surf B Biointerfaces 105:207–214. https://doi.org/10.1016/j.colsurfb.2013.01.008
Bekirogullari M, Fragkopoulos IS, Pittman JK et al (2017) Production of lipid-based fuels and chemicals from microalgae: an integrated experimental and model-based optimization study. Algal Res 23:78–87. https://doi.org/10.1016/j.algal.2016.12.015
Bellou S, Baeshen MN, Elazzazy AM et al (2014) Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol Adv 32(8):1476–1493. https://doi.org/10.1016/j.biotechadv.2014.10.003
Bentahar J, Doyen A, Beaulieu L et al (2018) Investigation of β-galactosidase production by microalga Tetradesmus obliquus in determined growth conditions. J Appl Phycol 31:301–308. https://doi.org/10.1007/s10811-018-1550-y
Bhalamurugan GL, Valerie O, Mark L et al (2018) Valuable bioproducts obtained from microalgal biomass and their commercial applications: a review. Environ Eng Res 23(3):229–241. https://doi.org/10.4491/eer.2017.220
Bhattacharya M, Goswami S (2020) Microalgae-a green multi-product biorefinery for future industrial prospects. Biocatal Agric Biotechnol 101580. https://doi.org/10.1016/j.bcab.2020.101580
Borowitzka MA (2013) High-value products from microalgae-their development and commercialisation. J Appl Phycol 25(3):743–756. https://doi.org/10.1007/s10811-013-9983-9
Brasil BSAF, Siqueira FG, Salum TFC et al (2017) Microalgae and cyanobacteria as enzyme biofactories. Algal Res 25:76–89. https://doi.org/10.1016/j.algal.2017.04.035
Caporgno MP, Mathys A (2018) Trends in microalgae incorporation into innovative food products with potential health benefits. Front Nutr 5:58. https://doi.org/10.3389/fnut.2018.00058
Chen J, Wang Y, Benemann JR et al (2016) Microalgal industry in China: challenges and prospects. J Appl Phycol 28(2):715–725. https://doi.org/10.1007/s10811-015-0720-4
Chew KW, Yap JY, Show PL et al (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Chu WL, Phang SM, Miyakawa K et al (2002) Influence of irradiance and inoculum density on the pigmentation of Spirulina platensis. Asia Pac J Mol Biol Biotechnol 10(2):109–117
Cripwell R A, Van Zyl WH, Viljoen-Bloom M (2020) Fungal biotechnology: fungal amylases and their applications. Elsevier. doi: https://doi.org/10.1016/b978-0-12-809633-8.21082-0
Davies CM, Apte SC, Peterson SM et al (1994) Plant and algal interference in bacterial β-D-galactosidase and β-D-glucuronidase assays. Appl Environ Microbiol 60:3959–3964. https://doi.org/10.1128/AEM.60.11.3959-3964.1994
Demir BS, Teukel SS (2010) Purification and characterization of lipase from Spirulina platensis. J Mol Catal B Enzym 64:123–128. https://doi.org/10.1016/j.molcatb.2009.09.011
Dey PM, Kauss H (1981) α-Galactosidase of Poterioochromonas malhamensis. Phytochemistry 20:45–48. https://doi.org/10.1016/0031-9422(81)85216-8
Ejike CE, Collins SA, Balasuriya N et al (2017) Prospects of microalgae proteins in producing peptide-based functional foods for promoting cardiovascular health. Trends Food Sci Tech 59:30–36. https://doi.org/10.1016/j.tifs.2016.10.026
Ellatif SA, El-Sheekh MM, Senousy HH (2020) Role of microalgal ligninolytic enzymes in industrial dye decolorization. Int J Phytoremediation:1–12. https://doi.org/10.1080/15226514.2020.1789842
Enamala MK, Enamala S, Chavali M et al (2018) Production of biofuels from microalgae-a review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew Sust Energ Rev 94:49–68. https://doi.org/10.1016/j.rser.2018.05.012
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30(9):1525–1536. https://doi.org/10.1007/s10529-008-9740-3
Erpel F, Restovic F, Arce-Johnson P (2016) Development of phytase-expressing Chlamydomonas reinhardtii for monogastric animal nutrition. BMC Biotechnol 16(1):1–7. https://doi.org/10.1186/s12896-016-0258-9
Fabregas J, Herrero C (1990) Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J Ind Microbiol 5(4):259–263. https://doi.org/10.1007/BF01569683
Fagundes MB, Vendruscolo RG, Wagner R (2020) In: Jacob-Lopes E, Maroneze MM, Queiroz MI, Zepka LQ (eds) Sterols from microalgae. Handbook of microalgae-based processes and products. Academic Press. p 573–596. doi:https://doi.org/10.1016/b978-0-12-818536-0.00021-x.
Fernandes BD, Mota A, Teixeira JA et al (2015) Continuous cultivation of photosynthetic microorganisms: approaches, applications and future trends. Biotechnol Adv 33(6):1228–1245. https://doi.org/10.1016/j.biotechadv.2015.03.004
Geada P, Vasconcelos V, Vicente A et al (2017) In: Rastogi RP, Madamwar D, Pandey A (eds) Microalgal biomass cultivation. Algal green chemistry. Elsevier. p 257-284. doi: 10.1016/B978-0-444-63784-0.00013-8.
Geada P, Rodrigues R, Loureiro L et al (2018) Electrotechnologies applied to microalgal biotechnology-applications, techniques and future trends. Renew Sust Energ Rev 94:656–668. https://doi.org/10.1016/j.rser.2018.06.059
Gimpel JA, Henríquez V, Mayfield SP (2015) In the metabolic engineering of eukaryotic microalgae: potential and challenges come with great diversity. Front Microbiol 6:1376. https://doi.org/10.3389/fmicb.2015.01376
Giordano M, Wang Q (2018) In: Vaz S Jr (ed) Microalgae for industrial purposes, Biomass and green chemistry. Springer, Cham, pp 133–167. https://doi.org/10.1007/978-3-319-66736-2_6
Girard J-M, Roy M-L, Hafsa MB et al (2014) Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res 5:241–248. https://doi.org/10.1016/j.algal.2014.03.002
Godet S, Herault J, Pencreach G et al (2012) Isolation and analysis of a gene from the marine microalga Isochrysis galbana that encodes a lipase-like protein. J Appl Phycol 24:1547–1553. https://doi.org/10.1007/s10811-012-9815-3
Graziani G, Schiavo S, Nicolai MA et al (2013) Microalgae as human food: chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct 4(1):144–152. https://doi.org/10.1039/C2FO30198A
Handa V, Sharma D, Kaur A et al (2020) Biotechnological applications of microbial phytase and phytic acid in food and feed industries. Biocatal Agri Biotechnol 25:101600. https://doi.org/10.1016/j.bcab.2020.101600
Hess SK, Lepetit B, Kroth PG et al (2018) Production of chemicals from microalgae lipids–status and perspectives. Eur J Lipid Sci Tech 120(1):1700152. https://doi.org/10.1002/ejlt.201700152
Ho SH, Huang SW, Chen CY et al (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198. https://doi.org/10.1016/j.biortech.2012.10.015
Hong JW, Jo SW, Yoon HS (2015) Research and development for algae-based technologies in Korea: a review of algae biofuel production. Photosynth Res 123(3):297–303. https://doi.org/10.1007/s11120-014-9974-y
Hubert F, Poisson L, Loiseau C et al (2017) Lipids and lipolytic enzymes of the microalga Isochrysis galbana. Oilseeds fats crop lipids 24: D407. Doi: 10.1051 / ocl / 2017023
Husain Q (2010) β galactosidases and their potential applications: a review. Crit Rev Biotechnol 30:41–62. https://doi.org/10.3109/07388550903330497
Jacob-Lopes E, Maroneze MM, Deprá MC et al (2019) Bioactive food compounds from microalgae: an innovative framework on industrial biorefineries. Curr Opin Food Sci 25:1–7. https://doi.org/10.1016/j.cofs.2018.12.003
Japar AS, Takriff MS, Yasin NHM (2017) Harvesting microalgal biomass and lipid extraction for potential biofuel production: a review. J Environ 5(1):555–563. https://doi.org/10.1016/j.jece.2016.12.016
Jha D, Jain V, Sharma B et al (2017) Microalgae based pharmaceuticals and nutraceuticals: an emerging field with immense market potential. Chembioeng Rev 4(4):257–272. https://doi.org/10.1002/cben.201600023
Joshi S, Kumari R, Upasani VN (2018) Applications of algae in cosmetics: an overview. Int J Innov Res Sci Eng Technol 7(2):1269. https://doi.org/10.15680/IJIRSET.2018.0702038
Junior WGM, Gorgich M, Corrêa PS et al (2020) Microalgae for biotechnological applications: cultivation, harvesting and biomass processing. Aquac 735562. https://doi.org/10.1016/j.aquaculture.2020.735562
Klanbut K, Peerapornpisarn Y, Khanongnuch C et al (2002) Phytase from some strains of thermophilic blue-green algae. J Phycol S2:57–60
Koller M, Salerno A, Tuffner P et al (2012) Characteristics and potential of micro algal cultivation strategies: a review. J Clean Prod 37:377–388. https://doi.org/10.1016/j.jclepro.2012.07.044
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6:52–63. https://doi.org/10.1016/j.algal.2014.09.002
Kombrink E, Weober G (1980) Identification and subcellular localization of starch-metabolizing enzymes in the green alga Dunaliella marina. Planta 149:130–137. https://doi.org/10.1007/BF00380873
Lafarga T, Fernández-Sevilla JM, González-López C et al (2020) Spirulina for the food and functional food industries. Food Res Int 109356. https://doi.org/10.1016/j.foodres.2020.109356
Lee YK (1997) Commercial production of microalgae in the Asia-Pacific rim. J Appl Phycol 9(5):403–411. https://doi.org/10.1023/A:1007900423275
Levasseur W, Perré P, Pozzobon V (2020) A review of high value-added molecules production by microalgae in light of the classification. Biotechnol Adv 107545. https://doi.org/10.1016/j.biotechadv.2020.107545
Levi C, Gibbs M (1984) Starch degradation in synchronously grown Chlamydomonas reinhardtii and characterization of the amylase. Plant Physiol 74:459–463. https://doi.org/10.1104/pp.74.3.459
Li X, Li S, Liang X et al (2020) Applications of oxidases in modification of food molecules and colloidal systems: laccase, peroxidase and tyrosinase. Trends Food Sci Tech 103:78–93. https://doi.org/10.1016/j.tifs.2020.06.014
Lockau W, Massalsky B, Dirmeier A (1988) Purification and partial characterization of a calcium-stimulated protease from the cyanobacterium, Anabaena variabilis. Eur J Biochem 172:433–438. https://doi.org/10.1111/j.1432-1033.1988.tb13906.x
Maeda Y, Yoshino T, Matsunaga T et al (2018) Marine microalgae for production of biofuels and chemicals. Curr Opin Biotech 50:111–120. https://doi.org/10.1016/j.copbio.2017.11.018
Manoj BS, Sushma CM, Karosiya A (2018) Western Ghats terrestrial microalgae serve as a source of amylase and antioxidants enzymes. J Pharmacogn Phytochem 7:1555–1560
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31(8):1532–1542. https://doi.org/10.1016/j.biotechadv.2013.07.011
Markou G, Angelidaki I, Georgakakis D (2012) Microalgal carbohydrates: an overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl Microbiol Biot 96(3):631–645. https://doi.org/10.1007/s00253-012-4398-0
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14(1):217–232. https://doi.org/10.1016/j.rser.2009.07.020
Matos ÂP (2017) The impact of microalgae in food science and technology. J Am Oil Chem Soc 94(11):1333–1350. https://doi.org/10.1007/s11746-017-3050-7
Medina JDC, Woiciechowski AL, Guimarães LRC et al (2016) In: Pandey a, Negi S, Soccol CR (eds) peroxidases. Current developments in biotechnology and bioengineering: production, isolation and purification of industrial products. Elsevier, p. 217-232. doi: https://doi.org/10.1016/B978-0-444-63662-1.00010-5
Mehta D, Satyanarayana T (2016) Bacterial and archaeal α-amylases: diversity and amelioration of the desirable characteristics for industrial applications. Front Microbiol 7:1129. https://doi.org/10.3389/fmicb.2016.01129
Mobin S, Alam F (2017) Some promising microalgal species for commercial applications: a review. Energy Procedia 110:510–517. https://doi.org/10.1016/j.egypro.2017.03.177
Mohanan N, Satyanarayana T (2019) Amylases. In: Schmidt TM (ed) Encyclopedia of microbiology. Fourth, p. 107-126
Moradi S, Ziaei R, Foshati S et al (2019) Effects of spirulina supplementation on obesity: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med 47:102211. https://doi.org/10.1016/j.ctim.2019.102211
de Morais WGJ, Gorgich M, Corrêa PS et al (2020) Microalgae for biotechnological applications: cultivation, harvesting and biomass processing. Aquac 735562. https://doi.org/10.1016/j.aquaculture.2020.735562
Murphy CD, Moore RM, White RL (2000) Peroxidases from marine microalgae. J Appl Phycol 12:507–513. https://doi.org/10.1023/A:1008154231462
Nanni B, Balestreri E, Dainese E et al (2001) Characterization of a specific Phycocyanin hydrolysing protease purified from Spirulina platensis. Microbiol Res 156:259–266. https://doi.org/10.1078/0944-5013-00110
Nascimento TC, Cazarin CBB, Maróstica JMR et al (2020a) Microalgae carotenoids intake: influence on cholesterol levels, lipid peroxidation and antioxidant enzymes. Food Res Int 128:108770. https://doi.org/10.1016/j.foodres.2019.108770
Nascimento TC, Nass PP, Fernandes AS et al (2020b) Exploratory data of the microalgae compounds for food purposes. Data Brief 29:105182. https://doi.org/10.1016/j.dib.2020.105182
Naumoff DG (2011) Hierarchical classification of glycoside hydrolases. Biochem Mosc 76:622–635. https://doi.org/10.1134/s0006297911060022
Niven GW (1995) The characterization of two aminopeptidase activities from the cyanobacterium Anabaena flosaquae. Biochim Biophys Acta Protein Struct Mol Enzymol 1253:193–198. https://doi.org/10.1016/0167-4838(95)00175-0
Nur MMA, Buma AG (2019) Opportunities and challenges of microalgal cultivation on wastewater, with special focus on palm oil mill effluent and the production of high value compounds. Waste Biomass Valorization 10(8):2079–2097. https://doi.org/10.1007/s12649-018-0256-3
Oesterhelt C, Vogelbein S, Shrestha RP et al (2008) The genome of the thermoacidophilic red microalga Galdieria sulphuraria encodes a small family of secreted class III peroxidases that might be involved in cell wall modification. Planta 227:353–362. https://doi.org/10.1007/s00425-007-0622-z
Olguín EJ (2012) Dual purpose microalgae-bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol Adv 30(5):1031–1046. https://doi.org/10.1016/j.biotechadv.2012.05.001
Otto B, Schlosser D, Reisser W (2010) First description of a laccase-like enzyme in soil algae. Arch Microbiol 192:759–768. https://doi.org/10.1007/s00018-009-0169-1
Otto B, Beuchel C, Liers C et al (2015) Laccase-like enzyme activities from chlorophycean green algae with potential for bioconversion of phenolic pollutants. FEMS Microbiol Lett 362:1–8. https://doi.org/10.1093/femsle/fnv072
Overbaugh JM, Fall R (1982) Detection of glutathione peroxidases in some microalgae. FEMS Microbiol Lett 13:371–375. https://doi.org/10.1111/j.1574-6968.1982.tb08290.x
Overbaugh JM, Fall R (1985) Characterization of a selenium-independent glutathione peroxidase from Euglena gracilis. Plant Physiol 77:437–442. https://doi.org/10.1104/pp.77.2.437
Patias LD, Maroneze MM, Siqueira SF et al (2018) Single-cell protein as a source of biologically active ingredients for the formulation of Antiobesity foods. Alternative and Replacement Foods:317–353. https://doi.org/10.1016/b978-0-12-811446-9.00011-3
Patil K, Mahajan RT (2016) Enzymatic study of fresh water macro and micro algae isolated from Jalgaon, Maharashtra. Int J Pharm Bio Sci 7:207–215. https://doi.org/10.4172/2155-952X.C1.068
Patil KJ, Mahajan RT, Mahajan SR et al (2001) Enzyme profile of fresh water uncultured algae belonging to Bhusawal region, Maharashtra. J Chem biosphere 2(1): 33-28. Doi: https://doi.org/10.20546/ijcrbp.2016.301.013
Pignolet O, Jubeau S, Vaca-Garcia C et al (2013) Highly valuable microalgae: biochemical and topological aspects. J Ind Microbiol Biot 40(8):781–796. https://doi.org/10.1007/s10295-013-1281-7
Pragya N, Pandey KK, Sahoo PK (2013) A review on harvesting, oil extraction and biofuels production technologies from microalgae. Renew Sust Energ Rev 24:159–171. https://doi.org/10.1016/j.rser.2013.03.034
Rahman KM (2020) In: Alam MA, Jing-Liang X, Zhongming W (eds) Food and high value products from microalgae: market opportunities and challenges. Microalgae biotechnology for food, health and high value products. Springer, Singapore, . p 3-27. doi: 10.1007/978-981-15-0169-2_1
Raja R, Hemaiswarya S, Ganesan V et al (2016) Recent developments in therapeutic applications of cyanobacteria. Crit Rev Microbiol 42(3):394–405. https://doi.org/10.3109/1040841X.2014.957640
Raposo MFJ, de Morais RMSC, de Morais AMMB (2013a) Health applications of bioactive compounds from marine microalgae. Life Sci 93(15):479–486. https://doi.org/10.1016/j.lfs.2013.08.002
Raposo MFDJ, De Morais RMSC, Bernardo de Morais AMM (2013b) Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs 11(1):233–252. https://doi.org/10.3390/md11010233
Rizwan M, Mujtaba G, Memon SA et al (2018) Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renew Sust Energ Rev 92:394–404. https://doi.org/10.1016/j.rser.2018.04.034
Rocha CM, Genisheva Z, Ferreira-Santos P et al (2018) Electric field-based technologies for valorization of bioresources. Bioresour Technol 254:325–339. https://doi.org/10.1016/j.biortech.2018.01.068
Rodrigues DB, Menezes CR, Mercadante AZ et al (2015) Bioactive pigments from microalgae Phormidium autumnale. Food Res Int 77:273–279. https://doi.org/10.1016/j.foodres.2015.04.027
Savvidou MG, Sotiroudis TG, Kolisis FN (2016) Cell surface and cellular debris-associated heat-stable lipolytic enzyme activities of the marine alga Nannochloropsis oceanica. Biocatal Biotransformation 34:24–32. https://doi.org/10.1080/10242422.2016.1212843
Sharma KM, Kumar R, Panwar S et al (2017) Microbial alkaline proteases: optimization of production parameters and their properties. J Genet Eng Biotechnol 15(1):115–126. https://doi.org/10.1016/j.jgeb.2017.02.001
Sharma A, Ahluwalia O, Tripathi AD et al (2020) Phytases and their pharmaceutical applications: mini-review. Biocatal Agric Biotechnol 23:101439. https://doi.org/10.1016/j.bcab.2019.101439
da Silva Vaz B, Moreira JB, de Morais MG et al (2016) Microalgae as a new source of bioactive compounds in food supplements. Curr Opin Food Sci 7:73–77. https://doi.org/10.1016/j.cofs.2015.12.006
Silva PEC, Souza FASD, Barros RC et al (2017) Enhanced production of fibrinolytic protease from microalgae Chlorella vulgaris using glycerol and corn steep liquor as nutrient. Ann Microbiol Res 1: 9-19. Doi: 10.36959/958/564
Spier MR, Peron-schlosser B, Paludo LC et al (2020) Microalgae as enzymes biofactories. In: Jacob-Lopes E, Queiroz MI, Maroneze MM, Zepka LQ (eds) Handbook of microalgae-based processes and products. Academic, New York, pp 687–706. https://doi.org/10.1016/B978-0-12-818536-0.00025-7
Spolaore P, Joannis-Cassan C, Duran E et al (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96. https://doi.org/10.1263/jbb.101.87
Strohmeier U, Gerdes C, Lockau W (1994) Proteolysis in heterocyst-forming cyanobacteria: characterization of a further enzyme with trypsin-like specificity, and of a prolyl endopeptidase from Anabaena variabilis. Z Naturforsch C J Biosci 49:70–78. https://doi.org/10.1515/znc-1994-1-212
Suwal S, Bentahar J, Marciniak A et al (2019) Evidence of the production of galactooligosaccharide from whey permeate by the microalgae Tetradesmus obliquus. Algal Res 39:101470. https://doi.org/10.1016/j.algal.2019.101470
Tang DYY, Khoo KS, Chew KW et al (2020) Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour Technol 304:122997. https://doi.org/10.1016/j.biortech.2020.122997
Um BH, Kim YS (2009) A chance for Korea to advance algal-biodiesel technology. J Ind Eng Chem 15(1):1–7. https://doi.org/10.1016/j.jiec.2008.08.002
Vidya CH, Kumar BS, Chinmayee CV et al (2020) Purification, characterization and specificity of a new GH family 35 galactosidase from Aspergillus awamori. Int J Biol Macromol 156:885–895. https://doi.org/10.1016/j.ijbiomac.2020.04.013
Vilchez C, Garbayo I, Lobato MV et al (1997) Microalgae-mediated chemicals production and wastes removal. Enzyme Microb Technol 20(8):562–572. https://doi.org/10.1016/S0141-0229(96)00208-6
Vonshak A (1997) Microalgal biotechnology: new development in production facilities and products. In 2: Asia-Pacific marine biotechnology conference and 3. Asia-Pacific conference on algal biotechnology, Phuket (Thailand)
Yada E, Nagata H, Noguchi Y et al (2005) An arginine specific protease from Spirulina platensis. Mar Biotechnol 7:474–480. https://doi.org/10.1007/s10126-004-4115-9
Yen HW, Hu IC, Chen CY et al (2013) Microalgae-based biorefinery-from biofuels to natural products. Bioresour Technol 135:166–174. https://doi.org/10.1016/j.biortech.2012.10.099
Yong SK, Lim BH, Saleh S et al (2016) Optimisation, purification and characterization of extracellular lipase from Botryococcus sudeticus (UTEX 2629). J Mol Catal B Enzym 126:99–105. https://doi.org/10.1016/j.molcatb.2016.02.004
Zanette CM, Mariano AB, Yukawa YS et al (2019) Microalgae mixotrophic cultivation for β-galactosidase production. J Appl Phycol 31:1597. https://doi.org/10.1007/s10811-018-1550-y
Zepka LQ, Jacob-Lopes E, Goldbeck R et al (2010) Nutritional evaluation of single-cell protein produced by Aphanothece microscopica Nägeli. Bioresour Technol 101(18):7107–7111. https://doi.org/10.1016/j.biortech.2010.04.001
Zepka LQ, Jacob-Lopes E, Roca M (2019) Catabolism and bioactive properties of chlorophylls. Curr Opin Food Sci 26:94–100. https://doi.org/10.1016/j.cofs.2019.04.004
Zhao R, Zhao R, Tu Y et al (2018) A novel α-galactosidase from the thermophilic probiotic Bacillus coagulans with remarkable protease-resistance and high hydrolytic activity. PLoS One 13:e0197067. https://doi.org/10.1371/journal.pone.0197067
Zhu LD, Hiltunen E (2016) Application of livestock waste compost to cultivate microalgae for bioproducts production: a feasible framework. Renew Sust Energ Rev 54:1285–1290. https://doi.org/10.1016/j.rser.2015.10.093
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Lasta, P., da Silva, P.A., Caetano, P.A., Pinheiro, P.N., Zepka, L.Q., Jacob-Lopes, E. (2022). Microalgae Application in Chemicals, Enzymes, and Bioactive Molecules. In: Inamuddin, Ahamed, M.I., Prasad, R. (eds) Application of Microbes in Environmental and Microbial Biotechnology. Environmental and Microbial Biotechnology. Springer, Singapore. https://doi.org/10.1007/978-981-16-2225-0_14
Download citation
DOI: https://doi.org/10.1007/978-981-16-2225-0_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-2224-3
Online ISBN: 978-981-16-2225-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)