Abstract
Background
Fucoxanthin isolated from edible seaweeds and its metabolite fucoxanthinol have been recently found to have anti-obesity effects, but the mechanism is not fully understood.
Aim of study
We investigated the effects of these carotenoids on the absorption of triglycerides in conscious rats implanted with cannulae into a lymph duct and the portal or jugular vein.
Methods
A duodenal infusion of 1 ml of test oil emulsion with or without 2 mg of fucoxanthin or fucoxanthinol was administered in the lymph duct and the portal (Experiment 1) or the jugular vein (Experiment 2) cannulated rats. The test oil contained 10% soybean oil (Experiment 1) and pre-digested 10% soybean oil (Experiment 2). The inhibitory activities of these carotenoids on pancreatic lipase activity were measured in vitro.
Results
Increases in lymphatic and blood triglyceride levels were much lower in the two carotenoid-treated groups than in the carotenoid-free group, indicating that these carotenoids inhibit triglyceride absorption. The total amounts of triglycerides released into the lymph after 4 h in the carotenoid-free, fucoxanthin and fucoxanthinol groups were 113.5, 59.4 and 53.1 μmol, respectively. The inhibitory effects of carotenoids were completely abolished after an infusion of pre-digested soybean oil containing carotenoids. Furthermore, these carotenoids inhibited pancreatic lipase activity in vitro. Regarding absorptive route, we found that fucoxanthinol, but not fucoxanthin, appeared in lymph fluid, whereas neither carotenoid was detected in portal blood.
Conclusion
These results show that these two marine carotenoids inhibit lipase activity in the gastrointestinal lumen and suppress triglyceride absorption, and fucoxanthin was converted to fucoxanthinol in the intestine and released into the lymph.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fucoxanthin is a major marine carotenoid found in edible seaweeds, such as Undaria pinnatifida and Sargassum fulvellum, and is metabolised to fucoxanthinol in vivo [1]. Its structure, including an allelic bond and a 5,6-monoepoxide [7, 14], differs from those of common carotenoids, such as β-carotene and lycopene. Fucoxanthin and fucoxanthinol are reported to exert anti-carcinogenic [10] and anti-inflammatory effects [22] as well as apoptotic effects in cancer cells [7, 11] and radical scavenging activity [19]. The beneficial effects of long-term ingestion of fucoxanthin and fucoxanthinol in anti-obesity studies have also been reported. Recently, Hosokawa et al. [13] showed that dietary fucoxanthin and fucoxanthinol suppress increases in white adipose tissue (WAT) in KKAy mice, a model of obesity and diabetes. However, the effects of these carotenoids on postprandial serum triglyceride (TG) levels have not been investigated.
Hypertriglyceridaemia is a risk factor for cardiovascular disease and is part of the definition of metabolic syndrome [9]. Consequently, the suppression of postprandial hypertriglyceridaemia may be an effective solution to prevent metabolic syndrome. Orlistat is an agent clinically used for the management of obesity. This drug strongly inhibits pancreatic lipase activity and suppresses lipid absorption [8]. However, this inhibitory activity is so intense that the absorption of some vitamins may also be inhibited. Furthermore, orlistat treatment may result in a greater amount of faecal fat in the large intestine, which increases the risk of various large intestinal disorders, such as carcinogenesis [4, 5]. Plant polyphenols have also been reported to mildly suppress postprandial hypertriglyceridaemia in humans and animals [25, 27]. A mixture of catechins dose dependently inhibited pancreatic lipase activity in vitro and suppressed the increase in postprandial serum TG levels in rats and mice [26].
The aims of this study were to examine the effects of fucoxanthin and fucoxanthinol on TG absorption from emulsified soybean oil and to elucidate the absorption and metabolism of these carotenoids using rats with lymph duct and portal vein cannulae. To elucidate a mechanism for the effects of carotenoids on the absorption of TG, digested soybean oil was administered to rats cannulated into the jugular vein and the inhibitory activities of these carotenoids on pancreatic lipase activity were measured in vitro.
Materials and methods
Chemicals
Fucoxanthin was extracted from U. pinnatifida as described previously [7]. Fucoxanthinol was prepared from fucoxanthin by hydrolysis with porcine pancreas lipase, according to a previous report [14]. All reagents and chemicals not specified were of the highest commercially available grade.
Preparation of test emulsions for duodenal infusion
To prepare the non-pre-digested test emulsion used in Experiments 1 and 2, soybean oil (100 mg/ml in saline; Wako Pure Chemicals, Osaka, Japan) and sodium taurocholate (10 mg/ml in saline) were emulsified with or without carotenoid (2 mg/ml fucoxanthin or fucoxanthinol) using a supersonic wave crusher (150 W for 90 s; SONICATOR 5202, Ohtake Seisakusyo, Aichi, Japan). The pre-digested soybean oil used in Experiment 2 was prepared as follows [18]: a lipid emulsion containing 100 mg of soybean oil and 10 mg of taurocholate in 2.5 ml of 50 mM Tris buffer (pH 8.2, containing 20 mM CaCl2) was hydrolysed with porcine pancreatic lipase (activity for TG hydrolysis, 750–1,400 units/g; Wako Pure Chemicals) at 37 °C for 4 h. The hydrolysis products were extracted with five volumes of chloroform–methanol (2:1, v/v) according to the method of Folch et al. [3]. The complete hydrolysis of TG was confirmed by thin-layer chromatography (TLC) [18]. The extracts were then dried and re-emulsified with or without each carotenoid in saline.
Animals and diets
Male Wistar ST rats (280–300 g; Japan SLC, Shizuoka, Japan) were used. The rats were kept as described by Matsumoto et al. [15]. This study was approved by the Hokkaido University Animal Committee. The animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals.
Experiment 1: lymphatic and portal absorption of TG and carotenoids
Thirty acclimated rats, weighing 280-300 g, were implanted with portal, lymph, and duodenal cannulae under sodium pentobarbital anaesthesia (40 mg/kg body weight Nembutal; Abbott, North Chicago, IL, USA). The portal cannula (polyethylene tube, sp 28; i.d., 20.4 mm; o.d., 0.8 mm; Natsume Seisakusyo, Tokyo, Japan) was directly inserted into the portal vein [19]. The lymph duct cannula (SV-35; i.d., 0.5 mm; o.d., 0.8 mm; Natsume Seisakusyo) was implanted into the thoracic lymph duct [18], and the duodenal cannula (silicon tube, Silascon No.00; Kaneka Medix, Tokyo, Japan) was inserted through an intestinal fistula at 1 cm distal to the pylorus [15]. After the operation, the rats were placed in individual restraining cages. An iso-osmotic solution containing 139 mmol/l glucose and 85 mmol/l NaCl was infused continuously at a rate of 3 ml/h through a duodenal catheter during the 24-h recovery period and experimental periods, except during the infusion of test emulsion. The rats that had been operated on were allowed to recover for 24 h. Then, we confirmed whether the lymph flow was at least 1 ml/h. Of the 30 operated rats, 24 were in good condition and were used for the experiments.
After the 24-h recovery period, 1 ml of test emulsion containing soybean oil with or without each carotenoid (2 mg/ml fucoxanthin or fucoxanthinol) was infused into the duodenum for 2 min, and portal blood (0.3 ml) was collected before and at 15, 30, 60, 90, 120, and 240 min after infusion, and then centrifuged to isolate serum. Lymph fluid was collected in a test tube before and at 30, 60, 90, 120, 180, and 240 min after infusion. The collected serum and lymph were frozen immediately and kept at −80 °C until subsequent analyses. TG concentrations in the lymph fluid were measured via enzymatic assay (TG-EN; Kainos Laboratories, Tokyo, Japan).
Experiment 2: absorption of pre-digested test emulsions
Thirty-six acclimated rats, weighing 280-300 g, were implanted with cannulae into the jugular vein and duodenum under sodium pentobarbital anaesthesia (40 mg/kg body weight Nembutal; Abbott, North Chicago). A cannula (silicon tube, Silascon No.00) was inserted directly into the right jugular vein, and the duodenal cannula was implanted in the same manner as described in Experiment 1. After a 24-h recovery period, 1 ml of test emulsion containing soybean oil or pre-digested soybean oil with or without each carotenoid (2 mg/ml fucoxanthin or fucoxanthinol) was infused into the duodenum for 2 min, and jugular blood (0.3 ml) was collected before and at 15, 30, 60, 90, 120, and 240 min after infusion, and then centrifuged to isolate serum. Serum TG concentrations were measured via enzymatic assay (TG-EN; Kainos Laboratories).
Effect of fucoxanthin and fucoxanthinol on lipase activity in vitro
Pancreatic lipase activity was determined according to the rate of release of oleic acid from triolein [12]. Pancreatic juice was collected from three acclimated rats under pentobarbital anaesthesia through a catheter inserted into the bile-pancreatic duct. The pancreatic juice was diluted tenfold in buffer containing 125 mmol/l NaCl, 4 mmol/l KCl, and 30 mmol/l HEPES at pH 7.0. A suspension of 90 μmol of triolein and 9.45 μmol of taurocholate in 9 ml of HEPES was sonicated for 3 min (triolein emulsion). Fucoxanthin, fucoxanthinol or orlistat (positive control) at concentrations of 0.02-2 μmol/ml were added to the triolein emulsion. The test mixture (200 μl), consisting of 100 μl of pancreatic juice and 100 μl of triolein emulsion, was then incubated at 37 °C for 30 min. The amount of oleic acid released was determined using a commercially available kit (NEFA kit; Wako Pure Chemicals).
HPLC–MS analysis of serum and lymph samples
Fucoxanthin and fucoxanthinol in serum and lymph samples (100 μl) were extracted as described by Matsumoto et al. [15]. A high-performance liquid chromatography (HPLC) system was fitted with a 5-μm C-18 Waters Puresil TM column (150 × 4.6 mm2; Waters), and the temperature was maintained with the column oven set at 40 °C. Absorbance was monitored at 450 nm. Solvent A (water:methanol:trifluoroacetic acid, 70:30:0.1) and B (methanol:trifluoroacetic acid, 100:0.1) were run at a flow rate of 0.2 ml/min using a linear gradient from 10 to 30% for solvent B for 20 min, then reduced linearly to 10% for solvent B for the next 5 min and maintained at the initial condition. Fucoxanthin, fucoxanthinol, and their metabolites were then identified and quantified with a mass spectrometry (MS) system equipped with an electric spray ionisation (ESI) interface (QCC, Finiganmat, Milford, MA, USA). The temperatures of the capillary heater and the vaporisation heater were maintained at 100 and 350 °C, respectively. The flow rate of the sheath gas (nitrogen) was 60 arb. ESI–MS was carried out in scan mode from m/z: 50 to 2,000 [M + H]+ and in selected ion monitoring (SIM) mode m/z: 659 [M + H]+ for fucoxanthin and m/z: 640 [M + H]+ for fucoxanthinol.
Statistics
The concentrations of fucoxanthin and fucoxanthinol were calculated from the peak area of each mass spectrum in combination with calibration curves. In area under the curve (AUC) analysis, the values were calculated areas from which the region is between the curve of the TG concentrations and elapsed time. This value was used for the index as absorbed TG. Statistical analyses were performed using one-way analysis of variance (ANOVA). Values are expressed as the mean ± standard error of the mean (SEM). Tukey–Kramer tests were used for comparisons amongst groups. A difference of p < 0.05 was considered statistically significant. All data were analysed using STATCEL2 (OMS, Saitama, Japan), which is an add-in application for Excel (Microsoft, Redmond, WA, USA).
Results
Effect of carotenoids on lymph flow rate and absorption of TG
The lymph flow rate of the control group reached a peak value at 60 min after infusion of the test emulsion (Fig. 1a). Notably, the increase in lymph flow rate was slower in the fucoxanthin- and fucoxanthinol-treated groups than in the control group. The lymph flow rates at 60 min after infusion in the fucoxanthin group and at 30 and 60 min after infusion in the fucoxanthinol group were significantly lower than those in the control group. However, total lymph flow (0-240 min) did not differ amongst groups (Fig. 1b).
Changes in lymph flow rate (a), total lymph flow (b), lymphatic TG output (c), and total lymphatic TG output (d) in rats receiving a duodenal infusion of 1 ml of 10% soybean oil, with or without 2 mg of fucoxanthin or fucoxanthinol (Experiment 1). Lymph was collected at 30, 60, 90, 120, 180, and 240 min after the infusion of each emulsion. Values represent the mean ± SEM (n = 6). Statistical analyses were performed using one-way ANOVA. Differences amongst treatment groups were analysed using the Tukey–Kramer test and were considered significant at p < 0.05
Lymphatic TG absorption reached a peak value at 90 min in the control group and at 120 min in both carotenoid-treated groups (Fig. 1c). Compared to the control, the lymphatic TG absorption rates at 60 and 90 min in the fucoxanthin group and at 60, 90, and 120 min in the fucoxanthinol group were significantly lower. The total lymphatic absorption of TG (0-240 min) in the carotenoid-treated groups was less than half that observed in the control group (Fig. 1d).
Effect of carotenoids on TG increase in systemic blood after infusion with pre-digested TG
Triglyceride concentrations in jugular blood continued to increase until 30 min after the infusion of non-pre-digested soybean oil with or without fucoxanthin or fucoxanthinol (Fig. 2a). In rats infused with the control emulsion, serum TG concentrations at each time point from 30 to 120 min were significantly higher than those of carotenoid-treated rats. In AUC analysis (0–240 min) for serum TG concentrations, the AUC values for the carotenoid-treated groups were significantly lower than those of the control group (Fig. 2b).
Changes in TG concentration in jugular blood after infusion with non-pre-digested soybean oil (a) and subsequent AUC values (b). TG concentrations in jugular blood after infusion with pre-digested soybean oil (c) and subsequent AUC values (d) in rats infused with fucoxanthin or fucoxanthinol emulsion into the duodenum (Experiment 2). Jugular blood was collected at 15, 30, 60, 90, 120, 180, and 240 min after the infusion of each carotenoid emulsion (2 mg fucoxanthin or fucoxanthinol in 1 ml of 10% soybean oil or pre-digested soybean oil). Values represent the mean ± SEM (n = 6). Statistical analyses were performed using one-way ANOVA. Differences amongst treatment groups were analysed using the Tukey–Kramer test and were considered significant at p < 0.05
After the infusion of pre-digested TG emulsion, serum TG concentrations in the control and two carotenoid-treated groups reached peak values at 15 min (Fig. 2c), but no significant difference was observed amongst groups at any time point or in terms of AUC values (Fig. 2d).
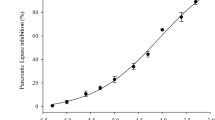
Effect of fucoxanthin and fucoxanthinol on pancreatic lipase activity in vitro
The hydrolytic activity of rat pancreatic lipase on triolein emulsion was analysed in the presence of each carotenoid or orlistat (Fig. 3). Fucoxanthin and fucoxanthinol inhibited the hydrolysis of triolein. The molecular concentrations corresponding to 50% inhibition of enzymatic activity (IC50) by both carotenoids (660 and 764 nmol/l, respectively) were approximately 100-fold higher than the IC50 of orlistat (6.8 nmol/l). However, the inhibitory activities of fucoxanthin and fucoxanthinol on rat lipase activity were similar.
Lymphatic and portal absorption of fucoxanthin and fucoxanthinol
Intact fucoxanthin was not detected in either lymph fluid or portal blood at any time point after the infusion of a lipid emulsion including fucoxanthin. However, fucoxanthinol was detected in the lymph, but not in portal blood, after the infusion of an emulsion containing fucoxanthin or fucoxanthinol into the duodenum. A broad peak was detected very near to the peak of fucoxanthinol. The m/z of this peak was 640 [M + H], which is identical to that of fucoxanthinol, and was assumed to represent isomerised fucoxanthinol in the intestine.
The concentration of fucoxanthinol and isomerised fucoxanthinol quantified by HPLC–MS in the lymph increased gradually and reached a peak value at 120 min (Fig. 4a, b). The amounts of fucoxanthinol and isomerised fucoxanthinol released into the lymph at 240 min after the infusion of fucoxanthin (3.1 μmol) or fucoxanthinol (3.2 μmol) were 0.33 ± 0.14 and 0.44 ± 0.05 μmol, respectively.
Changes in fucoxanthin, fucoxanthinol, and isomerised fucoxanthinol released into the lymph in rats receiving a duodenal infusion of fucoxanthin (a) or fucoxanthinol (b) (Experiment 1). Lymph was collected at 30, 60, 90, 120, 180, and 240 min after infusion (2 mg of fucoxanthin or fucoxanthinol in 1 ml of 10% soybean oil per rat). Values represent the mean ± SEM (n = 6). Statistical analyses were performed using one-way ANOVA. Differences amongst treatment groups were analysed using the Tukey–Kramer test and were considered significant at p < 0.05
Discussion
The present study demonstrates that two carotenoids, fucoxanthin and fucoxanthinol, derived from edible seaweed strongly suppress TG absorption. Two approaches were used to investigate the effects of fucoxanthin and fucoxanthinol on TG absorption. One approach was to measure the lymphatic release of TG and the other was to measure TG increase in systemic blood. In the study of lymphatic release, the quantitative aspects of TG absorption were observed. Polyphenols from oolong tea (500 mg/kg) are reported to suppress the release of TG into lymph fluid by 53% [26]. In this study, fucoxanthin and fucoxanthinol (2 mg) decreased the total amount of TG released into the lymph by 50% for 4 h, showing that the suppressive effects of marine carotenoids are more potent than those of tea catechins. In contrast to the lymph results, systemic blood TG concentrations have no quantitative aspect, but can be used to examine absorption under physiological conditions. TG concentrations in the blood increased much faster than those in the lymph after lipid infusion, which may reflect the fact that jugular vein-cannulated rats can be examined under conditions more representative of the physiological norm than lymph duct-cannulated rats. The former rats were able to move freely [6], whereas the latter could not, which may influence gut physiology, including intestinal motility and mucosal blood flow. Using pre-digested oil (Experiment 2), the peak TG concentrations in blood were observed just 15 min after infusion, which reflects a very rapid absorption of lipids without digestion and also reflects absorption under physiological conditions [16].
Both carotenoids inhibited the increase in blood TG concentrations by 50% from 30 to 60 min after infusion, as described above. When fucoxanthin and fucoxanthinol were infused with pre-digested soybean oil, this inhibitory effect was completely abolished, demonstrating that the suppression of TG absorption in the gastrointestinal tract is due to the effects of these carotenoids on TG digestion. Both carotenoids inhibited rat pancreatic lipase activity in vitro. Therefore, carotenoids suppress TG absorption via the inhibition of pancreatic lipase in the intestinal lumen. Although the inhibitory capacity of these carotenoids was much lower than that of orlistat, an anti-obesity agent used in clinical practice, orlistat treatment is associated with side effects, such as diarrhoea. Therefore, carotenoids may be a safer, milder alternative to prevent postprandial hyperlipidemia. Ingested fat is usually emulsified in the stomach or upper small intestine, which is important for TG digestion by lipase [21]. Catechin is reported to inhibit emulsification in the gastrointestinal tract and to suppress lipid absorption [20, 23]. Thus, it may be informative to examine the effects of fucoxanthin and fucoxanthinol on emulsification.
Fucoxanthin and fucoxanthinol absorption in the lymph and portal blood was also examined. After an infusion of fucoxanthin or fucoxanthinol, fucoxanthinol and its isomer were detected in lymph fluid, which is the first observation for lymphatic absorption of these carotenoids. Until now, fucoxanthinol and amarouciaxanthin A have only been detected in circulating blood 1 h after the intake of fucoxanthin in mice and humans [2, 24]. Fucoxanthin was metabolised to fucoxanthinol by Caco-2 cells, but was metabolised to amarouciaxanthin by HepG2 cells [1]. Taken together, these results indicate that fucoxanthin may be converted to fucoxanthinol in the intestine.
Previous studies have demonstrated that highly polar lipids, such as medium chain fatty acids, are released into portal blood, but not into the lymph [17]. In this study, fucoxanthinol, a metabolite of fucoxanthin, was detected only in lymph and was not detected in the portal vein. Regarding this, it is thought that fucoxanthinol is readily contained in chylomicrons, because of its low molecular polarity, and is released into lymph. Part of the free fucoxanthinol is thought to be released into the portal vein. Although the detection limit of HPLC–MS used in this study is 1 fmol and is capable of measuring a small amount of carotenoids, none was detected in this experiment. Compared with the amount of fucoxanthinol released into lymph, it was thought that the amount released into the portal vein was very small.
No previous study has reported that other carotenoids suppress the absorption of TG. Although other carotenoids are thought to have the same effect, fucoxanthin and fucoxanthinol have distinct structures, which might affect lipid metabolism. Further study should examine the relationship between molecular structure and lipid metabolism.
Fucoxanthinol inhibits adipocyte differentiation in 3T3-L1 cells, and this effect was more potent than that of fucoxanthin [14]. However, in this study, the amount of fucoxanthinol released into the lymph after a fucoxanthin infusion was very similar to that after fucoxanthinol itself. Furthermore, the inhibitory effects of fucoxanthinol and fucoxanthin on lipase activity were similar. Thus, the putative physiological effects of fucoxanthinol may also be achieved via the infusion of fucoxanthin. The actual mechanism for fucoxanthin- and fucoxanthinol-mediated inhibition requires further investigation.
The maximum concentration that can emulsify fucoxanthin and fucoxanthinol was used in the injection volume of these carotenoids. One-tenth of the additive amount in vivo suppressed TG degradation in vitro. Although the amount injected, which is less than that used in this experiment, should have the same effect, further study is needed. As 10 kg of dry seaweed contains only a few milligrams of fucoxanthin, it would be difficult to consume sufficient seaweed to have the effect observed in this experiment. Thus, marine carotenoids should be provided in food supplements.
In conclusion, fucoxanthin or fucoxanthinol inhibited lymphatic TG absorption and suppressed the increase in TG concentration in systemic blood. These effects were likely the result of lipase inhibition in the gastrointestinal lumen. We also found that fucoxanthin is converted into fucoxanthinol in the gastrointestinal tract and released into the lymph. Our results indicate that these carotenoids may be useful to safely and mildly suppress lipid absorption in patients with hypertriglyceridaemia.
References
Asai A, Sugawara T, Ono H, Nagao A (2004) Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab Dispos 32:205–211
Asai A, Yonekura L, Nagao A (2008) Low bioavailability of dietary epoxyxanthophylls in humans. Br J Nutr 100:273–277
Folch J, Lees M, Sloane Stanley GH (1956) A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Garcia SB, Barros LT, Turatti A, Martinello F, Modiano P, Ribeiro-Silva A, Vespúcio MV, Uyemura SA (2006) The anti-obesity agent Orlistat is associated to increase in colonic preneoplastic markers in rats treated with a chemical carcinogen. Cancer Lett 240:221–224
Guerciolini R, Radu-Radulescu L, Boldrin M, Dallas J, Moore R (2001) Comparative evaluation of faecal fat excretion induced by orlistat and chitosan. Obes Res 9:364–367
Harris KL, Felts JM (1970) Kinetics of chylomicron triglyceride removal from plasma in rats: a comparison of the anesthetized and the unanesthetized states. J Lipid Res 11:75–81
Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K (2004) Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARgamma ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta 1675:113–119
Hvizdos KM, Markham A (1999) Orlistat: a review of its use in the management of obesity. Drugs 58:743–760
Karpe F (1999) Postprandial lipoprotein metabolism and atherosclerosis. J Intern Med 246:341–355
Kim JM, Araki S, Kim DJ, Park CB, Takasuka N, Baba-Toriyama H, Ota T, Nir Z, Khachik F, Shimidzu N, Tanaka Y, Osawa T, Uraji T, Murakoshi M, Nishino H, Tsuda H (1998) Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 19:81–85
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
Luddy FE, Barford RA, Herb SF, Magidman P, Riemenschneider RW (1964) Pancreatic lipase hydrolysis of triglycerides by a semimicro technique. J Am Oil Chem Soc 41:693–696
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K (2005) Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun 332:392–397
Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K (2006) Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int J Mol Med 18:147–152
Matsumoto M, Chiji H, Hara H (2005) Intestinal absorption and metabolism of a soluble flavonoid, alphaG-rutin, in portal cannulated rats. Free Radic Res 39:1139–1146
Mattson FH, Volpenhein RA (1964) The digestion and absorption of triglycerides. J Biol Chem 239:2772–2777
Mu H, Høy CE (2001) Intestinal absorption of specific structured triacylglycerols. J Lipid Res 42:792–798
Nishimukai M, Hara H, Aoyama Y (2003) Enteral administration of soyabean lecithin enhanced lymphatic absorption of triacylglycerol in rats. Br J Nutr 90:565–571
Nomura T, Kikuchi M, Kubodera A, Kawakami Y (1997) Protondonative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl (DPPH). Biochem Mol Biol Int 42:361–370
Raederstorff DG, Schlachter MF, Elste V, Weber P (2003) Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem 14:326–332
Ros E (2000) Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis 151:357–379
Shiratori K, Ohgami K, Ilieva I, Jin XH, Koyama Y, Miyashita K, Kase S, Ohno S (2005) Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res 81:422–428
Shishikura Y, Khokhar S, Murray BS (2006) Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem 54:1906–1913
Sugawara T, Baskaran V, Tsuzuki W, Nagao A (2002) Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J Nutr 132:946–951
Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, Ohtake Y (2007) Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem 55:4604–4609
Toyoda-Ono Y, Yoshimura M, Nakai M, Fukui Y, Asami S, Shibata H, Kiso Y, Ikeda I (2007) Suppression of postprandial hypertriglyceridemia in rats and mice by oolong tea polymerized polyphenols. Biosci Biotechnol Biochem 71:971–976
Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA (2001) Catechins are bioavailable in men and women drinking black tea throughout the day. J Nutr 131:1731–1737
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, M., Hosokawa, M., Matsukawa, N. et al. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur J Nutr 49, 243–249 (2010). https://doi.org/10.1007/s00394-009-0078-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-009-0078-y