Abstract
Fucoxanthin is a carotenoid present in the chloroplasts of brown seaweeds. When ingested, it is metabolized mainly to fucoxanthinol in the gastrointestinal tract by digestive enzymes. These compounds have been shown to have many beneficial health effects. The present study was designed to evaluate the molecular mechanisms of action of fucoxanthin and/or of its metabolite fucoxanthinol against viability of estrogen-sensitive MCF-7 and estrogen-resistant MDA-MB-231 breast cancer cell lines. Fucoxanthin and fucoxanthinol reduced the viability of MCF-7 and MDA-MB-231 cells in dose- and time-dependent manners as a result of increased apoptosis. Furthermore, fucoxanthinol-induced apoptosis was more potent than that of fucoxanthin and correlated, for MDA-MB-231 cells, with inhibitory actions on members of the NF-κB pathway p65, p50, RelB, and p52. Being overexpressed and regulated by NF-κB in different types of cancers, the transcription factor SOX9 was also decreased at the nuclear level by fucoxanthin and fucoxanthinol in MDA-MB-231. Taken together, the current results suggest that fucoxanthinol and fucoxanthin could be potentially effective for the treatment and/or prevention of different types of cancers, including breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer diagnosed in women worldwide. In the United States alone, it is estimated that 40,000 women will die of breast cancer and over 208,000 new cases will be diagnosed each year (Jemal et al. 2010). These statistics emphasize the urgent need for improvements in detection, diagnosis, and treatment of breast cancer. Several studies have linked diets rich in carotenoids with reduced risk of chronic diseases and cancers, including breast cancer (Lordan et al. 2011; Peng et al. 2011; Tanaka et al. 2012).
Fucoxanthin (Fx) is a naturally occurring brown- or orange-colored pigment that belongs to the class of non-provitamin A carotenoids present in the chloroplasts of brown seaweeds. It is the most abundant of all carotenoids, accounting for more than 10 % of the estimated total natural production of carotenoids (Matsuno 2001). It forms a complex with chlorophyll–protein and plays an important role in light harvesting and photoprotection for effective light use and upregulation of photosynthesis. Oral administration of Fx does not exhibit toxicity and mutagenicity under experimental conditions in mice (Beppu et al. 2009a, b). Carotenoids and Fx, being mostly fat soluble, follow the same intestinal absorption path as dietary fat. Ingested Fx is metabolized mainly to fucoxanthinol (Fxol), which is further converted to amarouciaxanthin A in the liver (Asai et al. 2004), thus the bioactive forms of Fx are Fxol and/or amarouciaxanthin A. Dietary Fx is hydrolyzed to Fxol in the gastrointestinal tract by digestive enzymes such as lipase and cholesterol esterase and then absorbed into intestinal cells (Sugawara et al. 2002). Fx and Fxol have been shown to have many beneficial health effects, including anti-mutagenic (Nishino et al. 2009), antidiabetic (Nishikawa et al. 2012), antiobesity (Maeda et al. 2005), anti-inflammatory (Kim et al. 2010b; Shiratori et al. 2005), and preventive actions on liver, breast, prostate, colon, and lung cancers (Das et al. 2005; Kotake-Nara et al. 2005; Le Marchand et al. 1993; Nishino et al. 2009; Slattery et al. 2000; Zhang et al. 1999). Most of these actions involve modulation of the nuclear factor kappaB (NF-κB) signaling pathway.
The transcription factor members of the NF-κB family found in most cells are p50, p65/RelA, c-Rel, p52, and RelB (Hayden and Ghosh 2004). NF-κB is regulated by two main pathways: the canonical and noncanonical pathways (Hayden and Ghosh 2008). In its resting state, NF-κB dimers are secured in the cytoplasm by IκB proteins which render the NF-κB transcription factors inactive. These IκB members α, β, and ε contain two conserved serine residues that become phosphorylated by IκB kinases (IKKs) upon NF-κB pathway activation, followed by proteasome-dependent degradation and NF-κB transcription factors activation (Karin and Ben-Neriah 2000). NF-κB members then translocate to the nucleus to form dimers likely to have distinct regulatory functions. Among the NF-κB target genes are inflammatory cytokines, anti-apoptotic proteins, and cell cycle regulators that promote cancer cells proliferation (Karin and Lin 2002). Indeed, NF-κB is activated by most carcinogens, leading to expression of anti-apoptotic gene products which allow survival and growth of tumors (Baker et al. 2011; Kumar et al. 2004; Zubair and Frieri 2013). It has been shown that p65 and p50 subunits of NF-κB are constitutively active and are overexpressed in breast cancer (Nakshatri et al. 1997), resulting in further transcription of anti-apoptotic genes (Fan et al. 2008).
The objective of the current research was to determine if Fx and/or Fxol have inhibitory effects on viability and on the NF-κB signaling pathway of breast cancer cell lines. Comparative studies have been undertaken in two different types of cells, the MCF-7 and MDA-MB-231 cell lines. Since MCF-7 cells inherently express high levels of estrogen receptor (ER)-α and MDA-MB-231 express very low, if any, ER-α or -β, we sought to determine if the presence of a functional ER altered the effects of Fx and/or Fxol treatments. Here, we report that Fxol has been shown to reduce cell viability, to increase apoptosis, and to regulate components of the NF-κB pathway in both cell lines from breast cancers.
Material and methods
Chemicals
Fucoxanthin (Fx) and fucoxanthinol (Fxol) were purchased from Wako Chemicals (Richmond, VA, USA).
Cell culture
Human breast cancer cell lines MCF-7 (hormone sensitive) and MDA-MB-231 (hormone resistant) were obtained from the American Type Culture Collection (ATCC; Bethesda, MD, USA). MCF-7 and MDA-MB-231 cells were grown in DMEM/F12 medium supplemented with 10 % heat-inactivated fetal bovine serum (Canadian origin) and 100 U/ml penicillin/streptomycin (Corning, Tewksbury, MA, USA). Cells were cultured at 37 °C and 5 % CO2.
Cell viability
The viability of Fx- or Fxol-treated cells were measured using CellTiter-Blue cell viability assay (Promega, Madison, WI, USA) according to manufacturer’s instructions. Following dose (20, 30, or 40 μM) and time-dependent (12, 24, or 48 h) treatments with Fx or Fxol of breast cancer cell lines, cell viability was estimated by measuring the amount of reduced resorufin by its fluorescence at 560Ex/590Em using a multimode microplate reader (Varioskan, Thermo Scientific, Waltham, MA, USA). IC50 values were determined from linear regression of fluorescence versus concentration and correspond to doses of Fx or Fxol necessary to reduce cell viability by 50 % for 12 h of treatment.
Apoptotic, necrotic, and healthy cells assay
The breast cancer cell lines were treated with 20 μM Fx or Fxol for 12 h, followed by staining with FITC-Annexin V, ethidium homodimer III, and Hoechst 33342 according to the manufacturer’s protocol (Biotium, Inc., Hayward, CA, USA). Fluorescence was assessed using an Axio Observer A1 inverted fluorescence microscope (Carl Zeiss, Gottingen, Germany) with FITC, rhodamine, and DAPI filters. Images were merged and analyzed using the ImageJ software (http://rsbweb.nih.gov/ij/).
Western blot analysis
The breast cancer cell lines were treated with 20 μM Fx or Fxol for 12 h, followed by protein extractions for nuclear and cytoplasmic proteins, using hypertonic buffer with phosphatase inhibitors (10 mM NaF, 1 mM Na3VO4, and 20 mM glycerol2-phosphate) and protease inhibitors (30 μg/ml aprotinin, 2.5 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 mM phenylmethyl fluoride) as described previously (Schreiber et al. 1989). Protein concentrations of protein extracts were evaluated using the Bradford method (Bradford 1976). Ten micrograms (for nuclear extracts) or 30 μg (for cytoplasmic extracts) of protein from each sample was separated by SDS–polyacrylamide gels (SDS–PAGE) and electrophoretically transferred onto a PVDF membrane. Membranes were incubated in blocking solution containing 5 % nonfat dry milk in TBST buffer (TBS buffer containing 0.1 % Tween 20) for 1 h at room temperature, followed by incubation with a specific primary antibody overnight at 4 °C. Specific proteins were detected using the following primary antibodies: polyclonal anti-CASPASE 3 (1:1,000, Cat.: 9662, Cell Signaling, Danvers, MA, USA), polyclonal anti-Poly(ADP-ribose) polymerase-1 (PARP) (1:1,000, Cat.: 9542, Cell Signaling), NF-κB family member antibody sampler kit (1:1,000, Cat.: 4766, Cell Signaling), monoclonal anti-Phospho-NF-κB p65 (Ser536) (1:1,000, Cat.: 3033, Cell Signaling), monoclonal anti-STAT3 (1:2,000, Cat.: 4904, Cell Signaling), polyclonal anti-STAT5 (1:1,000, Cat.: 9363, Cell Signaling), polyclonal anti-SOX9 (1:500, Cat.: AB5535, Millipore, Billerica, MA, USA), monoclonal anti-α-Tubulin (1:5,000, Cat.: 05-829, Millipore), and polyclonal anti-NCL (1:1,000, Cat.: 12247S, Cell Signaling). The membranes were washed three times in TBST buffer for 5 min, followed by incubation for 1 h with horseradish peroxidase-conjugated secondary antibody. The membranes were washed again and developed using an enhanced chemiluminescent detection system (Luminata Forte, Millipore) according to the manufacturer’s instructions. Images were taken using the FluorChem IS 8900 imaging system (Alpha Innotech, San Jose, CA, USA). The Western blotting results were referred to NCL (for nuclear extracts) and α-Tubulin level (for cytoplasmic extracts) as references.
Statistics
Experiments for cells viabilities were repeated at least three times, and the data were presented as means ± S.E.M. Statistical analysis of the data was performed using Student’s t test and ANOVA with GraphPad Prism (GraphPad Software Inc., San Diego, Ca, USA). P < 0.05 was considered significant.
Results
Fx and Fxol inhibit cell viability in human breast cancer cells
We first examined the effects of Fx and Fxol on cell viability of human breast cancer cell lines MCF-7 and MDA-MB-231 (Fig. 1). Fx and Fxol significantly decreased cell viability in a time- and dose-dependent manner for both cell lines. A two-way ANOVA test showed a significant effect of time and dose on cell viability (P < 0.0001). Fxol-induced suppression of cell viability was more rapid and more pronounced, compared to Fx. Indeed, IC50 values of 121.89 and 39.63 μM for Fx and Fxol, respectively, were calculated for MCF-7, whereas for MDA-MB-231, IC50 values of 141.54 and 33.59 μM for Fx and Fxol were determined for 12 h of treatment. In addition, these results also demonstrate a more efficient inhibitory action of Fxol on cell viability of MDA-MB-231, compared to MCF-7, for all times of treatments investigated. Only Fxol had significant inhibitory effects on viability at 12 h with doses of 30 and 40 μM of treatments in both breast cancer cell lines. Although giving nonsignificant viability reduction, we used the dose of 20 μM for 12 h as treatments with Fx or Fxol for the remaining of the study to obtain enough cells for protein extractions and the study of apoptotic markers by Western blot assays.
Fx and Fxol reduce cell viability of MCF-7 and MDA-MB-231 cell lines. Exponentially growing cells were incubated in the absence or presence of 20–40 μM of Fx or Fxol, for 12–48 h, followed by determination of cell viability as described in Material and methods. The results are presented as absolute fluorescence signal and are expressed as means ± standard error of mean deviation of four independent experiments in which each treatment was performed in quadruplicate. Comparisons to control were done using a multiple comparison test (*significant, P < 0.05)
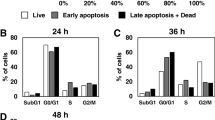
Reduction of breast cancer cell lines viability in response to Fxol involves increase of apoptotic pathways activities (Fig. 2). In MCF-7 cells, lacking caspase-3 expression (Jänicke 2009), PARP was cleaved in response to 20 μM Fx and Fxol stimulations for 12 h and resulted in cytoplasmic (Fig. 2a) and nuclear (Fig. 2b) accumulations of the cleaved fragment. For MDA-MB-231 cells, procaspase-3 was cleaved following Fxol treatment (Fig. 2a), whereas PARP cleavage in response to Fx and Fxol treatments resulted in nuclear accumulation of the cleaved form (Fig. 2b) with no cytoplasmic detection. Increased apoptosis of breast cancer cell lines in response to treatments for 12 h with 20 μM Fx or Fxol was also confirmed using fluorescence microscopy (Fig. 2b). For both cell lines, Fxol resulted in higher apoptotic levels compared to Fx treatment as shown with FITC-Annexin V green fluorescence (Fig. 2c). However, although a major difference was observed between Fx and Fxol on viability reduction of MDA-MB-231 cells (Fig. 1b, d), Fxol treatment resulted in a slightly higher level of apoptosis when compared to Fx (Fig. 2c, lower panels). In both cell lines, Fx and Fxol treatments did not result in increased levels of necrosis as shown with ethidium homodimer III red fluorescence. Thus, these changes correspond to increased apoptosis of breast cancer cells in response to Fx/Fxol.
Effects of Fx and Fxol on markers of apoptosis in breast cancer cell lines. MCF-7 and MDA-MB-231 cell lines were treated with 20 μM Fx or Fxol for 12 h. Cytoplasmic (a) and nucleic (b) proteins were extracted from control and treated cells, followed by determination of Caspase 3 and PARP cleavages using Western blot analysis as described in Material and methods and use of α-Tubulin and Nucleolin (NCL) as loading controls for cytoplasmic and nuclear extracts, respectively. In c, MCF-7 cells (left panel) and MDA-MB-231 cells (right panel) were stained with Hoechst 33342 (for viable cells in blue), FITC-Annexin V (for apoptotic cells in green), and ethidium homodimer III (for necrotic cells in red), followed by imaging with an inverted fluorescence microscope with total magnifications of ×200. The experiments where repeated three times
Regulatory effects of Fx and Fxol on the NF-κB signaling pathway
Since several NF-κB target genes are involved in inhibiting apoptosis in breast cancer cells (Nakshatri and Goulet 2002; Wu and Kral 2005; Zhou et al. 2005a), we examined whether Fx and/or Fxol inhibits members of the NF-κB pathway. In MCF-7 cells, Fxol, but not Fx, increased the nuclear levels of p65, p50, cRel, and p52 (Fig. 3b). In addition, the nuclear levels of phosphorylated p65 increased in response to Fxol. Since cytoplasmic concentrations of p65, p50, and p52 remained constant following Fxol treatments of MCF-7 cells (Fig. 3a), increased nuclear accumulations of these NF-κB members might be attributed to increases in their protein syntheses. However, decreased cytoplasmic concentrations of cRel in response to Fxol in MCF-7 cells suggest that its nuclear accumulation is mainly attributed to increased nuclear translocation. In MDA-MB-231 cells, Fxol contributed to decreased nuclear levels p65, p50, RelB, p52/p100, and phosphorylated-p65 (Fig. 3d). In this cell line, decreased nuclear levels of components of the NF-κB pathways were attributed to decreased synthesis, as shown from cytoplasmic protein extracts (Fig. 3c).
Effects of Fx and Fxol on members of the NF-κB pathway. MCF-7 and MDA-MB-231 cell lines were treated with 20 μM Fx or Fxol for 12 h and cytoplasmic and nuclear proteins were extracted from controls and treated cells. The levels of target proteins were determined using Western blot analysis as described in Material and methods. α-Tubulin and Nucleolin (NCL) were used as loading controls for cytoplasmic and nuclear extracts, respectively. Every experiment was repeated three times
Since SOX9 has been shown to be regulated by NF-κB at the transcriptional level in other cancer cells (Saegusa et al. 2012; Sun et al. 2013), we investigated whether Fx and/or Fxol could influence SOX9 expression in breast cancer cell lines. Although no effects on SOX9 could be observed in MCF-7 cells (Fig. 4a), SOX9 expression and nuclear accumulation were decreased in response to Fx and Fxol in MDA-MB-231 cells (Fig. 4b). SOX9 Western blots from MDA-MB-231 extracts show the presence of two forms of the protein. Such result might be attributed to SOX9 phosphorylation, as previously shown by protein kinase A in Sertoli-like cells (Malki et al. 2005). Therefore, Fx and Fxol treatments might result in loss of SOX9 phosphorylation, as seen with the disappearance of the upper band (Fig. 4b).
Effects of Fx and Fxol on SOX9 protein levels and nuclear translocation. MCF-7 and MDA-MB-231 cell lines were treated with 20 μM Fx or Fxol for 12 h and cytoplasmic and nuclear proteins were extracted from controls and treated cells. The levels of SOX9 were determined using Western blot analysis as described in Material and methods. Every experiment was repeated three times
Influences of Fx and Fxol on members of the STAT family
Since deregulation of STAT signaling cascades is associated with cellular proliferation and transformation (Kisseleva et al. 2002), we investigated whether Fx and/or Fxol could have any effects on expression and nuclear localization of members of the STAT family in breast cancer cells. Although Fxol had an inhibitory effect on STAT5 protein synthesis in MCF-7 cells (Fig. 5a), no changes in nuclear concentrations for STAT3 and STAT5 in response to Fx or Fxol could be observed in MCF-7 and STAT3 did not vary in MDA-MB-231 nuclear extracts (Fig. 5b, d). Unlike others (Lahusen et al. 2007; Yuan et al. 2010), we were not able to detect STAT5 at the protein level in MDA-MB-231 cells. However, Fx alone had an inhibitory effect on cytoplasmic accumulation of STAT3 in MDA-MB-231 (Fig. 5c), suggesting that nuclear translocation of STAT3 is increased in response to Fx in MDA-MB-231 cells to maintain a constant nuclear concentration (Fig. 5d). Together, these results suggest that inhibitory effects of Fx and Fxol on cell viability may be independent of the STAT signaling pathway.
Effects of Fx and Fxol on STAT members’ protein levels and nuclear translocation. MCF-7 and MDA-MB-231 cell lines were treated with 20 μM Fx or Fxol for 12 h and cytoplasmic and nuclear proteins were extracted from controls and treated cells. The levels of STAT3 and STAT5 proteins were determined using Western blot analysis as described in Material and methods. Results for STAT5 in MCF-7 were analyzed for densitometry using the ImageJ software (http://rsbweb.nih.gov/ij/) and expressed as a ratio between STAT5 and Tubulin followed by normalization to control. Every experiment was repeated three times
Discussion
In the current study, we demonstrated that Fxol show inhibitory effects on cell viability and apoptosis-inducing effects on two human breast cancer cell lines, MCF-7 and MDA-MB-231. Furthermore, the apoptosis-inducing activities of Fxol were more potent than that of Fx and were correlated, for hormone independent MDA-MB-231 cells, to inhibitory actions on members of the NF-κB pathway.
As shown in primary effusion lymphoma cell lines (Yamamoto et al. 2011), Fxol-induced suppression of cell viability was more pronounced than that of Fx for breast cancer cell lines MCF-7 and MDA-MB-231. Compared to Fx, Fxol also had a stronger antiproliferative effect on PC-3 human prostate cancer cells (Asai et al. 2004), MCF-7 and Caco-2 human colon cancer cells (Konishi et al. 2006), thus supporting our current results on breast cancer cells. Indeed, Fxol has been shown previously to reduce MCF-7 cells viability by 75 % following treatment with 25 μM Fxol for 48 h (Konishi et al. 2006). Differences with our results (87 % reduction with 20 μM Fxol for 48 h) may be attributed to variations in culture conditions and Fxol concentration, purity, and stability.
Hallmarks of the apoptotic process are cleavages of PARP, an enzyme implicated in DNA damage and repair mechanisms, and of Caspase-3, an enzyme activated during apoptosis both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways. During apoptosis, PARP is cleaved from its precursor having a mass of 116 kDa to yield an 85 kDa fragment (Kaufmann et al. 1993). PARP fragment migrates from the nucleus into the cytoplasm during late stages of apoptosis associated with severe nuclear fragmentation (Soldani et al. 2001), suggesting that apoptosis is more advanced in MCF-7 compared to MDA-MB-231 cells treated with Fxol. Others have shown that Fx induced Caspase-3, -7, and PARP cleavages and thus triggered apoptosis of human leukemia HL-60 and prostate cancer PC-3 cells (Kim et al. 2010a; Kotake-Nara et al. 2005). Fx and Fxol also induced cell cycle arrest in G1 phase and caspase-dependent apoptosis, inhibited the activation of NF-κB, and downregulated anti-apoptotic proteins in primary effusion lymphoma cells (Yamamoto et al. 2011). The present study clearly demonstrated that Fxol induced apoptosis of breast cancer cells MDA-MB-231 and MCF-7 by cleavages of procaspase-3 and PARP. In support to our results, Fxol isolated from Halocynthia roretzi induced apoptosis in human breast cancer cells (Konishi et al. 2006). Lack of difference in necrosis levels between treatments in breast cancer cells suggests that Fx and Fxol have no effects on necrotic pathways. Indeed, apoptosis and necrosis are two processes than can occur independently, sequentially, or simultaneously (Elmore 2007; Zeiss 2003). Presence of necrosis in MCF-7 cells may be attributed to suboptimal cell culture conditions. Thus, our results suggest that reduction of breast cancer cells viability in response to Fx and Fxol treatments may be attributed to increased apoptosis.
Estrogen receptors play an important role in breast cancer; women with ER-positive tumors have an overall better prognosis and are more likely to have their tumors respond to therapy. However, in up to 25 % of cases, ER-positive tumors are nonresponsive to therapy as a result of acquired resistance, possibly linked to constitutive NF-κB leading to estrogen-independent growth (Frasor et al. 2009; Kalaitzidis and Gilmore 2005; Zubair and Frieri 2013). In this study, we looked at possible differences in the inhibitory actions of Fx and/or Fxol on components of the NF-κB pathway between estrogen-sensitive MCF-7 and estrogen-resistant MDA-MB-231 breast cancer cell lines. In lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages, others have shown that Fx inhibited the nuclear translocation of p50 and p65 proteins, resulting in lower levels of nuclear transactivation by NF-κB transcription factors (Kim et al. 2010b; Lee et al. 2013). In the present study, major differences were observed in the inhibitory mechanisms between Fx and Fxol on the activation of members of the NF-κB pathway in breast cancer cell lines. Indeed, Fxol treatment decreased nuclear levels p65, p50, RelB, and p52/p100 in hormone independent MDA-MB-231 cells. Thus, Fxol treatment may contribute to reduce viability of aggressive estrogen-independent tumor growth and may involve inhibitions of nuclear translocation and transcriptional activity of members of the NF-κB pathway. Indeed, other NF-κB inhibitors such as celastrol and triptolide have been shown to reduce MDA-MB-231 cell viability (Kang et al. 2009; Kannaiyan et al. 2011; Liu et al. 2009). Further regulation of NF-κB members by phosphorylation, acetylation, and coactivator and corepressor interactions in response to Fx/Fxol remains to be investigated. Surprisingly, our results show increased nuclear localization of members of the NF-κB pathway (p65, p50, cRel) in response to Fxol in MCF-7 as opposed to MDA-MB-231 cells. Although others have shown that inhibition of NF-κB activation in MCF-7 cells results in reduced cell viability (Zhou et al. 2005b), our results suggest that decreased MCF-7 cells viability in response to Fx/Fxol may be independent of NF-κB inhibition. However, the balance between the expression of genes involved in the survival and apoptotic processes ultimately determines whether the cell undergoes apoptosis in response to NF-κB (Aggarwal 2000). Indeed, treatment of MCF-7 cells with Fxol resulted in increased apoptosis despite the fact that NF-κB transcription factors were activated, thus suggesting that other signaling pathways may be involved.
In breast cancer cells, SOX9, being regulated by NF-κB in other cell types (Saegusa et al. 2012; Sun et al. 2013), has been reported mainly in the cytoplasm of MDA-MB-231 cells, and was located mostly in the nucleus of MCF-7 cells (Chakravarty et al. 2011). We showed that nuclear levels of SOX9 were unchanged in MCF-7 cells when stimulated with Fx or Fxol, whereas SOX9 was located mainly in the nucleus of MDA-MB-231 cells. These differences with the Chakravarty et al. 2011 study may be attributed to differences in cell culture and potential cross-reaction of the antibody they used against SOX9. Interestingly, upregulation of SOX9 in response to retinoic acid has also been shown to induce cell growth arrest in breast cancer cells (Müller et al. 2010). This is in contrast to our results where treatments with Fx and Fxol resulted in decreased nuclear levels of SOX9. Indeed, SOX9 may rather be involved in MDA-MB-231 cells proliferation and downregulation of its phosphorylation may contribute to the inhibitory effects of Fx and Fxol on viability of estrogen resistant breast cancers.
In addition to having crucial roles in mammary gland development, STAT3/5 transcription factors also contribute to tumorigenesis when deregulated (Desrivières et al. 2006; Kisseleva et al. 2002). Indeed, constitutive activation of STAT3 has been linked to the growth and survival of human breast cancer cells (Garcia et al. 1997, 2001). Higher concentrations of Fx (50–150 μM) than what was used in the current study have been shown to inhibit STAT3 expression and phosphorylation in sarcoma tissue (Wang et al. 2012) and gastric adenocarcinoma cells (Yu et al. 2011). Although we have shown that Fx and Fxol had no effect on nuclear levels of STAT3/5 in breast cancer cells, the phosphorylation profiles of these transcription factors may be modulated to promote apoptosis. Indeed, decreased phosphorylation of STAT3 is associated to reduced invasiveness and survival of breast tumors (Hsieh et al. 2005).
In conclusion, Fx and Fxol effectively induced cell viability reduction and apoptotic cell death of breast cancer cells. Thus, dietary Fx or Fxol could be potentially effective for the treatment and/or prevention of different types of cancers, including breast cancer. Indeed, most dietary Fx may be converted to Fxol, and Fxol may exert a suppressive effect on cancer cells more efficiently than Fx in vivo. However, although there is a correlation between increased apoptosis and decreased nuclear accumulations of p65, RelB, p50, and p52 in MDA-MB-231 cells, more research will be required to clearly establish a regulatory action of Fxol on members of NF-κB to activate apoptosis.
References
Aggarwal BB. Apoptosis and nuclear factor-kappa B: a tale of association and dissociation. Biochem Pharmacol. 2000;60:1033–9.
Asai A, Sugawara T, Ono H, Nagao A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab Dispos Biol Fate Chem. 2004;32:205–11.
Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22.
Beppu F, Niwano Y, Sato E, Kohno M, Tsukui T, Hosokawa M, et al. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J Toxicol Sci. 2009a;34:693–8.
Beppu F, Niwano Y, Tsukui T, Hosokawa M, Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci. 2009b;34:501–10.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Chakravarty G, Rider B, Mondal D. Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. Cancer Biol Ther. 2011;11:71–83.
Das SK, Hashimoto T, Shimizu K, Yoshida T, Sakai T, Sowa Y, et al. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim Biophys Acta. 2005;1726:328–35.
Desrivières S, Kunz C, Barash I, Vafaizadeh V, Borghouts C, Groner B. The biological functions of the versatile transcription factors STAT3 and STAT5 and new strategies for their targeted inhibition. J Mammary Gland Biol Neoplasia. 2006;11:75–87.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516.
Fan Y, Dutta J, Gupta N, Fan G, Gélinas C. Regulation of programmed cell death by NF-kappaB and its role in tumorigenesis and therapy. Adv Exp Med Biol. 2008;615:223–50.
Frasor J, Weaver A, Pradhan M, Dai Y, Miller LD, Lin C-Y, et al. Positive cross-talk between estrogen receptor and NF-kappaB in breast cancer. Cancer Res. 2009;69:8918–25.
Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ Mol Biol J Am Assoc Cancer Res. 1997;8:1267–76.
Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513.
Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–224.
Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62.
Hsieh F-C, Cheng G, Lin J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem Biophys Res Commun. 2005;335:292–9.
Jänicke RU. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res Treat. 2009;117:219–21.
Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52.
Kang DW, Lee JY, Oh DH, Park SY, Woo TM, Kim MK, et al. Triptolide-induced suppression of phospholipase D expression inhibits proliferation of MDA-MB-231 breast cancer cells. Exp Mol Med. 2009;41:678–85.
Kannaiyan R, Manu KA, Chen L, Li F, Rajendran P, Subramaniam A, et al. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis Int J Program Cell Death. 2011;16:1028–41.
Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63.
Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7.
Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–85.
Kim K-N, Heo S-J, Kang S-M, Ahn G, Jeon Y-J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol Vitro. 2010a;24:1648–54.
Kim K-N, Heo S-J, Yoon W-J, Kang S-M, Ahn G, Yi T-H, et al. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur J Pharmacol. 2010b;649:369–75.
Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24.
Konishi I, Hosokawa M, Sashima T, Kobayashi H, Miyashita K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp Biochem Physiol Toxicol Pharmacol. 2006;142:53–9.
Kotake-Nara E, Asai A, Nagao A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005;220:75–84.
Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-kappaB: its role in health and disease. J Mol Med Berl Ger. 2004;82:434–48.
Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res. 2007;67:7256–65.
Le Marchand L, Hankin JH, Kolonel LN, Beecher GR, Wilkens LR, Zhao LP. Intake of specific carotenoids and lung cancer risk. Cancer Epidemiol Biomark Prev. 1993;2:183–7.
Lee J-Y, Lee M-S, Choi H-J, Choi J-W, Shin T, Woo H-C, et al. Hexane fraction from Laminaria japonica exerts anti-inflammatory effects on lipopolysaccharide-stimulated RAW 264.7 macrophages via inhibiting NF-kappaB pathway. Eur J Nutr. 2013;52:409–21.
Liu J, Jiang Z, Xiao J, Zhang Y, Lin S, Duan W, et al. Effects of triptolide from Tripterygium wilfordii on ERalpha and p53 expression in two human breast cancer cell lines. Phytomedicine Int J Phytother Phytopharm. 2009;16:1006–13.
Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs. 2011;9:1056–100.
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005;332:392–7.
Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Méjean C, et al. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–809.
Matsuno T. Aquatic animal carotenoids. Fish Sci. 2001;67:771–83.
Müller P, Crofts JD, Newman BS, Bridgewater LC, Lin C-Y, Gustafsson J-A, et al. SOX9 mediates the retinoic acid-induced HES-1 gene expression in human breast cancer cells. Breast Cancer Res Treat. 2010;120:317–26.
Nakshatri H, Goulet Jr RJ. NF-kappaB and breast cancer. Curr Probl Cancer. 2002;26:282–309.
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet Jr RJ, Sledge Jr GW. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–39.
Nishikawa S, Hosokawa M, Miyashita K. Fucoxanthin promotes translocation and induction of glucose transporter 4 in skeletal muscles of diabetic/obese KK-A(y) mice. Phytomedicine. 2012;19:389–94.
Nishino H, Murakoshi M, Tokuda H, Satomi Y. Cancer prevention by carotenoids. Arch Biochem Biophys. 2009;483:165–8.
Peng J, Yuan J-P, Wu C-F, Wang J-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs. 2011;9:1806–28.
Saegusa M, Hashimura M, Suzuki E, Yoshida T, Kuwata T. Transcriptional up-regulation of Sox9 by NF-κB in endometrial carcinoma cells, modulating cell proliferation through alteration in the p14(ARF)/p53/p21(WAF1) pathway. Am J Pathol. 2012;181:684–92.
Schreiber E, Matthias P, Müller MM, Schaffner W. Rapid detection of octamer binding proteins with « mini-extracts », prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419.
Shiratori K, Ohgami K, Ilieva I, Jin X-H, Koyama Y, Miyashita K, et al. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2005;81:422–8.
Slattery ML, Benson J, Curtin K, Ma KN, Schaeffer D, Potter JD. Carotenoids and colon cancer. Am J Clin Nutr. 2000;71:575–82.
Soldani C, Lazzè MC, Bottone MG, Tognon G, Biggiogera M, Pellicciari CE, et al. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res. 2001;269:193–201.
Sugawara T, Baskaran V, Tsuzuki W, Nagao A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J Nutr. 2002;132:946–51.
Sun L, Mathews LA, Cabarcas SM, Zhang X, Yang A, Zhang Y, et al. Epigenetic regulation of SOX9 by the NF-κB signaling pathway in pancreatic cancer stem cells. Stem Cells. 2013;31:1454–66.
Tanaka T, Shnimizu M, Moriwaki H. Cancer chemoprevention by carotenoids. Mol. 2012;17:3202–42.
Wang J, Chen S, Xu S, Yu X, Ma D, Hu X, et al. In vivo induction of apoptosis by fucoxanthin, a marine carotenoid, associated with down-regulating STAT3/EGFR signaling in sarcoma 180 (S180) xenografts-bearing mice. Mar Drugs. 2012;10:2055–68.
Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res. 2005;123:158–69.
Yamamoto K, Ishikawa C, Katano H, Yasumoto T, Mori N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett. 2011;300:225–34.
Yu R-X, Hu X-M, Xu S-Q, Jiang Z-J, Yang W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur J Pharmacol. 2011;657:10–9.
Yuan T, Wang Y, Zhao ZJ, Gu H. Protein-tyrosine phosphatase PTPN9 negatively regulates ErbB2 and epidermal growth factor receptor signaling in breast cancer cells. J Biol Chem. 2010;285:14861–70.
Zeiss CJ. The apoptosis-necrosis continuum: insights from genetically altered mice. Vet Pathol. 2003;40:481–95.
Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547–56.
Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005a;12 Suppl 1:S37–46.
Zhou Y, Eppenberger-Castori S, Marx C, Yau C, Scott GK, Eppenberger U, et al. Activation of nuclear factor-kappaB (NFkappaB) identifies a high-risk subset of hormone-dependent breast cancers. Int J Biochem Cell Biol. 2005b;37:1130–44.
Zubair A, Frieri M. Role of nuclear factor-ĸB in breast and colorectal cancer. Curr Allergy Asthma Rep. 2013;13:44–9.
Acknowledgments
The current work was funded by the Canadian Breast Cancer Foundation (CBCF) (#5250 to L.J.M.), the New Brunswick Innovation Foundation (NBIF) (#IAR2012 and IAR2013-029 to L.J.M.), and the Natural Sciences and Engineering Research Council (NSERC) of Canada (#386557-2012 to L.J.M.).
Conflict of interest
The authors declare that there is no conflict of interest that would prejudice there impartiality.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rwigemera, A., Mamelona, J. & Martin, L.J. Inhibitory effects of fucoxanthinol on the viability of human breast cancer cell lines MCF-7 and MDA-MB-231 are correlated with modulation of the NF-kappaB pathway. Cell Biol Toxicol 30, 157–167 (2014). https://doi.org/10.1007/s10565-014-9277-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-014-9277-2