Abstract
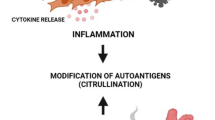
Rheumatoid arthritis (RA) is a debilitating, systemic autoimmune disease that affects people around the world. The disease is characterized by chronic inflammation of the joints, which eventually results in cartilage and bone damage. An increasing number of patients with RA worldwide are seeking help from complementary and alternative medicine (CAM) to alleviate the severity of the disease and to improve physical conditions. Among these treatments, traditional Chinese medicine (TCM) is regarded as a powerful treatment option, and it has been used for RA therapy for thousands of years in China. TCM is characterized by a holistic theory that emphasizes maintaining the balance of the patient’s whole body using Chinese herbal medicines (CHMs) with multiple bioactive ingredients. Some studies have revealed that many antiarthritic CHMs may exert anti-inflammatory and immunomodulatory effects by regulating the production of pro-inflammatory cytokines and immuno-related pathways. However, the precise molecular mechanisms underlying the anti-RA activities of CHMs have not been fully elucidated. Moreover, safety issues have also blocked the development of CHMs; therefore, it is of great significance for clinicians, researchers, and pharmaceutical companies to share responsibility by regulating the clinical use of CHMs, strengthening the basic toxicology research, and establishing a strict quality control system to ensure the safe use of CHMs and decrease the number of toxic cases. The present chapter illustrates the clinical utilization of CHMs acting on RA, elucidates their mechanisms of action, analyzes their limitations and problems, and discusses their development and application prospects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chinese herbal medicine
- Clinical utilization
- Molecular mechanism rheumatoid arthritis
- Traditional Chinese medicine
16.1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology. The overall prevalence of RA is approximately 0.5–1 % worldwide (Gibofsky 2014). Substantial variation existed regarding the incidence and prevalence of RA, implying its dynamic characteristic of the epidemiology (Gabriel and Michaud 2009), which is also a reflection of a well-accepted hypothesis that RA is caused by an environmental exposure or “trigger” in individuals who are genetically susceptible (Gibofsky 2012a). RA is manifested by synovial inflammation, insidious pain, morning stiffness, and joint swelling. It could result in erosion of the cartilage and bone and eventually cause joint deformity (Doan and Massarotti 2005). Moreover, patients with RA are more likely to suffer from myocardial infarction, atherosclerosis, stroke, and other complications (Avina-Zubieta et al. 2008), and they also tend to suffer from disability without appropriate and regular treatment. The management of RA should be commensurate with the characteristics and degree of the disease, which demands an integrated approach including both rheumatologists and orthopedic surgeons (Longo et al. 2015). Currently, the therapeutic strategies for the treatment of RA can be mainly divided into two categories. One category contains disease-modifying antirheumatic drugs (DMARDs), which consist of conventional DMARDs, including leflunomide, methotrexate (MTX), and sulfasalazine (Gibofsky 2012a). Extensive clinical experience has indicated that compared to leflunomide, the application of MTX, the “anchor” DMARD, may improve a patients’ ability to remain on long-term therapy (Donahue et al. 2012). The second category contains biological agents, including tumor necrosis factor (TNF) inhibitors and non-TNF biologics (abatacept, rituximab, tocilizumab), which target the underlying pathophysiology of the disease and may alter disease progression (Gibofsky 2012b). Although these remedies have benefited many RA patients, their poor efficacies, high prices, and adverse effects are of common concern (Li et al. 2015a). Currently, an increasing number of RA patients have been seeking help from complementary and alternative medicine (CAM) for the alleviation of severe diseases and improvement of physical conditions.
Traditional Chinese medicine (TCM), which is based on empirical applications of experience from thousands of years, has become a crucial component of the modern medical system and has been extensively used as CAM in clinical practice. TCM is characterized by a holistic theory, which emphasizes maintaining the balance of the patients’ whole body using Chinese herbal medicines (CHMs) that consist of multiple bioactive ingredients based on syndrome (ZHENG in Chinese) differentiation (Li et al. 2015b; Zhang et al. 2010). According to the theory of TCM, RA is an impediment disease (“Bi” syndrome), which is a group of diseases caused by the invasion of wind, cold, dampness, or heat pathogen into the human body (He et al. 2014). Compared to western medicine, CHMs excel in their lower prices, higher security, and feasibility of long-term administration (Jiang et al. 2012). Regarding the administration, CHMs used in clinics are usually classified into drugs for external use, such as CHM bath, acupoint application therapy, and ion introduction, and for internal use, such as single herbs, herbal formulae, and Chinese patent medicines (Qi et al. 2010). These have been regarded as indispensable strategies to alleviate the conditions and to enhance the life quality of RA patients.
The present chapter illustrates the clinical utilization of CHMs for RA, elucidates their mechanisms of action, analyzes their limitations and problems, and discusses their development and application prospects.
16.2 Clinical Utilization and Efficacy of CHMs in the Treatment of RA
TCM has been applied in the treatment of RA since ancient times in China. Several pathological factors, including weakness, the insufficiency of vital qi, and the invasion of cold and dampness, are always related to RA (Liu and Liu 2011). TCM practitioners determine the appropriate therapeutic schedules based on the categorization of patients through syndrome differentiation, such as a hot pattern, cold pattern, and deficiency pattern (Yuan et al. 2015). Moreover, the anti-inflammatory and antiarthritic activities of multiple CHMs applied in the treatment of RA have been proven through animal experiments and clinical trials (Venkatesha et al. 2011). In this section, we would like to illustrate the clinical utilizations and efficacies of CHMs in the treatment of RA.
16.2.1 Application of CHMs for External Use in the Treatment of RA
16.2.1.1 CHM Bath
A CHM bath is an ancient therapeutic approach in RA therapy and has been regarded as a supplementary method to assist with conventional therapeutic strategies, such as medications, acupuncture, etc., to enhance their therapeutic effectiveness. A CHM bath is a combination of heat therapy and medication, that is, to immerse the whole body or limbs of RA patients in a CHM soup, which exerts antiarthritic effects by dilating the capillaries in the skin and subcutaneous tissues, accelerating blood circulation, and improving drug absorption and infiltration into the lesion site. Moreover, when patients are soaking in the CHM liquid, their active or passive exercises may contribute to the improvement of their physical functions (Li 2001). In TCM theory, CHM baths can expel evil wind, remove dampness, and disperse cold by warming the meridians, which coincides well with the pathogenesis of RA (Zhu et al. 2011). Clinically, there are no fixed formulae for CHM baths. Clinicians make prescriptions according to the physical condition and disease severity of the RA patients. Herbs that have functions of dispelling wind and dampness, stimulating blood circulation, removing blood stasis, and causing muscle and joint relaxation are frequently used (Li 2001). Among them, Carthami flos (Honghua), Angelicae pubescentis Radix (Duhuo), Lonicerae japonicae Caulis (Rendongteng), and Salviae miltiorrhizae Radix et Rhizoma (Danshen) are representative herbs (Zhu et al. 2011). Interestingly, growing evidence shows that CHM baths can improve patients’ joint function and quality of life and enhance the efficiency of conventional medical treatment in combined applications (Christie et al. 2007). However, although there are no obvious toxicities or side effects, patients suffering from dermatosis, acute inflammation, malignant tumors, severe cardiac insufficiency, and hypertension should avoid CHM baths (Li 2001).
16.2.1.2 Acupoint Application Therapy
Acupoint application therapy prevents and treats diseases under the guidance of a unique theory of TCM, the so-called preventive treatment of diseases. This therapy is performed by applying a mixed herbal cone cake into acupoints on the human body (Yang et al. 2015), and it has been well accepted by RA patients both alone and in combination with other therapies.
Clinically, Qubi analgesic gel paste acupoint application, midnight-noon ebb-flow acupoint application, and Leima adhesive plaster are the three most common therapeutic strategies in the treatment of RA, and they can improve the patients’ balance ability and achieve good therapeutic effects (Wang et al. 2013; Gao et al. 2013). Moreover, Chinese herbs such as Aconite radix (Chuanwu), Sinomenii caulis (Qingfengteng), Moschus (Shexiang), and Carthami flos (Honghua) that can dispel wind and dampness can activate blood circulation and remove stasis and are frequently applied to produce herbal cone cakes and act on Dazhui, Tsusanli, Waiguan, Yangliquan, and other related acupoints (Du et al. 2013). It has been shown that acupuncture combined with the acupoint application of CHMs may improve immunologic function, enhance the physical conditions of RA patients, and achieve better therapeutic effects than the sole application of acupuncture (Chen et al. 2014).
16.2.1.3 Ion Introduction of CHM
Ion introduction of CHM electrically transports ionic particles into tissues through the skin by the application of certain devices (Kim et al. 2009). This technique can be used not only to deliver molecules into the body but also to monitor drugs and biomarkers in the clinical environment (Sieg and Wascotte 2009). Ion introduction serves as a complementary therapy in the treatment of many diseases, such as topical anesthesia, endodontics, and temporomandibular joint disorders (Girenes and Ulusu 2014).
Ion introduction of CHM is often applied in combination with other therapies, including conventional medical treatments and acupuncture, in RA therapy. According to TCM theory, the obstruction may cause pain. Chinese medicinal herbs that can promote blood circulation, remove blood stasis, dispel the wind, remove meridian obstructions, dispel cold, and remove dampness are often combined with certain devices to deliver the active ingredients of the herbs deeper into the joints and to achieve better efficacy (Zhang 2002). Growing clinical evidence has shown that ion introduction of CHM may accelerate the functional recovery of RA patients and enhance the therapeutic efficacy of CHM with little side effects (Zhang et al. 2011). However, this therapy is not suitable for patients suffering from severe heart disease, active tuberculosis, hyperpyrexia, and bleeding disorders (Fan and Xia 2007).
16.2.2 Application of CHMs for Internal Use in the Treatment of RA
16.2.2.1 Extracts of CHM Administration
In recent years, numerous studies have been conducted to investigate the prominent active components contained in Chinese medicinal herbs, following that extracts of CHMs have received more popularity due to its better efficacy and fewer adverse effects with the development of pharmaceutical technology. The detailed information about these herbs is provided below.
Tripterygium wilfordii Hook F (TWHF), also known as “Lei Gong Teng” in China, have been widely applied in the treatment of several autoimmune and inflammatory diseases such as ankylosing spondylitis, psoriasis, and especially RA. It has been reported that its ethanol/ethyl acetate extract and chloroform-methanol extract could maximize therapeutic benefit and minimize toxicity (Bao and Dai 2011). Clinical trials assessed by the attainment of ACR20 response criteria had proven that TWHF extract administration resulted in greater therapeutic efficacy than a conventionally used western drug, sulfasalazine, when the treatment for RA lasted for more than 24 weeks (Goldbach-Mansky et al. 2009). Moreover, during a 6-month study, the treatment with ethanol/ethyl alcohol extract (180 mg/day) of TWHF could significantly improve the clinical signs and syndromes of RA patients, such as joint pain, joint swelling, overall well-being as well as in several indicators of inflammation including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and pro-inflammatory cytokine interleukin (IL)-6, suggesting great enhancement in active RA achieved by TWHF extracts (Macfarlane et al. 2011). In recent years, several bioactive ingredients extracted from TWHF have been further prepared into capsules, pellets, and powders for the treatment of RA (Lv et al. 2015), including Tripterygium wilfordii tablets, tripterygium glycoside tablets, and triptolide tablets, all of which have been extensively applied in clinics for RA therapy.
Sinomenii caulis (Qingfengteng), recorded by the Chinese Pharmacopoeia 2005 (Anonymous 2005), is an ancient Chinese medicinal herb and has been utilized to treat rheumatic diseases for more than a thousand years. It contains many active alkaloids, among which sinomenine (SIN) has been proved to be predominant in anti-inflammatory, antirheumatic, as well as immunosuppressive effects (Zhao et al. 2005). SIN preparation has been recorded in drug standard set by the Chinese Health Ministry and has been widely used in RA therapy in clinics (Chinese Pharmacopoeia 2005). Studies have been performed to compare the efficacy of SIN preparation with those of nonsteroidal anti-inflammatory drugs (NSAIDs). It has been indicated that SIN treatments could be more desirable regarding the total cases of RA patients whose clinical manifestations were significantly enhanced than NSAIDs. Moreover, SIN preparations possess better efficacy in improving the major syndromes of RA such as morning stiffness, joint pain, and swelling and clinical indicators of RA including ESR and CRP with dermato-mucosal adverse effects, which could be completely under the control by antihistamine reagents (Xu et al. 2008). SIN preparations are valuable remedies to treat RA in clinics.
Total glucosides of paeony (TGP) is composed of active components obtained from the roots of a conventional Chinese medicinal herb named Paeoniae radix Aiba (Baishao). It has been approved by the State Food and Drug Administration of China as a disease-modifying drug since 1998 and has been utilized in the treatment of RA for centuries (He and Dai 2011). In clinics, it is frequently applied in combination with MTX or leflunomide. It has been indicated that this combination remedy could greatly improve the registered indexes of RA patients such as the time of morning stiffness, the number of swelling joints, grip strength, ESR, CRP, and blood rheumatoid factor when the treatment lasted for 8 weeks (Zhang et al. 2007). Moreover, patients in combination therapy could achieve a better European League against rheumatism response than the sole application of western medicine, and the addition of TGP could significantly alleviate the severe hepatotoxicity resulting from MTX or leflunomide, observed in RA patients who have received therapy for 12 weeks (Chen et al. 2013).
16.2.2.2 Chinese Patent Medicine
Chinese patent medicine refers to CHMs made in the forms of pills, pulvis, emplastrum and unguentum, pellets and capsules, etc., which have the characteristics of being convenient to carry and store and have been regarded as the “essence” of TCM (Gao et al. 2014). In the clinical setting, Chinese patent medicines, such as the Xinfeng capsules, Simiao pills, Yi Shen Juan Bi pill (YLB), and Biqi capsule, are frequently used in the treatment of RA (Zhang et al. 2010).
The Xinfeng capsule is an extensively used commercial antiarthritic Chinese patent medicine and is composed of several herbs, including Astragali radix (Huangqi), Coicis semen (Yiyiren), TWHF, Scolopendra (Wugong), etc., which can invigorate the function of the spleen and resolve dampness. Clinical trials have proved that it could significantly reduce the total score of pain and swelling in involved joints and the level of uric acid and high-sensitivity CRP and also improve RA disease activity index and serum iron reserve (Huang et al. 2013a, b). Also, it has been found to exert amelioration effects in the abarticular pathologic changes in patients with active RA (Liu et al. 2014), indicating that it is a reliable therapeutic remedy.
The Simiao pill, consisting of Phellodendri chinensis Cortex (Huangbo), Atractylodis rhizoma (Cangzhu), Achyranthis bidentatae Radix (Niuqi), and Coicis semen (Yiyiren), is a well-known Chinese patent medicine for the treatment of RA with a precise compatibility. In clinics, modified Simiao pills are widely used for accurate individual RA therapies according to the syndromes and signs of RA patients. It has been demonstrated that modified Simiao pill could greatly improve the index of blood uric acid, blood leukocyte count, score of clinical symptoms, etc., and also has been proved to exert a better therapeutic efficacy than western medicine such as MTX with the advantages of low prices and readily availability (Zhao et al. 2013), indicating that it is a promising formula in clinics.
YLB is the pill form of a formula that was first prepared by the National TCM master Zhu Liangchun, and it is composed of 20 herbs including Zaocys (Wushaoshe), Scorpio (Quanxie), Scolopendra (Wugong), and Corydalis rhizoma (Yanhusuo), which can expel evil wind and remove dampness. It has been proven to have superior efficacy in the treatment of RA patients with kidney deficiency patterns (Zhou et al. 2007). Clinical trials have demonstrated that YLB could achieve more improvement on life quality and syndromes of RA patients such as arthralgia, joint pain, and joint tenderness than MTX and sulfasalazine after 24-week treatment although with relatively lower ACR20 and ACR50 responses (He et al. 2008).
Biqi capsule is composed of several traditional Chinese medicinal herbs such as Codonopsis radix (Dangshen), Astragali radix (Huangqi), and Salviae miltiorrhizae Radix et Rhizoma (Danshen) and has become an important Chinese patent drug for RA therapy in clinics. Studies have shown that Biqi capsule possesses favorable therapeutic outcomes especially for RA patients with qi deficiency and blood stasis syndrome including alleviating the degree of joint pain, the tender joint number, and the swollen joint number and shortening the morning stiffness time with no obvious adverse reaction (Liu et al. 2006). Moreover, its combination use with western medicine such as MTX showed better clinical efficacy than the application of Biqi capsule and MTX alone, indicating that it could be regarded as an effective treatment program for RA (Jie et al. 2012).
16.2.2.3 TCM Herbal Formulae
The administration of TCM herbal formulae, which are complex mixtures of herbs with multiple bioactive ingredients, is a notable feature of treatment based on holistic principles. The increased efficacy and decreased toxicity of TCM herbal formulae may arise as a result of complex synergistic or antagonistic interactions among different formula components, which meet the requirements of complex disease treatment in a systematic manner (Mao et al. 2015). Regarding the RA treatment, several classic TCM herbal formulae, such as Fangji Huangqi decoction (FHD), Wutou decoction (WTD), Guizhi Shaoyao Zhimu decoction (GSZD), Baihu Guizhi decoction (BGD), and Duhuo Jisheng decoction (DJD), have been extensively used for thousands of years (Liu and Liu 2011). Also, proved recipes prescribed by experienced clinicians have also obtained much recognition due to their satisfactory therapeutic effects. In clinics, the compositions and doses of these formulae are adjusted by clinicians by the syndromes and signs of RA patients.
FHD, composed of Stephaniae tetrandrae Radix (Fangji), Astragali radix (Huangqi), Atractylodis macrocephalae Rhizoma (Baizhu), Glycyrrhizae radix et Rhizoma (Gancao), Zingiberis rhizoma (Shengjiang), and Jujubae fructus (Dazao), is an ancient and effective remedy for the treatment of painful inflammatory disorders, such as RA, as well as the pain and edema caused by abdominal pain. In TCM theory, this remedy can relieve the symptoms of RA patients by replenishing qi to invigorate the spleen and eliminate dampness. Studies have indicated that FHD could significantly shorten the time of morning stiffness, alleviate joint pain, and exert a protective effect on the lung, liver, and kidney, of which mechanisms may be related to peripheral nociceptive pathway such as prostaglandins (Lin et al. 2015). Its components Stephaniae tetrandrae Radix (Fangji) and Astragali radix (Huangqi) play the most prominent role in this formula.
WTD, originating from the “Synopsis of Golden Chamber” (Chinese name: Jin Gui Yao Lue), is a famous TCM formula for the treatment of RA patients with cold pattern and joint pain with a history of more than a thousand years (Dai et al. 2014). It contains five herbs, including Aconite radix (Chuanwu), Ephedrae herba (Mahuang), Astragali radix (Huangqi), Paeoniae radix Aiba (Baishao), and Glycyrrhizae radix et Rhizoma (Gancao). In clinics, a prominent effectiveness can be observed when this formula is used for the treatment of RA characterized by an acute onset of severe pain. The formula exerts protective effects against joint destruction, inhibits the swelling of the limbs, and alleviates the severity of the disease. Numerous studies have shown that WTD in a combination of western medicine could enhance the therapeutic efficacy, shorten the treatment course of western medicine, as well as improve the life quality of RA patients in clinics (Hu 2011).
GSZD, originally recorded in the “Synopsis of Golden Chamber,” is a classic formula in RA therapy. It comprises the following nine herbs: Cinnamomi ramulus (Guizhi), Paeoniae radix Aiba (Baishao), Glycyrrhizae radix et Rhizoma (Gancao), Ephedrae herba (Mahuang), Zingiberis rhizoma Recens (Shengjiang), Atractylodis macrocephalae Rhizoma (Baizhu), Anemarrhenae rhizoma (Zhimu), Saposhnikoviae radix (Fangfeng), and Aconiti lateralis Radix Preparata (Fuzi). The formula has been regarded as a vital formula in the treatment of chronic RA, which is manifested by joint deformation, body weight loss, dizziness, nausea, vomiting, fatigue, shortness of breath, and pain in multiple joints. Studies have shown that it could significantly alleviate the progression of the disease, improve related indexes such as CRP and ESR, and improve patients’ quality of life (Xu et al. 2004).
BGD is another classic formula recorded in the “Synopsis of Golden Chamber” for RA treatment. In contrast to WTD, this remedy is superior to treat RA with the hot pattern, which is characterized by severe pain with hot, red, and inflamed joints, and the joint pain could be alleviated by applying cold to the joints (Lu et al. 2012). It could significantly alleviate syndromes of RA patients with hot pattern and improve their quality of life.
16.3 Pharmacological Mechanisms of CHMs Acting on RA
TCM, as a comprehensive and unique medical system, has excited worldwide interest. As a major component of TCM, CHMs are characterized by their complex nature. Subsequently, the ingredient profiling and molecular mechanisms of CHMs have not been fully elucidated, despite the considerable efforts made by many research groups. These limitations have hindered the application of CHMs in mainstream medicine and the modernization of TCM (Cooper 2007). In this section, we focus on the recent progress in elucidating the underlying mechanisms of CHMs acting on RA.
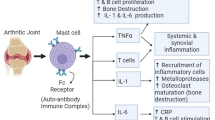
TWHF has an established history of its application in the treatment of RA. It has been proven to contain more than 70 components. Among them, tripdiolide, triptonide, and triptolide have been reported to exert the immunomodulatory and anti-inflammatory effects validated in both in vitro and in vivo studies (Qiu and Kao 2003). For instance, triptolide functions as an anti-inflammatory agent by inhibiting the production of nitric oxide (NO) and the expression of inducible nitric oxide synthase (iNOS), which lead to the inhibition of nuclear transcription-kB (NF-kB) and Jun N-terminal kinase (JNK) activation, TNF-α-induced cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) production, as well as lymphocyte proliferation (Wang et al. 2004; Zhang et al. 2004; Shao et al. 2004). Then, triptolide may play a protective role on cartilage by interfering with collagen-induced arthritis augmented expression of key enzymes including matrix metalloproteinases (MMPs)-13 and MMPs-3 in the pathological changes of cartilage (Lin et al. 2007). Also, its inhibitory effects on proMMPs-1 and proMMPs-3 and the simultaneous upregulation of tissue inhibitor of metalloproteinases (TIMPs) in IL-1 – treated synovial fibroblasts – may also contribute to the protective role of triptolide on cartilage (Lin et al. 2001). As one of the vital manifestations during RA progression, focal bone destruction within affected joints often severely influences the quality of patients’ life. A recent study of Liu et al. has indicated that triptolide may attenuate bone destruction in RA partially by regulating receptor activator of NF-kB ligand/receptor activator of NF-kB/osteoprotegerin (RANKL/RANK/OPG) signal pathway to inhibit osteoclast formation (Liu et al. 2013a, b, c). The authors also indicated that triptolide might exert therapeutic effects on angiogenesis, an essential event in the development of RA, by the downregulation of angiogenic activators and the inhibition of mitogen-activated protein kinase downstream signal pathway activation (Kong et al. 2013). Moreover, Lu et al. found that triptolide could effectively inhibit the bioactivity of IL-18 and its receptor in phorbol 12-myristate 13-acetate-stimulated RA synovial fibroblasts, highlighting a potential mechanism in RA therapy (Lu et al. 2008).
In addition to triptolide, celastrol is another single compound derived from TWHF with anti-inflammatory and bone protection properties. A recent study has reported that celastrol may directly inhibit the formation and function of osteoclast and be used as a novel medication management of RA in preventing bone destruction (Gan et al. 2015). Li et al. also found that celastrol could suppress the migration and invasion of fibroblast-like synoviocytes (FLS) by inhibiting Toll-like receptor (TLR) 4/NF-kB-mediated MMP-9 expression (Li et al. 2013a, b).
SIN is an alkaloid isolated from the stem of Chinese medicinal plant named Sinomenii caulis (Qingfengteng) and has been extensively used to treat RA diseases in China and Japan (Xu et al. 2008). Growing evidence show the anti-inflammatory and arthritis amelioration effects of SIN (Liu et al. 2005). For example, the administration of SIN could suppress inflammation response and joint destruction by targeting myeloid differentiation primary response protein 88 signaling (Mu et al. 2013). SIN could attenuate the formation of osteoclast and M. tuberculosis H37Ra-induced bone loss by mediating RANKL signaling pathways (Li et al. 2013). SIN exerts the inhibitory effects on cell invasion and migration abilities in a concentration-dependent manner by suppressing the expression of CD147, MMP-2, and MMP-9 in activated human monocytic THP-1 cells (Ou et al. 2011).
TGP, derived from the root of a Chinese herb Paeoniae radix Aiba (Baishao), contains more than 90 % paeoniflorin and has received wide popularities in China, Korea, and Japan due to its prominent anti-inflammatory, hepatoprotective, and immunomodulatory effects in the treatment of RA (Chang et al. 2009). It has been proved that TPG exerts anti-inflammatory effects through inhibiting the production of inflammatory mediators and chemokines and leukocyte migration. Also, TGP ameliorates synovitis, a major pathological change occurred in RA patients, by regulating the balance of differentiation and function of Th1/Th2 cells and secretion of pro-inflammatory cytokines originated from lymphocytes, macrophages, and FLS (He and Dai 2011). Notably, TGP also exerts protective effects on joint destruction by reducing the secretion of cartilage degradation enzymes MMPs including MMP-1 and MMP-3, which mainly account for the degradation of cartilage (Zhang and Dai 2012).
Curcumin, derived from the rhizome of Curcuma longa L., Zingiberaceae, has remarkable pharmacological and biological activities such as anti-inflammation and antioxidation against various chronic diseases, including RA (Recio et al. 2012). It has been indicated that curcumin may possess inhibitory effects on the growth and apoptosis of synovial fibroblasts, which was related to the proteolytic activation of caspase-3 and caspase-9 and modulation of poly(ADP-ribose) polymerase protein. Park et al. demonstrated that curcumin could reduce the expression of COX-2, a type of COX prominently involved in the process of inflammatory, partially by inhibiting PGE2 release (Park et al. 2007). Shakibaei et al. also revealed that curcumin might alleviate or reverse the breakdown of degenerative articular chondrocytes stimulated by IL-1β by antagonizing the activation of caspase-3 and matrix production (Shakibaei et al. 2005). Huang et al. also found the anti-inflammatory effects of curcumin, which might be associated with its suppression on the NF-kB and inflammatory loop (Huang et al. 2013). Notably, curcumin is frequently used in combination with other medications in RA therapy including resveratrol and MTX to benefit therapeutic efficiencies in clinical practice. For example, the combination of curcumin with resveratrol could antagonize the destruction of human articular chondrocytes in RA patients by activating the extracellular-regulated protein kinase signaling pathway, which might be associated with the differentiation and survival of chondrocyte (Shakibaei et al. 2011). Curcumin could synergistically support the therapy of MTX through its influence on arachidonic acid, thromboxanes, neutrophils, and lymphocytes. Interestingly, curcumin could also circumvent the hematological toxicities induced by MTX via suppressing the delivery of IL and leukotrienes, sufficiently making up this vital deficiency induced by western medicine (Banji et al. 2011).
The dry root of Anemone flaccida Fr. Schmidt (Diwu) is extensively used in clinical prescriptions in RA therapy, mainly excelling in anti-inflammatory, healing fractures and benefiting bone destruction (Han et al. 2013). Triterpenoid saponin W3 (TSW3), the major active ingredient in this plant, exerts anti-inflammatory, immunomodulatory, and analgesia effects (Cheng et al. 2008). Osteoclast plays an important role in the pathogenesis of RA and might result in excessive bone resorption within inflamed joints (Tanaka et al. 2001). The studies have indicated that TSW3 could inhibit the RANKL-induced osteoclast differentiation through downregulating the expression of a signaling adaptor molecule TNF receptor-associated factor (TRAF) 6, leading to the activation of mitogen-activated protein kinases (MAPKs) and NF-kB pathways, as well as the downregulation of two osteoclastogenic transcription factors including c-Fos and nuclear factor of activated T cells (Kong et al. 2015a, b). Also, the total saponin (TS) derived from this plant possesses antiarthritic effects and is undergoing the clinical trial in phase III in the treatment of RA (Huang et al. 2014). It has been clarified that the inhibitory effects of TS on the RANKL-induced osteoclast differentiation and bone destruction were mediated by inhibiting TRAF6 expression and suppressing JNK and p38 MAPKs and NF-kB activation. Subsequently, it downregulated the expression of a c-Fos and nuclear factor of activated T cells, which functions similarly in the way of W3 (Kong et al. 2015). Moreover, Liu et al. indicated that TS could attenuate focal and system bone destruction partially by modulating RANKL/RANK/OPG signal pathway, which has been demonstrated to play a crucial role in the process of bone loss and to inhibit the release of pro-osteoclastogenic cytokines (Liu et al. 2015).
Nobiletin, a citrus polymethoxy flavonoid, possesses numerous pharmacological activities including anti-inflammatory against various arthritic diseases. MMPs, the synthesis and secretion of which are mainly mediated by pro-inflammatory cytokines including IL-1, TNF-α, and IL-6, play an essential role in the destruction of matrix components in the pathological process of RA (Woessner 1991). Recent studies have indicated that nobiletin may suppress the secretion of proMMP-1, proMMP-3, and proMMP-9 partially by inhibiting the production of pro-inflammatory cytokines (Lin et al. 2003). Also, nobiletin also exerts inhibitory effects on PGE2 production in human synovial fibroblasts, which is an important inflammatory mediator due to its upregulation of vascular permeability (Ishiwa et al. 2000). Moreover, nobiletin could prevent cartilage destruction against RA through interfering with the expression of a disintegrin and metalloproteinase with thrombospondin-like motifs (ADAMTS)-4 and ADAMTS-5 (Imada et al. 2008). Murakami et al. proved that nobiletin could inhibit the osteoclastogenesis induced by RANKL possibly by suppressing MAPKs and blocking the differentiation of two key transcription factors including activator protein-1 and NF-kB (Murakami et al. 2007).
WTD is a classic formula for the treatment of RA, especially RA patients with the cold pattern, as previously described (Hu 2011). However, its pharmacological mechanisms have not been fully clarified. Recent studies have indicated that the anti-inflammatory effects of WTD were closely associated with its inhibition of pro-inflammatory cytokines including IL-β and TNF-α and its regulation on the TLR2/TRAF6/Faslg signal pathway (Xu et al. 2010). Accumulating studies have indicated that the pathological process of RA may frequently be accompanied by chronic inflammatory pain (Chiu et al. 2012). Members of the transient receptor potential (TRP) ion channel family especially TRP vanilloid type 1 (TRPV1), TRP ankyrin type 1 (TRPA1), and TRP melastatin type 8 (TRPM8) ion channels are closely involved in the induced inflammatory nociceptive responses (Sousa-Valente et al. 2014). Wang et al. found that WTD could possess antinociceptive property through decreasing mechanical and thermal hypersensitivities, partially resulting from its suppression of the expression of TRPV1, TRPA1, and TRPM8 (Wang et al. 2015). Interestingly, network pharmacology-based approaches have also been used to investigate the underlying mechanisms of WTD. Zhang et al. pointed out that WTD could alleviate RA possibly by reversing the imbalance of the nervous, endocrine, and immune systems, which markedly influence the pathological progression of RA (Zhang et al. 2015).
GSZD excels in the treatment of RA with hot pattern (Xu et al. 2004). An integrative method that combines both network analysis and experimental validation has also been applied to investigate the pharmacological mechanisms of GSZD acting on RA. The authors identified a candidate GSZD-targeted signal axis and found that GSZD plays a role in the treatment of RA partially by regulating inflammation-immune system imbalance (Guo et al. 2016).
Huo Luo Xiao Ling (HLXL) Dan, composed of Angelicae sinensis Radix (Danggui), Olibanum (Ruxiang), Salviae miltiorrhizae Radix et Rhizoma (Danshen), and Myrrha (Moyao), is a well-known herbal formula, and its modified versions have been applied to treat RA (Yu et al. 2013). Pharmacological studies have indicated that HLXL could alleviate the severity of ongoing inflammatory in RA, which might be associated with its alteration in T cells by regulating the antibody response against the RA-associated antigen (the mycobacterial heat-shock protein 65) and the serum level of NO (Yang et al. 2011). HLXL also exerts protective effects against arthritic bone destruction in adjuvant arthritis model, which was mediated by its regulatory effects on the mediators of bone remodeling (Nanjundaiah et al. 2013).
FHD extract possesses antinociceptive, anti-inflammatory, and immune-modulatory activities, some of which was validated in rodents with the acetic acid-induced writhing response, carrageenan-induced edema test, and formalin-induced licking test (Chen 2012). Regarding its antinociceptive effect, a recent study has demonstrated that the pretreatment with FHD extract could decrease acetic acid-induced writhing response and significantly prevent the late phase of formalin-induced licking response (Taber et al. 1969). Also, FHD produces marked antinociceptive activities in a dose-dependent way, which might be associated with the peripheral systems of pain pathway (Lin et al. 2015). Importantly, growing evidence show that almost all herbs in FHD may possess anti-inflammatory effects synergistically by regulating NF-kB, iNOS, and COX-2/prostaglandin pathway and by inhibiting the release of pro-inflammatory cytokines (Lin et al. 2015).
Wen Luo Yin (WLY), originated from the classic formula Guizhi Fuzi decoction and ZhuFu decoction, has been extensively applied in the treatment of RA with a cold pattern in clinics, especially excelling in the RA patients presented by obvious pain. WLY could alleviate pain, inflame joint swelling, and also inhibit the excessive secretion of synoviocytes. These possibly may be due to the decreased number of Golgi apparatus, rough surface endoplasmic reticulum, dense bodies, matrix filaments, and vacuoles, which are involved in the ultrastructures of synoviocytes (Li et al. 2002). Angiogenesis has been considered as an important event in affecting inflammatory and immune responses and further interfering with the pathological process of RA (Thairu et al. 2011). Liu and the group have reported that WLY exerts significant anti-angiogenic effects by suppressing the expression of numerous of angiogenic activators, including TNF-α, IL-1β, IL-17, vascular endothelial growth factor (VEGF), VEGF receptor, angiopoietins, and epidermal growth factor. All these participate in the progression of neovasculature by mutual interaction in the sera of collagen-induced rats in human FLS of RA and human umbilical vein endothelial cells induced by IL-1β, indicating that WLY is a promising therapeutic remedy in RA (Liu et al. 2013).
These previous studies have successfully identified the biological activities and targets of a herb or herbal formula and elucidated their molecular mechanisms for RA treatment. Many research techniques, such as flow cytometry analysis, cell differentiation assays, quantitative real-time PCR, Western blot analysis, cell migration assays, cell proliferation assays, and luciferase assays, have been commonly performed in the experimental studies. With the rapid progress in bioinformatics, system biology, and polypharmacology, network pharmacology has attracted much attention because it can reveal the underlying complex interactions between an herbal formula and cellular proteins, as well as can influence their interactions on the function and behavior of the system. It shifts the “one target, one drug” paradigm to the “network target, multicomponent” strategy (Li et al. 2007). Above all, the combination of the conventional experimental approaches and network pharmacology strategies can provide a powerful means of modern research on TCM in the future.
16.4 Safety and Adverse Effects of CHMs Acting on RA
CHMs perform well in clinical practice and have a bright future in the treatment of RA. However, the safety of CHMs has been a widespread concern due to their complex chemical nature and lack of proper evaluation methods, especially after recent consecutive reports of adverse drug reactions. Because CHMs are often used in humans for an extended period, continuous surveillance of patient safety during CHM treatments may be a convenient and powerful way to detect any potential harm caused by CHM therapy.
HLXL is a TCM formula for the treatment of a variety of immune disorders, including RA. By feeding HLXL to Lewis rats for 6 weeks consecutively, its toxicity was assessed. During the experiments, abnormal behavioral changes and standard manifestations of toxicity were documented (Zhang et al. 2009). Moreover, the blood biochemistry and histopathological variations of the tissues were examined to assess its toxicity. No adverse reactions or toxicity from this formula was observed at normal doses when taking all of the parameters together. Therefore, HLXL is reliable in RA therapy (Yang et al. 2011).
Fuzi (the lateral root of Aconitum carmichaeli) is a well-known herb for its bilateral effects, including both effectiveness and toxicity. Aconitine, mesaconitine, and hypaconitine are mainly responsible for its high toxicity. In clinical practice, Fuzi is processed by hydrolyzing toxic components into nontoxic derivatives, resulting in a toxicity reduction (Huang et al. 2007). Studies have been conducted to investigate whether the therapeutic efficacy of Fuzi remains after processing. In adjuvant-induced arthritic rats, it has been proven that processed Fuzi with 120 min decoctions could achieve the same therapeutic efficacy as the products processed for less time (Tong et al. 2013), indicating a non-interdependent relationship between its therapeutic effectiveness and toxicity.
Regarding TWHF, recent studies have reported that the efficacy and toxicity of this CHM are dose dependent (Zhao et al. 2015). The adverse effects of TWHF may be involved in many aspects of human body, such as gastrointestinal tract disturbances, dermatosis, reproductive system malfunction in both males and females, acute hepatotoxicity, and nephrotoxicity (Bao and Dai 2011). It could also lead to the blood system adverse events including the white blood cell decreasing, hemoglobin decreasing, and platelet decreasing (Li et al. 2015c). Triptolide is regarded to be the main contributor to these toxicities. Terpene triptonide and alkaloids in TWHF have been proved to be of no toxicological concern at the dosage of 20-fold of the therapeutic dose. Detoxification of TWHF could be achieved by metabolic eliminations, leading to less reactive metabolites. Moreover, safety issues should be taken into more consideration when it is coadministered with other cyclophosphamide inhibitors or glutathione-depleting agents (Li et al. 2015). TWHF could be a promising drug with the further in-depth investigation of its efficacy and toxicity.
Insect medicine excels in expelling evil wind and removing dampness in RA therapy. Scorpio, centipede, and Agkistrodon are representative insects. However, modern pharmacological studies indicate that homologous proteins such as toxic protein and histamine-like substance in these insects could exert toxic effects or allergic reactions if not properly used (Li and Liao 2011).
Asarum is a key herb in the treatment of arthrodynia. Volatile oils are its active constituent. The safrole containing in volatile oils could lead to several side effects, such as respiratory paralysis or arrhythmias (Li and Liao 2011).
Given all the issues mentioned above, it is clinically important to harness CHM advantages and bypass the disadvantages. The adverse effects of CHMs can be confined within a reasonable range by appropriate methods such as the standardization of CHM production, CHM processing, proper combination, and correct dose administration based on different pathological conditions. Moreover, the more information we obtain about the safety and adverse events of CHM therapies, the greater the likelihood that physicians trained in conventional medicine will be encouraged to use such medicines. Therefore, further studies are required to generate data and information on the adverse effects of existing CHM therapies for the treatment of RA.
16.5 Perspective
CHMs have been recognized extensively to benefit RA patients due to their efficacies, minimal adverse effects, affordable price, therapeutic effects, and possibility of long-term application. However, the following limitations and issues need our consideration.
First, TCM is characterized by individualized treatment. Clinicians make prescriptions according to the syndromes and signs of patients and classify them into different patterns such as hot pattern, cold pattern, deficiency pattern, etc. It is important to clarify the molecular mechanisms of pattern and CHMs. Gaps in knowledge regarding the characteristics and mechanisms of CHMs acting on different patterns of RA still exist due to the complexities of CHMs and limitations of investigative techniques.
Second, cognized consensus standard regarding the safety of CHMs has not been completed. The efficacy of CHMs against RA has long been based on empiricism. Although the appropriate compatibility of herbs could enhance the therapeutic efficacy and reduce toxicity such as the coadministration of Bupleuri radix (Chaihu) and Pinelliae rhizoma (Banxia) (Liu et al. 2013a, b, c), its safety cannot be fully guaranteed. The application of several modern research tools, such as gene expression microarrays, proteomics, and biological molecular networks, may shed light on a holistic understanding of CHMs. Thus, offer an interface where CHMs and conventional medicine can find common ground to investigate the mechanisms of action of therapeutic products and to enhance their practical use for the ultimate benefit of the patients (Venkatesha et al. 2011).
Finally, efforts are needed to standardize the safety assessment of CHMs, in-depth toxicity-related studies of CHMs, and safer advanced preparation technology, such as nano-drug delivery and other targeted drug delivery. Also, refinement in trial-related issues, such as sample size, explicit inclusion/exclusion criteria, consistent standards for assessing the outcome of therapeutic intervention, and proper statistical analysis are other issues for consideration (Efthimiou and Kukar 2010).
Thus, the future studies that combine the routine application of CHMs and advanced technologies will be essential to explore fully the therapeutic effects, mechanisms, and safety of CHMs acting on RA. There is a promise that CHMs may receive increasing therapeutic approvals and may benefit RA patients in a more effective way.
Abbreviations
- BGD:
-
Baihu Guizhi decoction
- CAM:
-
Complementary and alternative medicine
- CHMs:
-
Chinese herbal medicines
- COX-2:
-
Cyclooxygenase-2
- CRP:
-
C-reactive protein
- DJD:
-
Duhuo Jisheng decoction
- DMARDs:
-
Disease-modifying antirheumatic drugs
- ESR:
-
Erythrocyte sedimentation rate
- FHD:
-
Fangji Huangqi decoction
- FLS:
-
Fibroblast-like synoviocytes
- GSZD:
-
Guizhi Shaoyao Zhimu decoction
- HLXL:
-
Huo-Luo-Xiao-Ling Dan
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
Jun N-terminal kinases
- MAPKs:
-
Mitogen-activated protein kinases
- MMPs:
-
Matrix metalloproteinases
- MTX:
-
Methotrexate
- NF-kB:
-
Nuclear transcription-kB
- NO:
-
Nitric oxide
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- OPG:
-
Osteoprotegerin
- PGE2:
-
Prostaglandin E2
- RA:
-
Rheumatoid arthritis
- RANK:
-
Receptor activator of NF-kB
- RANKL:
-
Receptor activator of NF-kB ligand
- SIN:
-
Sinomenine
- TCM:
-
Traditional Chinese medicine
- TGP:
-
Total glucosides of paeony
- TIMPs:
-
Tissue inhibitor of metalloproteinases
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TRAF:
-
TNF receptor-associated factor
- TRP:
-
Transient receptor potential
- TRPA:
-
TRP ankyrin type 1
- TRPM8:
-
TRP melastatin type 8
- TRPV1:
-
TRP vanilloid type 1
- TS:
-
Total saponin
- TSW3:
-
Triterpenoid saponin W3
- TWHF:
-
Tripterygium wilfordii Hook F
- VEGF:
-
Vascular endothelial growth factor
- WLY:
-
Wen Luo Yin
- WTD:
-
Wutou decoction
- YLB:
-
Yi Shen Juan Bi pill
References
Anonymous (2005) Caulis sinomenii. In: Chinese Pharmacopoeia Committee (ed) Chinese pharmacopoeia, vol 1. Chinese Chemical Industry Press, Beijing, p 135
Avina-Zubieta JA, Choi HK, Sadatsafavi M et al (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 59(12):1690–1697. doi:10.1002/art.24092
Banji D, Pinnapureddy J, Banji OJ et al (2011) Evaluation of the concomitant use of methotrexate and curcumin on Freund’s complete adjuvant-induced arthritis and hematological indices in rats. Indian J Pharmacol 43(5):546–550. doi:10.4103/0253-7613.84970
Bao J, Dai SM (2011) A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: mechanism, efficacy, and safety. Rheumatol Int 31(9):1123–1129. doi:10.1007/s00296-011-1841-y
Chang Y, Wei W, Zhang L (2009) Effects and mechanisms of total glucosides of paeony on synoviocytes activities in rat collagen-induced arthritis. J Ethnopharmacol 121(1):43–48. doi:10.1016/j.jep.2008.09.028
Chen GX (2012) Clinical trials of the combination of modified Fangji-Huangqi decoction and Duhuo-Jishen decoction in the treatment of RA in 32 cases. Guiding J Trad Chin Med Pharm 18(4):58–59 (In Chinese)
Chen Z, Li XP, Li ZJ et al (2013) Reduced hepatotoxicity by total glucosides of paeony in combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. Int Immunopharmacol 15(3):474–477. doi:10.1016/j.intimp.2013.01.021
Chen ZP, Chen PD, Zhuang LX (2014) Acupoint herbal application combined with electro-acupuncture in the treatment of knee osteoarthritis. J Clin Acupunct Moxibustion 5(5):28–30 (In Chinese)
Cheng S, Du Y, Bing F et al (2008) Synthesis of flaccidoside II, a bidesmosidic triterpene saponin isolated from Chinese folk medicine Di Wu. Carbohydr Res 343(3):462–469
Chiu IM, von Hehn CA, Woolf CJ (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15(8):1063–1067. doi:10.1038/nn.3144
Christie A, Jamtvedt G, Dahm KT et al (2007) Effectiveness of nonpharmacological and nonsurgical interventions for patients with rheumatoid arthritis: an overview of systematic reviews. Phys Ther 87(12):1697–1715
Cooper EL (2007) The immune system and complementary and alternative medicine. Evid Based Complement Alternat Med 4(1):5–8. doi:10.1093/ecam/nem093
Dai PM, Wang Y, Ye L et al (2014) Pharmacokinetic comparisons of benzoylmesaconine in rats using ultra-performance liquid chromatography-tandem mass spectrometry after administration of pure benzoylmesaconine and Wutou decoction. Molecules 19(10):16757–16769. doi:10.3390/molecules191016757
Doan T, Massarotti E (2005) Rheumatoid arthritis: an overview of new and emerging therapies. J Clin Pharmacol 45(7):751–762
Donahue KE, Jonas DE, Hansen RA et al (2012) Drug therapy for rheumatoid arthritis in adults: an update. Agency for Healthcare Research and Quality (US), Rockville. Report No.: 12-EHC025-EF
Du J, Li YJ, Li Q et al (2013) Efficacy of Hanbi powder acupoint application with massage for knee osteoarthritis. J Nurs (12):1–4 (In Chinese)
Efthimiou P, Kukar M (2010) Complementary and alternative medicine use in rheumatoid arthritis: proposed mechanism of action and efficacy of commonly used modalities. Rheumatol Int 30(5):571–586. doi:10.1007/s00296-009-1206-y
Fan DH, Xia B (2007) Observation on therapeutic effect of heat needle combined with herb iontophoresis and western medicine on rheumatoid arthritis. Chin Acupunct Moxibustion 27(10):731–734 (In Chinese)
Gabriel SE, Michaud K (2009) Epidemiological studies of incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 11(3):229. doi:10.1186/ar2669
Gan K, Xu L, Feng X et al (2015) Celastrol attenuates bone erosion in collagen-induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7. Int Immunopharmacol 24(2):239–246. doi:10.1016/j.intimp.2014.12.012
Gao J, Zhang T, Zhang Q et al (2013) Effect of midnight-noon ebb-flow of acupoint application on balance ability in patients with knee osteoarthritis. Chin J Pract Nurs 29(29):26–28 (In Chinese)
Gao F, Leng J, Fu CM et al (2014) Interpretation of contemporary positioning of traditional Chinese medicine injections and analysis of key problems. China J Chin Mater Med 39(17):3416–3419 (In Chinese)
Gibofsky A (2012a) Comparative effectiveness of current treatments for rheumatoid arthritis. Am J Manag Care 18(13):303–314
Gibofsky A (2012b) Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care 18(13):295–302
Gibofsky A (2014) Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care 20(7):128–135
Girenes G, Ulusu T (2014) An in vitro evaluation of the efficacy of a novel iontophoresis fluoride tray on remineralization. J Clin Exp Dent 6(4):327–334. doi:10.4317/jced.51376
Goldbach-Mansky R, Wilson M, Fleischmann R et al (2009) Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med 151(4):229–240
Guo QY, Mao X, Zhang YQ, et al (2016) Guizhi-Shaoyao-Zhimu decoction attenuates rheumatoid arthritis partially by reversing inflammation-immune system imbalance. J Trans Med 14(1):165
Han LT, Fang Y, Li MM et al (2013) The antitumor effects of triterpenoid saponins from the Anemone flaccida and the underlying mechanism. Evid Based Complement Alternat Med 2013:517931. doi:10.1155/2013/517931
He DY, Dai SM (2011) Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol 2:10. doi:10.3389/fphar.2011.00010
He Y, Lu A, Zha Y et al (2008) Differential effect on symptoms treated with traditional Chinese medicine and western combination therapy in RA patients. Complement Ther Med 16(4):206–211. doi:10.1016/j.ctim.2007.08.005
He YT, Ou AH, Yang XB et al (2014) Traditional Chinese medicine versus Western medicine as used in China in the management of rheumatoid arthritis: a randomized, single-blind, 24-week study. Rheumatol Int 34(12):1647–1655
Hu HZ (2011) A clinical analysis of treating rheumatic arthralgia with Wutou decoction. Clin J Chin Med 3(2):20 (In Chinese)
Huang QA, Zhang YM, He Y et al (2007) Studies on hydrolysis of aconitine. China J Chin Mater Med 32(20):2143–2145 (In Chinese)
Huang CB, Liu J, Chen X et al (2013a) Treatment of rheumatoid arthritis by xinfeng capsule: an efficacy observation. Zhongguo Zhong Xi Yi Jie He Za Zhi 33(12):1599–1602 (In Chinese)
Huang G, Xu Z, Huang Y et al (2013b) Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J Clin Immunol 33(3):550–557. doi:10.1007/s10875-012-9839-0
Huang XJ, Tang JQ, Li MM et al (2014) Triterpenoid saponins from the rhizomes of Anemone flaccida and their inhibitory activities on LPS-induced NO production in macrophage RAW264.7 cells. J Asian Nat Prod Res 16(9):910–921. doi:10.1080/10286020.2014.954554
Imada K, Lin N, Liu C et al (2008) Nobiletin, a citrus polymethoxy flavonoid, suppresses gene expression and production of aggrecanases-1 and -2 in collagen-induced arthritic mice. Biochem Biophys Res Commun 373(2):181–185. doi:10.1016/j.bbrc.2008.05.171
Ishiwa J, Sato T, Mimaki Y et al (2000) A citrus flavonoid nobiletin suppresses production and gene expression of matrix metalloproteinase 9/gelatinase B in rabbit synovial fibroblasts. J Rheumatol 27(1):20–25
Jiang M, Lu C, Chen G et al (2012) Understanding the molecular mechanism of interventions in treating rheumatoid arthritis patients with corresponding traditional Chinese medicine patterns based on bioinformatics approach. Evid Based Complement Alternat Med 2012:129452. doi:10.1155/2012/129452
Jie HY, Wu QF, Ding ZX (2012) Clinical study of Biqi capsule combined with methotrexate for treatment of rheumatoid arthritis. Zhongguo Zhong Xi Yi Jie He Za Zhi 32(2):195–198 (In Chinese)
Kim HE, Kwon HK, Kim BI (2009) Application of fluoride iontophoresis to improve remineralization. J Oral Rehabil 36(10):770–775. doi:10.1111/j.1365-2842.2009.01992.x
Kong X, Zhang Y, Liu C et al (2013) Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS ONE 8(10), e77513. doi:10.1371/journal.pone.0077513
Kong X, Wu W, Yang Y et al (2015a) Total saponin from Anemone flaccidaFr. Schmidt abrogates osteoclast differentiation and bone resorption via the inhibition of RANKL-induced NF-kB, JNK, and p38 MAPKs activation. J Transl Med 13:91. doi:10.1186/s12967-015-0440-1
Kong X, Yang Y, Wu W et al (2015b) Triterpenoid saponin W3 from Anemone flaccida suppresses osteoclast differentiation through inhibiting activation of MAPKs and NF-kB pathways. Int J Biol Sci 11(10):1204–1214. doi:10.7150/ijbs.12296
Li ZY (2001) Nursing care of treating rheumatoid arthritis by medicated bath with Chinese traditional herbs. Acta Acad Med Militaris Tertiae 23(6):685–685 (In Chinese)
Li N, Liao YH (2011) Discussion about the application of toxic traditional Chinese medicine in the treatment of rheumatoid arthritis. Guiding J Tradit Chin Med Pharm 17(3):99–100 (In Chinese)
Li S, Lu A, Jia H (2002) Therapeutic actions of the Chinese herbal formulae with cold and heat properties and their effects on ultrastructures of synoviocytes in rats of collagen-induced arthritis. J Tradit Chin Med 22(4):296–302
Li S, Zhang ZQ, Wu LJ (2007) Understanding ZHENG in traditional Chinese medicine in the context of the neuro-endocrine-immune network. IET Syst Biol 1(1):51–60
Li G, Liu D, Zhang Y et al (2013a) Celastrol inhibits lipopolysaccharide-stimulated rheumatoid fibroblast-like synoviocyte invasion through suppression of TLR4/NF-kB-mediated matrix metalloproteinase-9 expression. PLoS ONE 8(7), e68905. doi:10.1371/journal.pone
Li X, He L, Hu Y et al (2013b) Sinomenine suppresses osteoclast formation and Mycobacterium tuberculosis H37Ra-induced bone loss by modulating RANKL signaling pathways. PLoS ONE 8(9), e74274. doi:10.1371/journal.pone.0074274
Li XX, Du FY, Liu HX et al (2015a) Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicity and nephrotoxicity. J Ethnopharmacol 162:238–243. doi:10.1016/j.jep.2015.01.004
Li Y, Li R, Ouyang Z et al (2015b) Herb network analysis for a famous TCM doctor’s prescriptions on the treatment of rheumatoid arthritis. Evid Based Complement Alternat Med 2015:451319. doi:10.1155/2015/451319
Li ZX, Ma DM, Yang XH et al (2015c) Meta-analysis of blood system adverse events of Tripterygium wilfordii. Zhongguo Zhong Yao Za Zhi 40(2):339–345 (In Chinese)
Lin N, Sato T, Ito A (2001) Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f. suppresses the production and gene expression of pro–matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum 44(9):2193–2200
Lin N, Sato T, Takayama Y et al (2003) Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol 65(12):2065–2071
Lin N, Liu C, Xiao C et al (2007) Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol 73(1):136–146
Lin YC, Chang CW, Wu CR (2015) Antinociceptive, anti-inflammatory and toxicological evaluation of Fang-Ji-Huang-Qi-Tang in rodents. BMC Complement Altern Med 15:10. doi:10.1186/s12906-015-0527-5
Liu J, Liu RL (2011) The potential role of Chinese medicine in ameliorating extra-articular manifestations of rheumatoid arthritis. Chin J Integr Med 17(10):735–737. doi:10.1007/s11655-011-0872-2
Liu ZQ, Kelvin C, Zhou H et al (2005) The pharmacokinetics and tissue distribution of sinomenine in rats and its protein binding ability in vitro. Life Sci 77(25):3197–3209
Liu W, Zhang L, Xu Z (2006) Clinical observation on treatment of rheumatoid arthritis with the Biqi capsule. Zhongguo Zhong Xi Yi Jie He Za Zhi 26(2):157–159 (In Chinese)
Liu C, Kong X, Li X et al (2013a) Wen Luo Yin inhibits angiogenesis in collagen-induced arthritis rat model and in vitro. J Ethnopharmacol 149(2):478–489. doi:10.1016/j.jep.2013.07.002
Liu C, Zhang Y, Kong X et al (2013b) Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based Complement Alternat Med 2013:626038. doi:10.1155/2013/626038
Liu CF, Tan SF, Wang DH et al (2013c) Study on efficacy of compatibility between Aconiti radix cocta and Pinelliae rhizoma by the uniform design method. Zhongguo Zhong Yao Za Zhi 38(13):2169–2175 (In Chinese)
Liu J, Cao Y, Huang C, Wang Y et al (2014) Use of xinfeng capsule to treat abarticular pathologic changes in patients with rheumatoid arthritis. J Tradit Chin Med 34(5):532–538
Liu C, Yang Y, Sun D et al (2015) Total saponin from Anemone flaccida Fr. Schmidt prevents bone destruction in experimental rheumatoid arthritis via inhibiting osteoclastogenesis. Rejuvenation Res 18(6):528–542. doi:10.1089/rej.2015.1688
Longo UG, Petrillo S, Denaro V (2015) Current concepts in the management of rheumatoid hand. Int J Rheumatol 2015:648073. doi:10.1155/2015/648073
Lu Y, Wang WJ, Leng JH et al (2008) Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Inflamm Res 57(6):260–265. doi:10.1007/s00011-007-7128-9
Lu C, Xiao C, Chen G et al (2012) Cold and heat pattern of rheumatoid arthritis in traditional Chinese medicine: distinct molecular signatures identified by microarray expression profiles in CD4-positive T cell. Rheumatol Int 32(1):61–68. doi:10.1007/s00296-010-1546-7
Lv QW, Zhang W, Shi Q et al (2015) Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomized, controlled clinical trial. Ann Rheum Dis 74(6):1078–1086. doi:10.1136/annrheumdis-2013-204807
Macfarlane GJ, El-Metwally A, De Silva V et al (2011) Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: a systematic review. Rheumatology (Oxford) 50(9):1672–1683. doi:10.1093/rheumatology/ker119
Mao X, Zhang Y, Lin N (2015) Application perspectives of traditional Chinese medicine in the treatment of liver cancer. Cancer Transl Med 1:101–107
Mu H, Yao RB, Zhao LJ et al (2013) Sinomenine decreases MyD88 expression and improves inflammation-induced joint damage progression and symptoms in rat adjuvant-induced arthritis. Inflammation 36(5):1136–1144. doi:10.1007/s10753-013-9648-5
Murakami A, Song M, Katsumata S et al (2007) Citrus nobiletin suppresses bone loss in ovariectomized ddY mice and collagen-induced arthritis in DBA/1J mice: possible involvement of receptor activator of NF-kappaB ligand (RANKL)-induced osteoclastogenesis regulation. Biofactors 30(3):179–192
Nanjundaiah SM, Lee DY, Berman BM et al (2013) Chinese herbal formula Huo-Luo-Xiao-Ling Dan protects against bone damage in adjuvant arthritis by modulating the mediators of bone remodeling. Evid Based Complement Alternat Med 2013:429606. doi:10.1155/2013/429606
Ou Y, Li W, Li X et al (2011) Sinomenine reduces invasion and migration ability in fibroblast-like synoviocytes cells co-cultured with activated human monocytic THP-1 cells by inhibiting the expression of MMP-2, MMP-9, CD147. Rheumatol Int 31(11):1479–1485. doi:10.1007/s00296-010-1506-2
Park C, Moon DO, Choi IW (2007) Curcumin induces apoptosis and inhibits prostaglandin E2 production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med 20(3):365–372
Qi F, Li A, Inagaki Y et al (2010) Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 4(6):297–307
Qiu D, Kao PN (2003) Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D 4(1):1–18
Recio MC, Andujar I, Rios JL (2012) Anti-inflammatory agents from plants: progress and potential. Curr Med Chem 19(14):2088–2103
Shakibaei M, Schulze-Tanzil G, John T (2005) A curcumin protects human chondrocytes from IL-1β-induced inhibition of collagen type II and β1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat 187(5–6):487–497
Shakibaei M, Mobasheri A, Buhrmann C (2011) Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr 6(2):171–179. doi:10.1007/s12263-010-0179-5
Shao XT, Feng L, Yao HP et al (2004) Effect of triptolide on TNF-α-induced activation of NF-kB and expression of COX-2 and iNOS in human rheumatoid arthritis synovial fibroblasts. Zhejiang Daxue Xuebao Yixue Bao 33(2):160–165 (In Chinese)
Sieg A, Wascotte V (2009) Diagnostic and therapeutic applications of iontophoresis. J Drug Target 17(9):690–700. doi:10.3109/10611860903089750
Sousa-Valente J, Andreou AP, Urban L et al (2014) Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br J Pharmacol 171(10):2508–2527. doi:10.1111/bph.12532
Taber RI, Greenhouse DD, Rendell JK et al (1969) Agonist and antagonist interactions of opioids on acetic acid-induced abdominal stretching in mice. J Pharmacol Exp Ther 169:29–38
Tanaka S, Nakamura K, Oda H (2001) The osteoclast: a potential therapeutic target of bone and joint destruction in rheumatoid arthritis. Mod Rheumatol Jpn Rheum Assoc 11(3):177–183. doi:10.3109/s101650170001
Thairu N, Kiriakidis S, Dawson P et al (2011) Angiogenesis as a therapeutic target in arthritis in 2011: learning the lessons of the colorectal cancer experience. Angiogenesis 14(3):223–234. doi:10.1007/s10456-011-9208-2
Tong P, Wu C, Wang X et al (2013) Development and assessment of a complete-detoxication strategy for Fuzi (lateral root of Aconitum carmichaeli) and its application in rheumatoid arthritis therapy. J Ethnopharmacol 146(2):562–571. doi:10.1016/j.jep.2013.01.025
Venkatesha SH, Rajaiah R, Berman BM et al (2011) Immunomodulation of autoimmune arthritis by herbal CAM. Evid Based Complement Alternat Med 2011:986797. doi:10.1155/2011/986797
Wang B, Ma L, Tao X et al (2004) Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum 50(9):2995–303
Wang H, Fang JQ, Fang F et al (2013) Effects of Qubi analgesic gel paste acupoint application on collagen-induced arthritis inflammatory rats. J Zhejiang Chin Med Univ 37(5):613–616 (In Chinese)
Wang C, Liu C, Wan H et al (2015) Wu-Tou decoction inhibits chronic inflammatory pain in mice: participation of TRPV1 and TRPA1 ion channels. Biomed Res Int 2015:328707. doi:10.1155/2015/328707
Woessner JF Jr (1991) Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J 5(8):2145–2154
Xu Q, Wang J, Liu YH (2004) Clinical observation on treatment of rheumatoid arthritis with United Chinese medicine and western medicine. China J Mod Med 14(8):140–145 (In Chinese)
Xu M, Liu L, Qi C et al (2008) Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: a systematic review and meta-analysis. Planta Med 74(12):1423–1429
Xu S, Li L, Zhang W, Wang Y (2010) Comparative study of the regulation of TLR/TRAF signaling pathway of classic prescriptions that deals with arthralgia syndrome based on the relevant theory of prescription and syndrome. Zhongguo Zhong Yao Za Zhi 35(8):1025–1029 (In Chinese)
Yang YH, Rajaiah R, Lee DY et al (2011) Suppression of ongoing experimental arthritis by a Chinese herbal formula (Huo-Luo-Xiao-Ling Dan) involves changes in antigen-induced immunological and biochemical mediators of inflammation. Evid Based Complement Alternat Med 2011:642027. doi:10.1155/2011/642027
Yang XC, Yin T, Gao Q et al (2015) The immunomodulatory effect of acupoint application for childhood asthma: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2015:896247. doi:10.1155/2015/896247
Yu H, Lee DY, Nanjundaiah SM et al (2013) Microarray analysis reveals the molecular basis of antiarthritic activity of Huo-Luo-Xiao-Ling Dan. Evid Based Complement Alternat Med 2013:524746. doi:10.1155/2013/524746
Yuan HY, Zhang XL, Zhang XH et al (2015) Analysis of patents on anti-rheumatoid arthritis therapies issued in China. Expert Opin Ther Pat 25(8):909–930. doi:10.1517/13543776.2015.1044972
Zhang LJ (2002) Observation and nursing of treating rheumatoid arthritis by herbal ion infusion. Acta Acad Med Militaris Tertiae 24(7):792–792, 795 (In Chinese)
Zhang W, Dai SM (2012) Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int Immunopharmacol 14(1):27–31. doi:10.1016/j.intimp.2012.06.001
Zhang N, Xu YJ, Zhang ZX (2004) Regulatory function of nuclear factor kappa B on lymphocyte proliferation and apoptosis in bronchial asthmatic rats and effect of triptolide on the regulation. Zhongguo Zhongxiyi Jiehe Zazhi 24(5):435–438 (In Chinese)
Zhang DC, Ye HL, Zhu DB (2007) The prospective efficacy of total glucosides of paeony combined with leflunomide in treating rheumatoid arthritis: a report of 40 cases. J Bengbu Med Coll 32(3):307–308 (In Chinese)
Zhang RX, Fan AY, Zhou AN et al (2009) Extract of the Chinese herbal formula Huo- Luo-Xiao-Ling- Dan inhibited adjuvant arthritis in rats. J Ethnopharmacol 121(3):366–371. doi:10.1016/j.jep.2008.11.018
Zhang P, Li J, Han Y et al (2010) Traditional Chinese medicine in the treatment of rheumatoid arthritis: a general review. Rheumatol Int 30(6):713–718. doi:10.1007/s00296-010-1370-0
Zhang C, Jiang M, Lu A (2011) A traditional Chinese medicine versus western combination therapy in the treatment of rheumatoid arthritis: two-stage study protocol for a randomized controlled trial. Trials 12:137. doi:10.1186/1745-6215-12-137
Zhang Y, Bai M, Zhang B et al (2015) Uncovering pharmacological mechanisms of Wu-tou decoction acting on rheumatoid arthritis through systems approaches: drug-target prediction, network analysis, and experimental validation. Sci Rep 5:9463. doi:10.1038/srep09463
Zhao ZZ, Liang ZT, Zhou H et al (2005) Quantification of sinomenine in Caulis sinomenii collected from different growing regions and wholesale herbal markets by a modified HPLC method. Biol Pharm Bull 28(1):105–109
Zhao J, Zha Q, Jiang M et al (2013) Expert consensus on the treatment of rheumatoid arthritis with Chinese patent medicines. J Altern Complement Med 19(2):111–118. doi:10.1089/acm.2011.0370
Zhao QH, Li XY, Feng Q et al (2015) Evaluation of the dosage-based efficacy-toxicity correlation of Tripterygium wilfordii against immune inflammation in mice. Zhongguo Zhong Yao Za Zhi 40(6):1139–1143 (In Chinese)
Zhou DH, Zhou ZQ, Zhu WH (2007) Zhu Liangchun’s experience prescription in 38 cases treatment of rheumatoid arthritis. J New Chin Med 39(9):71–72 (In Chinese)
Zhu DL, Zeng DZ, Wang BH et al (2011) Observation of curative effects of Chinese medicine bath on rheumatoid arthritis. Mod J Integr Tradit Chin West Med 20(7):816–817 (In Chinese)
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Mao, X., Guo, Q., Lu, A., Zhang, Y., Lin, N. (2016). Advances in Chinese Herbal Medicine for Rheumatoid Arthritis: Clinical Utilization and Efficacy, Mechanism of Action, and Safety. In: Tsay, HS., Shyur, LF., Agrawal, D., Wu, YC., Wang, SY. (eds) Medicinal Plants - Recent Advances in Research and Development. Springer, Singapore. https://doi.org/10.1007/978-981-10-1085-9_16
Download citation
DOI: https://doi.org/10.1007/978-981-10-1085-9_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-1084-2
Online ISBN: 978-981-10-1085-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)