Abstract
Tripterygium wilfordii Hook F (TwHF) is a Chinese herb with immunosuppressive effects and an established history of use in the treatment of rheumatoid arthritis (RA). Numerous preclinical studies have demonstrated that the extracts from the root of TwHF inhibit the expression of proinflammatory cytokines, proinflammatory mediators, adhesion molecules, and matrix metalloproteinases by macrophages, lymphocytes, synovial fibroblasts, and chondrocytes. TwHF also induces apoptosis in lymphocytes and synovial fibroblasts and inhibits their proliferation. Except numerous uncontrolled clinical trials, there are some prospective, double-blind, randomized, and placebo/sulfasalazine-controlled trials, also demonstrating greater improvement in RA disease activity by TwHF extract than placebo/sulfasalazine. Radiographic progression in RA may also be retarded by TwHF. Therefore, the immunosuppressive, cartilage protective, and anti-inflammatory effects of TwHF extracts are well demonstrated, and TwHF extract is an alternative disease modifying anti-rheumatic drug (DMARD) for the patients with RA refractory to conventional therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In traditional Chinese medicine, extracts of the root of the medicinal plant Tripterygium wilfordii Hook F (TwHF) (known in China as “lei gong teng” or “thunder god vine”), a member of Celastraceae family, have been widely used in China to treat a broad spectrum of autoimmune and inflammatory diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), ankylosing spondylitis, psoriasis, and idiopathic IgA nephropathy.
Initially, a crude water extract (decoction) of TwHF was used, and the dose was adjusted based on the weight of the plant material from which the extract was made. Patients treated with the decoction of TwHF appeared to experience therapeutic benefit, but frequently developed adverse effects and, occasionally, severe toxicity. Subsequently, efforts were made to improve the extraction procedure in order to minimize toxicity and maximize therapeutic benefit. As a result, two new preparations of TwHF were developed in China in the 1970s. One was an ethanol/ethyl acetate extract, and the second was a chloroform–methanol extract known as T2 [1]. Both of these extracts of TwHF have been claimed to exert better therapeutic effects and cause fewer adverse events; these preparations have become commercially available and have therefore, been most widely used in China recently [2, 3]. The availability of these preparations has made it possible to carry out numerous clinical trials of TwHF in a variety of autoimmune and inflammatory diseases, in particular, RA [4, 5].
TwHF has been documented to contain more than 70 components, including diterpenes, triterpenes, glycosides, and alkaloids [6, 7], and 95% of them are terpenoids. Three diterpenoids—triptolide, tripdiolide, and triptonide—are the most abundant and account for the immunosuppressive and anti-inflammatory effects observed with the root extracts in both in vitro and in vivo studies [8–10]. Furthermore, the diterpene triepoxide triptolide is the major active component [11, 12]. In recent years, there has been considerable interest in the use of TwHF extracts and of the main bioactive constituent to treat a variety of autoimmune and inflammation-related conditions. A lot of studies describing the chemistry and the pharmacology of TwHF have been published, mostly in Chinese. Here, we briefly reviewed the immunosuppressive and anti-inflammatory effects, the efficacy, and safety of TwHF extracts/triptolide in the treatment of RA.
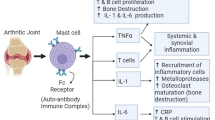
Effects on inflammatory mediators and immune system
Proinflammatory mediators
The direct anti-inflammatory effects of TwHF were observed in the croton oil-induced ear swelling, carrageenan-induced paw edema, and air pouch model of carrageenan-stimulated acute inflammation in animals [13–15]. Significantly lower volumes of the air pouch exudate, lower white blood cell counts with lower percentages of neutrophils, and lower concentrations of inflammatory mediators, including prostaglandin E2 (PGE2), tumor necrosis factor α (TNF-α), and nitrite in the exudate, were found in the animals treated orally with the TwHF extract. Correspondingly, spontaneous in vitro production of PGE2, TNF-α, and nitrite by exudate cells was significantly reduced in TwHF-treated animals. TwHF only inhibited PGE2 production and downregulated expression of the cyclooxygenase (COX)-2 gene at the inflammatory site without interfering with the COX-1-regulated PGE2 production in the non-inflammatory organs [15].
In vitro production of PGE2 and expression of COX-2 mRNA by a variety of human cells was examined [7]. The extracts of TwHF exerted an inhibitory effect on lipopolysaccharide-induced PGE2 production comparable to the effect of dexamethasone. The inhibition of PGE2 production was confirmed by suppression of COX-2 expression, whereas COX-1 expression was not affected [16]. In addition, TwHF extract inhibited nitric oxide (NO) production by inhibition of transcription of the inducible nitric oxide synthase (iNOS) gene [17] and also inhibited matrix metalloproteinases (MMP)-3 and matrix metalloproteinases (MMP)-13 by blocking mRNA transcription [18]. Inhibition of lipoxygenase was also noted [19]. Similarly, triptolide suppressed expression of COX-2 and the precursor forms of MMP-1 and MMP-3 and inhibited production of PGE2 and NO and activity of lipoxygenase [16, 20–22]. Transcription of the genes for iNOS and COX-2 is activated by the transcription factor nuclear factor-kappa B (NF-κB). TwHF extract and triptolide were found to inhibit binding of NF-κB to DNA or transactivation of NF-κB but did not interfere with the p38 mitogen-activated protein (MAP) kinase pathway [18, 23].
Proinflammatory cytokines
In RA, proinflammatory cytokines produced by lymphocytes and macrophages, which infiltrated in synovium, play a pivotal role. Numerous studies indicate that extracts of TwHF suppress production of cytokines, including TNF-α, interleukin-2 (IL-2), interferon (IFN)-γ, IL-6 and IL-8 from T lymphocytes, and macrophages in response to antigen and mitogen stimulation [1, 12, 24–27]. Suppression of IL-2 production was due to inhibition of IL-2 mRNA expression and also promotion of IL-2 mRNA degradation [28].
Pure triptolide also inhibited T-cell proliferation and production of TNF-α, IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, and IL-8 by activated T cells [1, 18, 20, 21, 23, 29]. A recent study showed that triptolide inhibits lipopolysaccharide-induced dendritic cells (DC) production of proinflammatory proteins, including macrophage inflammatory proteins (MIP)-1α, (MIP)-1β, monocyte chemoattractant protein-1 (MCP-1), thymus and activation-regulated chemokines (TARC), regulated upon activation of normal T cell expressed and secreted factor (RANTES), and IFN-γ inducible protein-10 (IP-10) possibly via inhibition of NF-κB activation and the signal transducer and activator of transcription 3 (Stat3) phosphorylation [30].
Adhesion molecules
During inflammation, adhesion molecules on either leukocytes or endothelium mediate the adhesion of circulating cells to endothelium. An extract from TwHF has been demonstrated to inhibit the secretion and expression of intracellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1), and E-selectin by activated human synovial fibroblasts or endothelial cells from human umbilical cord veins [31], as well as the production of the cell surface molecules CD18, CD11c, and CD14 [25, 32]. The adhesion molecules attract inflammatory cells such as T cells to the site of inflammation.
Humoral immune responses
TwHF extract has been reported to inhibit formation of antibody against sheep red blood cells in mice [13]. Both TwHF extract and triptolide decreased the anti-collagen antibody titers in mice/rats with type II collagen-induced arthritis (CIA) [33, 34]. Similarly, treatment of RA patients with TwHF extract for 12 weeks significantly reduced the production of IgM and IgM-rheumatoid factor by non-stimulated or pokeweed mitogen-stimulated peripheral blood mononuclear cells separated from these patients [35]. In vitro, TwHF extract inhibited proliferation and production of immunoglobulins by pokeweed mitogen-stimulated peripheral blood mononuclear cells or purified human B cells in response to stimulation with Staphylococcus aureus, indicating the TwHF extract directly affected the function of B cells as potently as that of T cells [26, 36].
Cell proliferation and apoptosis
Induction of apoptosis in T or B cells, or inhibition of their proliferation, reduces inflammation triggered by these cells. Therefore, compounds that enhance apoptosis or inhibit T-cell proliferation or B-cell proliferation may be useful for treating inflammatory or autoimmune diseases. Consistent with the effect on the proinflammatory signals, TwHF extract strongly inhibited T-cell proliferation and B-cell proliferation [1, 26, 37] and induced apoptosis in T cells [38]. Triptolide also induced apoptosis in certain T cell types and in dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation [39, 40]. Triptolide also inhibited proliferation of T and B cells [41].
Effects on synovial fibroblasts and chondrocytes
During the past decade, a body of evidence has accumulated illustrating that RA-synovial fibroblasts (RASFs) are active drivers of joint destruction in RA. In this destructive process, RASFs actively drive inflammation and degradation of the joint by producing inflammatory cytokines and matrix-degrading molecules, such as MMPs. TwHF extract inhibited PGE2 production by IL-1β-stimulated RASFs in a concentration-dependent manner and also inhibited COX-2 protein and mRNA expression in a similar fashion to dexamethasone. However, TwHF extract did not act as a glucocorticoid receptor agonist. TwHF extract suppressed IL-1β-induced NF-κB activity, but did not have a significant influence on activating protein-1 activity [42]. In addition, TwHF extract inhibited lipopolysaccharide-induced chemokine CCL5 production by RASFs [43] and induced apoptosis of RASFs by increase in caspase-3 activity [44, 45].
Similarly, triptolide induced apoptosis of RASFs [45], inhibited proliferation of RASFs, and suppressed PGE2 production by selectively suppressing the gene expression and production of COX-2 by RASFs, but not those of COX-1 [16, 20, 41, 46]. Triptolide also suppressed the production and gene expression of vascular endothelial growth factor, pro-matrix metalloproteinases-1, pro-matrix metalloproteinases-3, and pro-matrix metalloproteinases-9 and augmented those of tissue inhibitors of metalloproteinases-1 and metalloproteinases-2 [20, 47]. Triptolide inhibited the expression of interleukin-18 (IL-18) and its receptor in phorbol l2-myristate 13-acetate (PMA)-stimulated RASFs [48]. Taken together, both TwHF and triptolide decrease the number of RASFs by induction of apoptosis and inhibition of proliferation and inhibit the function of RASFs to produce PGE2, proinflammatory cytokines and MMPs.
A body of evidence demonstrates that the chondrocyte itself plays an important role in cartilage destruction by production of MMPs. Both TwHF extract and triptolide inhibited mRNA and protein expression of cytokine-induced MMP-3 and MMP-13 in stimulated chondrocytes by impairing activating protein-1 (AP-1) and NF-κB binding activities [18, 49]. Triptolide was effective at low doses and blocked the induction of MMP-13 by IL-1 in human and bovine cartilage explants. TwHF extract and triptolide also suppressed IL-1-, IL-17-, and TNF-α-induced expression of aggrecanase-1 in bovine chondrocytes. Thus, triptolide could protect cartilage from MMP- and aggrecanase-driven breakdown [49].
Effects on arthritis models
A number of in vivo studies have suggested that components of TwHF function to suppress various immune and inflammatory responses. TwHF extract also performed well in animal models of RA [7], including collagen-induced arthritis (CIA) in mice [33] and adjuvant-induced arthritis in rats [50]. TwHF extract reduced the incidence and severity of CIA in mice as well as the titers of anti-collagen antibodies. Both incidence and severity of arthritis were reduced, even when oral administration was delayed until 3 weeks after immunization with collagen [33], suggesting the preventative effect as well as therapeutic effect of TwHF in CIA. Treatment with TwHF extract also inhibited proliferative responses and the IL-2, IFN-γ, IL-1β, and TNF-α by in vitro collagen II-rechallenged lymph node cells from these animals [33, 51]. Treatment with TwHF extract downregulated the expression levels of receptor activator of NF-κB ligand (RANKL) in synovium, subchondral, and trabecular bone from rats with adjuvant-induced arthritis [52]. There is no evidence that the extracts of TwHF contain glucocorticoids or that the in vivo effects of these extracts are mediated by release of glucocorticoids. Thus, the anti-inflammatory effects of TwHF were also noted in adrenalectomized animals [53].
Similarly, triptolide lowered the arthritic scores, histopathological arthritis severity score, and in vivo cell-mediated immunity to collagen, delayed the onset of CIA, induced apoptosis of synoviocytes in CIA rats, and reduced the expressions of TNF-α, IL-6, NF-κB, and COX-2 in paw cartilage of CIA rats [34, 54, 55]. Triptolide significantly inhibited expression of MCP-1, MIP-1α, and RANTES at both mRNA and protein levels in adjuvant-induced arthritis rats [56]. Furthermore, triptolide displayed an immunosuppressive effect on CIA by downregulating collagen II-specific Th17 cells, which was confirmed in vitro that triptolide significantly inhibited the transcription of IL-17 mRNA and the generation of Th17 cells from murine splenocytes and purified CD4(+) T cells by inhibiting IL-6-induced phosphorylation of STAT3, a key signaling molecule involved in the development of Th17 cells [57]. It is suggested that the enteric mucosal immune system, including Peyer’s patch, is one of the primary targets of the immunosuppressive activity of triptolide [58, 59].
Therapeutic efficacy in RA
There have been numerous human clinical trials of TwHF extracts, and one that also included triptolide, for RA. Most of them have been reported from China and three from the US [60–62]. Although efficacy has been claimed in trials, few of them have been substantiated by randomized controlled trials. In 1989, the first, prospective, double-blind, crossover study of the T2 chloroform/methanol extract of TwHF for the treatment of RA was published by Chinese researchers Tao et al. [63]. Seventy RA patients were randomized to two parallel groups. Group A was treated with T2 extract (60 mg/day) for 12 weeks and with placebo for the subsequent 4 weeks. Group B was treated with placebo for 12 weeks followed by T2 for 4 weeks. In Group A, 27 of 35 patients completed the 12-week T2 treatment and 24 completed the subsequent 4-week placebo treatment. In Group B, 31 of 35 completed the 12-week placebo treatment and 27 completed the subsequent 4-week T2 treatment. In a per protocol analysis, Group A compared to Group B showed statistically significant improvement after 12 weeks in tenderness score, swelling count, duration of morning stiffness, mean grip strength, 15 m walking time, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), IgG, IgM, IgA, and patient’s and physician’s global assessments.
A US group (2002) randomized 35 patients with long-standing RA, in whom conventional therapy had failed, to receive either a high dose (360 mg/day) of a TwHF ethanol/ethyl alcohol extract, a low dose (180 mg/day) or placebo for 20 weeks [61]. At baseline, the patients in the high-dose group had suffered RA for longer time than those in either of the other two groups. Eight of 11 patients in the high-dose group, seven of 12 in the low-dose group, and six of 12 in the placebo group completed 20 weeks of treatment. The number of patients completing 4 weeks of treatment and qualifying for the modified intention to treat analysis was 10 in the high-dose group, 10 in the low-dose group, and 12 in the placebo group. Of these eight (80%) in the high-dose group, four (40%) in the low-dose group and none in the placebo group achieved an improvement meeting ACR 20 criteria. High dose was more effective than low dose and low dose was more effective than placebo. Five patients in the high-dose group and one patient in the low-dose group met criteria for ACR 50, and one patient in the high-dose group met criteria for ACR 70. For those patients who did reach ACR 20, the mean time taken was 7 weeks in the high-dose group and 12 weeks in the low-dose group. The individual components of ACR 20, namely number of tender joints, number of swollen joints, patient assessed pain, patient’s and physician’s global assessments, patient-rated physical function, and ESR and CRP all showed significant improvements in the high-dose group relative to the placebo group by 4 weeks of treatment. The secondary measure, duration of morning stiffness, declined from 145 min at baseline to 37 min at 4 weeks and then to 26 min by 20 weeks in the high-dose group. RF titer also decreased significantly within group for the high dose.
Recently, a randomized trial was conducted at 11 US centers to compare the benefits and side effects of TwHF ethanol/ethyl alcohol extract (180 mg/day) with those of sulfasalazine (2 g/day) for the treatment of active RA [62]. During the 6-month study, treatment with TwHF extract resulted in rapid improvement in clinical signs and symptoms of RA, including joint pain, joint swelling, and measures of overall well-being, and in markers of inflammation, such as CRP, ESR, and the proinflammatory cytokine interleukin-6. Compared with sulfasalazine, 2 g/day (an approved standard therapy for RA), TwHF led to statistically significantly greater improvement in terms of patients achieving ACR 20, ACR 50, and ACR 70 responses and to moderate-to-good improvement in DAS 28. Significant differences from baseline and significantly larger improvements in the TwHF group compared with the sulfasalazine group were apparent at 2 weeks of therapy and persisted throughout the study for HAQ disability assessment, pain, the patient’s and physician’s global assessment of health, ESR, and CRP level. Improvements in number of swollen and tender joints were statistically significantly greater in the TwHF group than in the sulfasalazine group starting from 8 weeks of therapy. The largest improvement in CRP and ESR occurred within the first 2 weeks of treatment with TwHF. Plasma interleukin-6 levels were significantly lower after 4 weeks of treatment with TwHF and remained low at 24 weeks. In addition, reductions in rheumatoid factor levels were more pronounced at 4 and 24 weeks in patients who received TwHF compared with patients who received sulfasalazine. Beneficial effects were observed in RA as early as 2 weeks after initiation of TwHF. Moreover, radiographic progression was lower in TwHF group. These data suggest that earlier and greater improvement in RA disease activity is achieved by treatment with TwHF than sulfasalazine.
In addition to oral usage, sixty-one patients with RA participated in a randomized, double-blind, placebo-controlled trial of a topically applied tincture of TwHF [64]. The tincture was applied up to six times per day to the swollen or tender joints. As a result, 6 weeks of daily topical TwHF application is superior to placebo for reducing RA disease activity.
Adverse effects
The major side effects of the different preparations of TwHF have been reported to be similar. The most common side effects of TwHF are gastrointestinal tract disturbances, especially diarrhea, leukopenia, thrombocytopenia, rash, skin pigmentation, and malfunction of the male and female reproductive system [2, 7, 61, 65]. However, the number of patients withdrawing from the clinical trials due to adverse side effects was similar in the TwHF treatment and placebo groups [61]. Most of these adverse reactions ceased either spontaneously or after dose adjustment. Yu [66] described 101 RA patients treated with TwHF extract T2 for 3–13 years, with a mean treatment duration of 5.8 years. Most side effects of long-term TwHF treatment could be resolved by dose adjustment, except for amenorrhea. Of note, the association with dysmenorrhea is well documented, and development and reversibility of amenorrhea is correlated with the age of the patient and accumulated quantity of the drug taken.
Summary
TwHF is a Chinese herb with immunosuppressive effects and an established history of use in the treatment of RA. Numerous preclinical studies have demonstrated immunosuppressive, cartilage protective, and anti-inflammatory effects in vitro and in vivo, and the diterpinoids appear to account for most of this activity, in particular the triptolide. The main mode of action of the TwHF extracts and triptolide is inhibition of expression of proinflammatory genes such as those for IL-2, iNOS, TNF-α, COX-2, and IFN-γ. An additional anti-inflammatory mechanism by which TwHF may work is its inhibitory effect on the expression of cellular adhesion molecules expressed by human neutrophils, synovial fibroblasts, and endothelial cells. TwHF may also induce apoptosis in lymphocytes and synovial fibroblasts, inhibit their proliferation, and suppress the production of MMPs by synovial fibroblasts and chondrocytes. The efficacy and safety of certain types of TwHF extracts were confirmed in human clinical trials in China and also in the US. Although TwHF has toxic potential, careful extraction procedures have generated preparations with an acceptable frequency of adverse reactions, which are largely related to the gastrointestinal tract and amenorrhea. Considering antifertility activity and potent therapeutic efficacy, we recommend that TwHF extract is a good alternative therapy for postmenopausal RA patients.
References
Tao X, Cai JJ, Lipsky PE (1995) The identity of immunosuppressive components of the ethyl acetate extract and chloroform methanol extract (T2) of Tripterygium wilfordii Hook. F. J Pharmacol Exp Ther 272:1305–1312
Su DF, Song YJ, Li RL (1989) Report of 270 patients with rheumatoid arthritis treated with ethyl acetate extract of Tripterygium wilfordii Hook F. Zhong Yao Yao Li Yu Lin Chuang 5(3):40–42 (in Chinese)
Yu DY (1982) Clinical observation of 144 cases of rheumatoid arthritis treated with glycoside of radix Tripterygium wilfordii. Zhong Yi Za Zhi (7):32–35 (in Chinese)
Lipsky PE, Tao XL (1997) A potential new treatment for rheumatoid arthritis: thunder god vine. Semin Arthr Rheum 26:713–723
Canter PH, Lee HS, Ernst E (2006) A systematic review of randomised clinical trials of Tripterygium wilfordii for rheumatoid arthritis. Phytomedicine 13:371–377
Zhang L, Zhang ZX, An DK (1990) The achievements in the studies of chemical components of Tripterygium wilfordii Hook family. Zhongguo Yao Ke Da Xue Xue Bao 21(4):251–256 (in Chinese)
Tao X, Lipsky PE (2000) The Chinese anti-inflammatory and immunosuppressive herbal remedy Tripterygium wilfordii Hook F. Rheum Dis Clin North Am 26:29–50
Zheng JR, Fang JL, Gu KX, Xu LF, Gao JW, Guo HZ et al. (1987) Screening of active anti-inflammatory-immunosuppressive and antifertile compositions from Tripterygium wilfordii. I. Screening of 8 components from total glucosides of Tripterygium wilfordii (TII). Zhongguo Yi Xue Ke Xue Yuan Xue Bao 9:317–322 (in Chinese)
Brinker AM, Ma J, Lipsky PE, Raskin I (2007) Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 68:732–766
Qiu D, Kao PN (2003) Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook f. Drugs R D 4:1–18
Zheng JR, Fang JL, Gu KX, Ti YQ, Xu LF, Gao JW et al. (1987) Screening of active anti-inflammatory-immunosuppressive and antifertile components from Tripterygium wilfordii. II. Screening of 5 monomers from total glucosides of Tripterygium wilfordii (TII). Zhongguo Yi Xue Ke Xue Yuan Xue Bao 9:323–328 (in Chinese)
Chen BJ (2001) Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma 42:253–265
Li LZ, Chert SF, Wang FJ (1986) Effect of triptolide on inflammation and immune function. Zhongguo Yao Li Xue Tong Bao 2:25–28 (in Chinese)
Li LZ, Chen SF, Wang FJ (1982) Pharmacological study of the ethyl acetate extract of Tripterygium wilfordii Hook f. Zhong Cao Yao 13:27–32 (in Chinese)
Tao X, Ma L, Mao Y, Lipsky PE (1999) Suppression of carrageenan-induced inflammation in vivo by an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F. Inflamm Res 48:139–148
Tao X, Schulze-Koops H, Ma L, Cai J, Mao Y, Lipsky PE (1998) Effects of Tripterygium wilfordii Hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthr Rheum 41:130–138
Guo W, Ma L, Tao X (2001) In vitro inhibitive effects of Tripterygium wilfordii on NO production, iNOS activity, and iNOS-mRNA expression in chondrocytes of patients with rheumatoid arthritis. Zhonghua Yi Xue Za Zhi 81:1035–1037 (in Chinese)
Sylvester J, Liacini A, Li WQ, Dehnade F, Zafarullah M (2001) Tripterygium wilfordii Hook f extract suppresses proinflammatory cytokine-induced expression of matrix metalloproteinase genes in articular chondrocytes by inhibiting activating protein-1 and nuclear factor-κB activities. Mol Pharmacol 59:1196–1205
Li RW, Lin GD, Myers SP, Leach DN (2003) Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol 85:61–67
Lin N, Sato T, Ito A (2001) Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook, f., suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthr Rheum 44:2193–2200
Zhou HF, Niu DB, Xue B, Li FQ, Liu XY, He QH et al (2003) Triptolide inhibits TNF-α, IL-1β and NO production in primary microglial cultures. Neuroreport 14:1091–1109
Wang B, Ma L, Tao X, Lipsky PE (2004) Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthr Rheum 50:2995–3003
Chang DM, Chang WY, Kuo SY, Chang ML (1997) The effects of traditional antirheumatic herbal medicines on immune response cells. J Rheumatol 24:436–441
Luk JM, Lai W, Tam P, Koo MWL (2000) Suppression of cytokine production and cell adhesion molecule expression in human monocytic cell line THP-1 by Tripterygium wilfordii polysaccharide moiety. Life Sci 67:155–163
Tao XL, Davis LS, Lipsky PE (1991) Effect of an extract of Chinese herbal remedy Tripterygium wilfordii Hook. f. on human immune responses. Arthr Rheum 34:1274–1281
Lee GI, Ha JY, Min KR, Nakagawa H, Tsurufuji S, Chang IM, Kim Y (1995) Inhibitory effects of oriental herbal medicines on IL-8 induction in lipopolysaccharide-activated rat macrophages. Planta Med 61:26–30
Wu F, Zhu L, Cui L, Wang X, Zhang S (1993) Effect of Tripterygium wilfordii glycosides (TII) on IL-2 and IL-2R gene transcription. Zhonghua Wei Sheng Wu Xue He Mian Yi Xue Za Zhi 13:193–197 (in Chinese)
Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S et al (1999) Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T- cells and NFkappaB transcriptional activation. J Biol Chem 274:13443–13450
Chan MA, Kohlmeier JE, Branden M, Jung M, Benedict SH (1999) Triptolide is more effective in preventing T cell proliferation and interferon-gamma production than is FK506. Phytother Res 13:464–467
Liu Q, Chen T, Chen G, Li N, Wang J, Ma P, Cao X (2006) Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-κB activation. Biochem Biophys Res Commun 345:1122–1130
Chang DM, Kuo SY, Lai JH, Chang ML (1999) Effects of anti-rheumatic herbal medicines on cellular adhesion molecules. Ann Rheum Dis 58:366–371
Luk JM, Tam P, Fan ST, Koo MWL (2000) Immunosuppressive effects of Tripterygium wilfordii polysaccharide on LPS-stimulated human monocytes. Transpl Proc 32:2013–2015
Gu WZ, Brandwein SR, Banerjee S (1992) Inhibition of type II collagen induced arthritis in mice by an immunosuppressive extract of Tripterygium wilfordii Hook f. J Rheumatol 19:682–688
Gu WZ, Brandwein SR (1998) Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol 20:389–400
Tao XL, Shi YP, Cheng XH (1988) Mechanism of treating rheumatoid arthritis with Tripterygium wilfordii Hook. Effects on secretion of total IgM and IgM-rheumatoid factor by peripheral blood mononuclear cells. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 10:361–364 (in Chinese)
Ye WH, Tao XL, Zhang NZ (1990) Mechanism of treating rheumatoid arthritis with polyglycosides of Tripterygium wilfordii Hook. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 12:217–221 (in Chinese)
Li XW, Weir MR (1990) Radix Tripterygium wilfordii—a Chinese herbal medicine with potent immunosuppressive properties. Transplantation 50:82–86
Ho LJ, Chang DM, Chang ML, Kuo SY, Lai JH (1999) Mechanism of immunosuppression of the antirheumatic herb TWHf in human T cells. J Rheumatol 26:14–24
Yang Y, Liu Z, Tolosa E, Yang J, Li L (1998) Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology 40:139–149
Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, Ma P, Cao X (2004) Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun 319:980–986
Tong KK, Yang D, Yuk-Tat E, Peter C, Chiu KY, Yau KS et al (1999) Downregulation of lymphocyte activity and human synovial fibroblast growth in rheumatoid arthritis by triptolide. Drug Dev Res 47:144–153
Maekawa K, Yoshikawa N, Du J, Nishida S, Kitasato H, Okamoto K et al (1999) The molecular mechanism of inhibition of interleukin-1beta-induced cyclooxygenase-2 expression in human synovial cells by Tripterygium wilfordii Hook F extract. Inflamm Res 48:575–581
Liu NT, Yang MH, Luo WF, Wei J, Yuan GH (2006) Inhibitory effects of methotrexate and tripterygium glycosides on chemokine CCL5 production in synoviocytes from patients with rheumatoid arthritis. Di Si Jun Yi Da Xue Xue Bao 27:1113–1115 (in Chinese)
Zhou GW, Shen Q, Jin RJ, Wang XD, Zeng YX, Ye CY (2007) Effects of Tripterygium glycosides (TG) on apoptosis of synovial fibroblasts in rheumatoid arthritis. Zhejiang Lin Chuang Yi Xue 9:1034–1035 (in Chinese)
Kusunoki N, Yamazaki R, Kitasato H, Beppu M, Aoki H, Kawai S (2004) Triptolide, an active compound identified in a traditional Chinese herb, induces apoptosis of rheumatoid synovial fibroblasts. BMC Pharmacol 4:2
Liu H, Liu ZH, Chen ZH, Yang JW, Li LS (2000) Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol Sin 21:782–786
Zhang Q, Shi Y, Tan W, Wang F (2008) Effect of triptolide on the changes of VEGF and MMP-9 levels in the rheumatoid arthritis fibroblast-like synoviocyte line, MH7A. Nanjing Yi Ke Da Xue Xue Bao 28:902–905 (in Chinese)
Mao X, Sun S, Pei Z, Zhang L (2009) Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Xi Bao Yu Feng Zi Mian Yi Xue Za Zhi 25:606–611 (in Chinese)
Liacini A, Sylvester J, Zafarullah M (2005) Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes. Biochem Biophys Res Commun 327:320–327
Yu KT, Nuss G, Boyce R, Jariwala N, Owens G, Pennetti A et al (1994) Inhibition of IL-1 release from human monocytes and suppression of streptococcal cell wall and adjuvant-induced arthritis in rats by an extract of Tripterygium wilfordii Hook. Gen Pharmacol 25:1115–1122
Asano K, Matsuishi J, Yu Y, Kasahara T (1998) Hisamitsu T. Suppressive effects of Tripterygium wilfordii Hook f., a traditional Chinese medicine, on collagen arthritis in mice. Immunopharmacology 39:117–126
Luo B, Hu Y, Zhang M, Tu S, Zeng K (2006) Effect of glycoside of Tripterygium wilfordii Hook. F on expression of receptor activator of nuclear factor-κB ligand and osteoprotegerin in joints of rat with adjuvant-induced arthritis. Yi Yao Dao Bao 25:395–397 (in Chinese)
Zhang BH, Wang LY, Zheng EZ (1985) Study on the pharmacological action of total alkaloids in Tripterygium hypoglaucum Hutch. Zhong Cao Yao 16:360–363 (in Chinese)
Xiao C, Zhou J, He Y, Jia H, Zhao L, Zhao N, Lu A (2009) Effects of triptolide from Radix Tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-κB: a study on induced arthritis in rats. Chin Med 4:13
Tu S, Hu Y, Weng G, Lu F, Zhang M, Lai X, Liu P (2006) Triptolide induces apoptosis of rat synoviocytes in collagen-induced arthritis. Zhongguo Bin Li Sheng Li Za Zhi 22:630–633
Wang Y, Wei D, Lai Z, Le Y (2006) Triptolide inhibits CC chemokines expressed in rat adjuvant-induced arthritis. Int Immunopharmacol 6:1825–1832
Wang Y, Jia L, Wu CY (2008) Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol 68:383–390
Zhou J, Xiao C, Zhao LH, Jia HW, Zhao N, Lu C et al (2006) The effect of triptolide on CD4 + and CD8 + cells in Peyer’s patch of SD rats with collagen induced arthritis. Int Immunopharmacol 6:198–203
Xiao C, Lu C, Zhao L, Liu Z, Zhang W, He Y et al (2006) The effects of triptolide on enteric mucosal immune responses of DBA/1 mice with collagen-induced arthritis. Planta Med 72:1268–1272
Tao X, Cush JJ, Garret M, Lipsky PE (2001) A phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii Hook F in rheumatoid arthritis. J Rheumatol 28:2160–2167
Tao X, Younger J, Fan FZ, Wang B, Lipsky PE (2002) Benefit of an extract of Tripterygium wilfordii Hook F in patients with rheumatoid arthritis: a double-blind, placebo-controlled study. Arthr Rheum 46:1735–1743
Goldbach-Mansky R, Wilson M, Fleischmann R, Olsen N, Silverfield J, Kempf P et al. (2009) Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: a randomized trial. Ann Intern Med 151:229–240, W49–W51
Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP et al (1989) A prospective, controlled, double-blind, cross-over study of Tripterygium wilfordii Hook F in treatment of rheumatoid arthritis. Chin Med J (Engl) 102:327–332
Cibere J, Deng Z, Lin Y, He Y, Wang Z, Thorne A et al (2003) A randomized double blind placebo controlled trial of topical Tripterygium wilfordii in rheumatoid arthritis: Reanalyais using logistic regression. J Rheumatol 30:465–467
Wen C (1993) The effects of Tripterygium wilfordii Hook F on reproductive organs and hormones. Zhongguo Zhong Xi Yi Jie He Za Zhi 13:376–377 (in Chinese)
Yu DY (1987) Experience in long term treatment of rheumatoid arthritis with Tripterygium wilfordii Hook. Jiangsu Yi Yao 12:661–662 (in Chinese)
Acknowledgments
This study was supported in part by the grants from National Natural Science Foundation of China (No. 30972729) and Shanghai Natural Science Foundation (No. 09ZR1410700).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bao, J., Dai, SM. A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: mechanism, efficacy, and safety. Rheumatol Int 31, 1123–1129 (2011). https://doi.org/10.1007/s00296-011-1841-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-011-1841-y