Abstract
Sinomenine (SIN) is the active principle of the Chinese medical plant Sinomenium acutum which is widely used for the treatment of rheumatoid arthritis (RA) in China. Recently, several groups indicated that myeloid differentiation primary response protein 88 (MyD88) might be associated with disease progression of RA. Here, we observed the effect of SIN on MyD88 expression and showed its therapeutic role in RA. First, immunohistochemical staining in clinical specimens showed that MyD88 was mainly located in characteristic pathological structures of RA synovial tissues. Second, we found that MyD88 was overexpressed in the synovial tissues of the rats with adjuvant-induced arthritis (AIA). Treatment with SIN markedly decreased the expression of MyD88 in AIA rats. Finally, we provided evidences that SIN suppressed inflammation response and inflammation-induced joint destructive progression and arthritis symptoms in AIA rats. Therefore, SIN is an effective therapeutic agent for RA. Targeting MyD88 signaling may provide new methods for the treatment of RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic inflammatory disease, which characteristically presents a symmetric polyarthritis associated with swelling and pain in multiple joints. It has been revealed that the dysregulation of inflammatory processes are implicated in the pathogenesis and pathological progression of RA [1, 2]. Chronic articular inflammation results in synovial hyperplasia, cartilage degradation, and bone damage [3]. Recently, myeloid differentiation primary response protein 88 (MyD88) has been noticed for its key role in the inflammation response [4]. As reported, MyD88 is a universal adapter that links the toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) family to the downstream activation of nuclear factor-κB (NF-κB) [5–7]. The activation of these signaling pathways induces the expression of many downstream genes that are involved in the regulation of inflammation responses. Since MyD88 plays a key role in these pathways, targeting MyD88 may provide new methods for the treatment of RA.
The active principle of this herb is sinomenine (SIN) (7,8-didehydro-4-hydroxy-3,7-dimethoxy-17-methylmorphinane-6-one). It has been reported that SIN exhibits a wide range of pharmacological actions such as anti-inflammatory, immunosuppressive, and analgesic [8]. Several groups demonstrated that SIN could inhibit NF-κB binding activity and suppress cytokine expression in arthritis rats [9, 10].
We proposed that SIN could affect MyD88 expression and then suppress the downstream inflammatory signal transduction. In this study, we mainly examined whether SIN decreased MyD88 expression in the synovial tissues of rats with adjuvant-induced arthritis. In addition, we further study the role of SIN in inflammation-induced joint damage progression and joint symptom in adjuvant-induced arthritis (AIA) rats.
MATERIALS AND METHODS
Clinical Specimens
Paraffin-embedded synovial tissues and pathology reports were retrieved from the Department of Pathology of the Jinling Hospital (Nanjing, China). Synovial samples from 16 patients [eight with RA and eight with osteoarthritis (OA)] were included in the study for immunocytochemical analysis of MyD88 protein. Informed consent was obtained from the patients. The use of human synovial tissues was approved by the ethics committees of the hospitals.
Animal Preparation
A total of 30 male Sprague–Dawley rats weighing 120–140 g were used. The rats were housed in warm circumstance with the temperature at 22–24 °C and housed five per cage with food pellets and water ad libitum. All rats were acclimated to this environment for 2 days before the experiments. All experimental procedures were in keeping with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health and were approved by the Animal Care and Use Committee of Secondary Military Medical University.
Establishment of Model and Experimental Groups
AIA rat model was induced by an injection of 100 μl complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO, USA) into the plantar surface of right hind paw [11]. The arthritis was set up at day 1, and the rats were murdered at day 30. The synovial tissues were resected quickly. One synovial of each rat was stored in liquid nitrogen until used. The other ones were fixed in 10 % buffered formalin and were embedded in paraffin.
All rats were assigned randomly into three groups, including the AIA + SIN group, the AIA group, and the control group. (1) The rats in the AIA + SIN group (n = 10) were induced arthritis and orally administrated with SIN (100 mg/kg; Zhengqing Pharmaceuticals Limited Co., Hunan, China) once daily on days 7–30. (2) The rats in the AIA group (n = 10) were modeled and orally administrated with saline (100 mg/kg) once daily on days 7–30. (3) The rats in the control group (n = 10) were injected with saline (same volume as used for the CFA injection) and orally administrated with saline (100 mg/kg) once daily on days 7–30. We used the effective dosage of SIN as described [12].
Examination of the Joint Damage
To examine the synovial hypertrophied and cartilage damage, we observed the joint morphologically after murdering the rats and opening their joints. To further assess the joint damage and deformity of the rats, radiographic analysis was performed on days 28 after anesthetization (10 % chloral hydrate) using a dental X-ray machine (X-MIND DC; Satelec). We assessed osteoporosis, cartilage loss, and erosion of the AIA rat joint [13].
Evaluation of Arthritis Severity
The severity of arthritis was assessed on days 7, 14, 21, and 28, respectively. As described previously, a macroscopic scoring system was used to assess the severity of arthritis [14]. The severity of inflammation in each paw was scored as follows: score 1, detectable swelling and redness; score 2, moderate swelling and redness; and score 3, severe swelling and redness. The sum of the scores for all four paws in each mouse was used as an arthritis index. The maximum of arthritis index is 12 for each rat. Rats with a total score of >2 were considered to have arthritis. The severity of arthritis was evaluated by two pathologists blinded to the experimental design.
Thermal and Pressure Pain Tests
Thermal and pressure pain tests were conducted on day 14, 21, and 28. The paw withdrawal threshold was used to assess the inflammatory pain of the rats. The paw withdrawal threshold in response to innocuous mechanical stimuli was determined using an Electro Von Frey anesthesia meter (Model 2390CE, IITC Life Science, Inc.). Each rat was placed singly under an inverted ventilated Plexiglas cage with a metal mesh floor, allowing access to the plantar surface of the hind paw and allowed to acclimatize for 10 min. The von Frey hair was used to press perpendicular to the plantar surface of the hind paw, and mechanical stimulation was increased in a graded manner until the hind paw was withdrawn. Four trials were conducted at intervals of several seconds. The force (in gram) applied to the paw was recorded.
The paw withdrawal threshold of noxious heat stimuli was measured with a paw stimulator analgesia meter (Model 390, IITC Life Science, Inc.). Each rat was placed on a 6-mm-thick glass floor under an inverted clear Plexiglas cage and was allowed to acclimatize for 10 min. The movable radiant heat source under the glass floor was focused on the plantar surface of the hind paw when in contact with the glass floor. To avoid tissue damage of the rats, withdrawal latencies were measured automatically, and a cutoff time was set at 20 s. The light intensity was preset to obtain a baseline latency of approximately 10 s. Four trials were conducted at least 5-min intervals, and the average value of the latencies was recorded.
Immunohistochemical Staining
Four-micrometer-thick sections were used for immunohistochemical assay, which was performed with an anti-MyD88 polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), according to the previous study [15]. The specificity of immunohistochemistry reaction was evaluated by replacement of the primary antibody with nonspecific rabbit IgG. The sections were also subjected to hematoxylin and eosin (H&E) staining.
Western Blot
To prepare the total proteins, the frozen synovial tissues were homogenized in cold RIPA lysis buffer (Beyotime, Jiangsu, China) containing 1 mM phenylmethylsulphonyl fluoride and centrifuged at 13,000×g for 5 min at 4 °C to remove debris. Equal amounts of protein per line were separated by 10 % SDS–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membranes were blocked for 2 h at room temperature with 5 % nonfat milk in Tris-buffered saline containing 0.1 % Tween 20 (TBST) and were incubated overnight at 4 °C with primary antibodies, including anti-MyD88 (1:200; Santa Cruz Biotechnology, Inc.) and anti-β-actin (1:1,000; Cell Signaling Technology, Beverly, MA, USA). After washing with TBST (3 × 10 min), the membranes were incubated with goat anti-rabbit IgG secondary antibody (1:1,000; Cell Signaling Technology, Beverly, MA, USA) for 2 h at room temperature. The blotted protein bands were developed using an ECL detection reagent (Amersham Biosciences, Bucks, UK), according to the manufacturer’s instructions, and were exposed to X-ray film.
Quantitative Real-Time PCR
Total RNA was isolated from rat synovial tissues with TRIzol reagent (Invitrogen, Carlsbad, CA). The concentration of total RNA was determined by using a spectrophotometer (OD260/280 = 1.8–2.2). Then, the RNA was reverse transcripted to cDNA using PrimeScript™ RT reagent kit (TaKaRa). Quantitative real-time PCR analysis was performed using the DA7600 Sequence Detection System (DaAn Gene, Guangzhou, China), applying real-time SYBR Green PCR technology. The reaction mixtures contained 10 μl of Real-time PCR Master Mix (Toyobo, Osaka, Japan), 1 μl of cDNA, 1 μl of each forward and reverse primer (10 μM), and 7 μl of DEPC-treated water. The PCR thermal cycling was carried out with a 5-min initial denaturation step at 95 °C, followed by 40 three-step cycles: a denaturation step (95 °C, 15 s), an annealing step (60 °C, 30 s), and an extension step (72 °C, 30 s). The experimental results were analyzed by the 2−ΔΔCt method.
Primer sequences used in this study were as follows: MyD88 forward and reverse primers, 5′-GGACTGCCAGAAATACATACGC-3′ and 5′-CTTTGTCTGTGGGACACTGCTC-3′; TLR2 forward and reverse primers, 5′-GCCCTCAGTCTTGGAGTGTC-3′ and 5′-TAACACAGGGCGCCTAAGAG-3′; TLR4 forward and reverse primers, 5′-CAGGATGATGCCTCTCTTGC-3′ and 5′-TGATCCATGCATTGGTAGGTAA-3′; and GAPDH forward and reverse primers, 5′-TGTTGCCATCAACGACCCCTT-3′ and 5′-CTCCACGACATACTCAGCA-3′.
Enzyme-Linked Immunosorbent Assay
The rat synovial tissues of three groups were homogenized in lysis buffer, and protein concentrations were determined as described previously. Levels of inflammatory cytokines (TNF-α from Diaclone Research, France; IL-1β and IL-6 from BioSource Europe SA, Belgium) were quantified using ELISA kits specific for rat, according to the manufacturer’s instructions. Briefly, supplied standards were used to generate the standard curves. Then, samples were added to the wells coated with anti-TNF-α, anti-IL-1β, and anti-IL-6 antibodies. After incubation for 2 h at 37 °C, the wells were washed, and horseradish peroxidase-conjugated streptavidin was added to all wells for 1 h at 37 °C. After the color reaction with substrate, the optical density was measured at 450 nm using an enzyme-labeling instrument. The cytokine contents in the synovial tissues were expressed as picogram per milligram of total protein.
Statistical Analysis
All experiments were repeated at least three times, and the results were analyzed by SPSS 16.0. Data are presented as means ± standard error of the mean (SEM) and evaluated by Student’s t test or one-way ANOVA followed by Tukey HSD post hoc comparisons. A p value of <0.05 was considered statistically significant.
RESULTS
The Expression and Distribution of MyD88 in Synovial Tissues of RA Patients
To evaluate the expression and distribution of MyD88 in the synovial inflammation, we performed immunohistochemistry analyses on synovial tissue sections from RA and OA patients. Representative staining of MyD88 was shown in Fig. 1. Compared with the OA synovial tissue, we found that MyD88 is highly expressed in the RA synovial tissue. The MyD88 staining is mainly located in the characteristic pathology structures of the RA synovial tissues, involving in multilayered synovial lining tissues, new capillary vessels, and lymphoid follicles [16, 17].
Expression and distribution of MyD88 in synovial tissues from RA and OA patients. Representative staining of serial sections from the individual samples. a, c H&E staining of the synovial tissues from RA. e H&E staining of the synovial tissues from OA. b, d MyD88 staining of the synovial tissues from RA. f MyD88 staining of the synovial tissues from OA. Scale bar = 100 μm.
SIN Decreases MyD88 Expression in the Synovial Tissues of AIA Rats
We examined MyD88 expression in animal models and further studied the effect of SIN on MyD88 expression. First, we performed immunohistochemical staining for MyD88 on rat synovial tissue sections. As shown in Fig. 2a, increased MyD88 staining was observed in the AIA group than in the control group. Consistent with the immunohistochemical staining in clinical samples, MyD88 was also located in the characteristic synovial pathology structures. Compared with the AIA group, there was a decrease in MyD88 expression in the AIA + SIN group.
Treatment with SIN decreases MyD88 expression in the synovial tissues of AIA rats. a Representative staining for MyD88 was shown. Scale bar = 100 μm. b Representative immunoblots showing the expression of MyD88 protein. β-Actin expression was used a loading control. c Statistical analysis of the protein levels of MyD88. Data were presented in mean ± SEM. ***p < 0.001, compared to the control group; ### p < 0.001, compared to the AIA group. d The relative mRNA levels of MyD88 gene. Data were presented in mean ± SEM. **p < 0.01; ***p < 0.001, compared to the control group. ## p < 0.01, compared to the AIA group; n = 5 per group.
Next, we further examined the protein levels of MyD88 in different groups by western blot analysis. As shown in Fig. 2b, c, the protein level of MyD88 was markedly increased in the AIA group than in the control group. SIN significantly decreased the expression of MyD88 in the synovial tissues of AIA rats. There was no significant change in MyD88 expression between the AIA + SIN group and the control group.
The changes in MyD88 protein may result from the alterations in protein synthesis in the synovial, so we determined the mRNA level of MyD88 gene by real-time PCR (Fig. 2d). As expected, increased mRNA level of MyD88 was detected in the AIA group, and treatment with SIN markedly reversed the increase. Together, these findings suggested that MyD88 was overexpressed in the RA synovial and that SIN suppressed MyD88 expression in AIA rats.
SIN Decreases TLR2/TLR4 Expression in the Synovial Tissues of AIA Rats
To study the mechanism of regulating MyD88 expression, we detected the mRNA levels of TLR2 and TLR4 by real-time PCR. As shown in Fig. 3, the mRNA levels of TLR2 and TLR4 were higher in the AIA group than those in the control group. Compared with the AIA group, we found that SIN could decrease the mRNA expression of TLR2 and TLR4.
SIN Decreased Synovitis of AIA Rats
To assess the role of SIN in synovitis, we performed H&E staining in rat synovial tissue sections. Representative picture was shown in Fig. 4a. Serious proliferated synovial lining cells, hyperplastic fibrous tissues, infiltrated inflammatory cell, and lymphoid follicles were observed in the AIA group. SIN treatment significantly overcame these pathology changes. Next, we tested the expression of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in rat synovial tissues by ELISA. As shown in Fig. 4b–d, the protein levels of TNF-α, IL-1β, and IL-6 were higher in the AIA group than those in the control group. In the AIA + SIN group, there was a decreased trend in the expression of cytokines (TNF-α, IL-1β, and IL-6). Moreover, the decreases in the protein levels of TNF-α and IL-1β were significant. These results suggested that SIN improved synovitis in AIA rats.
Treatment with SIN decreases synovitis of AIA rats. a Representative H&E staining was shown. b ELISA analysis of protein levels of inflammatory cytokines. Data were presented in mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001, compared to the control group. # p < 0.05; ### p < 0.001, compared to the AIA group; n = 5 per group.
SIN Improves Damage Progression in AIA Rats
To investigate whether SIN could improve synovial hypertrophied and cartilage damage, joints were observed on day 30 after the rats were murdered. As shown in Fig. 5a, in the AIA group, synovial and cartilage edges were markedly blurred. Synovial hypertrophied notably and attacked cartilage in the AIA rats. In the SIN + AIA group, there was no markedly synovial or cartilage change observed by naked eyes. Therefore, SIN could reduce cartilage damage.
To observe whether SIN could reduce the joint damage and deforming, we performed radiographic analysis on day 28. As shown in Fig. 5b, severe joint damage, including joint space narrowing, osteoporosis, and cartilage and bone erosions, was found in AIA rats. As shown in the AIA + SIN group, the joint space got narrowing but not had notable osteoporosis and cartilage or bone erosion. These findings suggested that SIN protected joints from damage and deformity in AIA rats.
SIN Improves Joint Symptoms in AIA Rats
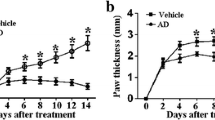
First, in AIA rats, signs of inflammation, including bilateral paw swelling and redness, were observed on days 7 and beyond. Compared with the AIA group, there were significant decreases in the arthritis index in the AIA + SIN group on days 14, 21, and 28 (Fig. 6a).
SIN alleviates joint symptoms in the AIA rats. a SIN improves joint swelling and redness in AIA rats. Data were presented in mean ± SEM. *p < 0.05, compared to the AIA group. b SIN reduces mechanical and heat pain in AIA rats. Data were presented in mean ± SEM. **p < 0.01; ***p < 0.001, compared to the control group. ## p < 0.01; ### p < 0.001, compared to the AIA group.
Next, we examined the role of SIN in pain behavior in AIA rats. The rats in different groups were subjected to thermal and pressure pain tests on days 14, 21, and 28. As shown in Fig. 6b, on day 14, there was no difference in the hind paw withdrawal threshold to mechanical and heat stimuli between the AIA and AIA + SIN groups. On days 21 and 28, the hind paw withdrawal threshold to mechanical and heat stimuli was markedly decreased in the AIA + SIN group compared to the AIA group. The hind paw withdrawal threshold was significantly different between the control and AIA groups at all three time points. Together, SIN could improve joint symptoms in AIA rats.
DISCUSSION
There are three important findings in this study. First, we detected increased MyD88 expression in RA synovial tissues. Second, we demonstrated that SIN treatment markedly decreased MyD88 expression in the synovial tissues of AIA rats. Third, we provided evidences that SIN suppressed inflammation damage of synovial tissues and improved joint symptoms in AIA rats.
It is well-known that TLRs and IL-1 family are involved in the disease progression of RA [3]. MyD88 is a critical adaptor protein that transmits signals for TLRs and IL-1 family [7]. Gutiérrez-Cañas et al. found that MyD88 mRNA expression was increased in cultured human RA fibroblast-like synoviocytes (FLS) compared with osteoarthritis FLS [12]. Here, for the first time, we described the distribution character of MyD88 in RA synovial. We also demonstrated that the protein and mRNA levels of MyD88 were markedly increased in the synovial tissues of AIA rats. Moreover, we also showed that the distribution of MyD88 was closely associated with characteristic pathological structures of RA synovial tissues in both human and rat samples. Together, we proposed that MyD88 is a key player in the aberrant signaling networks of RA.
The mechanism involving in the treatment of RA by SIN is still not well-known. Some groups showed the suppressive effect of SIN on cytokines in RA. Zhou et al. found that SIN suppressed the serum levels of IL-1β and IL-6 in collagen-induced arthritis rats [13]. Wang et al. found that SIN decreased the mRNA expression of TNF-α and IL-1β by inhibiting the NF-κB binding activity in the macrophages and synoviocytes of AIA rats [18]. It is worth mentioning that MyD88 plays a key role in triggering the NF-κB signaling pathway and regulates the production of inflammatory cytokines. So, we put eyes on the relationship between SIN and MyD88. We demonstrated that SIN could affect MyD88 protein level by affecting its gene expression in the synovial tissues of AIA rats. Besides, we found that TLR2/TLR4 mRNA was overexpressed in AIA synovial, and SIN decreased it. These findings suggested that MyD88 expression may be regulated by the TLR2/TLR4 pathway. Furthermore, we found that SIN reduced the protein levels of TNF-α, IL-1β, and IL-6 in the synovial tissues of AIA rats. Therefore, there was a positive correlation between changes in MyD88 and pro-inflammatory cytokines following SIN treatment. Collectively, we hypothesized that the role SIN reduced the production of inflammatory cytokines is relative to its role of affecting the MyD88 signaling pathway. The relationship between SIN and MyD88 should attract more attention in studying arthritis. In further study, we propose to test this hypothesis.
Our data show that SIN suppresses these inflammation-induced joint damage events, including synovial hyperplasia, cartilage degradation, and bone damage, and improves the symptoms of swelling and pain in AIA rats. In support of our findings, it has been reported that SIN has the capacity to protectively antagonize cartilage degradation and relieve joint pain in RA [19, 20]. It is well-known that articular inflammation is closely related with disease procession of RA. Many groups have revealed that the production of inflammatory cytokines results in joint damage procession and clinical symptoms [1, 3]. So, the role of SIN in synovitis and joint symptoms may closely associate with a decrease of the cytolines production. Besides, Guerrero et al. indicated that TLR2/MyD88 was involved in the cascade of joint hypernociception [21]. Hutchinson et al. also proposed that MyD88 might be a target for the development of novel drugs to control pain in arthritis [22]. These studies support our finding that MyD88 plays a key role in controlling arthritis pain. According to these findings, we considered that the protective mechanism of SIN might be closely related with the MyD88 and cytokines signaling pathways. The specific mechanism of it still needs further study.
In conclusion, the therapeutic effect of SIN in arthritis might be associated with the MyD88 pathway. Therefore, SIN may be an ideal therapeutic agent for RA. Understanding MyD88-related mechanisms may provide new methods to the treatment of RA.
REFERENCES
Choy, E.H., and G.S. Panayi. 2001. Cytokine pathways and joint inflammation in rheumatoid arthritis. The New England Journal of Medicine 344: 907–916.
Sweeney, S.E., and G.S. Firestein. 2004. Rheumatoid arthritis: Regulation of synovial inflammation. The International Journal of Biochemistry & Cell Biology 36: 372–378.
McInnes, I.B., and G. Schett. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews Immunology 7: 429–442.
Joosten, L.A., M.I. Koenders, R.L. Smeets, M. Heuvelmans-Jacobs, M.M. Helsen, K. Takeda, S. Akira, E. Lubberts, F.A. van de Loo, and W.B. van den Berg. 2003. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: Critical role of myeloid differentiation factor 88. Journal of Immunology 171: 6145–6153.
Brown, J., H. Wang, G.N. Hajishengallis, and M. Martin. 2011. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. Journal of Dental Research 90: 417–427.
Choe, J.Y., B. Crain, S.R. Wu, and M. Corr. 2003. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. The Journal of Experimental Medicine 197: 537–542.
Janssens, S., and R. Beyaert. 2002. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends in Biochemical Sciences 27: 474–482.
Kok, T.W., P.Y. Yue, N.K. Mak, T.P. Fan, L. Liu, and R.N. Wong. 2005. The anti-angiogenic effect of sinomenine. Angiogenesis 8: 3–12.
Wang, A.L., Z. Li, M. Yuan, A.C. Yu, X. Zhu, and M.O. Tso. 2007. Sinomenine inhibits activation of rat retinal microglia induced by advanced glycation end products. International Immunopharmacology 7: 1552–1558.
Chen, D.P., C.K. Wong, P.C. Leung, K.P. Fung, C.B. Lau, C.P. Lau, E.K. Li, L.S. Tam, and C.W. Lam. 2011. Anti-inflammatory activities of Chinese herbal medicine sinomenine and Liang Miao San on tumor necrosis factor-alpha-activated human fibroblast-like synoviocytes in rheumatoid arthritis. Journal of Ethnopharmacology 137: 457–468.
Liang, Y., J.Q. Fang, J.Y. Du, and J.F. Fang. 2012. Effect of electroacupuncture on activation of p38MAPK in spinal dorsal horn in rats with complete Freund’s adjuvant-induced inflammatory pain. Evidence-based Complementary and Alternative Medicine 2012: 568273.
Gutiérrez-Cañas, I., Y. Juarranz, B. Santiago, A. Arranz, C. Martinez, M. Galindo, M. Payá, R.P. Gomariz, and J.L. Pablos. 2006. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology 45: 527–532.
Zhou, H., Y.F. Wong, J. Wang, X. Cai, and L. Liu. 2008. Sinomenine ameliorates arthritis via MMPs, TIMPs, and cytokines in rats. Biochemical and Biophysical Research Communications 376: 352–357.
Ahmed, A.S., J. Li, M. Ahmed, L. Hua, T. Yakovleva, M.H. Ossipov, G. Bakalkin, and A. Stark. 2010. Attenuation of pain and inflammation in adjuvant-induced arthritis by the proteasome inhibitor MG132. Arthritis and Rheumatism 62: 2160–2169.
Dong, Y., J. Wang, Z. Sheng, G. Li, H. Ma, X. Wang, R. Zhang, G. Lu, Q. Hu, H. Sugimura, and X. Zhou. 2009. Downregulation of EphA1 in colorectal carcinomas correlates with invasion and metastasis. Modern Pathology 22: 151–160.
Tsubaki, T., N. Arita, T. Kawakami, T. Shiratsuchi, H. Yamamoto, N. Takubo, K. Yamada, S. Nakata, S. Yamamoto, and M. Nose. 2005. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthritis Research & Therapy 7: R825–R836.
Walsh, D.A., M. Wade, P.I. Mapp, and D.R. Blake. 1998. Focally regulated endothelial proliferation and cell death in human synovium. The American Journal of Pathology 152: 691–702.
Wang, Y., Y. Fang, W. Huang, X. Zhou, M. Wang, B. Zhong, and D. Peng. 2005. Effect of sinomenine on cytokine expression of macrophages and synoviocytes in adjuvant arthritis rats. Journal of Ethnopharmacology 98: 37–43.
Ju, X.D., M. Deng, Y.F. Ao, C.L. Yu, J.Q. Wang, J.K. Yu, G.Q. Cui, and Y.L. Hu. 2010. Protective effect of sinomenine on cartilage degradation and chondrocytes apoptosis. Yakugaku Zasshi: Journal of the Pharmaceutical Society of Japan 130: 1053–1060.
Xu, M., L. Liu, C. Qi, B. Deng, and X. Cai. 2008. Sinomenine versus NSAIDs for the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Planta Medica 74: 1423–1429.
Guerrero, A.T., T.M. Cunha, W.A. Jr Verri, R.T. Gazzinelli, M.M. Teixeira, F.Q. Cunha, and S.H. Ferreira. 2012. Toll-like receptor 2/MyD88 signaling mediates zymosan-induced joint hypernociception in mice: Participation of TNF-alpha, IL-1beta and CXCL1/KC. European Journal of Pharmacology 674: 51–57.
Hutchinson, M.R., L.C. Loram, Y. Zhang, M. Shridhar, N. Rezvani, D. Berkelhammer, S. Phipps, P.S. Foster, K. Landgraf, J.J. Falke, K.C. Rice, S.F. Maier, H. Yin, and L.R. Watkins. 2010. Evidence that tricyclic small molecules may possess toll-like receptor and myeloid differentiation protein 2 activity. Neuroscience 168: 551–563.
ACKNOWLEDGMENTS
We would particularly like to thank Zi-xiang Cong who has guided us through all stages of the project. This work was supported by grants from the Jinling Hospital of Nanjing (grant no. 2012014).
Conflict of Interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mu, H., Yao, RB., Zhao, LJ. et al. Sinomenine Decreases MyD88 Expression and Improves Inflammation-Induced Joint Damage Progression and Symptoms in Rat Adjuvant-Induced Arthritis. Inflammation 36, 1136–1144 (2013). https://doi.org/10.1007/s10753-013-9648-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-013-9648-5