Abstract
Differentiated thyroid carcinoma (DTC) is uncommon in the pediatric population but its incidence has been increasing, especially in 15–19 year olds, the most commonly affected age group. Papillary thyroid carcinoma represents 90% or more of cases in children, who typically present with larger tumors, a higher prevalence of regional lymph node disease, and an increased rate of pulmonary metastases compared with adults. Despite more advanced disease, patients with pediatric DTC paradoxically have very low disease-specific mortality, even in the presence of distant metastases at diagnosis. Whenever feasible, children with DTC should be cared for at centers with comprehensive and multidisciplinary thyroid cancer programs. Initial surgery performed by a high-volume thyroid surgeon is the most critical step to improve long-term disease free survival and to limit surgical morbidity. Concerns about late effects, such as secondary malignancies, has prompted reconsideration of universal radioactive iodine (RAI) ablation. Rather, a more conservative approach has evolved, recognizing that patients can take years to respond to previous RAI therapy and also acknowledging that pediatric DTC becomes, not uncommonly, an incurable yet indolent disease. Postoperative staging is used to individualize treatment and recent American Thyroid Association guidelines were created specifically for the management of pediatric DTC. More research needs to be done to better understand the genomics of pediatric DTC and to improve risk-stratification systems to determine who may or may not benefit from more aggressive treatment and postoperative surveillance during childhood.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Childhood
- Pediatric
- Adolescent
- Thyroid cancer
- Epidemiology
- Mutation
- Papillary
- Follicular
- Poorly differentiated
- Hereditary
- Radioactive iodine
- Surgery
- Tyrosine kinase inhibitor
Introduction
Differentiated thyroid carcinoma (DTC) is the most common endocrine malignancy, with papillary thyroid carcinoma (PTC) representing 90% or more of cases in children [1,2,3,4,5]. Similar to adults, children with PTC commonly present with a palpable thyroid nodule or may be identified incidentally in the context of imaging studies. Tumor size is larger in children compared with older patients (70% of children aged 0–9 years have a tumor ≥2 cm) [5,6,7]. In further contrast with adults, pediatric PTC is frequently associated with clinically apparent, malignant cervical lymphadenopathy and may present as a diffusely infiltrative carcinoma without a discrete nodule (Fig. 21.1). Rarely, children may also be diagnosed via imaging that demonstrates pulmonary metastases that may initially be attributed to an infectious etiology (Fig. 21.2). Symptoms related to neoplastic involvement of adjacent aerodigestive structures is rare (4% or less of pediatric PTC patients) [7]. Often multifocal and bilateral, PTC is associated with regional metastases in 80% or more of childhood cases at diagnosis in some retrospective series [4, 8,9,10,11,12,13,14,15]. Children with significant cervical lymph node disease are at the highest risk of hematogenously-spread lung metastases, [10, 11] (Fig. 21.2) which have been identified in up to 25% of patients in some published pediatric case series [3, 5, 12, 16,17,18,19]. Other sites of distant metastatic disease can include the brain, bones, and other solid organs but such events are exceedingly rare in children. Paradoxically, despite having more extensive disease at diagnosis, pediatric PTC is usually a well-differentiated tumor with an indolent clinical course, which translates to an extremely low disease-specific mortality [1, 17, 20,21,22].
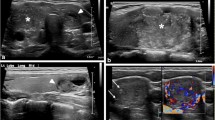
Clinical presentation of pediatric papillary thyroid carcinoma. A 6-year-old boy with palpable cervical lymphadenopathy (asterisk) and a firm asymmetric goiter. Ultrasound demonstrates a primary tumor with scattered microcalcifications that is diffusely infiltrating both thyroid lobes and the isthmus (bottom left) as well as malignant neck lymphadenopathy (asterisk) in both the right (top left) and left (top right) lateral neck. CT neck with contrast obtained as part of pre-operative staging also documents the extent of disease and its anatomic relationship to underlying aerodigestive structures such as the trachea. CA carotid artery, JV jugular vein, T trachea
Pulmonary metastases of pediatric papillary thyroid carcinoma. (a) Chest X-ray demonstrating a diffuse, miliary pattern of disease in a child; this pattern may initially be mistaken for an infectious etiology. (b) Axial post-contrast computed tomography image showing the micronodular pulmonary metastases typical of pediatric disease. (c) Post-treatment thyroid scan with diffuse and intense pulmonary uptake of 131I. This pattern is associated with an increased risk of pulmonary fibrosis. (d) Post-treatment thyroid scan of a different patient showing multifocal nodular pulmonary uptake, thyroid bed uptake (arrowhead), and residual cervical lymph node disease (arrow)
Follicular thyroid carcinoma (FTC) represents a distinct histopathological entity from PTC. In children, the diagnosis of FTC is almost always made following the pathologic identification of capsular and/or vascular invasion in a nodule surgically removed after an indeterminate fine needle aspiration biopsy (FNAB). Given the rarity of FTC in children and because it remains poorly studied in this age group, the bulk of this chapter, especially the treatment algorithms, will focus on PTC.
The excellent prognosis of pediatric DTC, in concert with substantive concerns regarding the potential for long-term sequelae related to overaggressive treatment during childhood, make the optimal management of pediatric DTC a challenging endeavor. Moreover, the existence of a very small population of patients with advanced disease who may benefit from systemic therapy poses a further challenge to care, given that these agents remain largely untested in children with DTC. Only recently have formal guidelines for management been developed by the American Thyroid Association (ATA) [23].
Epidemiology and Prognosis
In 2017, it is estimated that there will be 56,870 new thyroid cancer diagnosed in the United States, [24] of which 1.4% are expected to occur in individuals <20 years of age [25]. Pediatric thyroid cancer incidence is significantly lower in blacks versus whites, and the diagnosis is much more common in females [1, 6, 22, 25]. From 2010 to 2014, the age-adjusted incidence rate for children ages 0–14 years was two case/million/year in males and five cases/million/year in females; adolescents (ages 15–19) are the most commonly affected pediatric age group (29 cases/million/year) and the female: male ratio is 5.4:1 in this population [25]. More broadly, thyroid cancer is the fifth most common cancer diagnosed in adolescence, representing 11% of all cancers [24]. In adolescents, incidence rates have been rising over the decades, [1, 6, 22, 25, 26] a trend that cannot solely be explained by an increased diagnosis of small tumors.
Children with DTC have an excellent prognosis and survival over decades is typical, even for those patients with distant metastases [1, 22]. Remission rates are high, especially in the current era of high-resolution neck ultrasonography and more comprehensive initial thyroid cancer surgery. In the pediatric population, 10-year survival is almost universally 100% [1, 19, 20, 27,28,29,30,31,32]. Children diagnosed before age 10 years appear to have a higher risk of recurrence and ultimately death from their disease, [17, 21, 33,34,35,36] although a significant difference in tumor biology between the very young and older children has not been confirmed in other studies [7, 11, 37,38,39]. A subgroup of pediatric patients will ultimately die of their cancer or succumb to treatment-related complications [20, 28, 29, 40, 41]. For children with distantly metastatic disease, it is those with micronodular lung metastases and iodine-avid disease who maintain the best prognosis [29, 42]. Improved risk stratification that incorporates knowledge about clinical presentation, somatic mutational analysis, and response to treatment are needed to better identify those children who are at highest risk for ultimate disease-specific death and to avoid overzealous treatment of others who are unlikely to die from their cancer .
Risk Factors
Exposure of the thyroid to radiation (primarily in the context of medical therapy for cancer) is the major established risk factor for the development of thyroid carcinoma, although irradiation not including the thyroid region can also increase the risk [43]. Children, particularly those younger than age 5 years, appear to be especially sensitive to the tumorigenic effects of ionizing radiation [44]. Thyroid cancer is one of the most frequently diagnosed subsequent primary malignancies in childhood cancer survivors, with at least a fivefold increase in risk [45, 46]. This risk appears to decline once the thyroid dose exceeds 2000 cGy, [44, 47] suggesting that sublethal irradiation of the thyroid imparts the greatest risk. Radiation-induced tumors do not appear to be more aggressive compared with sporadic, non-radiation induced disease [48, 49]. The latency period between radiation exposure and diagnosis is typically long, with a median time to occurrence around 19 years [45]. Surprisingly, the risk of thyroid malignancy in childhood cancer survivors appears to be increased even in those who have not received therapeutic irradiation and this may reflect, at least in part, additional increased cancer risk among recipients of alkylating chemotherapy [50, 51].
Exposure to ionizing radiation can also occur via the ingestion of radionuclides, epitomized by the large environmental exposure to radioactive iodine (RAI) resulting from the Chernobyl, and possibly, the more recent Fukushima nuclear accidents [2, 52]. Additional environmental exposures may increase the risk of thyroid cancer in children living in geographic areas with volcanic activity [53]. The small activities of 123I/131I used for diagnostic studies and the treatment of hyperthyroidism appear to be below the threshold needed for tumorigenesis [54]. The use of 131I-Metaiodobenzylguanidine (131I-MIBG) to treat children with neuroblastoma has also been implicated in the development of benign and malignant thyroid neoplasia, [55] although common genetic factors and mechanisms of tumorigenesis between these two malignancies may also play a role [56, 57].
There has been some conjecture regarding a link between thyroid autoimmunity and the risk of DTC. However, whether or not a strong association between autoimmune thyroid disease and DTC exists in children remains unknown. The prevalence of PTC was 3% in one study of 375 children with autoimmune thyroiditis, [58] and another study of children with goiter did not clearly identify an increased risk of PTC in children with positive thyroid peroxidase antibodies compared with those with normal titers [59]. In a retrospective review of 32 children operated for Graves disease, DTC was identified in 22% of patients (including four diagnosed preoperatively); [60] three and two patients had lymph node and pulmonary metastases, respectively.
Although rare in the developed world, iodine deficiency is associated with an increased risk of thyroid neoplasia (specifically FTC) [61, 62] and, similar to many other cancers, obesity may be an additional risk factor [63]. Finally, although accounting for a small minority of pediatric DTC, germline variants in several genes have been associated with syndromic and non-syndromic hereditary DTC and are discussed in greater detail later in this chapter.
Histopathologic Variants
DTC comprises two major histopathologic variants, PTC and FTC, which differ in their clinical and metastatic presentations. Anaplastic (undifferentiated) thyroid carcinomas and poorly differentiated thyroid carcinomas are exceedingly rare in childhood, as are primary thyroid lymphomas and metastases to the thyroid gland.
Subtypes of PTC include classic/conventional, encapsulated, follicular (fvPTC), tall cell, oncocytic, columnar cell, diffuse sclerosing (dsPTC), hobnail, cribriform-morular and solid/trabecular variants [64, 65]. Recently, based on a retrospective analysis of clinical outcomes, the noninvasive encapsulated follicular variant of PTC has been reclassified in adults as a low risk tumor called NIFTP (noninvasive follicular thyroid neoplasm with papillary-like nuclear features [66]. It is unclear if these data can be extrapolated to pediatric patients with similar histology as no patients <age 21 years was in the NIFTP group.
In children, classic PTC is the most common variant (48%) followed by (in one recent study) dsPTC (16%), fvPTC (14.5%), encapsulated PTC (13%), tall cell PTC (13%), poorly differentiated (6.5%) and solid PTC (2%) [67]. In contrast with adults, histologic subtype does not appear to independently predict event-free survival, [67] although some studies have shown a higher risk of recurrence in children with classic PTC compared with fvPTC [18]. Solid variant PTC is more common in children with a history of radiation exposure [64]. The diffuse sclerosing variant, characterized by diffuse and often bilateral involvement of the thyroid, demonstrates extensive squamous metaplasia, abundant psammoma bodies, stromal fibrosis, and prominent lymphocytic infiltration. It is often accompanied by chronic lymphocytic thyroiditis in the background thyroid, extensive regional nodal metastases, and a higher frequency in younger patients [68, 69].
FTC is broadly divided into minimally invasive and widely invasive forms [70]. In 2017, the World Health Organization reclassified FTC into three groups: (1) minimally invasive (capsular invasion only) (2) encapsulated angioinvasive, and (3) widely invasive [65]. Similar to adults, minimally invasive FTC in children may be a low-risk malignancy, [30, 71] but bone and lung metastases have been described in minimally invasive tumors with vascular invasion, underscoring the fact that any degree of vacular invasion may confer a risk for metastatic disease in pediatric FTC [72]. The oncocytic (Hürthle-cell) variant of FTC is rare in the pediatric population [31, 71].
Molecular Mechanisms of Disease
Somatic Genomic Alterations
Abundant work over recent decades has established the primacy of aberrations in signaling through receptor tyrosine kinase (RTK) pathways, predominantly via the cognate RAS-RAF-MEK-ERK and PI3K-AKT-mTOR pathways, in the molecular pathogenesis of DTC. Recent genomic and transcriptome analyses [73, 74] have demonstrated that activating pathogenic variants in one of these two pathways are common, identified in 96.5% of PTCs [73] and 73% of follicular thyroid neoplasms (FTC and follicular adenomas) [74]. These somatic alterations typically are mutually exclusive and the mutational burden of DTC is low compared with other carcinomas. Moreover, the relative balance of RAF- versus RAS-mediated signaling has been linked to the extent of thyrocyte differentiation and differences in expression of genes responsible for iodine uptake and metabolism, which has potential treatment implications [73].
In pediatric PTC, gene rearrangements are the most common molecular event, especially after previous radiation exposure, [75,76,77,78] but they are not limited to children with that well-established risk factor [79,80,81]. Fusions involving the REarranged during Transfection (RET) proto-oncogene and the neurotropic tyrosine receptor kinase (NTRK) gene are the most common [77, 79,80,81,82,83,84]. Gene rearrangements of peroxisome proliferator-activated receptor gamma (PPARG) and paired box gene 8 (PAX8) and fusions involving the v-raf murine sarcoma viral oncogene homolog B (BRAF) and anaplastic lymphoma kinase (ALK) genes have also been identified, [77, 81, 84,85,86,87] although the PAX8/PPARG fusion is much more commonly associated with FTC [88]. BRAF V600E point mutations are also prevalent, [78,79,80,81, 86, 89, 90] although not as common as in adults. Rat sarcoma (RAS) mutations are also identified in pediatric PTC and FTC, [77, 78, 80, 81, 86, 90] although these have also been identified among benign follicular adenomas; [74] thus, at least from a diagnostic perspective, their relevance is debatable. BRAF mutations are more common in older pediatric patients, [84, 86, 87, 89] and it appears that BRAF-mutated PTC may not be more clinically aggressive in the pediatric population, as has been suggested for adult PTC [78, 79, 81, 86, 90, 91]. TERT promoter, PIK3CA, and PTEN mutations are rare [89,90,91].
Hereditary DTC
Hereditary DTC is rare and <5% of pediatric thyroid cancers are associated with an underlying germline mutation [78]. A family history of PTC is present in 4% of pediatric cases [7]. However, in the context of the high prevalence of PTC in the population at large, the presence of a single affected 1st degree relative with PTC does not indicate a familial etiology. Multiple genes and heritable tumor syndromes have been associated with both benign and malignant thyroid neoplasia: APC-Associated Polyposis (APC gene; OMIM[Online Mendelian Inheritance in Man®] #175100), Birt-Hogg-Dubé Syndrome (FLCN gene; OMIM #135150), the Carney complex (PRKAR1A gene; OMIM #160980), CHEK2-Related Cancer (CHEK2 gene; OMIM #604373), DICER1-pleuropulmonary blastoma familial tumor predisposition syndrome (DICER1 gene; OMIM #606241), familial nonmedullary thyroid carcinoma (FNMTC) (multiple genes; OMIM #188550), Li-Fraumeni syndrome (TP53 gene; OMIM #151623), PTEN [phosphatase and tensin homolog] Hamartoma Tumor Syndrome (PTEN gene; OMIM #601728), Pendred syndrome (SLC26A4 gene; OMIM #274600) and Werner syndrome (WRN gene; OMIM# 277700). Most recently, a germline compound heterozygous deletion in the USF3 gene was identified in a Cowden syndrome-like kindred with PTC, suggesting that this gene may also be involved in the predisposition to thyroid cancer [92]. Comprehensive reviews on the individual syndromes are available elsewhere, [65, 78, 93,94,95,96] and it should be emphasized that the association of DTC with some of these tumor predisposition syndromes does not necessarily indicate causality.
The PTEN hamartoma tumor syndrome is one of the more common syndromic causes of multinodular goiter and DTC (primarily PTC and fvPTC, but FTC is overrepresented relative to the general population), [95, 97,98,99] and the youngest reported case of thyroid cancer associated with a PTEN mutation was in a 7-year-old [98]. A clue to this diagnosis in a child presenting with thyroid neoplasia is macrocephaly, present in 98% of children with a germline PTEN mutation, [99] although macrocephaly has also been reported in 42% of DICER1 mutation carriers [100]. Multinodular goiter is predominant in patients with DICER1 mutations, especially in females, and the risk of DTC is also increased [101, 102]. In contrast with children at risk for hereditary medullary thyroid carcinoma, it is not recommended that children at risk for DTC due to an underlying disease-predisposing gene mutation undergo prophylactic thyroidectomy. In these cases, screening ultrasounds may be considered (based on expert opinion) in the DICER1-Syndrome, PTEN hamartoma tumor syndrome, Carney complex, and APC-associated polyposis, [95, 102,103,104,105,106] noting that the latter syndrome is uniquely associated with the cribriform-morular variant of PTC [107].
Evaluation, Treatment, and Follow Up
Once described as a “fatal disease with few exceptions,” [108] malignant thyroid disease is now recognized to have an excellent long-term prognosis in the majority of patients, especially in children. The evaluation and treatment of pediatric DTC has certainly evolved over the years. Historically, surgery was less-than comprehensive, relying more on palpation and limited by the lack of pre-operative high resolution ultrasonography and other cross-sectional imaging that are commonly utilized today. There has also shift in the utilization of RAI. It was once held that 131I would “clean up” whatever disease was not removed surgically and RAI was used almost universally to treat children with DTC until the thyroid scan became negative or as long as the thyroglobulin (Tg) remained detectable (once this tumor marker became available for clinical use). More recently, the concept of iodine-refractory disease in pediatric DTC has been better appreciated and current guidelines suggest a more conservative approach to RAI treatment, even among those with iodine-avid cancer [23].
Initial Work Up and Staging (Fig. 21.3)
Once the diagnosis of thyroid cancer is established, a comprehensive pre-operative assessment is critical to understand the extent of cervical disease to facilitate the optimal surgical approach. Universally recommended, assuming it is not already done, is a high-resolution cervical ultrasound (US) performed by an experienced ultrasonographer to interrogate the thyroid and lateral neck lymph nodes [23, 109, 110]. If suspicious adenopathy is identified, US-guided FNAB should be undertaken to confirm disease and the extent of regional metastases. In the presence of cystic lymph nodes, which are highly suggestive of PTC metastases, measurement of Tg on fluid from the needle washout may help to secure the diagnosis of malignant involvement [111, 112]. In children with bulky cervical disease, a primary tumor that appears fixed to underlying aerodigestive structures, or vocal cord paralysis, contrast-enhanced computed tomography (CT) of the neck is recommended to help guide further the surgical approach [23, 109]. Only a minority of children will have pulmonary metastases, typically only those with bulky cervical disease, [10] and the detection of pulmonary metastatic disease does not change the initial therapeutic approach. In addition, postoperative staging with RAI, if indicated, will effectively identify most children with pulmonary metastases, even those with negative baseline radiographic imaging [16]. Therefore, current guidelines do not suggest routine chest imaging in all pediatric DTC patients, although some centers do obtain a pre-operative CXR in high risk children because the finding of a significant disease burden may alter the approach to subsequent RAI therapy [109]. However, a CXR is not sensitive enough to identify small-volume micronodular pulmonary metastases, [16] and for that reason, some also consider chest CT, especially if neck CT is also planned. Although not recommended by all, some experts do obtain baseline Tg and thyroglobulin autoantibodies (TgAb) after confirmation of DTC [109].
Initial evaluation and treatment of biopsy-proven pediatric papillary thyroid carcinoma. 1If residual/recurrent PTC is suspected during long-term follow up of low-risk patients, and assuming neck US is negative, further evaluation and treatment with RAI is recommended. 2Assumes negative TgAb; if TgAb is positive and there is no evidence of iodine-avid disease on the diagnostic scan, consideration can be given to deferring RAI treatment, especially in intermediate-risk patients. 3RAI only if surgery deemed unsafe or not feasible. ATA American Thyroid Association, CT computed tomography, CXR chest X-ray, FNAB fine needle aspiration biopsy, LN lymph node, LT4 levothyroxine, RAI radioactive iodine (131I), Tg thyroglobulin, TgAb thyroglobulin antibody, TSH thyroid stimulating hormone, US ultrasound
Surgery
The surgical approach to pediatric DTC is not significantly different from adult cases and so will not be discussed in detail. In general, given the high rate of lymph node and pulmonary metastases, total thyroidectomy is recommended in most cases [23]. However, in children with small, incidentally discovered tumors, no previous radiation exposure, and no evidence of contralateral disease or cervical lymph node disease, thyroid lobectomy ± ipsilateral central neck dissection may result in similar long-term outcomes [7, 22, 32, 113, 114].
Current data suggest that the single most important factor for improving long-term disease-free survival is the extent of the initial surgery, with more complete surgery decreasing or eliminating persistent/recurrent disease and the need for additional treatment [11, 14, 17, 18, 20, 115,116,117]. Older studies, however, are confounded by the frequent prescription of RAI. Compartment-oriented neck dissection is advised for biopsy-proven lymph node disease and central neck dissection (CND) , in the absence of documented lymph node metastases, can be selectively considered in children and determined by intraoperative findings, [23] recognizing that complication rates are higher with central neck dissection [118]. In the setting of a unifocal PTC > 1 cm, [119] an ipsilateral CND, with pursuit of contralateral CND only if intra-operative findings suggest central compartment disease, may help to balance the risks and benefits in pediatric patients. In every case, the decision to perform a CND should be driven by the experience of the surgeon and his or her ability to localize the parathyroid glands. Preservation of parathyroid function (including the liberal use of parathyroid autotransplantation) and avoidance of significant recurrent laryngeal nerve injury is paramount, even if it means leaving residual microscopic disease. Surgical complications are not insignificant in children with thyroid cancer, [2, 7, 19, 117, 118, 120] and there are lower complication rates and shorter hospital length of stay when surgery is performed by a high-volume thyroid surgeon [23, 121,122,123,124,125]. Therefore, it is strongly preferred that thyroid surgery, especially when lymph node dissection is required, always be performed by a surgeon experienced in the management of pediatric DTC.
Postoperative Staging
Several prognostic staging systems have been used for DTC, and a thorough discussion of this topic is beyond the scope of the current chapter. The pathological TNM (tumor-node-metastasis) classification developed by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) is the international reference staging system for thyroid cancer, [126] and this has recently been updated to the eighth edition, which will be implemented in January 2018. Underscoring the excellent prognosis, the highest TNM stage that anyone with pediatric-onset DTC can have is stage II, distinguished from stage I disease only by the presence of distant metastases. Therefore, most children, even those with extensive locoregional disease, are considered to have stage I disease. Another used staging system for PTC, the MACIS score, may also be useful in children and adolescents [127, 128].
Utilizing AJCC 7th edition TNM staging, a novel risk categorization for PTC (ATA Pediatric Low-, Intermediate-, and High-risk) was introduced in the inaugural ATA pediatric guidelines [23] (Table 21.1) and subsequently validated in other studies [4, 36, 39]. This risk stratification is intended to identify those at risk of persistent cervical disease and distant metastases in order to determine which patients would benefit from more intensive postoperative staging, management, and surveillance. Children with ATA Pediatric Intermediate- or High-risk PTC benefit from an initial postoperative diagnostic RAI scan and a TSH-stimulated Tg to identify persistent locoregional or distantly metastatic disease, [23, 109] whereas those in the ATA Pediatric Low-risk category can be more conservatively monitored, understanding that it is plausible for children initially identified in one risk category to move to a different risk level after appropriate surgery and RAI and during long-term follow up (Fig. 21.3). As increasingly employed in adults, [129] dynamic risk stratification based on the response to initial therapy may also predict outcomes in pediatric DTC [4]. Limitations of the ATA pediatric risk categorization is that it does not fully incorporate the identification of extrathyroidal extension, margin status, primary tumor and metastatic lymph node size, histologic variant or knowledge regarding tumor mutational status. Furthermore, the extent of pre-operative staging and the experience of the surgeon also impacts the validity of this system.
Radioactive Iodine Therapy
Employed for the treatment of thyroid cancer since the 1940s, RAI has had an important role in the evaluation and management of children with DTC. Almost universally administered in the past, the pendulum has now swung in the opposite direction and 131I therapy is currently used much more selectively in children and only after incorporating new data obtained from initial postoperative staging [23, 109] (Fig. 21.3). This more conservative approach has, in part, arisen from knowledge that there are real long-term risks from the overzealous prescription of 131I during childhood, such as pulmonary fibrosis in young children with a large lung disease burden and the potential for the late development of a second primary malignancy (primarily hematological and salivary gland malignancies) [20, 41, 42, 130,131,132,133,134,135,136]. Furthermore, better understanding that death from pediatric DTC remains quite rare, experts have come to realize that very aggressive treatment during childhood may not ultimately improve disease-specific survival, especially during an era when novel targeted therapeutics have become quickly incorporated into the treatment of advanced DTC.
RAI is no longer given exclusively to ablate normal remnant thyroid tissue, since the serum Tg can become undetectable within 5–7 years after no RAI therapy [137] and because the risks of routine remnant ablation largely outweigh the potential benefits in children, especially in those operated on by high-volume surgeons. Currently, surgery is preferred over RAI to treat residual/recurrent cervical disease that is amenable to surgical resection, [23, 109] and long-term control of cervical disease has been shown to be excellent in the reoperative setting [138].
The greatest challenge in pediatric DTC remains how to determine when additional 131I therapy is warranted in children with iodine-avid, distantly metastatic disease and a previous response to RAI. Repeated courses of 131I can induce remission in some, but not all children, with pulmonary metastases, of whom 50% or more will have persistent disease despite RAI [42, 139,140,141]. Children with small-volume, iodine-avid micronodular (<1 cm) lung disease are those most likely to respond to treatment, [142] and they may ultimately become disease-free whereas others, especially those with a more extensive metastatic disease burden, may never become cancer free as assessed by Tg levels [42, 139, 142, 143].
Furthermore, the Tg response to 131I treatment may not be attained for up to 15–18 months [144] and studies have shown a continued decline in serum Tg levels years after the last dose of RAI has been administered [139, 143]. Recognizing these issues and asserting that an undetectable serum Tg level should no longer be the sole goal of treatment in children with pulmonary disease, the ATA guidelines have recommended longer intervals between 131I courses, suggesting that treatment be given no sooner than 12 months after the last dose [23]. In all cases, the decision to prescribe 131I to any child with DTC should be individualized and incorporate knowledge regarding prognosis, tumor avidity for RAI, and previous response to therapy (Fig. 21.4).
Follow up of the pediatric patient with known or suspected residual/recurrent papillary thyroid carcinoma after initial surgery and RAI. 1 Assumes a negative TgAb; in TgAb-positive patients, the singular presence of TgAb cannot be interpreted as a sign of disease unless the titer is clearly rising over time; a declining TgAb titer would suggest ongoing response to treatment. 2Further evaluation and treatment with RAI should only be considered after a reasonable period of time (1–2 years) has elapsed in order not to overtreat a patient who may have a delayed clinical response to prior RAI. 3Repeated courses of 131I should be considered only if iodine-avid disease is proven/suspected and there was a previous clinical response to 131I therapy. CT computed tomography, FNAB fine needle aspiration biopsy, LT4 levothyroxine, RAI radioactive iodine, Tg thyroglobulin, TgAb thyroglobulin antibody, US ultrasound
A diagnostic thyroid scan and a stimulated Tg level (assuming the patient is TgAb negative) are recommended for children with DTC who require such staging [23, 109] (Fig. 21.3). Obtaining these data can identify those children with no evidence of disease (defined as those with an undetectable serum Tg and the absence of structurally-apparent disease) who can avoid unnecessary RAI exposure, children with extensive RAI-avid cervical disease who may benefit from re-operation, and those with RAI-avid distant metastases, who may need to have their planned administered 131I activity adjusted because of either the extent of disease or the intensity of the pulmonary uptake. Diagnostic thyroid scans using 123I are generally preferred [145, 146]. One challenge in young patients is the fact that iodine-avid DTC may not be visualized on the diagnostic scan [146] (Fig. 21.5). In these cases, the presence of disease is suggested by an elevated Tg out of proportion to the scan findings and what would be expected for normal remnant thyroid tissue. It has been proposed that a stimulated Tg is >10 ng/mL would be a reasonable cutoff for empirically treating patients at higher risk for residual/metastatic disease [23]. However, the exact Tg threshold above which treatment should be given has not been well studied in children. A recent study suggested that the novel application of a TSH/Tg ratio may help to inform the decision regarding 131I treatment [147]. Another study in pediatric DTC patients, already treated with surgery and RAI, showed that all children with lung metastases had a stimulated Tg > 10 ng/mL. Thus, this cutoff may indeed be appropriate to identify higher risk patients who might benefit from further evaluation and treatment [148].
Diagnostic (a) and post-treatment (b) radioactive iodine scans in a high-risk 15-year-old patient with a stimulated thyroglobulin of 229 ng/mL. The diagnostic 123I scan (a) shows asymmetric upper cervical uptake (arrow) and subtle nodular uptake in the lungs on the posterior view. The scan obtained after treatment with high dose 131I (b) demonstrates intense left upper cervical uptake with star artifact (arrow), subtle left thyroid bed uptake (arrowhead), and diffuse and nodular uptake in the lungs. The addition of SPECT/CT imaging documents the iodine-avidity of the pulmonary metastases (c) and confirms the location of the upper cervical uptake to be in a retropharyngeal lymph node (d), which is well demonstrated (arrow) on CT neck after intravenous contrast (e)
In order to facilitate RAI scanning and possible treatment, the TSH level should be above 30 mIU/mL and the ATA guidelines suggest an approach using short-term thyroid hormone withdrawal, except in select patients for whom recombinant human TSH (rhTSH) should be considered [23]. In children, an appropriately elevated TSH can almost always be achieved after 2–3 weeks of thyroid hormone withdrawal, [149, 150] which is usually extremely well-tolerated. The use of rhTSH for 131I treatment may result in a lower absorbed dose to the blood compared with withdrawal [151] and its efficacy appears to be non-inferior to thyroid hormone withdrawal after 43 months of follow up [152]. A low iodine diet is generally advised for 1–2 weeks to facilitate uptake by remaining thyroid tissue; for children who received intravenous contrast during CT, it is advisable to wait 2–3 months or to confirm appropriate 24-h urine iodine levels first.
In pediatric DTC, there are no standardized approaches to determining the administered 131I activity, which is generally based on a weight (child’s weight in kg/70 kg) or body surface area (child’s BSA m2/1.6 m2 for females or 1.9 m2 for males) adjustment of the typical prescribed activity used in adults for a similar extent of disease [153,154,155]. For example, for empiric therapy, a child with pulmonary metastases would be administered an activity that is proportionately equivalent in an adult to a 150–200 mCi (5.55–7.4 GBq) dose (or less, if significant diffuse pulmonary uptake is present (Fig. 21.1), in order to minimize the risk of pulmonary fibrosis). Others determine the activity based on body weight alone (2.0–2.5 mCi/kg; 74–92.5 MBq/kg) [153]. Dosimetric studies to limit whole body retention at 48 h to less than 80 mCi (2.96 GBq) and blood/bone marrow exposure to less than 200 cGy should be considered in children anticipated to have significant diffuse lung uptake, patients with more widespread distant metastases, and children who may have limited bone marrow reserve due to prior cancer therapy [23, 145, 156, 157]. However, this approach is more time consuming, is not available at all centers, and has not yet been demonstrated to improve outcomes or minimize treatment-related morbidity.
In all children treated with 131I, a post-treatment scan is advised 4–7 days subsequent to therapy to identify other sites of persistent disease that were not apparent on the diagnostic study [16, 157] (Fig. 21.5). The incorporation of single-photon emission computed tomography (SPECT)/CT with nuclear scintigraphy has substantially improved our ability to localize disease and distinguish benign from malignant sites of RAI uptake [158, 159] (Fig. 21.5). One must also be aware that 50% of children may have findings on the post-treatment thyroid scan, notably thymic uptake, that are not representative of iodine-avid disease [160]. Although the empiric treatment with 131I of adults who have RAI scan-negative, structural disease or Tg positive, imaging negative disease has not been proven to be effective, [161, 162] whether or not similar empiric treatment of children with an abnormal Tg and a negative diagnostic scan results in similar long-term outcomes has not been studied.
Thyroid Stimulating Hormone Suppression and Follow Up
Thyroid stimulating hormone (TSH) is believed to be a thyrocyte mitogen. The role of exogenous thyroid hormone to treat thyroid cancer in children (by lowering TSH) was first reported in 1937, [163] and TSH suppression is universally employed in the management of DTC today. All children who have undergone total thyroidectomy for malignant thyroid disease are replaced with thyroid hormone at age-appropriate doses, [164] and the degree of initial TSH suppression depends on the child’s ATA risk category [23] (Table 21.1). In general, the TSH goal can be loosened and the TSH allowed to rise to the low normal range in patients who have no evidence of disease after a 1–3 year period of follow up, depending on the original extent of disease. In children who have had thyroid lobectomy, the TSH is measured 4–6 weeks after surgery and supplementation considered if the TSH is in the upper half of the normal range or overtly elevated, understanding that thyroid hormone supplementation can be stopped and the TSH reevaluated after a period of follow up in the child who continues to have no evidence of cancer. The potential risks of TSH suppression in children (such as negative effects on growth and bone age, bone mineralization, cognition, behavior, and the heart) [165, 166] are unstudied but are assumed to be minimal in otherwise healthy children.
Follow-up of children with DTC should be lifelong, given that the probability of recurrence continues to increase over time and because clinical disease may not be identified until decades after initial treatment [20, 21, 32]. For those with persistent disease despite appropriate initial therapy, early recognition that thyroid cancer may become a chronic disease, albeit one with low morbidity and mortality, may justify a more restrained approach to treatment. In addition to tailored TSH suppression, and not dissimilar from adult recommendations, the long-term surveillance of pediatric DTC includes the periodic assessment of Tg and TgAb levels, routine neck US, and the selective use of diagnostic thyroid scans and other cross sectional imaging of the neck ± chest [23, 129] (Table 21.1 and Fig. 21.4). The intensity and type of follow up is primarily based on the postoperative ATA pediatric risk categorization and dynamic risk re-stratification, modifying the follow-up regimen as new data become available.
In adults, 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) imaging can be helpful in the prognostic evaluation of advanced disease and the determination of who would not benefit from empiric 131I [167,168,169]. There are no similar published studies in children, who are more likely to have well-differentiated tumors and hence unlikely to have significant FDG-avid disease, although even in the presence of FDG-avid lung disease, younger adults are still likely to demonstrate RAI uptake [170].
Even if 131I therapy is not utilized, Tg levels and their trend over time serve as a useful indicator of disease status, assuming there are no interfering TgAb [137, 171, 172]. Extrapolating from adult studies, children who have had only a lobectomy for low-risk disease should also be able to be followed similarly with Tg levels and cervical US [173]. If the TSH-suppressed Tg is undetectable after primary therapy has been completed, there appears to be very little added value in obtaining a TSH-stimulated Tg, [174,175,176] and there is now movement away from obtaining a routine TSH-stimulated Tg in children in the absence of other indicators of disease [23]. Nevertheless, as demonstrated in adult studies, [175,176,177] an undetectable TSH-stimulated Tg after initial treatment that includes total thyroidectomy and 131I is highly predictive of long-term remission. Clinical recurrences primarily occur in cervical lymph nodes, [175, 176] underscoring the critical importance of cervical US during long-term disease surveillance [148] (Fig. 21.4). As alluded to earlier, the exact Tg values that indicate residual, clinically relevant disease in children that would warrant more intensive surveillance or treatment remains poorly studied in children, although a stimulated Tg >10 ng/mL is likely to represent an actionable threshold [23, 148].
In children with detectable TgAb, the antibody titer itself can be followed, and it is the trend of this analyte over time (using the same assay and lab) that is more important than its absolute value [178, 179]. Even with successful treatment of DTC, TgAb may persist for a median of 3 years after treatment [180]. Therefore, in patients with +TgAb, greater emphasis should be placed on structural and functional imaging to identify disease .
Systemic Therapy for Advanced Disease
In children, the development of progressive DTC that warrants systemic treatment outside of repeated courses of 131I is very rare. The definition of RAI-refractory disease, better established in adults, [181] remains poorly defined in the pediatric population. It is now understood that RAI-refractory DTC does indeed occur in children, and contemporary guidelines clearly state that repeated courses of RAI should not be given when there was no documented response to previous 131I therapy [23]. In such cases, disease may remain quite indolent for years while continuing TSH suppression. In the rare event of a child with progressive DTC needing alternative approaches to care, consultation with providers who are experienced in the use of systemic therapy in children is recommended. A review of the currently available, molecularly targeted agents for the treatment of DTC is beyond the purview of this chapter and is covered elsewhere. Of the FDA-approved therapies, single agent sorafenib has been studied in phase I and phase II clinical trials in pediatric patients with refractory solid tumors or leukemias, [182,183,184,185] but no children with DTC were enrolled into these trials and there is only limited and anecdotal experience using sorafenib to treat advanced PTC in children [186, 187]. Lenvatinib remains largely unstudied in the pediatric population and there have been no published reports on its use in children. Currently, a phase I/II study in children, including an expanded cohort with 131I-refractory DTC, is recruiting (ClinicalTrials.gov Identifier NCT02432274).
Conclusion
Rare in children but increasing in incidence, pediatric DTC presents with more locally-advanced and distant disease compared with adults. Despite this more advanced clinical presentation, the prognosis for patients with pediatric-onset disease is excellent and disease-specific mortality remains low, even in the presence of pulmonary metastases. This is most likely due to the fact that DTC in children is typically a well-differentiated tumor that most often is iodine-avid and responsive to TSH suppression. Given the excellent prognosis and anticipated indolent nature of the cancer, therapy for pediatric DTC should be individualized and geared towards balancing goals of disease eradication with limiting treatment-related morbidity, particularly in non-progressive residual disease. It is preferred that, whenever possible, children with DTC be cared for at centers with established programs in the multidisciplinary management of this disease; most critical is the identification of a high-volume surgeon to undertake such cases, which should improve oncologic outcomes and minimize postoperative complications. Therapy with 131I is becoming less frequently prescribed and is now tailored to the individual patient and based on the likelihood of having disease that is expected to respond to RAI therapy, recognizing that aggressive treatment may not improve the already low disease-specific mortality observed. Further research geared towards better understanding the optimal use of RAI and predicting long-term outcomes based on clinical presentation and tumor mutational status is needed.
References
Hogan AR, Zhuge Y, Perez EA, Koniaris LG, Lew JI, Sola JE. Pediatric thyroid carcinoma: incidence and outcomes in 1753 patients. J Surg Res. 2009;156(1):167–72.
Demidchik YE, Saenko VA, Yamashita S. Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arq Bras Endocrinol Metabol. 2007;51(5):748–62.
Borson-Chazot F, Causeret S, Lifante JC, Augros M, Berger N, Peix JL. Predictive factors for recurrence from a series of 74 children and adolescents with differentiated thyroid cancer. World J Surg. 2004;28(11):1088–92.
Sung TY, Jeon MJ, Lee YH, Lee YM, Kwon H, Yoon JH, et al. Initial and dynamic risk stratification of pediatric patients with differentiated thyroid cancer. J Clin Endocrinol Metab. 2017;102(3):793–800.
Silva-Vieira M, Santos R, Leite V, Limbert E. Review of clinical and pathological features of 93 cases of well-differentiated thyroid carcinoma in pediatric age at the Lisbon Centre of the Portuguese Institute of Oncology between 1964 and 2006. Int J Pediatr Otorhinolaryngol. 2015;79(8):1324–9.
Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164(6):1481–5.
Spinelli C, Strambi S, Rossi L, Bakkar S, Massimino M, Ferrari A, et al. Surgical management of papillary thyroid carcinoma in childhood and adolescence: an Italian multicenter study on 250 patients. J Endocrinol Investig. 2016;39(9):1055–9.
Zimmerman D, Hay ID, Gough IR, Goellner JR, Ryan JJ, Grant CS, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery. 1988;104(6):1157–66.
Frankenthaler RA, Sellin RV, Cangir A, Goepfert H. Lymph node metastasis from papillary-follicular thyroid carcinoma in young patients. Am J Surg. 1990;160(4):341–3.
Wada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H, et al. Treatment strategy of papillary thyroid carcinoma in children and adolescents: clinical significance of the initial nodal manifestation. Ann Surg Oncol. 2009;16(12):3442–9.
Machens A, Lorenz K, Nguyen Thanh P, Brauckhoff M, Dralle H. Papillary thyroid cancer in children and adolescents does not differ in growth pattern and metastatic behavior. J Pediatr. 2010;157(4):648–52.
Newman KD, Black T, Heller G, Azizkhan RG, Holcomb GW 3rd, Sklar C, et al. Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children’s Cancer Group. Ann Surg. 1998;227(4):533–41.
Popovtzer A, Shpitzer T, Bahar G, Feinmesser R, Segal K. Thyroid cancer in children: management and outcome experience of a referral center. Otolaryngol Head Neck Surg. 2006;135(4):581–4.
Savio R, Gosnell J, Palazzo FF, Sywak M, Agarwal G, Cowell C, et al. The role of a more extensive surgical approach in the initial multimodality management of papillary thyroid cancer in children. J Pediatr Surg. 2005;40(11):1696–700.
Mao XC, Yu WQ, Shang JB, Wang KJ. Clinical characteristics and treatment of thyroid cancer in children and adolescents: a retrospective analysis of 83 patients. J Zhejiang Univ Sci B. 2017;18(5):430–6.
Bal CS, Kumar A, Chandra P, Dwivedi SN, Mukhopadhyaya S. Is chest x-ray or high-resolution computed tomography scan of the chest sufficient investigation to detect pulmonary metastasis in pediatric differentiated thyroid cancer? Thyroid. 2004;14(3):217–25.
Demidchik YE, Demidchik EP, Reiners C, Biko J, Mine M, Saenko VA, et al. Comprehensive clinical assessment of 740 cases of surgically treated thyroid cancer in children of Belarus. Ann Surg. 2006;243(4):525–32.
Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007;48(6):879–88.
Klein Hesselink MS, Nies M, Bocca G, Brouwers AH, Burgerhof JG, van Dam EW, et al. Pediatric differentiated thyroid carcinoma in the Netherlands: a nationwide follow-up study. J Clin Endocrinol Metab. 2016;101(5):2031–9.
Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34(6):1192–202.
Mihailovic J, Nikoletic K, Srbovan D. Recurrent disease in juvenile differentiated thyroid carcinoma: prognostic factors, treatments, and outcomes. J Nucl Med. 2014;55(5):710–7.
Golpanian S, Perez EA, Tashiro J, Lew JI, Sola JE, Hogan AR. Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int. 2016;32(3):201–8.
Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–59.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER cancer statistics review, 1975-2014, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Bethesda: National Cancer Institute; 2017 [cited 2017 July 18]. Available from: https://seer.cancer.gov/csr/1975_2014/.
Pole JD, Zuk AM, Wasserman JD. Diagnostic and treatment patterns among children, adolescents, and young adults with Thyroid Cancer in Ontario: 1992–2010. Thyroid. 2017;27(8):1025–33.
Spoudeas HA, editor. Paediatric endocrine tumours. West Sussex: Novo Nordisk Ltd; 2005.
Vassilopoulou-Sellin R, Goepfert H, Raney B, Schultz PN. Differentiated thyroid cancer in children and adolescents: clinical outcome and mortality after long-term follow-up. Head Neck. 1998;20(6):549–55.
Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–9.
Ito Y, Miyauchi A, Tomoda C, Hirokawa M, Kobayashi K, Miya A. Prognostic significance of patient age in minimally and widely invasive follicular thyroid carcinoma: investigation of three age groups. Endocr J. 2014;61(3):265–71.
Golpanian S, Tashiro J, Sola JE, Allen C, Lew JI, Hogan AR, et al. Surgically treated pediatric nonpapillary thyroid carcinoma. Eur J Pediatr Surg. 2016;26(6):524–32.
Sugino K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, et al. Papillary thyroid carcinoma in children and adolescents: long-term follow-up and clinical characteristics. World J Surg. 2015;39(9):2259–65.
Landau D, Vini L, A'Hern R, Harmer C. Thyroid cancer in children: the Royal Marsden Hospital experience. Eur J Cancer. 2000;36(2):214–20.
Harach HR, Williams ED. Childhood thyroid cancer in England and Wales. Br J Cancer. 1995;72(3):777–83.
Alzahrani AS, Xing M. Impact of lymph node metastases identified on central neck dissection (CND) on the recurrence of papillary thyroid cancer: potential role of BRAFV600E mutation in defining CND. Endocr Relat Cancer. 2013;20(1):13–22.
Lazar L, Lebenthal Y, Segal K, Steinmetz A, Strenov Y, Cohen M, et al. Pediatric thyroid cancer: postoperative classifications and response to initial therapy as prognostic factors. J Clin Endocrinol Metab. 2016;101(5):1970–9.
O'Gorman CS, Hamilton J, Rachmiel M, Gupta A, Ngan BY, Daneman D. Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid. 2010;20(4):375–80.
Lazar L, Lebenthal Y, Steinmetz A, Yackobovitch-Gavan M, Phillip M. Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr. 2009;154(5):708–14.
Pires BP, Alves PA Jr, Bordallo MA, Bulzico DA, Lopes FP, Farias T, et al. Prognostic factors for early and long-term remission in pediatric differentiated thyroid carcinoma: the role of sex, age, clinical presentation, and the newly proposed American Thyroid Association Risk Stratification System. Thyroid. 2016;26(10):1480–7.
Schlumberger M, De Vathaire F, Travagli JP, Vassal G, Lemerle J, Parmentier C, et al. Differentiated thyroid carcinoma in childhood: long term follow-up of 72 patients. J Clin Endocrinol Metab. 1987;65(6):1088–94.
Hebestreit H, Biko J, Drozd V, Demidchik Y, Burkhardt A, Trusen A, et al. Pulmonary fibrosis in youth treated with radioiodine for juvenile thyroid cancer and lung metastases after Chernobyl. Eur J Nucl Med Mol Imaging. 2011;38(9):1683–90.
Pawelczak M, David R, Franklin B, Kessler M, Lam L, Shah B. Outcomes of children and adolescents with well-differentiated thyroid carcinoma and pulmonary metastases following (1)(3)(1)I treatment: a systematic review. Thyroid. 2010;20(10):1095–101.
Tucker MA, Jones PH, Boice JD Jr, Robison LL, Stone BJ, Stovall M, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. The Late Effects Study Group. Cancer Res. 1991;51(11):2885–8.
Bhatti P, Veiga LH, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res. 2010;174(6):741–52.
Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, et al. Temporal trends in treatment and subsequent Neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017;317(8):814–24.
Casagranda L, Oriol M, Freycon F, Frappaz D, Bertrand Y, Bergeron C, et al. Second malignant neoplasm following childhood cancer: a nested case-control study of a recent cohort (1987–2004) from the childhood cancer registry of the Rhone-Alpes region in France. Pediatr Hematol Oncol. 2016;33(6):371–82.
Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, Mertens AC, Liu Y, et al. Thyroid cancer in childhood cancer survivors: a detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166(4):618–28.
Naing S, Collins BJ, Schneider AB. Clinical behavior of radiation-induced thyroid cancer: factors related to recurrence. Thyroid. 2009;19(5):479–85.
Rose J, Wertheim BC, Guerrero MA. Radiation treatment of patients with primary pediatric malignancies: risk of developing thyroid cancer as a secondary malignancy. Am J Surg. 2012;204(6):881–6. discussion 6–7.
Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. Int J Cancer. 2007;121(10):2233–40.
Veiga LH, Bhatti P, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Chemotherapy and thyroid cancer risk: a report from the childhood cancer survivor study. Cancer Epidemiol Biomark Prev. 2012;21(1):92–101.
Tsuda T, Tokinobu A, Yamamoto E, Suzuki E. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology. 2016;27(3):316–22.
Russo M, Malandrino P, Moleti M, D'Angelo A, Tavarelli M, Sapuppo G, et al. Thyroid cancer in the pediatric age in sicily: influence of the volcanic environment. Anticancer Res. 2017;37(3):1515–22.
Angusti T, Codegone A, Pellerito R, Favero A. Thyroid cancer prevalence after radioiodine treatment of hyperthyroidism. J Nucl Med. 2000;41(6):1006–9.
Clement SC, van Eck-Smit BL, van Trotsenburg AS, Kremer LC, Tytgat GA, van Santen HM. Long-term follow-up of the thyroid gland after treatment with 131I-Metaiodobenzylguanidine in children with neuroblastoma: importance of continuous surveillance. Pediatr Blood Cancer. 2013;60(11):1833–8.
de Vathaire F, Francois P, Schlumberger M, Schweisguth O, Hardiman C, Grimaud E, et al. Epidemiological evidence for a common mechanism for neuroblastoma and differentiated thyroid tumour. Br J Cancer. 1992;65(3):425–8.
Buryk MA, Picarsic JL, Creary SE, Shaw PH, Simons JP, Deutsch M, et al. Identification of unique, heterozygous germline mutation, STK11 (p.F354L), in a child with an encapsulated follicular variant of papillary thyroid carcinoma within six months of completing treatment for neuroblastoma. Pediatr Dev Pathol. 2015;18(4):318–23.
Corrias A, Cassio A, Weber G, Mussa A, Wasniewska M, Rapa A, et al. Thyroid nodules and cancer in children and adolescents affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med. 2008;162(6):526–31.
Kambalapalli M, Gupta A, Prasad UR, Francis GL. Ultrasound characteristics of the thyroid in children and adolescents with goiter: a single center experience. Thyroid. 2015;25(2):176–82.
Kovatch KJ, Bauer AJ, Isaacoff EJ, Prickett KK, Adzick NS, Kazahaya K, et al. Pediatric thyroid carcinoma in patients with Graves’ disease: the role of ultrasound in selecting patients for definitive therapy. Horm Res Paediatr. 2015;83(6):408–13.
Aydin Y, Besir FH, Erkan ME, Yazgan O, Gungor A, Onder E, et al. Spectrum and prevalence of nodular thyroid diseases detected by ultrasonography in the Western Black Sea region of Turkey. Med Ultrason. 2014;16(2):100–6.
Niedziela M, Korman E, Breborowicz D, Trejster E, Harasymczuk J, Warzywoda M, et al. A prospective study of thyroid nodular disease in children and adolescents in western Poland from 1996 to 2000 and the incidence of thyroid carcinoma relative to iodine deficiency and the Chernobyl disaster. Pediatr Blood Cancer. 2004;42(1):84–92.
Xu L, Port M, Landi S, Gemignani F, Cipollini M, Elisei R, et al. Obesity and the risk of papillary thyroid cancer: a pooled analysis of three case-control studies. Thyroid. 2014;24(6):966–74.
Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5(1):51–6.
Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO classification of tumours of endocrine organs. Lyon: IARC Press; 2017.
Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2(8):1023–9.
Balachandar S, La Quaglia M, Tuttle RM, Heller G, Ghossein RA, Sklar CA. Pediatric differentiated thyroid carcinoma of follicular cell origin: prognostic significance of histologic subtypes. Thyroid. 2016;26(2):219–26.
Thompson LD, Wieneke JA, Heffess CS. Diffuse sclerosing variant of papillary thyroid carcinoma: a clinicopathologic and immunophenotypic analysis of 22 cases. Endocr Pathol. 2005;16(4):331–48.
Akaishi J, Sugino K, Kameyama K, Masaki C, Matsuzu K, Suzuki A, et al. Clinicopathologic features and outcomes in patients with diffuse sclerosing variant of papillary thyroid carcinoma. World J Surg. 2015;39(7):1728–35.
Sobrinho-Simoes M, Eloy C, Magalhaes J, Lobo C, Amaro T. Follicular thyroid carcinoma. Mod Pathol. 2011;24(Suppl 2):S10–8.
Vuong HG, Kondo T, Oishi N, Nakazawa T, Mochizuki K, Miyauchi A, et al. Pediatric follicular thyroid carcinoma – indolent cancer with low prevalence of RAS mutations and absence of PAX8-PPARG fusion in a Japanese population. Histopathology. 2017;71(5):760–8.
Enomoto K, Enomoto Y, Uchino S, Yamashita H, Noguchi S. Follicular thyroid cancer in children and adolescents: clinicopathologic features, long-term survival, and risk factors for recurrence. Endocr J. 2013;60(5):629–35.
Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90.
Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12(8):e1006239.
Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120(6):799–807.
Leeman-Neill RJ, Brenner AV, Little MP, Bogdanova TI, Hatch M, Zurnadzy LY, et al. RET/PTC and PAX8/PPARgamma chromosomal rearrangements in post-Chernobyl thyroid cancer and their association with iodine-131 radiation dose and other characteristics. Cancer. 2013;119(10):1792–9.
Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123(11):4935–44.
Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin N Am. 2017;46(2):389–403.
Prasad ML, Vyas M, Horne MJ, Virk RK, Morotti R, Liu Z, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in Northeast United States. Cancer. 2016;122(7):1097–107.
Cordioli MI, Moraes L, Cury AN, Cerutti JM. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr Relat Cancer. 2015;22(6):R311–24.
Picarsic JL, Buryk MA, Ozolek J, Ranganathan S, Monaco SE, Simons JP, et al. Molecular characterization of sporadic pediatric thyroid carcinoma with the DNA/RNA ThyroSeq v2 next-generation sequencing assay. Pediatr Dev Pathol. 2016;19(2):115–22.
Romei C, Ciampi R, Elisei R. A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol. 2016;12(4):192–202.
Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85(3):1170–5.
Cordioli MI, Moraes L, Bastos AU, Besson P, Alves MT, Delcelo R, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2016;27:182–8.
Cordioli MI, Moraes L, Carvalheira G, Sisdelli L, Alves MT, Delcelo R, et al. AGK-BRAF gene fusion is a recurrent event in sporadic pediatric thyroid carcinoma. Cancer Med. 2016;5(7):1535–41.
Nikita ME, Jiang W, Cheng SM, Hantash FM, McPhaul MJ, Newbury RO, et al. Mutational analysis in pediatric thyroid cancer and correlations with age, ethnicity, and clinical presentation. Thyroid. 2016;26(2):227–34.
Vanden Borre P, Schrock AB, Anderson PM, Morris JC, Heilmann AM, Holmes O, et al. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations, including kinase fusions. Oncologist. 2017;22(3):255–63.
Raman P, Koenig RJ. Pax-8-PPAR-gamma fusion protein in thyroid carcinoma. Nat Rev Endocrinol. 2014;10(10):616–23.
Ballester LY, Sarabia SF, Sayeed H, Patel N, Baalwa J, Athanassaki I, et al. Integrating molecular testing in the diagnosis and management of children with thyroid lesions. Pediatr Dev Pathol. 2016;19(2):94–100.
Alzahrani AS, Murugan AK, Qasem E, Alswailem M, Al-Hindi H, Shi Y. Single point mutations in pediatric differentiated thyroid cancer. Thyroid. 2017;27(2):189–96.
Onder S, Ozturk Sari S, Yegen G, Sormaz IC, Yilmaz I, Poyrazoglu S, et al. Classic architecture with multicentricity and local recurrence, and absence of TERT promoter mutations are correlates of BRAF (V600E) harboring pediatric papillary thyroid carcinomas. Endocr Pathol. 2016;27(2):153–61.
Ni Y, Seballos S, Fletcher B, Romigh T, Yehia L, Mester J, et al. Germline compound heterozygous poly-glutamine deletion in USF3 may be involved in predisposition to heritable and sporadic epithelial thyroid carcinoma. Hum Mol Genet. 2017;26(2):243–57.
Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al.. GeneReviews® [Internet]. Seattle: University of Washington; 1993–2017 [cited 2017]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1116/.
Bauer AJ. Clinical behavior and genetics of nonsyndromic, familial nonmedullary thyroid cancer. Front Horm Res. 2013;41:141–8.
Schultz KAP, Rednam SP, Kamihara J, Doros L, Achatz MI, Wasserman JD, et al. PTEN, DICER1, FH, and their associated tumor susceptibility syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23(12):e76–82.
Nagy R, Ringel MD. Genetic predisposition for nonmedullary thyroid cancer. Horm Cancer. 2015;6(1):13–20.
Ngeow J, Mester J, Rybicki LA, Ni Y, Milas M, Eng C. Incidence and clinical characteristics of thyroid cancer in prospective series of individuals with cowden and cowden-like syndrome characterized by germline PTEN, SDH, or KLLN alterations. J Clin Endocrinol Metab. 2011;96(12):E2063–71.
Smith JR, Marqusee E, Webb S, Nose V, Fishman SJ, Shamberger RC, et al. Thyroid nodules and cancer in children with PTEN hamartoma tumor syndrome. J Clin Endocrinol Metab. 2011;96(1):34–7.
Hansen-Kiss E, Beinkampen S, Adler B, Frazier T, Prior T, Erdman S, et al. A retrospective chart review of the features of PTEN hamartoma tumour syndrome in children. J Med Genet. 2017;54(7):471–8.
Khan NE, Bauer AJ, Doros L, Schultz KA, Decastro RM, Harney LA, et al. Macrocephaly associated with the DICER1 syndrome. Genet Med. 2016;19:244–8.
Rutter MM, Jha P, Schultz KA, Sheil A, Harris AK, Bauer AJ, et al. DICER1 mutations and differentiated thyroid carcinoma: evidence of a direct association. J Clin Endocrinol Metab. 2016;101(1):1–5.
Khan NE, Bauer AJ, Schultz KAP, Doros L, Decastro RM, Ling A, et al. Quantification of thyroid cancer and multinodular goiter risk in the DICER1 syndrome: a family-based cohort study. J Clin Endocrinol Metab. 2017;102(5):1614–22.
Doros L, Schultz KA, Stewart DR, Bauer AJ, Williams G, Rossi CT, et al. DICER1-related disorders Seattle: University of Washington; 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK196157/.
Eng C. PTEN hamartoma tumor syndrome. Seattle: University of Washington; 2001 [Updated 2016 Jun 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1488/.
Jasperson KW, Patel SG, Ahnen DJ. APC-associated polyposis conditions. Seattle: University of Washington; 1998 [Updated 2017 Feb 2]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1345/.
Stratakis CA, Salpea P, Raygada M. Carney complex. Seattle: University of Washington; 2003 [Updated 2015 Jan 29]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1286/.
Lam AK, Saremi N. Cribriform-morular variant of papillary thyroid carcinoma: a distinctive type of thyroid cancer. Endocr Relat Cancer. 2017;24(4):R109–R21.
Schreiner BF, Murphy WT. Malignant neoplasms of the thyroid gland. Ann Surg. 1934;99(1):116–25.
Waguespack SG, Francis G. Initial management and follow-up of differentiated thyroid cancer in children. J Natl Compr Cancer Netw. 2010;8(11):1289–300.
Kim J, Sun Z, Adam MA, Adibe OO, Rice HE, Roman SA, et al. Predictors of nodal metastasis in pediatric differentiated thyroid cancer. J Pediatr Surg. 2017;52(1):120–3.
Landry CS, Grubbs EG, Busaidy NL, Monroe BJ, Staerkel GA, Perrier ND, et al. Cystic lymph nodes in the lateral neck as indicators of metastatic papillary thyroid cancer. Endocr Pract. 2011;17(2):240–4.
Wang Y, Zhao H, Wang YX, Wang MJ, Zhang ZH, Zhang L, et al. Improvement in the detection of cystic metastatic papillary thyroid carcinoma by measurement of thyroglobulin in aspirated fluid. Biomed Res Int. 2016;2016:8905916.
Kluijfhout WP, Pasternak JD, van der Kaay D, Vriens MR, Propst EJ, Wasserman JD. Is it time to reconsider lobectomy in low-risk paediatric thyroid cancer? Clin Endocrinol. 2017;86(4):591–6.
Jin X, Masterson L, Patel A, Hook L, Nicholson J, Jefferies S, et al. Conservative or radical surgery for pediatric papillary thyroid carcinoma: a systematic review of the literature. Int J Pediatr Otorhinolaryngol. 2015;79(10):1620–4.
Jarzab B, Handkiewicz Junak D, Wloch J, Kalemba B, Roskosz J, Kukulska A, et al. Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med. 2000;27(7):833–41.
Sywak M, Cornford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140(6):1000–5. discussion 5–7.
Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Francis GL. Extensive surgery improves recurrence-free survival for children and young patients with class I papillary thyroid carcinoma. J Pediatr Surg. 1999;34(12):1799–804.
Machens A, Elwerr M, Thanh PN, Lorenz K, Schneider R, Dralle H. Impact of central node dissection on postoperative morbidity in pediatric patients with suspected or proven thyroid cancer. Surgery. 2016;160(2):484–92.
Lee KE, Chung IY, Kang E, Koo do H, Kim KH, Kim SW, et al. Ipsilateral and contralateral central lymph node metastasis in papillary thyroid cancer: patterns and predictive factors of nodal metastasis. Head Neck. 2013;35(5):672–6.
van Santen HM, Aronson DC, Vulsma T, Tummers RF, Geenen MM, de Vijlder JJ, et al. Frequent adverse events after treatment for childhood-onset differentiated thyroid carcinoma: a single institute experience. Eur J Cancer. 2004;40(11):1743–51.
Sosa JA, Tuggle CT, Wang TS, Thomas DC, Boudourakis L, Rivkees S, et al. Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrinol Metab. 2008;93(8):3058–65.
Tuggle CT, Roman SA, Wang TS, Boudourakis L, Thomas DC, Udelsman R, et al. Pediatric endocrine surgery: who is operating on our children? Surgery. 2008;144(6):869–77. discussion 77.
Al-Qurayshi Z, Hauch A, Srivastav S, Aslam R, Friedlander P, Kandil E. A national perspective of the risk, presentation, and outcomes of pediatric thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2016;142(5):472–8.
Thompson GB, Hay ID. Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg. 2004;28(12):1187–98.
Burke JF, Sippel RS, Chen H. Evolution of pediatric thyroid surgery at a tertiary medical center. J Surg Res. 2012;177(2):268–74.
AJCC. Chapter 8. Thyroid. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual, 7th ed. New York: Springer; 2010. p. 87–96.
Powers PA, Dinauer CA, Tuttle RM, Francis GL. The MACIS score predicts the clinical course of papillary thyroid carcinoma in children and adolescents. J Pediatr Endocrinol Metab. 2004;17(3):339–43.
Jang HW, Lee JI, Kim HK, Oh YL, Choi YL, Jin DK, et al. Identification of a cut-off for the MACIS score to predict the prognosis of differentiated thyroid carcinoma in children and young adults. Head Neck. 2012;34(5):696–701.
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133.
Albano D, Bertagna F, Panarotto MB, Giubbini R. Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatr Blood Cancer. 2017;64(11):26595.
Reiners C, Biko J, Haenscheid H, Hebestreit H, Kirinjuk S, Baranowski O, et al. Twenty-five years after Chernobyl: outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2013;98(7):3039–48.
Marti JL, Jain KS, Morris LG. Increased risk of second primary malignancy in pediatric and young adult patients treated with radioactive iodine for differentiated thyroid cancer. Thyroid. 2015;25(6):681–7.
Chen L, Shen Y, Luo Q, Yu Y, Lu H, Zhu R. Pulmonary fibrosis following radioiodine therapy of pulmonary metastases from differentiated thyroid carcinoma. Thyroid. 2010;20(3):337–40.
Iyer NG, Morris LG, Tuttle RM, Shaha AR, Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117(19):4439–46.
Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab. 2008;93(2):504–15.
Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003;89(9):1638–44.
Durante C, Montesano T, Attard M, Torlontano M, Monzani F, Costante G, et al. Long-term surveillance of papillary thyroid cancer patients who do not undergo postoperative radioiodine remnant ablation: is there a role for serum thyroglobulin measurement? J Clin Endocrinol Metab. 2012;97(8):2748–53.
Clayman GL, Agarwal G, Edeiken BS, Waguespack SG, Roberts DB, Sherman SI. Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid. 2011;21(12):1309–16.
Dottorini ME, Vignati A, Mazzucchelli L, Lomuscio G, Colombo L. Differentiated thyroid carcinoma in children and adolescents: a 37-year experience in 85 patients. J Nucl Med. 1997;38(5):669–75.
Samuel AM, Rajashekharrao B, Shah DH. Pulmonary metastases in children and adolescents with well-differentiated thyroid cancer. J Nucl Med. 1998;39(9):1531–6.
Xu L, Liu Q, Liu Y, Pang H. Parameters influencing curative effect of 131I therapy on pediatric differentiated thyroid carcinoma: a retrospective study. Med Sci Monit. 2016;22:3079–85.
Bal CS, Garg A, Chopra S, Ballal S, Soundararajan R. Prognostic factors in pediatric differentiated thyroid cancer patients with pulmonary metastases. J Pediatr Endocrinol Metab. 2014;28(7–8):745–51.
Biko J, Reiners C, Kreissl MC, Verburg FA, Demidchik Y, Drozd V. Favourable course of disease after incomplete remission on (131)I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging. 2011;38(4):651–5.
Padovani RP, Robenshtok E, Brokhin M, Tuttle RM. Even without additional therapy, serum thyroglobulin concentrations often decline for years after total thyroidectomy and radioactive remnant ablation in patients with differentiated thyroid cancer. Thyroid. 2012;22(8):778–83.
Luster M, Lassmann M, Freudenberg LS, Reiners C. Thyroid cancer in childhood: management strategy, including dosimetry and long-term results. Hormones (Athens). 2007;6(4):269–78.
Schoelwer MJ, Zimmerman D, Shore RM, Josefson JL. The use of 123I in diagnostic radioactive iodine scans in children with differentiated thyroid carcinoma. Thyroid. 2015;25(8):935–41.
Livhits MJ, Pasternak JD, Xiong M, Li N, Gosnell JE, Yeh MW, et al. Pre-ablation thyroglobulin and thyroglobulin to thyroid-stimulating hormone ratio may be associated with pulmonary metastases in children with differentiated thyroid cancer. Endocr Pract. 2016;22(11):1259–66.
Vali R, Rachmiel M, Hamilton J, El Zein M, Wasserman J, Costantini DL, et al. The role of ultrasound in the follow-up of children with differentiated thyroid cancer. Pediatr Radiol. 2015;45(7):1039–45.
Kuijt WJ, Huang SA. Children with differentiated thyroid cancer achieve adequate hyperthyrotropinemia within 14 days of levothyroxine withdrawal. J Clin Endocrinol Metab. 2005;90(11):6123–5.
Turpin S, Lambert R, Deal C. Timing of hormone withdrawal in children undergoing 131I whole-body scans for thyroid cancer. Horm Res Paediatr. 2016;86(6):410–5.
Hanscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C, et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47(4):648–54.
Handkiewicz-Junak D, Gawlik T, Rozkosz J, Puch Z, Michalik B, Gubala E, et al. Recombinant human thyrotropin preparation for adjuvant radioiodine treatment in children and adolescents with differentiated thyroid cancer. Eur J Endocrinol. 2015;173(6):873–81.
Jarzab B, Handkiewicz-Junak D, Wloch J. Juvenile differentiated thyroid carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer. 2005;12(4):773–803.
Hung W, Sarlis NJ. Current controversies in the management of pediatric patients with well-differentiated nonmedullary thyroid cancer: a review. Thyroid. 2002;12(8):683–702.
Dinauer C, Francis GL. Thyroid cancer in children. Endocrinol Metab Clin N Am. 2007;36(3):779–806, vii.
Lassmann M, Hanscheid H, Verburg FA, Luster M. The use of dosimetry in the treatment of differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2011;55(2):107–15.
Verburg FA, Reiners C, Hanscheid H. Approach to the patient: role of dosimetric RAI Rx in children with DTC. J Clin Endocrinol Metab. 2013;98(10):3912–9.
Barwick TD, Dhawan RT, Lewington V. Role of SPECT/CT in differentiated thyroid cancer. Nucl Med Commun. 2012;33(8):787–98.
Lee M, Lee YK, Jeon TJ, Chang HS, Kim BW, Lee YS, et al. Frequent visualization of thyroglossal duct remnant on post-ablation 131I-SPECT/CT and its clinical implications. Clin Radiol. 2015;70(6):638–43.
Mostafa M, Vali R, Chan J, Omarkhail Y, Shammas A. Variants and pitfalls on radioiodine scans in pediatric patients with differentiated thyroid carcinoma. Pediatr Radiol. 2016;46(11):1579–89.
Sabra MM, Grewal RK, Tala H, Larson SM, Tuttle RM. Clinical outcomes following empiric radioiodine therapy in patients with structurally identifiable metastatic follicular cell-derived thyroid carcinoma with negative diagnostic but positive post-therapy 131I whole-body scans. Thyroid. 2012;22(9):877–83.
Rosario PW, Mourao GF, Dos Santos JB, Calsolari MR. Is empirical radioactive iodine therapy still a valid approach to patients with thyroid cancer and elevated thyroglobulin? Thyroid. 2014;24(3):533–6.
Hurley JR. Historical note: TSH suppression for thyroid cancer. Thyroid. 2011;21(11):1175–6.
Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–751.
Bauer AJ. Approach to the pediatric patient with Graves’ disease: when is definitive therapy warranted? J Clin Endocrinol Metab. 2011;96(3):580–8.
Rivkees SA. Pediatric Graves’ disease: controversies in management. Horm Res Paediatr. 2010;74(5):305–11.
Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91(2):498–505.
Ciarallo A, Marcus C, Taghipour M, Subramaniam RM. Value of fluorodeoxyglucose PET/computed tomography patient management and outcomes in thyroid cancer. PET Clin. 2015;10(2):265–78.
Leboulleux S, El Bez I, Borget I, Elleuch M, Deandreis D, Al Ghuzlan A, et al. Post-radioiodine treatment whole body scan in the era of fluorodesoxyglucose positron emission tomography for differentiated thyroid carcinoma with elevated serum thyroglobulin levels. Thyroid. 2012;22(8):832–8.
Isoda T, BaBa S, Maruoka Y, Kitamura Y, Tahara K, Sasaki M, et al. Impact of patient age on the iodine/FDG “flip-flop” phenomenon in lung metastasis from thyroid cancer. Ann Nucl Med. 2016;30(8):518–24.
Evans C, Tennant S, Perros P. Thyroglobulin in differentiated thyroid cancer. Clin Chim Acta. 2015;444:310–7.
Nascimento C, Borget I, Troalen F, Al Ghuzlan A, Deandreis D, Hartl D, et al. Ultrasensitive serum thyroglobulin measurement is useful for the follow-up of patients treated with total thyroidectomy without radioactive iodine ablation. Eur J Endocrinol. 2013;169(5):689–93.
Momesso DP, Vaisman F, Yang SP, Bulzico DA, Corbo R, Vaisman M, et al. Dynamic risk stratification in patients with differentiated thyroid Cancer treated without radioactive iodine. J Clin Endocrinol Metab. 2016;101(7):2692–700.
Dominguez JM, Nilo F, Contreras T, Carmona R, Droppelmann N, Gonzalez H, et al. Neck sonography and suppressed thyroglobulin have high sensitivity for identifying recurrent/persistent disease in patients with low-risk thyroid cancer treated with total thyroidectomy and radioactive iodine ablation, making stimulated thyroglobulin unnecessary. J Ultrasound Med. 2017;36(11):2299–307.
Castagna MG, Brilli L, Pilli T, Montanaro A, Cipri C, Fioravanti C, et al. Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levels. J Clin Endocrinol Metab. 2008;93(1):76–81.
Han JM, Kim WB, Yim JH, Kim WG, Kim TY, Ryu JS, et al. Long-term clinical outcome of differentiated thyroid cancer patients with undetectable stimulated thyroglobulin level one year after initial treatment. Thyroid. 2012;22(8):784–90.
Klubo-Gwiezdzinska J, Burman KD, Van Nostrand D, Wartofsky L. Does an undetectable rhTSH-stimulated Tg level 12 months after initial treatment of thyroid cancer indicate remission? Clin Endocrinol. 2011;74(1):111–7.
Spencer C, Fatemi S. Thyroglobulin antibody (TgAb) methods – strengths, pitfalls and clinical utility for monitoring TgAb-positive patients with differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2013;27(5):701–12.
Verburg FA, Luster M, Cupini C, Chiovato L, Duntas L, Elisei R, et al. Implications of thyroglobulin antibody positivity in patients with differentiated thyroid cancer: a clinical position statement. Thyroid. 2013;23(10):1211–25.
Chiovato L, Latrofa F, Braverman LE, Pacini F, Capezzone M, Masserini L, et al. Disappearance of humoral thyroid autoimmunity after complete removal of thyroid antigens. Ann Intern Med. 2003;139(5 Pt 1):346–51.
Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–95.
Kim A, Widemann BC, Krailo M, Jayaprakash N, Fox E, Weigel B, et al. Phase 2 trial of sorafenib in children and young adults with refractory solid tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62(9):1562–6.
Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a Children’s Oncology Group Phase I Consortium report. Clin Cancer Res. 2012;18(21):6011–22.
Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-Oncology. 2014;16(10):1408–16.
Kim A, Dombi E, Tepas K, Fox E, Martin S, Wolters P, et al. Phase I trial and pharmacokinetic study of sorafenib in children with neurofibromatosis type I and plexiform neurofibromas. Pediatr Blood Cancer. 2013;60(3):396–401.
Iyer P, Mayer JL, Ewig JM. Response to sorafenib in a pediatric patient with papillary thyroid carcinoma with diffuse nodular pulmonary disease requiring mechanical ventilation. Thyroid. 2014;24(1):169–74.
Waguespack SG, Sherman SI, Williams MD, Clayman GL, Herzog CE. The successful use of sorafenib to treat pediatric papillary thyroid carcinoma. Thyroid. 2009;19(4):407–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Waguespack, S.G., Wasserman, J.D. (2018). Pediatric Differentiated Thyroid Carcinoma. In: Mallick, U.K., Harmer, C. (eds) Practical Management of Thyroid Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-91725-2_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-91725-2_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91724-5
Online ISBN: 978-3-319-91725-2
eBook Packages: MedicineMedicine (R0)