Abstract
Background
Thyroid cancer is the most common endocrine malignancy with relatively good prognosis in children. However, unlike adults, children usually present with more advanced disease and have a higher local recurrence and distant metastases. Thus surveillance for recurrence is a major goal of long-term follow-up.
Objective
This retrospective study evaluates the diagnostic value of ultrasound (US) imaging in the post-therapy surveillance of children with differentiated thyroid cancer.
Materials and methods
We reviewed the charts of 54 children (40 girls; mean age 14.3 ± 3.6 years) with differentiated thyroid cancer treated with total or near-total thyroidectomy. Forty children (29 girls and 11 boys) who had routine follow-up US examinations (112 studies) were included for the evaluation of US accuracy in the follow-up of pediatric differentiated thyroid cancer. Histopathology, stimulated thyroglobulin determination, post-therapy whole-body iodine scan and clinical follow-up were used as the standards of reference.
Results
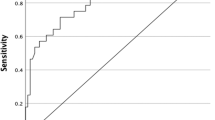
Mean period of follow-up was 34 months. The frequency of recurrence was 42% (17/40). Seventeen percent of the children had lung metastases either at presentation or on follow-up. In all cases of lung metastases, stimulated thyroglobulin level was greater than 10 ng/ml. The sensitivity was 85.7%, specificity 89.4%, negative predictive value 94.4% and positive predictive value 75% for US in detecting loco-regional recurrence in follow-up studies of pediatric differentiated thyroid cancer. In 17.3% (18/104) of studies, the results of stimulated thyroglobulin and US were discordant.
Conclusion
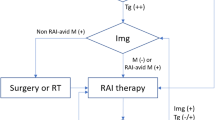
US showed very good sensitivity and specificity and a high negative predictive value for evaluation of loco-regional involvement in follow-up of pediatric differentiated thyroid cancer. Diagnostic whole-body iodine scan is indicated when serum anti-thyroglobulin Ab is high, or in cases of discordant findings between US and stimulated thyroglobulin levels, or when stimulated thyroglobulin levels are >10 ng/ml (to evaluate for lung metastasis).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thyroid cancer is the most common endocrine malignancy, affecting more than 60,000 people in 2014 in North America [1]. Of these cases only 2% occurred in children and adolescents [2]. The majority of thyroid cancers are of differentiated type, mainly papillary (85%) and to a lesser extent follicular (10%) [3, 4]. The standard treatment of differentiated thyroid carcinoma consists of total or near-total thyroidectomy, with resection of affected regional lymph nodes and in some cases dissection of the central compartment, followed by ablation of thyroid remnant and metastases using 131I followed by thyroid-stimulating hormone (TSH) suppression with levothyroxine [5, 6].
The long-term prognosis of differentiated thyroid cancer diagnosed in childhood is good, with overall 10-year survival rates of 80–95%. Hay et al. [7] showed a 2% cause-specific mortality rate for pediatric papillary thyroid cancer after 20 years of follow-up. Unlike adults, children with differentiated thyroid cancer may present with more advanced disease and have a higher local recurrence and distant metastases, even though their prognosis is favorable [8]. Surveillance for recurrence is therefore a major goal of long-term follow-up [9, 10].
Conventionally, stimulated serum thyroglobulin levels (using levothyroxine withdrawal or recombinant human TSH and diagnostic whole-body iodine [123I or 131I] scanning) are determined to detect thyroid tissue remnant and recurrence in the follow-up of patients with differentiated thyroid cancer. High-resolution US imaging at regular intervals after initial therapy has also been effective for evaluation of adults with thyroid cancer. It can detect recurrent cancer in the thyroid bed after thyroidectomy or in the lymph nodes of the head and neck before tumor becomes palpable on physical examination. US is also superior in sensitivity to diagnostic whole-body iodine scan for evaluation of loco-regional lymph node involvement [11, 12]. However, outcomes in children have been less studied [7].
The accurate interpretation of US in children with differentiated thyroid cancer following curative surgery or radioablative therapy can be complicated by the presence of large inflammatory lymph nodes in the neck. These reactive lymph nodes typically occur more commonly in children than in adults and make it particularly difficult to differentiate benign processes from local recurrence [11–13].
In adults, high-sensitivity US and stimulated thyroglobulin measurements are often sufficient for follow-up, with diagnostic whole-body iodine scan reserved for cases in which US and stimulated thyroglobulin are discordant; however, this has not been fully explored in pediatric differentiated thyroid cancer. Moreover, discrepant findings between stimulated thyroglobulin measurements and US have been reported in children with recurrent disease [13], indicating a need for further study of the role of US and diagnostic whole-body iodine scan in the follow-up of pediatric differentiated thyroid cancer. This study evaluated the diagnostic accuracy of US in the post-therapy follow-up of children with differentiated thyroid cancer and clarifies the clinical value of sonography, combined US and stimulated thyroglobulin measurements, and diagnostic whole-body iodine scan.
Materials and methods
Patients
Data were retrospectively reviewed from the charts of 54 children (40 girls, 14 boys) younger than 18 years who had histopathologically proven differentiated thyroid cancer at our institution between 2000 and 2011. All children were treated with total or near-total thyroidectomy and central neck dissection followed by radio-iodine ablation therapy. Children who had US for follow-up of recurrent disease at least 6 months after the thyroidectomy and radioablation therapy were selected for evaluation of US accuracy. Residual disease following initial treatment was not included in the calculation. The study was approved by the local institutional research ethics board.
Imaging
US was performed 6 months after thyroidectomy and radioablation therapy and then routinely every 6–12 months along with thyroglobulin measurements. US was performed using a Toshiba Aplio XG (Otawara-shi, Tochigi, Japan) equipped with 12.5- and 17.5-MHz linear probes (depending on patient age). US examination included the thyroid bed and both central and lateral neck compartments. US findings were not described based on the level of neck lymph nodes. The findings on US were categorized as (1) suspicious for malignancy or (2) negative or benign lymph nodes. Suspicion of malignant lymph node was based on the following criteria: hypoechoic appearance (compared with surrounding muscles), hyperechoic foci (from microcalcification or colloid formation), peripheral vascularization, and round-shape node (with long-to-short-axis ratio of less than 1.5) without hyperechoic hilum [14, 15] (Fig. 1). All studies were reviewed by two radiologists (A.D. with 30 years of experience in pediatric radiology and M.E. with 12 years of experience in adult and pediatric radiology) blinded to patient history, clinical data and diagnostic whole-body iodine scan findings. The agreement between the readers was estimated using a kappa statistic. Disagreement was resolved by consensus.
US in a 16-year-old boy with papillary carcinoma evaluated for recurrence/metastasis. Transverse US image shows a hypoechoic nodule with scattered hyperechoic foci suggestive of calcifications. These findings are suggestive of malignancy. Serum thyroglobulin level was high. Pathology confirmed that the lymph node was malignant
Biochemistry and diagnostic whole-body iodine scan
Stimulated serum thyroglobulin levels were measured 2–4 weeks after L-thyroxine withdrawal (such that TSH levels were >30 mU/L) using electrochemiluminescence assay (Beckman Coulter access 2, Chaska, MN). Serum TSH and anti-thyroglobulin antibody titers were measured using immunoradiometric assays. Stimulated thyroglobulin values were considered unreliable when serum anti-thyroglobulin titers were positive. Stimulated thyroglobulin level was interpreted as negative when it was undetectable (<1 ng/ml), whereas a stimulated thyroglobulin >10 ng/ml indicated a positive assay. Stimulated thyroglobulin values ranging 1–10 ng/ml were considered indeterminate [16].
Diagnostic whole-body iodine scan was performed 48 h after oral ingestion of 37–148 MBq 131I, whereas post-therapy whole-body iodine scan was obtained 7–10 days after a therapeutic dose of 131I (1,850–5,550 MBq). The children were asked to withdraw L-thyroxine for at least 2–4 weeks and avoid iodine in their diet for 1 week before the 131I ingestion. The TSH level was >30 mU/L in all children before the administration of 131I. All scintigraphic imaging was performed using a dual-head Millennium MG camera (Milwaukee, WI) equipped with a high-energy parallel-hole collimator with a 20% window centered on the 364-kEv photopeak. Planar images consisting of 10-min acquisitions were acquired to include head, neck and upper thorax in the field-of-view. All nuclear medicine scans were reviewed independently by two nuclear medicine physicians (M.C. with 25 years and R.V. with 12 years of experience in adult and pediatric nuclear medicine).
Standards of reference and statistical analysis
Histopathology was considered the gold standard to assess the results of US. In cases where histopathology was not available a combination of stimulated thyroglobulin levels >10 ng/ml and post-therapy whole-body iodine scan was used as the gold standard. According to the American Thyroid Association guidelines [9], patients were considered disease-free when there was no evidence of clinical findings suggestive of a malignancy, a negative US examination, negative whole-body iodine scan and undetectable stimulated thyroglobulin values (<1 ng/ml) in the absence of anti-thyroglobulin antibody titers.
US findings were considered true positive if histopathology confirmed the malignancy in the lymph nodes (7 cases) or if stimulated thyroglobulin level was more than 10 ng/ml with a positive post-therapy whole-body iodine scan or positive follow-up clinical/diagnostic imaging (17 cases). US findings were considered true negative when stimulated thyroglobulin level was less than 1 ng/ml and there was a negative post-therapy whole-body iodine scan (64 cases), and if the stimulated thyroglobulin was 1–10 ng/ml with a negative post-therapy whole-body iodine scan or negative follow-up clinical/diagnostic imaging without any treatment (4 cases). US findings were considered false positive if US was suggestive of malignancy but the histopathology did not confirm malignancy (1 case) or if the stimulated thyroglobulin level was less than 1 ng/ml and post-therapy whole-body iodine scan was negative (if performed) (5 cases). US findings were also interpreted as false positive if the stimulated thyroglobulin was less than 10 ng/ml and the enlarged lymph node disappeared on follow-up studies in the absence of treatment (2 cases). US findings were considered false negative when the US was negative or showed benign inflammatory lymph nodes but histopathology confirmed malignancy (1 case) or diagnostic and therapeutic whole-body iodine scan showed a focal uptake (1 case) or the stimulated thyroglobulin level was more than 10 ng/ml with a focal uptake in the neck on diagnostic whole-body iodine scan or post-therapy whole-body iodine scan (2 cases).
In eight US studies (out of 112 reviewed), we were not able to prove whether findings were true positive, false positive, true negative or false negative according to the aforementioned criteria. Because there was a lack of confirmatory tests (histopathology or post-therapy whole-body iodine scan), these eight cases were excluded from the statistical analysis. Data are expressed as the mean ± standard deviation (SD). We determined the sensitivity and specificity, as well as the positive and negative predictive values of US to detect loco-regional malignancy.
Results
Diagnosis and frequency of disease recurrence during follow-up
The clinical characteristics of 54 thyroid cancer patients (total group) and the US subgroup (40 patients) are summarized (Table 1). In 100/104 follow-up US examinations there was agreement between the readers in 96%, kappa = 0.90. Disagreements (4/104, 4%) were resolved by consensus. The initial pathological Tumor-Nodes-Metastasis (TNM) staging system for classification of malignant tumors is summarized in Table 2. Mean period of follow-up was 34 months (range 12–120 months). Histology was papillary in 70% of cases, follicular in 5%, and papillary-follicular variant in 25%.
The frequency of recurrence was 42% (17/40). Sites of recurrent disease were in lymph nodes (10 patients), lungs only (1 patient), and lymph nodes and lungs (6 patients). Of note, 17% of patients (7/40) showed lung metastases on diagnostic or post-therapy whole-body iodine scan. Stimulated thyroglobulin level was more than 10 in all children with lung metastases.
Diagnostic accuracy of US alone in the follow-up of differentiated thyroid cancer
One hundred four (104) US studies were evaluated in 40 children with differentiated thyroid cancer (Table 1). US was true positive in 24 cases and false positive in 8, whereas it was true negative in 68 cases and false negative in 4. The sensitivity was 85.7%, specificity 89.4%, negative predictive value 94.4% and positive predictive value 75% for US in detecting loco-regional recurrence in follow-up studies of pediatric differentiated thyroid cancer. On per-patient analysis, US was true positive in 12 children and false positive in 4, whereas it was true negative in 21 and false negative in 3. US yielded a sensitivity of 80%, specificity of 84%, negative predictive value of 87.5% and positive predictive value of 75% on per-patient analysis.
Combination of US and stimulated thyroglobulin measurements
Concordance between US and stimulated thyroglobulin measurements during follow-up was observed in 86/104 studies (82.6%). Of these, the US and stimulated thyroglobulin were both true positive in 20 studies and true negative in 64 studies. In two studies both US and stimulated thyroglobulin level were negative. In these two cases, US showed small lymph nodes (less than 1 cm) in the lateral neck region with normal internal architectures suggestive of inflammation. Diagnostic and post-therapy iodine scan were positive in both cases. In the other 18 studies, the findings of US and stimulated thyroglobulin were discordant.
Discussion
Although the long-term prognosis for children with differentiated thyroid cancer is generally quite good, tumor recurrence is still common, affecting 20–50%, sometimes decades after initial therapy [5, 6, 17]. Accurate surveillance with physical examination, serum stimulated thyroglobulin measurements and US imaging is therefore fundamental to long-term follow-up. US together with serum stimulated thyroglobulin measurements has proved effective at detecting recurrent disease [5, 6]. Indeed, the results of this study confirm that US combined with measurements of stimulated thyroglobulin levels can be considered the first-line modality for the follow-up surveillance of children with differentiated thyroid cancer. However in children with discordant stimulated thyroglobulin and US results, further evaluation with diagnostic whole-body iodine scan can be considered. When stimulated thyroglobulin level is higher than 10 ng/ml diagnostic whole-body iodine scan may also be indicated to determine the extent of disease and to explore the possibility of lung or osseous metastasis; whole-body iodine scan is also indicated when the results of stimulated thyroglobulin are not reliable because of the presence of serum anti-thyroglobulin Ab.
The reported frequency of loco-regional recurrence or de novo metastatic disease is variable. In adults, the reported frequency of recurrence is 3–13% [18, 19], whereas in pediatric patients the rate is much higher [20–25]. In the current study, 42% (17/40) of patients with differentiated thyroid cancer showed recurrence or metastasis during follow-up.
The sensitivity of US for detecting loco-regional lymph node metastasis was found to be superior to that of diagnostic whole-body iodine scan [26]. Unlike diagnostic whole-body iodine scan, US does not require the discontinuation of thyroid replacement medication and the inconveniences or risks associated with prolonged hypothyroidism. Combined with stimulated thyroglobulin measurements, US examination can be considered the first-line modality in following up with children with differentiated thyroid cancer. This is also significant considering that the incidence of recurrence is very low in cases in which US is negative and serum-stimulated thyroglobulin levels are undetectable (i.e. 2/104, or 1.9%, as determined in the current study).
A small but notable number of cases had discordant results between stimulated thyroglobulin and US. Because diagnostic whole-body iodine scan has a high positive predictive value and specificity, further evaluation with diagnostic whole-body iodine scan is helpful in these cases. We therefore consider diagnostic whole-body iodine scan to be of particular importance in the follow-up of high-risk children with a questionable US finding or a discrepancy between US findings and stimulated thyroglobulin level.
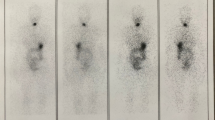
Numerous factors known to cause a false-negative or false-positive stimulated thyroglobulin level or US study have been proposed. For example, malignant thyroglobulin-producing lesions outside the anatomical region examined by US, or enlarged or inflamed lymph nodes, can result in false-negative or false-positive findings by US, respectively [11–13]. On the other hand, determination of serum thyroglobulin level alone may also be misleading. In a study by Park et al. [27], a 6.3% false-negative thyroglobulin determination was reported using post-therapy whole-body iodine scan as a reference standard. Possible causes for a false-negative thyroglobulin level include: (1) dedifferentiated thyroid cancer cells that can still concentrate iodine but cannot synthesize or release thyroglobulin [28], (2) the presence of interfering anti-thyroglobulin antibodies in the circulation [28, 29], (3) limitations in the functional sensitivity of routine thyroglobulin measurements, (4) decreased secretion of thyroglobulin from so-called stunned residual/recurrent tumor tissue [30], or (5) small volume of residual/recurrent tumor and amount of excreted thyroglobulin [31]. Indeed, Bachelot et al. [32] reported a relationship between the mass of residual tumor and the amount of excreted thyroglobulin and found that for each gram of tissue serum thyroglobulin increases 0.5–1.0 ng/ml. In contrast, a false-positive thyroglobulin-level may be the result of benign thyroid or non-thyroidal tissue producing thyroglobulin [33], a delayed conversion of thyroglobulin to undetectable levels because of the slow, progressive death of differentiated thyroid cancer after radioablation or the leakage of thyroglobulin from dead or dying cells. This slow, progressive death of differentiated thyroid cancer after radioablation therapy can be also seen on diagnostic whole-body iodine scan (Fig. 2).
Follow-up imaging in a 14-year-old girl who underwent total thyroidectomy for papillary carcinoma 1 year earlier. a Multiple foci of activity are noted in the neck on post-therapy whole-body iodine scan (arrows). b Six months later, after radioiodine therapy, a focus of persistent iodine uptake is seen on the diagnostic whole-body iodine scan in the right retroclavicular region (arrow). c Transverse sonogram done at the same time as (b) is negative for residual thyroid or nodes. Thyroglobulin value was undetectable and antithyroglobulin was negative. d After an additional 6 months without treatment, a diagnostic whole-body iodine scan is normal. Thyroglobulin value was undetectable. Only neck and chest regions of the diagnostic whole-body iodine scan are shown
Study limitations include the lack of pathology confirmation for all positive lymph nodes visualized on US. However, post-therapy whole-body iodine scan, diagnostic whole-body iodine scan and follow-up studies were available in the majority of cases to confirm or exclude malignancy. Eight cases were excluded from the final calculation for lack of confirmatory tests. We included all types of pathology in pediatric differentiated thyroid cancer. The behavior of the follicular type of the pediatric differentiated thyroid cancer can be different from that of the papillary or follicular variant of papillary types of differentiated thyroid cancer in children. Because the number of specific types of differentiated thyroid cancer (i.e. follicular type) in children is very limited, a multicenter study is needed to evaluate the frequency of recurrence and metastases as well as diagnostic accuracy of different modalities in these children.
Conclusion
Our study supports the recommendation that US combined with stimulated thyroglobulin level measurements can be considered the first-line modality in the follow-up surveillance of children with differentiated thyroid cancer. In cases of high antithyroglobulin Ab, or when discordant findings between US and serum stimulated thyroglobulin level are encountered, and when serum stimulated thyroglobulin levels are >10 ng/ml (in order to detect lung metastasis), further evaluation with diagnostic whole-body iodine scan can be considered.
References
National Cancer Institute (2014) The survival, epidemiology, and end result program: SEER stats fact sheet: thyroid cancer. http://seer.cancer.gov/statfacts/html/thyro.html. Accessed 7 Nov 2014
Hogan AR, Zhuge Y, Perez EA et al (2009) Pediatric thyroid carcinoma: incidence and outcomes in 1,753 patients. J Surg Res 156:167–172
Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167
Hundahl SA, Fleming ID, Fremgen AM et al (1998) A national cancer database report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer 83:2638–2648
Mazzaferri EL, Massoll N (2002) Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer 9:227–247
Jones MK (2002) Management of papillary and follicular thyroid cancer. J R Soc Med 95:325–326
Hay ID, Gonzalez-Losada T, Reinalda MS et al (2010) Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg 34:1192–1202
Parisi MT, Mankoff D (2007) Differentiated pediatric thyroid cancer: correlates with adult disease, controversies in treatment. Sem Nucl Med 37:340–356
Cooper DS, Doherty GM, Haugen BR et al (2009) Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214
O’Gorman CS, Hamilton J, Rachmiel M et al (2010) Thyroid cancer in childhood: a retrospective review of childhood course. Thyroid 20:375–380
Ko MS, Lee JH, Shong YK et al (2010) Normal and abnormal sonographic findings at the thyroidectomy sites in postoperative patients with thyroid malignancy. AJR Am J Roentgenol 194:1596–1609
Torlontano M, Attard M, Crocetti U et al (2004) Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab 89:3402–3407
Antonelli A, Miccoli P, Fallahi P et al (2003) Role of neck ultrasonography in the follow-up of children operated on for thyroid papillary cancer. Thyroid 13:479–484
Sohn YM, Kwak JY, Kim EK et al (2010) Diagnostic approach for evaluation of lymph node metastasis from thyroid cancer using ultrasound and fine-needle aspiration biopsy. AJR Am J Roentgenol 194:38–43
Leboulleux S, Girard E, Rose M et al (2007) Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab 92:3590–3594
Cailleux AF, Baudin E, Travagli JP et al (2000) Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J Clin Endocrinol Metab 85:175–178
Lin JD, Chao TC, Hsueh C et al (2009) High recurrent rate of multicentric papillary thyroid carcinoma. Ann Surg Oncol 16:2609–2616
Cirocchi R, Trastulli S, Sanguinetti A et al (2011) Recurrent differentiated thyroid cancer: to cut or burn. World J Surg Oncol 9:89
Brassard M, Borget I, Edet-Sanson A et al (2011) Long-term follow-up of patients with papillary and follicular thyroid cancer: a prospective study on 715 patients. J Clin Endocrinol Metab 96:1352–1359
Collini P, Mattavelli F, Pellegrinelli A et al (2006) Papillary carcinoma of the thyroid gland of childhood and adolescence: morphologic subtypes, biologic behavior and prognosis: a clinicopathologic study of 42 sporadic cases treated at a single institution during a 30-year period. Am J Surg Pathol 30:1420–1426
Merrick Y, Hansen HS (1989) Thyroid cancer in children and adolescents in Denmark. Eur J Surg Oncol 15:49–53
Chow SM, Law SC, Mendenhall WM et al (2004) Differentiated thyroid carcinoma in childhood and adolescence—clinical course and role of radioiodine. Pediatr Blood Cancer 42:176–183
Newman KD, Black T, Heller G et al (1998) Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Children’s Cancer Group. Ann Surg 227:533–541
Zimmerman D, Hay ID, Gough IR et al (1988) Papillary thyroid carcinoma in children and adults: long-term follow-up of 1,039 patients conservatively treated at one institution during three decades. Surgery 104:1157–1166
Palmer BA, Zarroug AE, Poley RN et al (2005) Papillary thyroid carcinoma in children: risk factors and complications of disease recurrence. J Pediatr Surg 40:1284–1288
Choi JW, Lee JH, Baek JH et al (2010) Diagnostic accuracy of ultrasound and 18-F-FDG PET or PET/CT for patients with suspected recurrent papillary thyroid carcinoma. Ultrasound Med Biol 36:1608–1615
Park EK, Chung JK, Lim IH et al (2009) Recurrent/metastatic thyroid carcinomas false negative for serum thyroglobulin but positive by posttherapy I-131 whole body scans. Eur J Nucl Med Mol Imaging 36:172–179
Ma C, Kuang A, Xie J et al (2005) Possible explanations for patients with discordant findings of serum thyroglobulin and 131I whole-body scanning. J Nucl Med 46:1473–1480
Spencer C, Petrovic I, Fatemi S (2011) Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J Clin Endocrinol Metab 96:1283–1291
Siddiqi A, Foley RR, Britton KE et al (2001) The role of 123I-diagnostic imaging in the follow-up of patients with differentiated thyroid carcinoma as compared to 131I-scanning: avoidance of negative therapeutic uptake due to stunning. Clin Endocrinol 55:515–521
Brendel AJ, Lambert B, Guyot M et al (1990) Low levels of serum thyroglobulin after withdrawal of thyroid suppression therapy in the follow up of differentiated thyroid carcinoma. Eur J Nucl Med 16:35–38
Bachelot A, Cailleux AF, Klain M et al (2002) Relationship between tumor burden and serum thyroglobulin level in patients with papillary and follicular thyroid carcinoma. Thyroid 12:707–711
Torrens JI, Burch HB (2001) Serum thyroglobulin measurement. Utility in clinical practice. Endocrinol Metab Clin North Am 30:429–467
Acknowledgments
We thank Dr. Rahim Moineddin for providing statistical analysis and Dr. Lianna Kyriakopoulou for laboratory and thyroglobulin interpretation. The authors also thank the editor and reviewers of this manuscript for their insightful comments and suggestions.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vali, R., Rachmiel, M., Hamilton, J. et al. The role of ultrasound in the follow-up of children with differentiated thyroid cancer. Pediatr Radiol 45, 1039–1045 (2015). https://doi.org/10.1007/s00247-014-3261-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-3261-0