Abstract
Differentiated thyroid cancer in children is a rare disease, accounting for only 1.4% of all pediatric malignancies. The diagnosis, biological behavior and treatment of differentiated thyroid cancer in children is different from that in adults. While there are many unresolved issues regarding approaches to management of differentiated thyroid cancer in the pediatric population, there is near universal consensus that treatment of this disease, which includes total thyroidectomy, central lymph node dissection at the time of initial surgery in those with nodal metastases, and the possible use of iodine-131 radiotherapy, is best performed by specialists including high-volume endocrine surgeons and experts with experience in calculating and administering radioactive iodine in children, when deemed appropriate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While thyroid cancer represents only 1.4% of all pediatric malignancies, 10% of thyroid cancers occur in those younger than 21 years [1, 2]. The biological behavior of differentiated thyroid cancer is different in children from that in adults. The well-differentiated papillary or follicular subtypes are the most commonly occurring pathological types in children, seen in 95% and 5%, respectively [3], compared to an occurrence rate of 70–80% and 15% in adults [4]. There are other differences, as well. Children typically present with advanced disease at diagnosis [5,6,7,8,9] (Fig. 1). The size of the primary tumor is frequently larger in children than in adults. Extensive regional nodal involvement at diagnosis is present in between 60% and 80% of children with differentiated thyroid cancer [2, 3, 5,6,7,8,9] compared to only 30% to 40% of adults with this disease [4]. Children with differentiated thyroid cancer have a higher rate of distant metastases than adults, typically to the lungs, where metastases are present in 20–25% of children [5,6,7,8,9] compared to less than 7% of adults [4]. Higher rates of both local and distant recurrences occur in children compared to adults with differentiated thyroid cancer. Despite this, children with differentiated thyroid cancer have a rapid response to therapy and an excellent prognosis that is significantly better than their adult counterparts with advanced disease. Overall survival in children with differentiated thyroid cancer is greater than 90%, with survival rates of 98% at 5 years and 15 years compared to 40% at 5 years and 20% at 10 years in adults with distant metastases [2, 4, 10, 11]. Unfortunately, progression-free survival is only between 65% and 70% for children with differentiated thyroid cancer, mandating lifelong surveillance [12,13,14].
Multifocal papillary thyroid carcinoma in a 15-year-old girl presenting with thyroid gland enlargement (American Thyroid Association pediatric high-risk group). a–c Transverse (a) and longitudinal (b, c) images from thyroid ultrasound demonstrate two masses, a 2.6×2.8-cm solid hyperechoic mass with microcalcifications and peripheral vascularity in the right lobe (asterisks, a and b) as well as a 1.2×1.6-cm mixed solid and cystic mass with microcalcifications in the left lobe (arrowheads, a and c). d Additionally, abnormally enlarged rounded lymph nodes with fine calcifications (arrows) and chaotic vascularity were present at left Level VI and right Levels III and IV; the largest lymph node, measuring 18×21 mm at Level IV, is shown on transverse ultrasound with gray-scale and color Doppler images. The constellation of findings is highly suspicious for thyroid carcinoma. Fine-needle aspiration (FNA) of the mass in the right lobe and the right Level IV lymph node confirmed metastatic papillary thyroid carcinoma. The girl underwent total thyroidectomy, right central neck dissection, and right modified radical neck dissection. Pathology was consistent with multifocal papillary thyroid carcinoma in the right lobe, classic type, with 17/22 lymph nodes positive for carcinoma (pathological classification pT3aN1b); and a hyperplastic nodule in the thyroid left lobe. Children with papillary thyroid carcinoma typically present with advanced disease at diagnosis, with extensive regional nodal involvement present in 60–80%
Despite extensive investigation and the recent publication of the American Thyroid Association (ATA)’s Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer [15], the treatment of pediatric differentiated thyroid cancer remains controversial for a variety of reasons. Despite its rising incidence [3, 10], pediatric differentiated thyroid cancer remains a rare entity, hence it is difficult to obtain a large single-institution series to evaluate. Moreover, there have been differences in treatment methods at various institutions as well as at the same institution over time, and as a result there has been no large retrospective study of pediatric patients with a well-defined treatment protocol nor the performance of a controlled prospective study in this patient population [8, 16]. Rather, most treatment plans have been derived from experiences in adults with papillary thyroid cancer [16].
The goals of initial treatment of differentiated thyroid cancer in both adults and children are to eradicate disease and extend disease-free survival. Typically, treatment has involved surgery in the form of thyroidectomy with lymph node dissection and the use of radioactive iodine (I-131) either to ablate remnant disease, allowing for surveillance with thyroglobulin levels, or to treat local and distant metastatic disease. But children are not small adults. In general, the complication rates from thyroidectomy are higher in children than in adults [17, 18]. Moreover, children are more sensitive to the effects of ionizing radiation and have a longer life expectancy during which the adverse effects of I-131 radiotherapy might manifest. Although death from differentiated thyroid cancer is low, recent studies reveal an increase in all-cause mortality for childhood survivors of this disease, predominantly from the development of secondary malignancies in those treated with I-131 radiotherapy [11, 19,20,21,22,23,24]. Thus, awareness of the complications of treatment assumes increasing importance, making it imperative to balance risks against potential gains from increasingly aggressive therapies.
This review provides an overview of the current treatment practices and the recent ATA guidelines [15] as well as a discussion of several ongoing challenges in managing this group of patients.
Initial treatment of pediatric differentiated thyroid cancer
Surgery
Current ATA pediatric guidelines [15] suggest that definitive surgical treatment for differentiated thyroid cancer is total or near-total thyroidectomy with central neck dissection at the time of initial surgery in all children with nodal metastases. But is total thyroidectomy necessary in all cases? Performance of central neck dissection — which involves the therapeutic or prophylactic resection of Level VI compartment lymph nodes — is also controversial because it is associated with an increased risk of hypoparathyroidism. Finally, who is best qualified to perform thyroid surgery in children with differentiated thyroid cancer to avoid complications?
In addition to postoperative pain, complications of total thyroidectomy include recurrent laryngeal nerve damage with resultant vocal cord paralysis or dysphagia, and either transient or permanent parathyroid dysfunction. Not only do children undergoing thyroidectomy have higher endocrine-specific complications than adults (9.1% compared to 6.3%), but children ages 0–6 years fare worse than those ages 5–12 years or those age 13–17 years, with surgical complication rates of 22% compared to 15% and 11%, respectively [17]. However when total thyroidectomy in children was performed by a high-volume endocrine surgeon, defined as a surgeon who performs more than 30 cervical endocrine procedures per year, overall outcomes were optimized and the risk of parathyroid dysfunction decreased to less than 2% [18, 25].
In adults, there are data correlating the size of the primary tumor with the risk of advanced disease features as well as with prognosis [26, 27]. Consequently, the 2015 ATA management guidelines for adults with thyroid nodules and differentiated thyroid cancer [28] state that in select cases a hemithyroidectomy is considered sufficient for those without metastases and with primary tumors <4 cm, and further that a lobectomy can be considered for low-risk patients without metastases whose tumors are less than 1 cm. However, for multifocal, bilateral or advanced thyroid cancer (infiltration of surrounding tissues, local or distant metastases), total thyroidectomy is mandated. Unfortunately, studies correlating primary tumor size with risk are not available in children. Rather, limited data in children who underwent less than total thyroidectomy demonstrated that not having a total thyroidectomy was one of the greatest risk factors for developing recurrent disease, increasing the relative risk of relapse by a factor of 10 in some cases [14, 29, 30]. Radical surgery was estimated to be the most significant factor for disease-free survival in this population [14].
Until prospective data on the safety and efficacy of lobectomy for selective use in children with low-risk pediatric differentiated thyroid cancer become available, total thyroidectomy is the recommended treatment in all children with this disease. Not only are children at increased risk of bilateral (30%) and multifocal (65%) disease but, as previously stated, they are at increased risk of recurrence necessitating a second surgery if a total or near-total thyroidectomy was not initially performed [11, 14, 15]. Further, surgical re-intervention for recurrent disease is associated with a higher complication rate in those initially treated with lobectomy compared to those initially treated with total thyroidectomy [31].
The performance of a total thyroidectomy allows for the use of I-123 or I-131 to detect and I-131 to treat remnant thyroid tissue, local and distant metastases. Serum thyroglobulin levels for the detecting residual or recurrent disease are more sensitive when all normal thyroid tissue has been removed or ablated with I-131 [8].
In addition to decreasing the risk of residual or recurrent locoregional disease, the performance of central neck dissection in those with nodal metastases decreases overall disease burden, which might increase the efficacy of subsequent I-131 treatment [16]. On the other hand, performance of central neck dissection increases the risk of developing hypoparathyroidism. While the extent of initial surgery has the greatest effect on improving long-term disease-free survival, it is important to balance the potential benefit of achieving surgical remission against the risks of more aggressive surgical procedures.
Radioiodine therapy: A risk-adaptive approach
The goals of I-131 radiotherapy are two-fold: (1) the ablation of remnant thyroid tissue following total thyroidectomy to facilitate disease surveillance with thyroglobulin levels, imaging or both; and (2) the treatment of residual thyroid cancer or its metastases. But the utility of postoperative I-131 radiotherapy remains controversial. Studies of both adults and children with advanced differentiated thyroid cancer who received postoperative I-131 radiotherapy showed improved survival, decreased disease progression and lower recurrence rates compared to those who did not receive I-131 treatment [30, 32,33,34,35,36,37]. While there is general agreement that I-131 radiotherapy should be used to treat residual disease not amenable to surgical resection as well as iodine-avid metastatic disease [36, 37], I-131 radiotherapy has not been proved to clearly benefit those with low-risk thyroid cancer after a complete surgical resection [38, 39]. Data show that while the use of radioiodine therapy in adults younger than 45 years with low-risk differentiated thyroid cancer increased from 3.3% to 38% between 1973 and 2007, overall survival rates remained constant and there has been an increasing incidence of secondary cancers in those with low-risk disease treated with I-131 radiotherapy [40]. Although one of the most serious sequelae is the development of secondary malignancies [11, 19,20,21,22,23,24,25], it is not the only adverse effect of I-131 radiotherapy. Acute adverse effects occurring at the time of treatment include mild nausea or emesis from radiation gastritis in approximately 50%, acute sialadenitis in as many as 30% and painful swelling of remnant disease or of metastases in 10–20% [33, 37, 41]. Transient, mild leukopenia and thrombocytopenia can occur in up to 66% of patients about 4–6 weeks post-therapy but blood counts typically normalize by 3 months [37, 42]. Secondary malignancies aside, there are numerous other late effects of I-131 radiotherapy (Table 1). These include chronic sialadenitis leading to xerostomia, which can have a negative effect on a person’s quality of life [37, 41, 43]. Females can experience short-term menstrual irregularities and transient amenorrhea, and while permanent infertility has not been reported, birth defects can occur in children conceived in the first 6 months following I-131 treatment [44,45,46]. In males, transient elevation of follicle-stimulating hormone, azoospermia, oligospermia and decreased spermatogenesis without effect on testosterone production have been reported [47, 48]. Therefore, ATA pediatric guidelines [15] recommend that attempts at conception be avoided for at least 4 months following I-131 radiotherapy in males and 12 months in females. Sperm banking should be considered when cumulative I-131 activities are expected to exceed 400 mCi (14.8 GBq). Finally, although rare, radiation pneumonitis and subsequent radiation fibrosis occur in approximately 3%. In people with differentiated thyroid cancer and lung metastases, the risk of pulmonary fibrosis correlates with the intensity of I-131 uptake on whole-body imaging and varies inversely with patient age. As many as 10% of children with lung metastases treated with I-131 radiotherapy develop pulmonary fibrosis compared with only 1% of adults [33, 37, 41]. As a result, dosimetry is recommended to guide I-131 radiotherapy in children with extensive pulmonary metastases.
Both the prevalence and the severity of adverse treatment effects appear to correlate with the cumulative activity of I-131 received [16]. Given the excellent prognosis as well as the life expectancy of children and adolescents with differentiated thyroid cancer, these side effects — particularly the risk of developing a secondary malignancy — must be strongly considered when contemplating treatment with radioactive iodine.
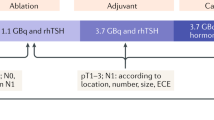
To maintain the low disease-specific mortality currently experienced by children with differentiated thyroid cancer as well as to reduce the potential complications of therapy, the ATA Pediatric Task Force on Thyroid Nodules and Differentiated Thyroid Cancer attempted to prospectively identify children in whom I-131 therapy would be indicated while limiting potential overtreatment in those unlikely to benefit. The task force defined three pediatric risk-stratification groups, based on the use of the tumor–lymph node–metastases (TMN) classification system. These pediatric risk-stratification groups do not define the risk of disease mortality in those with differentiated thyroid cancer but rather identify children at risk for persistent cervical disease; these groups are used to determine who should undergo postoperative staging for distant metastases and potential radioiodine treatment [15]. The ATA pediatric low-risk group [15] includes those in whom disease is confined to the thyroid gland with either no or non-assessed regional lymph node metastases (N0; NX) or children with incidental N1a nodal micro-metastases. Children in this group are at lowest risk for distant metastases (Fig. 2). The ATA pediatric intermediate-risk group [15] includes children with extensive metastases to Level VI nodes (N1a) or minimal metastases to unilateral, bilateral or contralateral levels I, II, III, IV or V cervical or superior mediastinal nodes (N1b). ATA pediatric intermediate-risk group patients are at low risk for distant metastases but at increased risk of incomplete resection and persistent cervical disease. The ATA pediatric high-risk group [15], children with regionally extensive (N1b) or locally invasive T4 tumors, are at highest risk for incomplete resection, persistent disease, and distant metastases. The ATA pediatric guidelines not only define these patient groups but detail initial postoperative staging, suggested thyroid stimulating hormone (TSH) goals, and surveillance management strategies for those with no evidence of disease following initial treatment [15].
Papillary thyroid carcinoma, American Thyroid Association (ATA) pediatric low-risk group. Imaging in a 16-year-old girl with Graves disease referred for I-131 ablation. a, b Longitudinal gray-scale (a) and color Doppler (b) images from a thyroid ultrasound, performed to estimate gland volume to determine I-131 treatment dose, reveal a hyperechogenic, well-defined solid nodule with a peripheral halo measuring 6×4 mm in the right lobe (a); there are no calcifications. The nodule did not demonstrate hypervascularity on Doppler ultrasound (b). The thyroid gland was heterogeneously echogenic and hypervascular, consistent with the diagnosis of Graves disease. The girl and family elected to proceed to total thyroidectomy. Pathology was consistent with papillary thyroid carcinoma, follicular variant (pathological classification pT1a). In accordance with the ATA pediatric guidelines, post-surgical I-123 whole-body staging scan was not indicated here; such cases can be followed with neck ultrasound and serial suppressed thyroglobulin levels
Previously, all children with differentiated thyroid cancer underwent post-surgical whole-body staging with either I-131 or, preferably, I-123. The recent ATA pediatric guidelines [15] suggest that the postoperative whole-body staging scan as well as radioiodine therapy be omitted for children in the pediatric low-risk group (Fig. 2). Rather, these children are initially assessed and subsequently monitored for persistent or recurrent disease with neck ultrasound and serial TSH-suppressed thyroglobulin levels following total thyroidectomy. On the other hand, children in either the pediatric intermediate or high-risk groups [15] undergo initial staging with a TSH-stimulated thyroglobulin level and an I-123 whole-body scan, preferably with single-photon emission computed tomography (SPECT)/CT of the neck (Fig. 3) following total thyroidectomy and central node dissection. In those with negative antithyroglobulin antibodies, the decision to administer I-131 therapy in the pediatric intermediate and high-risk groups is based on the results of these two studies, according to an algorithm presented in the ATA pediatric guidelines [15].
Multifocal papillary thyroid carcinoma with osseous metastases, American Thyroid Association pediatric high-risk group. An 18-year-old woman presented with left-side neck swelling. a Transverse gray-scale image from a thyroid ultrasound demonstrates a large nodule with fine calcifications in the left lobe (arrow) and isthmus of the thyroid gland. Multiple enlarged solid lymph nodes with fine calcifications and chaotic vascularity at left levels II, III and IV; right Level IV as well as Level VI (not shown) were also present, findings highly suspicious for metastatic papillary thyroid carcinoma. The diagnosis was confirmed on fine-needle aspiration of the left lobe mass. CT of the chest with intravenous contrast agent (not shown) also demonstrated Level VII (left paratracheal) lymphadenopathy but no lung metastases. The woman underwent total thyroidectomy, central neck dissection, and extended bilateral neck dissection. Pathology was consistent with metastatic multifocal papillary thyroid carcinoma, with extrathyroidal extension, lymphatic invasion, positive margins and 20/27 lymph nodes positive for carcinoma (pathological classification pT2 or T3 pN1b). b–f Anterior (b) and posterior (c) whole-body images from the post-surgical staging I-123 scan demonstrate three foci of radiotracer uptake in the head and neck (arrows, b). The intense focus seen in the posterior view (arrow, c) is a clue to the presence of cervical osseous metastatic disease. On axial fused single-photon emission computed tomography (SPECT)/CT images (d–f), abnormal foci of radiotracer uptake identified on whole-body imaging correspond to the right lateral aspect of the bony nasal bridge (arrow, d), left lingual tonsil (e) and C5 vertebral body (f). Stimulated thyroglobulin level was at 77 ng/mL. The woman underwent outpatient I-131 radiotherapy, receiving 202 mCi of I-131 orally. g, h Anterior (g) and posterior (h) whole-body images from a post-treatment scan obtained 7 days following I-131 administration demonstrate no additional metastatic foci. SPECT/CT of the thyroid bed is highly recommended to evaluate uptake on I-123 whole-body staging imaging. In this case, if SPECT/CT had not been obtained, the metastatic foci in the lingual tonsil and cervical spine might have been erroneously attributed to left Level II lymph node metastasis and uptake in the thyroid bed, respectively, resulting in administration of a lower than optimal treatment dose of I-131
According to the ATA pediatric guidelines [15], I-131 radiotherapy is not indicated in children in the pediatric intermediate- or high-risk groups who have no or minimal I-123 uptake in the thyroid bed and a stimulated thyroglobulin level <2 ng/mL unless the child had a T4 tumor or known residual microscopic cervical disease (Fig. 4). In those with no or minimal I-123 uptake in the thyroid bed but with a stimulated thyroglobulin level of 2–10 ng/mL, either I-131 therapy with post-treatment scan, LT4 suppression, or both can be considered (Fig. 5; [49]). I-131 radiotherapy is recommended in those with no or minimal I-123 uptake in the thyroid bed but with a stimulated thyroglobulin level >10 ng/mL as well as in those with distant metastases but no cervical uptake outside the thyroid bed. Finally, if there is cervical uptake outside the thyroid bed, either with or without distant metastases, then anatomical imaging should be obtained to identify residual disease amenable to surgical resection. If no such disease is identified, then I-131 therapy with a post-treatment scan is recommended. Post-treatment scans at 4–10 days following I-131 radiotherapy are highly recommended because there is a dose-related sensitivity of I-131 in disease detection, with new or additional lesions identified in up to 46% of cases [8, 9, 15] (Fig. 6; [50]).
Multifocal papillary thyroid carcinoma, American Thyroid Association (ATA) pediatric intermediate-risk group. a A 17-year-old girl presented with three thyroid nodules detected on neck ultrasound performed at an outside hospital, two in the right lobe (longitudinal gray-scale ultrasound [US] image, arrows) and a third in the left lobe (not shown). The nodules were heterogeneously hypoechoic and ill-defined and contained microcalcifications; the largest nodule, in the inferior right lobe, measured 2×1 cm. Initial fine-needle aspiration (FNA) demonstrated chronic thyroiditis. A second FNA performed 18 months later because of interval increase in size of the nodules demonstrated papillary thyroid carcinoma. The girl underwent total thyroidectomy and bilateral central neck dissection. Pathology was consistent with multifocal papillary thyroid carcinoma, classic type, with 3/9 central compartment lymph nodes positive for carcinoma (pathological classification pT1bN1a), in a background of lymphocytic thyroiditis. b Anterior whole-body image from the I-123 postsurgical scan demonstrates two foci of radiotracer uptake in the lower neck (arrow). According to the ATA pediatric guidelines, children in the intermediate- and high-risk groups undergo initial staging with a thyroid stimulating hormone (TSH)-stimulated thyroglobulin level and an I-123 whole-body scan following total thyroidectomy and central lymph node dissection. The decision to administer I-131 in these the two groups is based on the results of these tests. Although the stimulated thyroglobulin level was low (1 ng/mL), after discussion with the girl and her oncologist, an ablative I-131 dose was administered. Alternatively, this girl could have been managed with levothyroxine suppression and surveillance with neck ultrasound and suppressed thyroglobulin levels. c Anterior whole-body image from an I-131 post-treatment scan obtained 7 days following the administration of 101 mCi of I-131 demonstrates a single confluent focus of intense radiotracer uptake in the lower neck without distant metastases
Multifocal papillary thyroid carcinoma, American Thyroid Association pediatric high-risk group. A 14-year-old boy presented with a right supraclavicular mass. a, b Neck ultrasound demonstrates that the mass corresponds to an enlarged mixed cystic and solid supraclavicular lymph node (a, transverse image). In addition, the thyroid gland was enlarged with multifocal, mixed and solid masses (b, transverse image) and bilateral metastatic cervical lymph nodes (not shown). c, d Contrast-enhanced CT of the neck and chest confirm multifocal cystic and solid thyroid masses, with microcalcifications, retrosternal extension and extensive lateral and central lymphadenopathy (c, coronal image). The lymph nodes were both cystic and solid (arrows in c). Axial CT in lung window (d) demonstrates a single pleural-based nodule in the right middle lobe (arrow). Ultrasound-guided fine-needle aspiration of several of the abnormal lymph nodes as well as of the thyroid masses was consistent with metastatic papillary thyroid carcinoma. The boy underwent total thyroidectomy and bilateral complete neck dissections. Pathology confirmed multifocal papillary thyroid carcinoma, with 27/55 lymph nodes positive for carcinoma (pathological classification pT2N1b). e, f Anterior whole-body image from the I-123 postsurgical staging scan demonstrates three foci of radiotracer uptake in the lower neck, better demonstrated on oblique spot view (circle, f). Stimulated thyroglobulin level was 8.8 ng/mL. The boy was treated with I-131 radiotherapy. g Anterior whole-body image from a post-treatment scan obtained 7 days following I-131 radiotherapy demonstrates more intense radiotracer uptake in the previously identified sites of disease in the neck. Additionally, there was new intense uptake in the mediastinum demonstrated on lateral spot view (not shown) to represent physiological thymic uptake seen in up to 25% of children with differentiated thyroid cancer [49]. Diffuse liver uptake is a common finding on post-therapy scans and should not be mistaken for metastatic disease unless hepatic uptake is focal
Diffuse sclerosing papillary thyroid carcinoma with lung metastases, American Thyroid Association pediatric high-risk group. a An 8-year-old boy with history of medulloblastoma diagnosed at age 3 years and treated with gross total resection, chemotherapy and radiation therapy was noted to have an enlarged thyroid gland (arrows) on surveillance MRI of the spine (a, axial post-contrast T1-weighted image). b Transverse gray-scale ultrasound of the neck demonstrates a diffusely enlarged and heterogeneously echogenic thyroid gland with microcalcifications (arrow) and bilateral metastatic cervical lymph nodes (not shown). Fine-needle aspiration of the left lobe of the thyroid was reported as highly suspicious for papillary thyroid carcinoma. Non-contrast-enhanced CT of the chest (not shown) demonstrated multiple bilateral pulmonary nodules measuring up to 4 mm. The boy underwent total thyroidectomy with central neck, paratracheal and bilateral lateral modified radical neck dissections. Pathology was consistent with diffuse sclerosing variant of papillary thyroid carcinoma with angioinvasion, lymphatic invasion, perineural invasion and extensive extrathyroidal extension; 14/18 lymph nodes were positive for disease involvement. Oncoplex analysis of the tumor showed ALK-STRN fusion, a genetic mutation that has been reported in papillary thyroid carcinoma and that is predictive of crizotinib sensitivity. c Anterior whole-body image from the postsurgical dosimetric staging I-131 whole-body scan demonstrates two foci of radiotracer uptake in the neck and in diffuse pulmonary metastases (arrows). Recombinant thyroid stimulating hormone (rTSH)-stimulated thyroglobulin level was 330 ng/mL. The boy underwent I-131 radiotherapy. d Anterior whole-body image from a post-treatment scan obtained 7 days following I-131 administration demonstrates a similar distribution of metastatic disease, although pulmonary metastatic involvement is more conspicuous (arrows). Post-therapy scans demonstrate more disease in as many as 46% of patients [50]. e One year after I-131 radiotherapy, rTSH-stimulated thyroglobulin level remained markedly elevated and anterior whole-body I-123 scan demonstrates persistent but decreased uptake in the neck with resolution of the diffuse lung uptake. The boy was offered crizotinib therapy or a second round of I-131 radiotherapy
Patient preparation for postoperative staging and potential I-131 radiotherapy
More extensive patient preparation is necessary when performing an I-123 postoperative staging whole-body scan for differentiated thyroid cancer compared to I-123 imaging of benign thyroid disease. Thyrotropin levels above 30 mU/L are considered necessary not only for I-123 postoperative imaging but also when performing I-131 remnant ablation or I-131 therapy, when deemed appropriate. To achieve these high TSH levels, levothyroxine (LT4) should be discontinued for 3–6 weeks prior to planned staging I-123 whole-body imaging or I-131 radiotherapy although supplemental triiodothyronine (T3) can be administered up to 2 weeks prior. Because children are more sensitive to the effects of hypothyroidism than adults, the off-label use of intramuscular administration of recombinant human thyrotropin stimulation (rhTSH) — as opposed to performing thyroid hormone withdrawal as part of the preparative regimen for imaging — results in an unimpaired quality of life [51,52,53,54,55]. While rhTSH results in a lower radiation exposure to the body related to faster peripheral I-131 clearance in the euthyroid peripheral metabolic state [5], limited available data suggest that when used for I-131 radiotherapy in those with persistent local or metastatic disease, rhTSH might not result in an equally high radiation absorbed dose to the tumor compared to the hormone withdrawal method [16].
A low-iodine diet instituted 2 weeks prior to postoperative imaging as well as prior to I-131 radiotherapy has been shown to be effective in lowering the blood concentration of stable iodine, which competes with I-131 uptake [56, 57]. The use of a low-iodine diet increases the effectiveness of I-131 treatment by increasing I-131 uptake in remnant thyroid tissue as well as in metastases and is recommended to maximize therapeutic efficacy [15, 16]. Information regarding low-iodine diets can be found at Thyca.org.
If preoperative staging evaluation has included the use of intravenous iodinated contrast agent, it has been advised to wait 8–12 weeks before performing post-surgical I-123 whole-body staging or administering therapeutic I-131; however, a recent publication presents data that suggest that this interval can be as short as 4 weeks and that current guidelines be revisited [58].
Selection strategies for determining I-131 treatment activities
There are no standard administered activities for radioiodine treatment of children with differentiated thyroid cancer. There are two approaches to dose selection: empirical dosing in which fixed I-131 activities are administered that are sometimes based on patient weight, or the use of dosimetry in which whole-body and blood iodine clearance measures in addition to tumor surveys provide estimates of maximum tolerated doses to critical organs (bone marrow, lungs) [59, 60] or estimates of doses for maximal treatment efficacy [61, 62]. While the ATA pediatric guidelines [15] do not recommend for or against either approach; they do recommend that all I-131 treatment activities be calculated by experts with experience in dosing children.
At our institution, we typically use a risk-adaptive empiric strategy in which fixed I-131 activities, based on adult guidelines and adjusted for patient weight and additional safety factors dependent on patient age, are administered for initial I-131 treatments with some exceptions [8, 9]. We reserve dosimetry for younger children (those younger than 10 years); those who have undergone prior chemotherapy or radiotherapy or in whom thyroid cancer is a secondary malignancy (Fig. 6); those with extensive distant or pulmonary metastases; or when cumulative doses approach 250–500 mCi (9.3–18.5 GBq). Verburg and others [16, 42, 63,64,65], based on a strong body of literature, suggested that the dosimetric approach might be of greater benefit than administering fixed activities in those with advanced disease. However, determination of treatment activities is confounded by highly variable patient-specific factors such as I-131 avidity of tumor tissue, and tumor size and shape, among others, which might be as or more important than the administered activity for effective treatment [65].
Issues such as the management of known or suspected residual or recurrent disease identified 6–12 months after completion of initial treatment based on suppressed thyroglobulin levels, and the management of children with known distant metastatic disease following therapeutic I-131 radiotherapy, or of those with suspected or known non-iodine-avid disease, is beyond the scope of this manuscript. However, when a pediatric patient with known or suspected non-iodine-avid disease is encountered based on post-thyroidectomy I-123 whole-body scans, ultrasound, surgical, pathological, or clinical findings, imaging with fluorine-18 fluorodeoxyglucose (F-18 FDG) positron emission tomography (PET)/CT is recommended.
Surveillance and long-term follow-up for each of the ATA pediatric risk groups includes periodic physical examinations, neck ultrasound and laboratory testing of suppressed thyroglobulin levels. TSH-stimulated I-123 or I-131 whole-body imaging is no longer a routine part of these follow-up evaluations, except in very specific situations. The specific recommendations for TSH suppression and surveillance for each of the groups is well delineated in the ATA pediatric-specific guidelines [15].
Conclusion
Despite the aggressive nature of differentiated pediatric thyroid cancer, overall survival is excellent. However no treatment is without risk. It is imperative to be aware of the complications of treatment and balance these risks against potential gains from increasingly aggressive therapies and the very real lifelong possibility of recurrence.
References
Rivkees SA, Mazzaferri EL, Verburg FA et al (2011) The treatment of differentiated thyroid cancer in children: emphasis on surgical approach and radioactive iodine therapy. Endocr Rev 32:798–826
Pacini F (2002) Thyroid cancer in children and adolescents. J Endocrinol Invest 25:572–573
Hogan AR, Zhuge Y, Perez EA et al (2009) Pediatric thyroid carcinoma: incidence and outcomes in 1,753 patients. J Surg Res 156:167–172
Schlumberger MJ (1998) Papillary and follicular thyroid carcinoma. N Engl J Med 338:297–306
Dinauer CA, Breuer C, Rivkees SA (2008) Differentiated thyroid cancer in children: diagnosis and management. Curr Opin Oncol 20:59–65
Bauer AJ, Tuttle RM, Francis GL (2002) Differentiated thyroid carcinoma of children and adolescents. Endocrinologist 12:135–142
Waguespack SG, Wells SA (2007) Thyroid cancer. In: Bleyer AW, Barr RD (eds) Cancer in adolescents and young adults. Springer, New York, pp 259–270
Parisi MT, Mankoff D (2007) Differentiated pediatric thyroid cancer: correlates with adult disease, controversies in treatment. Semin Nucl Med 37:340–356
Parisi MT, Eslamy H, Mankoff D (2016) Management of differentiated thyroid cancer in children: focus on the American Thyroid Association pediatric guidelines. Semin Nucl Med 46:147–164
Vergamini LB, Frazier AL, Abrantes FL et al (2014) Increase in the incidence of differentiated thyroid cancer in children, adolescents, and young adults: a population-based study. J Pediatr 164:1418–1485
Hay ID, Gonzalez-Losada T, Reinalda MS et al (2010) Long-term outcome in 215 children and adolescents with papillary thyroid carcinoma treated during 1940–2008. World J Surg 34:1192–1202
LaQuaglia MP, Corbally MT, Heller G et al (1988) Recurrence and morbidity in differentiated thyroid cancer in children. Surgery 104:1149–1156
Newman KD, Black T, Heller G et al (1998) Differentiated thyroid cancer: determinants of disease progression in patients <21 years of age at diagnosis: a report from the Surgical Discipline Committee of the Surgical Committee of the Children’s Cancer Group. Ann Surg 227:533–541
Jarzab B, Handkiewicz-Junak D, Wloch J et al (2000) Multivariate analysis of prognostic factors for differentiated thyroid carcinoma in children. Eur J Nucl Med 27:833–841
Francis GL, Waguespack SG, Bauer AJ et al (2015) Management guidelines for children with thyroid nodules and differentiated thyroid cancer. The American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer. Thyroid 25:716–759
Verburg FA, Van Santen HM, Luster M (2017) Pediatric papillary thyroid cancer: current management challenges. Onco Targets Ther 10:165–175
Sosa JA, Tuggle CT, Wang TS et al (2008) Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrinol Metab 93:3058–3065
Tuggle CT, Roman SA, Wang TS et al (2008) Pediatric endocrine surgery: who is operating on our children? Surgery 144:869–877
Rubino C, de Vathaire F, Dottorini ME et al (2003) Second primary malignancies in thyroid cancer patients. Br J Cancer 89:1638–1644
Subramanian S, Goldstein DP, Parlea L et al (2007) Second primary malignancy risk in thyroid cancer survivors: a systematic review and meta-analysis. Thyroid 17:1277–1288
Brown AP, Chen J, Hitchcock YJ et al (2008) The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 93:504–515
Sawka AM, Thabane L, Parlea L et al (2009) Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid 19:451–457
Molenaar RJ, Sidana S, Radiviyevitch T et al (2018) Risk of hematologic malignancies after radioiodine treatment of well-differentiated thyroid cancer. J Clin Oncol 36:1831–1839
Verburg FA, Giovanella L, Iakovou et al (2018) I-131 as adjuvant treatment for differentiated thyroid carcinoma may cause an increase in the incidence of secondary haematological malignancies: an “inconvenient” truth. Eur J Nucl Med Mol Imaging 45:2247–2249
Bargen AE, Meyer-Rochow GY, Delbridge LW et al (2009) Outcomes of surgically managed pediatric thyroid cancer. J Surg Res 156:70–73
Verburg FA, Mader U, Luster M, Reiners C (2009) Primary tumor diameter as a risk factor for advanced disease features of differentiated thyroid carcinoma. Clin Endocrinol 7:291–297
Machens A, Holzhausen HJ, Dralle H (2005) The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma A comparative analysis. Cancer 103:2269–2273
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133
Mihailovic J, Nikoletic K, Srbovan D (2014) Recurrent disease in juvenile differentiated thyroid carcinoma: prognostic factors, treatments and outcomes. J Nucl Med 55:710–717
Handkiewicz-Junak D, Wloch J, Rosko J et al (2007) Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med 48:879–888
Bingol-Kologlu M, Tanyel FC, Senocak ME et al (2000) Surgical treatment of differentiated thyroid cancer in children. Eur J Pediatr Surg 10:347–352
Mazzaferri EL, Jhiang SM (1994) Long-term impact of initial surgery and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428
Reiners C, Farahati J (1999) 131I therapy of thyroid cancer patients. Q J Nucl Med 43:324–335
Mazzaferri EL, Kloos RT (2001) Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86:1433–1441
Reiners C, Biko J, Haenscheid H et al (2013) Twenty-five years after Chernobyl: outcome of radioiodine treatment in children and adolescents with very high-risk radiation-induced differentiated thyroid carcinoma. J Clin Endocrinol Metab 98:3039–3048
Robbins RJ, Schlumberger MJ (2005) The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med 46:28S–37S
Van Nostrand D (2009) The risks and benefits of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 19:1381–1391
Schlumberger M, Catargi B, Borget I et al (2012) Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med 366:1663–1673
Jonklaas J, Cooper DS, Ain KB et al (2010) Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid 20:1423–1424
Iyer NG, Morris LGT, Tuttle M et al (2011) Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer 117:4439–4446
Clement SC, Peeters RP, Ronckers CM et al (2015) Immediate and long-term adverse effects of radioiodine therapy for differentiated thyroid carcinoma — a systematic review. Cancer Treat Rev 41:925–934
Verburg FA, Hanscheid H, Biki J et al (2010) Dosimetry-guided high-activity (131)I therapy in patients with advanced differentiated thyroid carcinoma: initial experience. Eur J Nucl Med Mol Imaging 37:896–903
Walter MA, Turtschi CP, Schindler C et al (2007) The dental safety profile of high-dose radioiodine therapy for thyroid cancer: long term results of a longitudinal cohort study. J Nucl Med 48:1620–1625
Vini L, Hyer S, Al-Saadi A et al (2002) Prognosis for fertility and ovarian function after treatment with radioiodine for thyroid cancer. Postgrad Med J 78:92–93
Sawka AM, Lakra DC, Lea J et al (2008) A systemic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female cancer survivors. Clin Endocrinol 69:479–490
Garsi JP, Schlumberger M, Rubino C et al (2008) Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to the ovaries and outcome of pregnancies. J Nucl Med 49:845–852
Sawka AM, Lea J, Alshehri B et al (2008) A systematic review of the gonadal effects of therapeutic radioactive iodine in male thyroid cancer survivors. Clin Endocrinol 68:610–617
Hyer S, Vini L, O’Connell M et al (2002) Testicular dose and fertility in men following I (131) therapy for thyroid cancer. Clin Endocrinol 56:755–758
Mostafa M, Vali R, Chan J et al (2016) Variants and pitfalls on radioiodine scans in pediatric patients with differentiated thyroid carcinoma. Pediatr Radiol 46:1579–1589
Spies WG, Wojtowicz CH, Spies SM et al (1989) Value of post-therapy whole-body I-131 imaging in the evaluation of patients with thyroid carcinoma having undergone high-dose I-131 therapy. Clin Nucl Med 14:793–800
Schroeder PR, Haugen BR, Pacini F et al (2006) A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J Clin Endocrinol Metab 91:878–884
Iorcansky S, Herzovich V, Qualey RR et al (2005) Serum thyrotropin (TSH) levels after recombinant human TSH injections in children and teenagers with papillary thyroid cancer. J Clin Endocrinol Metab 90:6553–6555
Hoe FM, Charron M, Moshang T Jr (2006) Use of recombinant human TSH-stimulated thyroglobulin level and diagnostic whole body scan in children with differentiated thyroid cancer. J Pediatr Endocrinol Metabol 19:25–30
Lau WF, Zacharin MR, Waters K et al (2006) Management of paediatric thyroid carcinoma: recent experience with recombinant human thyroid stimulating hormone in preparation for radioiodine therapy. Intern Med J 36:564–570
Luster M, Handkiewicz-Junak D, Grossi A et al (2009) Recombinant thyrotropin use in children and adolescents with differentiated thyroid cancer: a multicenter retrospective study. J Clin Endocrinol Metab 94:3948–3953
Sonenberg M (2002) Low-iodine diet in the treatment of differentiated thyroid cancer with radioactive iodine. Endocrine 17:141–143
Park JT, Hennessey JV (2004) Two-week low iodine diet is necessary for adequate patient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid 14:57–63
Sohn SY, Choi JH, Kim NK et al (2014) The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid 24:872–877
Benua RS, Cicale NR, Sonnenberg R, Rawson RW (1962) The relation of radioiodine dosimetry to results and side effects in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med 87:171–182
Van Nostrand D, Atkins F (2011) Pediatric differentiated thyroid cancer: can the prescribed activity of I-131 be increased? J Clin Endocrinol Metab 96:2401–2403
Maxon HR, Thomas SR, Hertzberg VS et al (1983) Relation between effective radiation dose and outcome if radioiodine therapy for thyroid cancer. N Engl J Med 309:937–941
Maxon HR, Englaro EE, Thomas SR et al (1992) Radioiodine-131 radiotherapy for well-differentiated thyroid cancer — a quantitative radiation dosimetric approach: outcome and validation in 85 patients. J Nucl Med 33:1132–1136
Klubo-Gwiezdzinska K, Van Nostrand D, Atkins F et al (2011) Efficacy of dosimetric versus empiric prescribed activity of 131I for therapy of differentiated thyroid cancer. J Clin Endocrinol Metab 96:3217–3225
Dorn R, Kopp J, Vogt H et al (2003) Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med 44:451–456
Hanscheid H, Lassmann M, Luster M et al (2006) Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH stimulation or hormone withdrawal. J Nucl Med 47:648–654
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parisi, M.T., Khalatbari, H., Parikh, S.R. et al. Initial treatment of pediatric differentiated thyroid cancer: a review of the current risk-adaptive approach. Pediatr Radiol 49, 1391–1403 (2019). https://doi.org/10.1007/s00247-019-04457-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04457-7