Abstract
Ischemic heart disease is the leading cause of death worldwide. There has been a continued search for better therapeutic strategies that would reduce myocardial ischemia/reperfusion injury. Remote ischemic preconditioning (rIPC) was first introduced in 1993 by Przyklenk et al who reported that brief regional occlusion-reperfusion episodes in one vascular bed of the heart render protection to remote myocardial tissue. Subsequently, different studies have showed that rIPC applied to the kidney, liver, mesentery, and skeletal muscle, have all exhibited cardioprotective effects. The main purpose of this chapter is to summarize the advances in understanding the molecular mechanisms of rIPC, including those related to oxidative stress. Detailed understanding of the pathways involved in cardioprotection induced by rIPC is expected to lead to the development of new drugs to reduce the consequences of prolonged ischemia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ischemic heart disease is the leading cause of death and disability worldwide. Therefore, novel therapeutic strategies are required to protect the heart against ischemia/reperfusion injury, preserve myocardial function, prevent heart failure, and improve clinical outcomes in patients with ischemic heart disease.
In 1993, Przyklenk et al. [1] demonstrated that brief cycles of ischemia/reperfusion of the circumflex coronary artery protected remote virgin myocardium in the left anterior descending coronary artery territory. This observation extended the concept of classical ischemic preconditioning (IPC) described by Murry et al. [2] in 1986, to protect the heart at a distance or ‘remote ischemic preconditioning’ (rIPC). The rIPC phenomenon has been described in different organs and tissues such as kidney and brain, emerging as a strategy of inter-organ protection against the effects of acute ischemia/reperfusion injury.
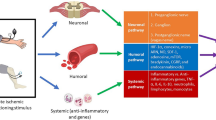
Thus, in the last 20 years, the concept of rIPC has evolved from being an experimental observation, whose underlying mechanisms continue to elude investigators, to a clinical application which offers the therapeutic potential to benefit patients with ischemic heart disease. Despite intensive investigation the mechanisms underlying rIPC remain unclear. The current hypothesis divides the mechanistic pathway of rIPC into three components (Fig. 17.1):
This figure shows the link between the neural pathway (green solid lines) and humoral pathway (broken red lines) in the mechanism of rIPC. Cycles of brief ischemia/reperfusion induced the local release of factors, which then activate local sensory afferent neurons. A study shown the participation of the neurons in the dorsal motor vagal nucleus (DMVN) in rIPC mechanism, this provides parasympathetic innervation of the left ventricle. The potential sites of cardioprotective factor(s) release include: (1) from the conditioned limb itself, (2) from the central nervous system, (3) from pre-/post-ganglionic parasympathetic nerve endings within the heart (broken green lines); and (4) from a non-conditioned remote organ/tissue receiving parasympathetic innervation
-
1.
Remote organ or tissue: rIPC stimulates the release of autacoids within the remote organ or tissue that activates a local afferent neural pathway [3].
-
2.
The connecting pathway: Different authors have described that the cardioprotective signal transference from the remote organ to the heart is the result of the action of humoral factors [4], neural pathways [5], or a neurohumoral interaction [6]. These hypotheses are not mutually exclusive and are probably part of the same mechanisms.
-
3.
Target organ: The protective factor activate an intracellular signaling pathways in the target organ which mediates the rIPC protective effects.
2 Neural Pathway of rIPC
The involvement of a neurogenic pathway in remote cardioprotection has been demonstrated by different authors [7, 8]. Pretreatment with the ganglion blocker hexamethonium abolished remote cardioprotection in rats through 15 min of mesenteric arterial occlusion [7]. Furthermore, rIPC activates a neural afferent pathway (Femoral and sciatic nerves and spinal cord) and the cardioprotective signal reaches the heart through the vagus nerve (efferent pathway) [9].
Thus, experimental and clinical studies have demonstrated that rIPC protection is dependent on an intact neural pathway to the remote organ or tissue with local resection of the neural pathway abolishing rIPC. The current paradigm has proposed that in response to the rIPC stimulus, autacoids such as adenosine [10], bradykinin [11] are produced in the remote organ or tissue resulting in the nitric oxide dependent stimulation of local afferent sensory nerves. In this sense, the administration of adenosine into the femoral artery resulted in the production of a cardioprotective plasma dialysate, in patients undergoing coronary angiography, confirming the findings in experimental animal studies that adenosine acted as a “trigger” for limb rIPC. On the other hand, it has suggested that the sensory arm of the neural pathway leading from the remote organ or tissue may be recruited by the activation of Transient receptor potential vanilloid (TRPV) [12]; and different experimental studies have demonstrated that the activation of these fibers by topical capsaicin or nociceptive stimuli can mimic the rIPC cardioprotection. However, the neural components of the pathway downstream of this sensory afferent neural via in the remote organ or tissue remain unclear. Jones et al. [13] described that cardioprotection elicited by peripheral nociception was blocked by spinal transection at T7 but not C7, suggesting that direct stimulation of cardiac nerves may be responsible for conveying the cardioprotective signal to the heart. Basalay et al demonstrated that rIPC activates a neural pathway, and the signal reaches the heart through the vagus nerves [14]. As we mentioned, and in accordance with the pioneer findings of the Gourine’s group, we showed that rIPC activates a neural afferent pathway (Femoral and sciatic nerves and spinal cord) and the cardioprotective signal reaches the heart through the vagus nerve (efferent pathway) and acetylcholine activates the classic ischemic preconditioning (IPC) phenomenon when acting on the muscarinic receptors [9]. However, the remotely activated signal transductions participating in the rIPC intracellular mechanism remain unclear.
3 Humoral Pathway of rIPC
The earliest experimental evidence for a blood-borne cardioprotective factor released by rIPC was provided in 1999 by Dickson et al. [4], who demonstrated that the cardioprotection elicited by IPC could be transferred via blood transfusion to a non-preconditioned animal . The existence of a circulating cardioprotective factor was first demonstrated in a model of porcine transplantation [15], where hind-limb preconditioning in an acceptor animal provided a significant cardioprotection to the subsequently transplanted and denervated donor heart. A similar type of study was performed by Kristiansen et al. [16] who demonstrated that hearts excised from a rat that had been received a rIPC protocol experienced a smaller infarct size, when it was subjected to a prolonged episode of ischemia and reperfusion on a Langendorff system . Subsequent studies confirmed the presence of a circulating factor and postulated a number of candidate, that including calcitonin gene-related peptide [17], opioids [18], endogenous cannabinoids [19], and hypoxia inducible factor-1α (HIF-1α) [20].
Although the actual identity of the factor remains unclear, some authors suggested that the factor may be a peptide <30 kDa [21]. In an isolated rabbit heart model [22], plasma from remotely preconditioned animals was cardioprotective when perfused into an isolated naïve heart. The authors concluded that the factor molecular weight is <15 kDa. Alternatively, the endogenous mediator may activate an afferent neural pathway within the remote organ to confer cardioprotection, as is the case with adenosine, bradykinin and CGRP.
Novel candidates for the blood-borne cardioprotective factors of rIPC were proposed, each with varying degrees of experimental evidence: including (1) stromal derived factor-1α or SDF-1α [23]; (2) exosomes [24]; nitrite [25]; (3) microRNA-144 [26]; (4) HIF-1α [20] Apolipoprotein α-I [27]. However, these studies have failed to demonstrate that the cardioprotective factor was actually responsible for the beneficial effect.
4 How Do the Neural and Humoral Pathways Interact to Mediate rIPC?
The neural and humoral pathways of rIPC could interact to mediate the protective effect, however the underlying mechanism of this relationship has not been describe (see Fig. 17.1 for a hypothetical scheme). Studies from Redington’s and Botker’s groups have suggested the link between these two pathways in the setting of rIPC. They use plasma dialysate harvested from animals or humans treated with rIPC to demonstrated a reduction in infarct size in a naïve animal hearts.
Redington et al. [28] have shown that the cardioprotective plasma dialysate can be produced in animals and human in response to neural stimulation that include direct nerve stimulation, transcutaneous electrical nerve stimulation, electro-acupuncture and topical capsaicin. Botker et al. [29] demonstrated that diabetic patients with a peripheral sensory neuropathy do not produce the cardioprotective plasma dialysate in response to rIPC protocol, when compared to diabetic patients without sensory neuropathy. Therefore, all this evidence suggests that the cardioprotective factor is produced downstream of the neural pathway. However, the question is: Where along the neural pathway is the cardioprotective factor released into the blood?; and which cell is responsible for its release?.
5 Myocardial Mechanisms of Remote Ischemic Preconditioning
Once the cardioprotective signal has been conveyed from the remote preconditioning organ to the heart, intracellular signal transduction mechanisms are recruited within cardiomyocytes . In this sense, some authors [6, 30] suggested that rIPC activates a signalling mechanism similar to that described for ischemic preconditioning (IPC), while others show that the cardioprotection conferred by rIPC follows a different pattern [31]. Recently, Heusch G carefully reviewed the signal transduction pathways involved in the different ischemic conditioning phenomena and noted that there are still some unsolved problems when studying the myocardial protection. Particularly, the absence of a temporal description of the cardioprotective signals involved [32].
A few years ago, Downey et al. proposed a classification of the signals participating in IPC that follows a logical/causal sequence of events and meets the temporal sequence of the preconditioning protocol [33, 34]. They defined a trigger as a factor released during the preconditioning ischemia periods that activates the cardioprotection phenomenon , and defined mediators as factors that transmit the cardioprotective signal during the prolonged myocardial ischemia to one or more end effectors, which are responsible for attenuating the irreversible injury during the lethal ischemic insult and/or during the subsequent reperfusion period. From this point of view, several studies evaluated the intracellular signalling pathway involved in the rIPC cardioprotection [35–37]. However, these authors have not considered the timing sequence of signalling activation.
In a recently study [38], we evaluated the signaling pathway that is activated at heart level, before myocardial ischemia. We hypothesized that pre-ischemic activation of muscarinic receptors induces Akt phosphorylation and through this pathway, the phosphorylation of the endothelial nitric oxide synthase (eNOS). As a consequence of a higher production of nitric oxide (NO), mitochondrial K+ ATP channels (mK+ ATP) open and mitochondrial production of hydrogen peroxide (H2O2) increases.
In order to demonstrate this hypothesis, we performed experiments in isolated rat hearts. As we expected, rIPC (Fig. 17.2) significantly reduce infarct size. The cervical vagal section (CVS) completely abolished the beneficial effect of rIPC, however the Sub-diaphragmatic vagal section (Sub-VS) did not modify the rIPC effect, thus demonstrating that denervation of other organs, different from the heart; do not contribute to the loss of rIPC.
Infarct size , expressed as a percentage of the left ventricular area. The rIPC significantly reduced the infarct size and this effect was abolished by the different treatments (CVS, L-NAME, 5-HD). rIPC remote ischemic preconditioning, CVS cervical vagal section, Sub-VS sub-diaphragmatic vagal section.*p < 0.05 vs. each other group
Since activation of muscarinic receptors can increase NO synthesis, we studied a possible involvement of NO in the observed infarct size reduction afforded by the rIPC. In this regard, administration of L-NAME, during the rIPC protocol, completely abolished the protective effect of rIPC, pointing out a central role of NO in the rIPC cardioprotection. Given that NO could induce the mK+ ATP channels to open, we administered 5-HD before the rIPC protocol. The mK+ ATP channels blocker completely abolished the effect of rIPC, thus providing evidence of an involvement of mK+ ATP channels in the rIPC.
Activation of the muscarinic receptors induces phosphorylation of Akt enzyme [39]. To address this issue in a rIPC group phosphrylation of Akt in cardiac tissue was determined at the end of the hindlimb preconditioning protocol (Fig. 17.3, Panel A). Importantly, rIPC induced a significant increase of the cardiac Akt phosphorylation, which was abolished by the CVS, in hearts that were not yet subjected to ischemia/reperfusion.
In Panel A, cardiac expression of phosphorylated Akt (Ser 473) can be observed in the Non-rIPC, rIPC and the rIPC + CVS groups, immediately after hindlimb rIPC. The rIPC induced a significant increase of the Akt phosphorylation, which was abolished by CVS. In Panel B of the same figure, expression of the phosphorylated eNOS (Ser 1177) can be observed. The rIPC induced a significant increase of the phosphorylation of this enzyme, which was abolished by CVS. *p < 0.05. rIPC remote ischemic preconditioning, CVS cervical vagal section
Since eNOS (Ser-1177) can be phosphorylated by Akt, we studied phosphor-eNOS expression. In these experiments, rIPC induced a significant increase in the phosphorylation of this enzyme, which was abolished by CVS, before the myocardial ischemia (Fig. 17.3, Panel B). Taken together, these results clearly indicate involvement of the Akt-eNOS pathway in the heart as triggers of the rIPC mechanism before the ischemia/reperfusion cardiac insult.
Reactive oxygen species (ROS ) have been shown to be toxic but also function as signalling molecules. It has been suggested that mitochondrial ROS production might play a relevant role in IPC [40], but this issue was not addressed in rIPC. Figure 17.4 (Panel A) shows a representative trace during an initial stabilization period of the reaction mixture and after the addition of isolated mitochondria from the following groups: Non-rIPC and rIPC. As it can be seen, an increased H2O2 release in the rIPC group is evident. Panel B shows that CVS, L-NAME and the mK+ ATP channels blockade with 5-HD attenuated the H2O2 release.
Panel A shows a representative trace during an initial stabilization period and after the addition of isolated mitochondria from Non-rIPC or rIPC rat hearts . Panel B shows the mitochondrial H2O2 production rate mean in the different study groups. CVS, L-NAME and 5-HD abolished the mitochondrial H2O2 production rate. rIPC remote ischemic preconditioning, CVS cervical vagal section. FL Fluorescence intensity. *p < 0.05
Clearly, rIPC induces activation of Akt enzyme and eNOS phosphorylation, mK+ ATP channels opening, and mitochondrial H2O2 production in the heart before the index myocardial ischemia. Therefore, they could be considered as rIPC triggers. In addition, the protective effect of rIPC was abolished by CVS but not by SVS, reinforcing the hypothesis of a parasympathetic vagal pathway .
Different authors have evaluated the possible intracellular mechanisms involved in rIPC pathway. However, most of these works have studied mechanisms at time points that are different from the ones used in this study: during early reperfusion [41], in late reperfusion [42], and also using different animal species [9, 43, 44], making difficult to establish a comparison. In agreement with our findings, Li SJ et al showed that rIPC induces the activation of the Pl3K/Akt and inhibition of GSK3β before myocardial ischemia [35]. However, the authors proposed that rIPC would induce release of a factor to the bloodstream, which activates the PI3K/Akt/GSK3β pathway at cardiac level, suggesting a myocardial mechanism similar to IPC. The results of our study support the existence of a vagal efferent pathway , and they extend the current knowledge by showing that rIPC could activate the Akt pathway. Furthermore, the denervation of other organs, different from the heart; do not contribute to the loss of rIPC, since that SVS did not abolish the rIPC cardioprotection.
Akt phosphorylation would lead to activation of the eNOS enzyme and, subsequently, to mK+ ATP channels opening and mitochondrial changes. The role of NO in the rIPC mechanism is difficult to evaluate because it could participate in the tissue or organ where the preconditioning stimulus is originated and/or at cardiac level as part of the cardioprotection signalling. In this last case, it is well known that the infusion of a drug able to increase the NO bioavailability puts the heart into a preconditioned state [45]. Our results demonstrate that the rIPC activation involves eNOS, since by administering L-NAME we abolished the protective effect, but more important they demonstrate that there is a cardiac eNOS phosphorylation immediately after the rIPC protocol, before myocardial ischemia. In addition, an increase in the NO production is capable of acting directly on the mitochondria inhibiting the respiratory chain and favoring ROS production [46], or activates the cGMP/PKG pathway, which was involved in the IPC cardioprotection mechanism.
Several authors showed that IPC induces mK+ ATP opening [47], however this fact has not been evaluated for rIPC. Our results shown that the administration of 5-HD abolished the rIPC protective effect, thus demonstrating an important role of these channels in rIPC. The mK+ ATP opening produces a higher K+ influx to the mitochondria, decreasing the mitochondrial membrane potential and leading to an increase of H2O2 mitochondrial productio n [40].
6 Role of Reactive Oxygen Species in rIPC Protection
ROS are thought to mediate the oxygen toxicity because of their greater chemical reactivity with regard to oxygen. They also operate as intracellular signalling molecules, a function that has been widely documented but is still controversial. In the setting of acute myocardial ischemia/reperfusion injury, oxidative stress plays a dual role. Its detrimental role is as a mediator of lethal reperfusion injury, however, its beneficial signalling role is believed to mediate the cardioprotective effects elicited by both IPC and postconditioning [48].
As mention, three components of rIPC can be distinguished: the signal generation, the transfer of the signal to the target organ, and its response to the transferred signal resulting in cardioprotection.
Weinbrenner et al. suggested a possible beneficial signalling role for ROS in the setting of rIPC. They showed that the administration of a free radical scavenger abolished the protection elicited by rIPC [49]. These results are in agreement with our findings in which rIPC induces a higher mitochondrial H2O2 production before the myocardial ischemia, and this effect is attenuates by the inhibition of the NO production with L-NAME and with the blockade of mK+ ATP channels with 5-HD.
In this regard, H2O2 could act as a second messenger of the rIPC protective signal. Besides, ROS production by the respiratory chain has been shown to activate mK+ ATP [50]. Furthermore, ROS generated by the mitochondria could activate other sensitive redox enzymes, among them, the PKCε, which is one of the most important kinases participating in the IPC mechanism [51].
It has been described in humans that a short episode of forearm ischemia/reperfusion performed with a blood pressure cuff is capable of modulating the composition of nitric oxide (NO•)/nitrite levels in the blood [52, 53]. Nitrite is not only the oxidation product of NO• but also a key reservoir for NO• in blood and cellular compartments [54, 55]. The half-life of nitrite in plasma is unknown but calculated for humans to be approximately 35 min [56].
In an elegant study, Rassaf et al. [57] evaluated in healthy volunteers whether the ischemic phase or the reactive hyperemia with the resulting shear stress during rIPC are responsible for the modulation of plasma nitrite levels. They determined that endothelial eNOS is responsible for nitrite generation during reactive hyperemia, which is then transported to the myocardium. In additional experiments, the authors assessed the response of the target organ to the transferred signal. Using myoglobin (Mb) knockout mouse, they demonstrated that the nitrite generated during reactive hyperemia is converted to bioactive NO• with subsequent modification of mitochondria by S-nitrosation, which then ultimately confer the cardioprotective effects [58]. Transfer experiments of plasma from healthy volunteers subjected to rIPC of the arm identified plasma nitrite as a cardioprotective agent in isolated Langendorff mouse heart preparations exposed to ischemia/reperfusion.
Finally, a higher H2O2 mitochondrial production before ischemia would protect the heart against an exacerbated production of ROS during reperfusion [39], being another important underlying mechanism of the rIPC protective effect. A scheme combining our results and current knowledge regarding the intracellular mechanisms activated by rIPC in the heart is depicted in Fig. 17.5.
Schematic illustration of the intracellular pathways activated by remote ischemic preconditioning before myocardial ischemia. Acetylcholine, released from cardiac vagal nerve endings, activates muscarinic receptors located in the cardiomyocyte plasma membrane, inducing the phosphorylation of Akt and eNOS enzymes. Subsequently, activation of soluble guanylate cyclase and protein kinase G could lead to mK+ ATP channels opening and increasing of H2O2 mitochondrial production. This way, H2O2 could act as a second messenger of the rIPC protective signal. Ach acetylcholine, eNOS endothelial nitric oxide synthase, NO nitric oxide, sGC soluble guanylate cyclase, PKG protein kinase G, mK + ATP mitochondrial K+ ATP channels
On the other hand, there is experimental evidence describing the protective properties of NO, despite the limitations of endocrine movement to a remote site. Endogenous NO seems to play a pivotal role in mechanism of rIPC in reducing liver damage, and this is abrogated by treatments with the NO scavenger carboxy-2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) and inhibited in the eNOS knockout mouse [59]. Tokuno et al. [55] have involved iNOS activation as a trigger for delayed rIPC of the heart using cerebral ischemia as preconditioning stimulus. The cardioprotective effect was seen 24 h later and was absent in iNOS knockout mice. Further studies demonstrated that NO is necessary for the development of ischemia-induced delayed protection against myocardial infarction [60]. Although it is clear that NOS and NO seem to participate in the process of rIPC, the mechanism for NO transport to a distant site and the nature of the endocrine rIPC mediator have remained unknown.
Rassaf et al. [57] evaluated the mechanism of rIPC and explore the possible identity of the circulating endocrine mediator. They first find in humans that the levels of plasma nitrite increase after brachial artery occlusion and release (reactive hyperemia). This is caused by eNOS activation with NO formation and oxidation to the more stable nitrite. The authors performed studies in mice and showed that nitrite levels increase. Inhibition of NO with cPTIO or in eNOS knockout mice prevents the rise in nitrite and rIPC effects on myocardial infarction. This association was confirmed by infusions of nitrite to match levels observed with rIPC. Finally, they infuse human plasma with and without rIPC into the isolated heart model of ischemia/reperfusion and showed that the increase nitrite account for effects.
Although these studies suggest that nitrite forms during rIPC and travels in the plasma to the heart, how is it then converted in the heart back into NO?. During ischemia, nitrite is reduced to NO and N2O3 by different nitrite reductase enzyme systems [61, 62]. Mitochondrial NO and S-nitrosothiols formed from nitrite dynamically and reversibly inhibit complex I during reperfusion, which limits ROS formation from complexes I and III. This ultimately prevents the opening of the mitochondrial permeability transition pore and the release of cytochrome c. It has been shown that the site of nitrosation is on cysteine 39 of the ND3 (NADH dehydrogenase, subunit 3) subunit of complex I. Several enzymes are required to convert nitrite into NO during organ ischemia. In the heart, deoxygenated myoglobin acts as a functional nitrite reductase. Nitrite-dependent NO formation is significantly decreased in myoglobin-deficient hearts and nitrite administration reduces myocardial infarction with abrogated effects in the myoglobin knockout mice. Rassaf et al. [57] showed that the effect of rIPC is inhibited in the myoglobin knockout mouse, providing additional evidence that the mediator factor of this effect is nitrite, which is produced in the extremity and travels in blood to the heart, where it is reduced by myoglobin to produce NO.
In a different experimental model of spinal cord ischemic injury, Dong et al. [63] showed that the beneficial effect of limb rIPC was attenuated by administration of a free-radical scavenger before rIPC. Additional, rIPC induced an increase in the activity of catalase and superoxide dismutase (SOD) in the serum. The increase in catalase and SOD activities was accompanied by a transient increase of serum malondialdehyde levels. The free radicals scavenger treatment abolished the increase in catalase and SOD activity induced by rIPC. This indicates that the increase in antioxidant enzyme activities is closely related to the ROS generated by rIPC.
7 Conclusions
Strong experimental evidence supports protection by rIPC from myocardial ischemia/reperfusion injury and other organs. However the mechanisms for local release of the protective signal at the remote site and the contributions of neuronal and humoral pathways are not yet clear, not only in signal release, but also in signal transfer to the target organ and protective signal transduction within the target organ. Thus, in this chapter we summarized the more recent advances in the molecular mechanisms of rIPC, particularly those related to oxidative stress.
A better understanding of the complex signaling involved in transduction of the rIPC signal from the remote organ and tissue to the protected target may allow the imminent discovery of novel pharmacological agents to directly activate the protective signaling pathways.
References
Przyklenk K, Bauer B, Ovize M, Kloner RA et al (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Wever KE, Warlé MC, Wagener FA, Van der Hoorn JW et al (2011) Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemia-reperfusion injury: the role of adenosine. Nephrol Dial Transplant 26:3108–3117
Dickson EW, Lorbar M, Porcaro WA, Fenton RA et al (1999) Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol 277:H2451–H2457
Mastitskaya S, Marina N, Gourine A, Gilbey MP et al (2012) Cardioprotection evoked by remote ischaemic preconditioning is critically dependent on the activity of vagal pre-ganglionic neurones. Cardiovasc Res 95:487–494
Hausenloy DJ, Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79:377–386
Kingma JG Jr, Simard D, Voisine P, Rouleau JR (2011) Role of the autonomic nervous system in cardioprotection by remote preconditioning in isoflurane-anaesthetized dogs. Cardiovasc Res 89:384–391
Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK et al (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:H1598–H1603
Donato M, Buchholz B, Rodríguez M, Pérez V et al (2013) Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. ExpPhysiol 98:425–434
Takaoka A, Nakae I, Mitsunami K, Yabe T et al (1999) Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning”. J Am Coll Cardiol 33:556–564
Schoemaker RG, van Heijningen CL (2000) Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol 278:H1571–H1576
Pickard J, Bøtker H, Crimi G, Davidson B et al (2015) Remote ischemic conditioning: from experimental observation to clinical application: report from the 8th Biennial Hatter Cardiovascular Institute Workshop. Basic Res Cardiol 110:453–467
Jones WK, Fan GC, Liao S, Zhang JM et al (2009) Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 120(11 Suppl):S1–S9
Basalay M, Barsukevich V, Mastitskaya S, Mrochek A et al (2012) Remote ischaemic pre- and delayed postconditioning similar degree of cardioprotection but distinct mechanisms. Exp Physiol 97:908–917
Skyschally A, Gent S, Amanakis G, Schulte C et al (2015) Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res 117:279–288
Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE et al (2005) Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol 288:H1252–H1256
Wolfrum S, Nienstedt J, Heidbreder M, Schneider K et al (2005) Calcitonin gene related peptide mediates cardioprotection by remote preconditioning. Regul Pept 127:217–224
Patel HH, Moore J, Hsu AK, Gross GJ (2002) Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol 34:1317–1323
Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P et al (2008) Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol 579:246–252
Kant R, Diwan V, Jaggi AS, Singh N et al (2008) Remote renal preconditioning-induced cardioprotection: a key role of hypoxia inducible factor-prolyl 4-hydroxylases. Mol Cell Biochem 312:25–31
Lang SC, Elsasser A, Scheler C, Vetter S et al (2006) Myocardial preconditioning and remote renal preconditioning–identifying a protective factor using proteomic methods? Basic Res Cardiol 101:149–158
Przyklenk K, Whittaker P (2011) Remote ischemic preconditioning: current knowledge, unresolved questions, and future priorities. J Cardiovasc Pharmacol Ther 16:255–259
Davidson SM, Selvaraj P, He D, Boi-Doku C, Yellon RL et al (2013) Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res Cardiol 108:377
Giricz Z, Varga ZV, Baranyai T, Sipos P et al (2014) Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 68:75–78
Rassaf T, Ferdinandy P, Schulz R (2014) Nitrite in organ protection. Br J Pharmacol 171:1–11
Li J, Rohailla S, Gelber N, Rutka J et al (2014) MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109:423
Hibert P, Prunier-Mirebeau D, Beseme O, Chwastyniak M et al (2013) Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS One 8:e77211
Redington KL, Disenhouse T, Strantzas SC, Gladstone R et al (2012) Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 107:241
Jensen RV, Stottrup NB, Kristiansen SB, Botker HE (2012) Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res Cardiol 107:285
Lim SY, Hausenloy DJ (2012) Remote ischemic conditioning: from bench to bedside. Front Physiol 3:27
Heinen NM, Pütz VE, Görgens JI, Huhn R et al (2011) Cardioprotection by remote ischemic preconditioning exhibits a signaling pattern different from local ischemic preconditioning. Shock 36:45–53
Heusch G (2015) Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res 116:674–699
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Downey JM, Krieg T, Cohen MV (2008) Mapping preconditioning's signaling pathways: an engineering approach. Ann NY Acad Sci 1123:187–196
Li SJ, Wu YN, Kang Y, Yin YQ et al (2010) Noninvasive limb ischemic preconditioning protects against myocardial I/R injury in rats. J Surg Res 164:162–168
Breivik L, Helgeland E, Aarnes EK, Mrdalj J et al (2011) Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol 106:135–145
Wolfrum S, Schneider K, Heidbreder M, Nienstedt J et al (2002) Remote preconditioning protects the heart by activating myocardial PKC epsilon-isoform. Cardiovasc Res 55:583–589
Donato M, Goyeneche MA, Garces M, Marchini T et al (2016) Myocardial triggers involved in activation of remote ischaemic preconditioning. Exp Physiol 101:708–716
Krieg T, Landsberger M, Alexeyev MF, Felix SB et al (2003) Activation of Akt is essential for acetylcholine to trigger generation of oxygen free radicals. Cardiovasc Res 58:196–202
Kalogeris T, Bao Y, Korthuis RJ1 (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2:702–714
Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A et al (2012) Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther 26:87–93
Dow J, Bhandari A, Simkhovich BZ, Hale SL et al (2012) The effect of acute versus delayed remote ischemic preconditioning on reperfusion induced ventricular arrhythmias. J Cardiovasc Electrophysiol 23:1374–1383
Brandenburger T, Huhn R, Galas A, Pannen BH et al (2014) Remote ischemic preconditioning preserves Connexin 43 phosphorylation in the rat heart in vivo. J Transl Med 12:228
Konstantinov IE, Li J, Cheung MM, Shimizu M et al (2005) Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation 79:1691–1695
Sun J, Aponte AM, Kohr MJ, Tong G et al (2013) Essential role of nitric oxide in acute ischemic preconditioning: S-nitros(yl)ation versus sGC/cGMP/PKG signaling? Free Radic Biol Med 54:105–112
Antunes F, Boveris A, Cadenas E (2004) On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc Natl Acad Sci U S A 101:16774–16779
Yao Z, Gross GJ (1993) Role of nitric oxide, muscarinic receptors, and the ATP-sensitive K+ channel in mediating the effects of acetylcholine to mimic preconditioning in dogs. Circ Res 73(6):1193–1201
D’Autréaux B, Toledano M (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824
Weinbrenner C, Schulze F, Sárváry L, Strasser RH (2004) Remote preconditioning by infrarenal aortic occlusion is operative via delta1-opioid receptors and free radicals in vivo in the rat heart. Cardiovasc Res 61:591–599
Tullio F, Angotti C, Perrelli MG, Penna C et al (2013) Redox balance and cardioprotection. Basic Res Cardiol 108:392
Fornazari M, de Paula JG, Castilho RF, Kowaltowski AJ (2008) Redox properties of the adenoside triphosphate-sensitive K+ channel in brain mitochondria. J Neurosci Res 86:1548–1556
Neye N, Enigk F, Shiva S, Habazettl H et al (2012) Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive Care Med 38:1381–1391
Birnbaum Y, Hale SL, Kloner RA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96:1641–1646
Gho BC, Schoemaker RG, Van den Doel MA, Duncker DJ et al (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200
Tokuno S, Hinokiyama K, Tokuno K, Löwbeer C et al (2002) Spontaneous ischemic events in the brain and heart adapt the hearts of severely atherosclerotic mice to ischemia. Arterioscler Thromb Vasc Biol 22:995–1001
Walsh SR, Tang T, Sadat U, Dutka DP et al (2007) Cardioprotection by remote ischaemic preconditioning. Br J Anaesth 99:611–616
Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G et al (2014) Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114:1601–1610
Chouchani ET, Methner C, Nadtochiy SM et al (2013) Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med 19:753–759
Abu-Amara M, Yang SY, Quaglia A, Rowley P et al (2011) Nitric oxide is an essential mediator of the protective effects of remote ischaemic preconditioning in a mouse model of liver ischaemia/reperfusion injury. Clin Sci (Lond) 121:257–266
Bolli R (2000) The late phase of preconditioning. Circ Res 87:972–983
Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC et al (2014) Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. J Biol Chem 289:10345–10358
Tejero J, Gladwin MT (2014) The globin superfamily: functions in nitric oxide formation and decay. Biol Chem 395:631–639
Dong HL, Zhang Y, Su BX, Zhu ZH et al (2010) Limb remote ischemic preconditioning protects the spinal cord from ischemia-reperfusion injury: a newly identified nonneuronal but reactive oxygen species-dependent pathway. Anesthesiology 112:881–891
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Donato, M., Paez, D.T., Evelson, P., Gelpi, R.J. (2016). Reactive Oxygen Species Are Involved in Myocardial Remote Ischemic Preconditioning. In: Gelpi, R., Boveris, A., Poderoso, J. (eds) Biochemistry of Oxidative Stress. Advances in Biochemistry in Health and Disease, vol 16. Springer, Cham. https://doi.org/10.1007/978-3-319-45865-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-45865-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45864-9
Online ISBN: 978-3-319-45865-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)