Abstract
Background
The mechanism underlying remote ischemic conditioning (RIC) remains unclear. We investigated whether RIC protects the heart through the activation of the adenosine receptor and the PI3K-Akt pathway at the onset of myocardial reperfusion.
Methods and results
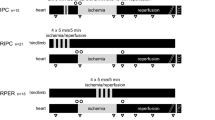
Domestic pigs (27–35 kg) were subjected to in situ left anterior descending coronary artery ischemia (60 min) followed by reperfusion (180 min) and randomised to the following: (1) Control- No additional intervention; (2) Remote ischemic preconditioning (RIPC)- Four-5 min cycles of lower limb ischemia/reperfusion were administered prior to myocardial ischemia; (3) RIPC + Wort or 8-SPT: Wortmannin (Wort 20 μg/kg, a PI3K inhibitor) or 8-sulfophenyltheophylline (8-SPT 10 mg/kg, an adenosine receptor inhibitor) were administered intravenously 30 s before myocardial reperfusion to RIPC-treated animals; (4) Remote ischemic perconditioning (RIPerC)- Four-5 min cycles of lower limb ischemia/reperfusion were applied 1 min before myocardial reperfusion; (5) RIPerC + Wort or 8-SPT: Wort or 8-SPT were given 30 s before myocardial reperfusion to RIPerC-treated animals. Both RIPC and RIPerC reduced myocardial infarct size (13.3 ± 2.2% with RIPC, 18.2 ± 2.0% with RIPerC versus 48.8 ± 4.2% in control:P < 0.05:N ≥ 5/group). Wortmannin abolished the infarct-limiting effects of RIPC (33.2 ± 6% with RIPC + Wort versus 13.3 ± 2.2% with RIPC:P < 0.05:N ≥ 5/group) but not RIPerC (18.0 ± 3.4% with RIPerC + Wort versus 18.2 ± 2.0% with RIPerC:P > 0.05:N ≥ 5/group). 8-SPT did not influence the infarct-limiting effects of either RIPC or RIPerC. Western blot analysis confirmed Wortmannin-sensitive PI3K and Akt activation at myocardial reperfusion in RIPC-treated hearts.
Conclusions
In the porcine heart, both RIPC and RIPerC both reduce myocardial infarct size and with RIPC but not RIPerC this cardioprotective effect is associated with the activation of the PI3K-Akt pathway at reperfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Remote ischemic conditioning (RIC) has emerged as a novel therapeutic strategy for protecting the heart against acute ischemia-reperfusion injury (IRI) in ischemic heart disease (IHD) patients undergoing either coronary artery bypass graft (CABG) surgery [7, 28, 30] or percutaneous coronary intervention (PCI) [14] and in those patients presenting with an acute myocardial infarction (AMI) [3, 23]. RIC of the heart describes the phenomenon in which the application of one or more brief cycles of non-lethal ischemia and reperfusion applied to an organ or tissue distal to the heart protects the myocardium against the detrimental effects of a sustained lethal episode of acute IRI [11, 13, 22]. Crucially, the RIC stimulus can be applied non-invasively to the upper or lower limb using a standard blood pressure cuff and can be instituted either prior to (remote ischemic preconditioning, RIPC), after the onset of myocardial ischemia (remote ischemic perconditioning, RIPerC) [24] or even at the time of myocardial reperfusion (remote ischemic postconditioning) [1]. The mechanistic pathways underlying RIC remain unclear with experimental studies suggesting a neuro-hormonal pathway linking the RIC stimulus to the heart where established myocardial pro-survival signalling pathways are activated [11, 13, 19]. Myocardial activation of the adenosine receptor and the downstream PI3K-Akt kinase pathway at the time of myocardial reperfusion is critical to direct myocardial preconditioning [8, 26] and postconditioning [29]. Whether this signalling pathway is also involved in RIPC or RIPerC is unknown and is investigated for the first time in this study.
Materials and methods
Porcine model of myocardial ischemia and reperfusion
All animals included in the study received proper care in compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of health. Approval from the Ethical Committee and the veterinary authorities of East Attica prefecture was obtained before this study was started.
Domestic Landrace pigs (27–35 kg) were fasted for 24 h before operation. All procedures were performed with animals under general anesthesia and have been previously published [15]. After premedication of the pigs with intramuscular ketamine (15 mg/kg) and midazolam (0.5 mg/kg), a catheter was inserted into a small ear vein. The pigs were intubated and ventilated mechanically with an Alpha Delta Siare electronic ventilator (15 ml/kg and 18–20 breaths per minute) and a gas mixture containing 40–45% oxygen. Induction of anesthesia was achieved with bolus intravenous injection of propofol (3 mg/kg), pancuronium (0.15 mg/kg) and fentanyl (17.5 μg/kg). Anesthesia, analgesia and muscle relaxation were maintained by administration of intravenous boluses of propofol (0.15 mg/kg) and pancuronium (0.07 mg/kg) every 20 min and fetanyl (15 μg/kg/h).
Through a right cervical incision the carotid artery was isolated, ligated, and the proximal portion cannulated. The catheter was connected to a transducer for continuous measurement of blood pressure. The left femoral artery was carefully prepared and a 2.0 silk suture was passed around the vessel to induce remote ischemia and reperfusion with the aid of a small piece of plastic tube and a clamp. Four 5 min-cycles ischemia/reperfusion applied before the index ischemia in all RIPC groups and 1 min before long reperfusion in all RIPerC groups. After median sternotomy and pericardiotomy the left anterior descending (LAD) coronary artery was exposed. A 2.0 silk suture was threaded through the myocardium around the mid-segment of the LAD. The two ends of the suture were passed through a 3-cm length of polyurethane tube to form a snare. The snare was pulled and clamped firmly to obstruct the LAD. Intraoperative monitoring included electrocardiography, continuous measurement of blood pressure, and pulse oximetry. Normal saline was infused into an ear vein as fluid replacement therapy during the procedure.
Experimental protocol
The animals were subjected to in situ left anterior descending (LAD) coronary artery ischemia (60 min) followed by myocardial reperfusion (180 min) at the end of which myocardial infarct size was determined. Animals were randomized to the following experimental protocols:
-
1. Control - No additional intervention.
-
2. RIPC- Four-5 min cycles of lower limb ischemia/reperfusion (achieved by clamping and declamping of the femoral artery) were applied immediately prior to the onset of myocardial ischemia.
-
3 and 4. RIPC + Wort or 8-SPT: Wortmannin (20 μg/kg, a PI3K inhibitor) or 8-sulfophenyltheophylline (8-SPT 10 mg/kg, a non-specific adenosine receptor inhibitor) were given intravenously 30 s before myocardial reperfusion to RIPC-treated animals.
-
5. RIPerC- Four-5 min cycles of lower limb ischemia/reperfusion were applied 1 min before the onset of myocardial reperfusion.
-
6 and 7. RIPerC + Wort or 8-SPT: Wortmannin or 8-SPT were given 30 s before myocardial reperfusion to RIPerC-treated animals.
-
8. Wort: Wortmannin (20 μg/kg, a PI3K inhibitor) alone was given intravenously 30 s before myocardial reperfusion to control animals.
In a separate group of animals subjected to the control and RIPC experimental protocols 1–4, and 8 listed above (N = 3 animals per group), myocardial samples were taken from the area at risk at 5 and 15 min of myocardial reperfusion and snap frozen in liquid nitrogen for subsequent Western blot analysis.
Myocardial infarct size measurement
At the end of the 3 h myocardial reperfusion period, the coronary snare was retightened and a 20 g needle connected to a syringe filled with monastral blue dye was inserted into the left ventricle at the apex. The dye was directly injected into the ventricular cavity of the beating heart and allowed to perfuse the coronary arteries that were still patent and their supplied myocardial walls. Therefore, the area at risk, which was supplied by the occluded coronary artery, was devoid of any monastral blue. The animals were then sacrificed with 20 ml Do-lethal and the hearts were excised, washed with normal saline and weighed. The hearts were then frozen at −20°C and 24 h later sliced into approximately 5-mm thick sections from apex to base. The slices were incubated in 1% triphenyl tetrazolium chloride (TTC) in isotonic phosphate buffer solution, pH 7.4 for 20 min at 37°C. TTC reacts with dehydrogenase enzymes and nicotinamide adenine dinucleotide in viable tissue; the infarcted area appears white as the non-staining region. The heart slices were then immersed in 10% formaldehyde solution for 24 h to enhance differences between the stained and unstained regions. The slices were then placed on a plexiglass holder and a cover glass was placed over the tissue. Four supports in the corners held the top glass with the cover sheet away from the bottom glass by 5 mm. Spring clamps pressed the glass down pressing the slices to a uniform 5 mm thickness between the plates. The areas of infarction and the area at risk were traced onto an acetate sheet, placed over the top glass plate. The tracings were subsequently scanned with the Abode Photoshop 6.0 and measured with the Scion Image program. The area at risk and areas of infarction were transformed into volumes by multiplying the areas by the slice thickness (5 mm). Infarct and area at risk volumes were expressed in cm3 and the percent of infarct to area at risk ratio (%I/R) was calculated [15].
Western blot analysis
Approximately 50 mg of frozen ventricular tissue was used for protein extraction. Tissue was homogenized on ice in 250 μl suspension buffer ([in mM] NaCl 100, Tris 10 [pH 7.6], EDTA 1 [pH 8], Sodium pyrophosphate 2, Sodium fluoride 2, b-Glycerophosphate 2; PMSF 0.1 mg/ml; and 1 μg/ml each of aprotinin, leupeptin, trypsin inhibitor and protease inhibitor). Samples were centrifuged and supernatant further diluted in 2x sample buffer ([in mM] Tris 100 (pH 6.8), DTT 200; and SDS 2%, bromophenol blue 0.2% and glycerol 20%) and subsequently boiled for 10 min at 100°C. The protein concentration was determined using the BioRad Bradford assay. A total protein of 30 μg for each sample was separated on a 12.5% SDS-PAGE gel and transferred to Hybond ECL nitrocellulose membranes (Amersham). Equal protein loading was confirmed by Ponceau red staining (Sigma) of membranes. The antibodies used were from the following sources: phospho-PI3 p85 (Tyr458)/p55 (Tyr 199), PI3 p85, phospho-Akt (Ser 473) and Akt (Cell Signaling Technology). The membranes were subsequently probed with a secondary anti-rabbit antibody conjugated to horseradish peroxides. Levels of phosphorylated proteins were normalized to their total protein levels. Proteins were detected using enhanced chemiluminescence ECL Western blotting detection reagent and bands were visualized by autoradiography. Relative densitometry was performed using Scion Image Release Beta 4.0.2. software and the values for phosphorylated PI3K and Akt were normalized to the values for total PI3K and Akt [20].
Statistical analysis
All values are expressed as mean ± SEM. Myocardial infarct sizes and relative densitometries for Western blots were analyzed by one-way ANOVA and Fisher’s protected least significant difference test for multiple comparisons. Differences were considered significant when P < 0.05.
Results
Exclusions
Eleven animals were excluded from the study for various reasons. Seven animals, in different groups, developed intractable ventricular arrhythmias at the time of sustained ischemia or reperfusion and four animals were excluded for technical reasons. Therefore, 60 pigs (45 in the infarct size protocol and 15 in the Western blotting protocol) successfully completed the study.
Baseline characteristics
Body weight, heart weights and area at risk volumes were similar in the experimental groups (Table 1). Hemodynamic variables were also similar in the groups (Table 2).
RIPC and RIPerC and myocardial infarct size
Both RIPC and RIPerC significantly reduced myocardial infarct size (13.3 ± 2.2% with RIPC, 18.2 ± 2.0% with RIPerC versus 48.8 ± 4.2% in control: P < 0.05: N ≥ 5/group) (Fig. 1). Wortmannin, the PI3K inhibitor, partially abolished the infarct-limiting effects of RIPC (33.2 ± 6.0% with RIPC + Wort versus 13.3 ± 2.2% with RIPC: P < 0.05: N ≥ 5/group) but not RIPerC (18.0 ± 3.4% with RIPerC + Wort versus 18.2 ± 2.0% with RIPerC:P > 0.05: N ≥ 5/group) (Fig. 1). 8-SPT, the adenosine receptor inhibitor, did not influence the infarct-limiting effects of either RIPC (10.4 ± 2.0% with RIPC + 8-SPT versus 13.3 ± 2.2% with RIPC:P > 0.05:N ≥ 5/group)(Fig. 1) or RIPerC (17.6 ± 4.0% with RIPerC + 8-SPT versus 18.2 ± 2.0% with RIPerC: P > 0.05: N ≥ 5/group)(Fig. 1). Wortmannin itself when administered at the onset of myocardial reperfusion did not influence infarct size (39.1 ± 5.6% with Wort versus 48.8 ± 4.2% in control: P > 0.05: N ≥ 5/group).
Both remote ischemic lower limb preconditioning (RIPC) and perconditioning (RIPerC) reduced myocardial infarct size (expressed as infarct size over the area at risk) in the in situ porcine heart. The infarct-limiting effect of RIPC but not RIPerC was abrogated by pre-treatment with Wortmannin (Wort), a PI3K-inhibitor. The non-specific adenosine receptor blocker, 8-sulfophenyltheophylline (8-SPT) did not affect the reduction in infarct size elicited by either RIPC or RIPerC. Wortmannin when administered alone at the time of myocardial reperfusion did not influence infarct size. *P < 0.05 compared to control. N ≥ 5 per group
RIPC and western blots analysis
RIPC resulted in a significant increase in phosphorylation of both myocardial PI3K and Akt at both 5 and 15 min myocardial reperfusion when compared to control (Figs. 2 and 3). Treatment with 8-SPT (non-selective adenosine receptor antagonist) and Wortmannin (the PI3K inhibitor) abrogated the phosphorylation induced by both RIPC (Figs. 2 and 3). Western blot samples were not obtained in animals treated with RIPerC based on the fact that 8-SPT and Wortmannin did not affect cardioprotection elicited by RIPerC.
Representative Western blots of phosphorylated and total PI3K with relative densitometries at 5 and 15 min reperfusion. In myocardial tissue harvested from hearts treated with remote ischemic lower limb preconditioning (RIPC), PI3K is significantly phosphorylated. This increase in phoshorylation is abrogated in the presence of Wortmannin (W), the PI3K inhibitor, and 8-sulfophenyltheophylline (8SPT), the non-specific adenosine receptor antagonist. *P < 0.05 compared to control. N = 3 per group
Representative Western blots of phosphorylated and total Akt with relative densitometries at 5 and 15 min reperfusion. In myocardial tissue harvested from hearts treated with remote ischemic lower limb preconditioning (RIPC), Akt is significantly phosphorylated. This increase in phoshorylation is abrogated in the presence of Wortmannin (W), the PI3K inhibitor, and 8-sulfophenyltheophylline (8SPT), the non-specific adenosine receptor antagonist. *P < 0.05 compared to control. N = 3 per group
Discussion
The main findings from the present study are as follows: (a) Remote ischemic preconditioning (RIPC) using limb ischemia and reperfusion reduced myocardial infarct size and this cardioprotective effect was associated with the activation of the PI3K-Akt pathway at the time of myocardial reperfusion within the heart; (b) However, remote ischemic perconditioning (RIPerC) using limb ischemia and reperfusion appeared to limit myocardial infarct size independently of the PI3K-Akt pathway at the time of reperfusion; (c) Neither RIPC or RIPerC cardioprotection were blocked by administration of the adenosine receptor antagonist at the time of reperfusion.
Despite intensive investigation, the mechanisms underlying the cardioprotection elicited by RIPC remain unclear [11, 13]. It has been postulated that a neuro-hormonal pathway links the remotely preconditioned organ or tissue to the heart, although the actual interplay between the neuronal system and the protective humoral factor remain unknown [19]. However, the myocardial signalling pathways activated by the neuro-hormonal pathway are believed to be similar to those recruited in direct myocardial ischemic preconditioning. For example, the activation of the PI3K-Akt pathway as part of the Reperfusion Injury Salvage Kinase (RISK) pathway [6, 9, 10] at the onset of myocardial reperfusion has been reported to underlie the cardioprotection elicited by direct myocardial preconditioning [8, 26] and postconditioning [2, 29].
In the current study, we have shown for the first time in the porcine heart that the PI3K-Akt component of the RISK pathway may be required for the infarct-limitation induced by remote ischemic lower limb preconditioning. We demonstrated increased phosphorylation of both PI3K and Akt at the onset of reperfusion in porcine hearts subjected to RIPC. Importantly, the pharmacological inhibition of PI3K and Akt abrogated the infarct-limiting effects of RIPC, demonstrating that these kinases are required for cardioprotection. A previous study using the rat had already demonstrated the activation of Erk1/2 in intestinal tissue (where the conditioning stimulus was applied) but not in the heart [12]. A recent study was published which has also linked remote ischemic limb preconditioning to the activation of the PI3K-Akt pathway at reperfusion within the murine heart, although in that study the kinase inhibitor was administered prior to the remote ischemic limb preconditioning protocol rather than at reperfusion as in our study [17]. Furthermore, a recent study has reported that perfusion of isolated rat heart at myocardial reperfusion with coronary effluent collected from an ischemic preconditioned rat heart limited myocardial infarct size through the activation of the PI3K-Akt pathway [4]. Our study was performed in the more clinical relevant in situ model of porcine myocardial IRI and also explored the role of adenosine as a potential upstream activator of the PI3K-Akt pathway.
A limitation of our study is the finding that, although 8-SPT, the non-specific adenosine receptor antagonist, was found to prevent the phosphorylation of PI3K and Akt in RIPC-treated hearts, it did not influence the reduction in MI size elicited by RIPC. The reason for this discrepancy is unclear. Another potential limitation of our study is the relatively small N numbers in each group. We investigated the role of adenosine receptor activation at the time of myocardial reperfusion whereas many of the studies implicating a role for the adenosine receptor as a mediator of RIPC have explored its contribution either in the remote organ or tissue or else in the heart but prior to myocardial ischemia [5, 18, 21, 27]. However, direct myocardial IPC has been reported to require endogenous adenosine receptor activation at reperfusion [26]. It may well be that RIPC does not require adenosine receptor activation at the onset of myocardial reperfusion.
The mechanisms underlying RIPerC have been less intensely investigated and remain somewhat unexplored. Our study is the first to examine the role of the adenosine receptor and the PI3K-Akt pathway in the setting of RIPerC. We found that RIPerC limited myocardial infarct size independent of both the adenosine receptor and the activation of the PI3K-Akt pathway. Another limitation of our study is that we did not collect tissue samples for Western blotting for PI3K and Akt in hearts treated with RIPerC. The reason for not performing these experiments was due to limited resources at the time and the fact that Wortmannin had not impacted on RIPerC cardioprotection. The failure to implicate the adenosine receptor in RIPerC cardioprotection is in contrast with the study which first described the phenomenon of RIPerC [16]. In that study, Kerendi and co-workers [16] reported that a single 5 min episode of renal artery occlusion and reflow applied prior to myocardial reperfusion limited myocardial infarct size and this cardioprotective effect was abolished by 8-SPT, implicating the involvement of the adenosine receptor. However, in that study the remote ischemic conditioning stimulus was applied to the kidney during myocardial ischemia and so it should really have been termed remote ischemic perconditioning and not postconditioning. In its truest sense, RIPost should be restricted to those situations in which the remote ischemic conditioning stimulus is applied at the onset of myocardial reperfusion as it was in a previous study by Andreka and co-workers [1]. The failure to implicate the PI3K-Akt pathway in hearts treated with RIPerC appears to support the findings of the study by Skyschally and co-workers [25], who found that direct myocardial ischemic postconditioning in the porcine heart was not mediated through the activation of the PI3K-Akt component of the RISK pathway.
From these findings it appears that RIPC and RIPerC may activate different intracellular pathways within the heart- further studies are required to dissect the signal transduction pathways in these cardioprotective strategies. The current paradigm for preventing lethal myocardial reperfusion injury suggests that the intervention should be applied at the immediate onset of myocardial reperfusion. However, if the protective effect of RIPerC using lower limb ischemia and reperfusion is dependant on the washout of a cardioprotective factor from the lower limb, then the protective factor will only be released 5–10 min into myocardial reperfusion, which may explain why different myocardial signalling pathways are involved.
In summary, we have demonstrated in the porcine heart that remote ischemic limb preconditioning limits myocardial infarct and this cardioprotective effect is associated with the activation of the PI3K-Akt pathway at reperfusion. Interestingly, remote ischemic limb perconditioning appears to reduce myocardial infarct size independently of the PI3K-Akt pathway. Further studies are required to investigate the myocardial signal transduction pathways underlying RIPerC. Furthermore, clinical proof of concept studies are now required to determine whether RIPerC, which is easier to implement, is beneficial in the clinical setting.
References
Andreka G, Vertesaljai M, Szantho G, Font G, Piroth Z, Fontos G, et al. Remote ischemic postconditioning protects the heart during acute myocardial infarction in pigs. Heart. 2007;93:749–52.
Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006;69:178–85.
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, et al. Remote ischemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34.
Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–45.
Ding YF, Zhang MM, He RR. Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. Sheng Li Xue Bao. 2001;53:7–12.
Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893–907.
Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–9.
Hausenloy DJ, Tsang A, Mocanu M, Yellon DM. Ischemic preconditioning protects by activating pro-survival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–6.
Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–60.
Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–34.
Hausenloy DJ, Yellon DM. Remote ischemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86.
Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. Ischemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res. 2008;78:108–15.
Heusch G, Schulz R. Remote preconditioning. J Mol Cell Cardiol. 2002;34:1279–81.
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–7.
Iliodromitis EK, Georgiadis M, Cohen MV, Downey JM, Bofilis E, Kremastinos DT. Protection from post-conditioning depends on the number of short ischemic insults in anesthetized pigs. Basic Res Cardiol. 2006;101:502–7.
Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–12.
Li J, Xuan W, Yan R, Tropak MB, Jean-St-Michel E, Liang W, et al. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of beta-catenin. Clin Sci (Lond). 2011;120:451–62.
Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–37.
Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–5.
Mocanu MM, Bell RM, Yellon DM. PI3 Kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–8.
Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–7.
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9.
Rentoukas I, Giannopoulos G, Kaoukis A, Kossyvakis C, Raisakis K, Driva M, et al. Cardioprotective role of remote ischemic periconditioning in primary percutaneous coronary intervention: enhancement by opioid action. JACC Cardiovasc Interv. 2010;3:49–55.
Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–90.
Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–8.
Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–9.
Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, et al. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning”. J Am Coll Cardiol. 1999;33:556–64.
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–64.
Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–2.
Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, et al. Remote ischemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–71.
Acknowledgements
We thank ELPEN Pharmaceutical Co. Inc. for continued support.
Funding
We wish to thank the British Heart Foundation for continued support.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hausenloy, D.J., Iliodromitis, E.K., Andreadou, I. et al. Investigating the Signal Transduction Pathways Underlying Remote Ischemic Conditioning in the Porcine Heart. Cardiovasc Drugs Ther 26, 87–93 (2012). https://doi.org/10.1007/s10557-011-6364-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-011-6364-y