Abstract
We have previously shown that remote ischemic preconditioning by limb ischemia (rIPC) or intra-arterial adenosine releases a dialyzable cardioprotective circulating factor(s), the release of which requires an intact neural connection to the limb and is blocked by pretreatment with S-nitroso-N-acetylpenicillamine (SNAP). Remote cardioprotection can be induced by other forms of peripheral stimulation including topical capsaicin, but the mechanisms of their signal transduction are incompletely understood. Rabbits were anesthetized by intravenous pentobarbital, intubated and ventilated, then randomized (4–7 animals in each group) to receive sham procedure, rIPC (4 cycles of 5 min lower limb ischemia, 5 min reperfusion), direct femoral nerve stimulation, topical capsaicin, pretreatment with intra-arterial SNAP + capsaicin, pretreatment with topical DMSO (a sensory nerve blocker) + topical capsaicin, or pretreatment with intra-arterial SNAP + femoral nerve stimulation, topical DMSO alone, or intra-arterial SNAP alone. Blood was then rapidly drawn from the carotid artery to produce the plasma dialysate which was used to perfuse a naïve heart from an untreated donor rabbit. The infarct size and recovery of LV-developed pressure and end-diastolic pressure were measured after 30 min of global ischemia and 120 min of reperfusion. Compared to sham, dialysate from rIPC, femoral nerve stimulation, and topical capsaicin groups all produced significant cardioprotection with significantly reduced infarct size, and improved the post-ischemic cardiac performance. Cardioprotection was not seen in the topical DMSO-capsaicin, SNAP + capsaicin, and SNAP + FNS groups. These results confirm the central role of peripheral nerves in the local signal transduction of remote cardioprotection. Direct electrical or peripheral neural stimulation evokes the release of cardioprotective substances into the bloodstream, with comparable effects to that of rIPC induced by limb ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the description of local ischemic preconditioning by Murry et al. [21], Przklenk [24] introduced the concept of remote preconditioning by showing protection in the left anterior coronary territory following a preconditioning stimulus in the circumflex coronary artery. Birnbaum et al. [1] extended this concept reporting “ischemic preconditioning at a distance”, whereby electrical stimulation of the gastrocnemius muscle in combination with reduced femoral arterial flow, but not electrical stimulation alone, reduced myocardial infarct size in rabbits.
Subsequently, transient limb ischemia (induced by short periods of ischemia by tourniquet or blood pressure cuff) has proven to be a simple method of inducing remote preconditioning (rIPC), with potent cardioprotection having been demonstrated in experimental animal models as well as clinically [2, 13]. These protective effects have since been demonstrated in other organs [4], and the technique has translated rapidly to positive clinical trials [14, 27].
Early studies of rIPC, showing abrogation by pretreatment with the ganglion blocker hexamethonium, suggested that an intact neural connection was required between the remote organ and the organ being protected [8]. However, our group has shown that remote preconditioning by transient limb ischemia in rabbits and humans is associated with release of a blood-borne, hydrophobic and small (molecular-mass <15 kDa) circulating factor(s), the effect of which can be blocked by the opiate receptor blocker, naloxone [25]. We subsequently established the role of PI3K/Akt/GSK3β signaling in the cardioprotection afforded by this stimulus of rIPC [18]. Interestingly, a subsequent study has shown that small, <30 kDa, hydrophobic molecules obtained from coronary effluent from hearts are cardioprotective in naïve hearts and act via a similar kinase pathway [3], and recent human data have invoked STAT5 signaling in the cardioprotective biology of rIPC by limb ischemia [10]. Intact neural pathways are required for adequate signal transduction within the remotely stimulated organ. We [26] and others [5] have shown that prior transection of the femoral nerve abolishes rIPC by lower limb ischemia and intra-arterial adenosine. We showed that this was mediated via abrogation of release of the circulating cardioprotective factor(s). Furthermore, similar abolition of cardioprotection was induced by pretreatment with the nitric oxide (NO) donor, S-nitroso-N-acetylpenicillamine (SNAP) [26], suggesting a central role for nitric oxide-sensitive nerve stimulation in the liberation of the humoral effectors of rIPC.
Recently, other methods of remote stimulation of cardioprotection have been described. For example, electroacupuncture stimulation of the limb confers cardioprotection [7], and in another study of nociceptive preconditioning [11], Jones and colleagues showed that stimulation of sensory C-fibers by topical capsaicin (0.1%) applied to the abdominal midline induced powerful cardioprotection in a mouse model. In the latter study, the authors showed that not only hexamethonium, but also transection of the spine at T7, but not C7, abolished in vivo cardioprotection by nociceptive preconditioning, leading them to state that their results “…support a neurogenic mechanism and rule out an essential diffusible humoral factor as the cause of cardioprotection…”. They suggested that cardioprotection was mediated through direct stimulation of cardiac nerves via dorsal root reflexes. However, they did not assess directly the presence of any blood-borne cardioprotective factor.
Our experimental model, whereby the preconditioning stimulus can be induced and modified in one animal and the subsequent release of cardioprotective factors (or otherwise) can be tested in a naïve heart of another, allows us to dissect out the often complex signaling biology of cardioprotection. We therefore used this system to examine the role of humoral cardioprotection after capsaicin-induced cardioprotection. Given that femoral nerve transection abrogates humoral release of cardioprotective factors by rIPC, we also tested whether femoral nerve stimulation (FNS) conversely induces rIPC.
Materials and methods
All animal protocols were approved by the Animal Care and Use Committee of the Hospital for Sick Children in Toronto and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996).
Donor rabbits
New Zealand white rabbits (male: weight 3–3.5 kg) were premedicated with 0.25 mg/kg akmezine (containing ketamine, acepromazine and atropine). After 10 min, pentobarbital sodium (30 mg/kg) was injected via a cannula placed in a marginal ear vein and the animal was intubated and ventilated. All rabbits were anticoagulated with heparin (100 IU/kg) via a marginal ear vein. The left carotid artery of sham and treated rabbits was exposed and cannulated with a 5-Fr catheter to collect blood for dialysate preparation (see below).
Experimental design
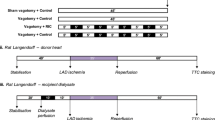
The rabbits were allocated to nine groups (Fig. 1a): group 1 (sham), animals were anesthetized for 50 min; group 2 (rIPC), remote ischemic preconditioning was induced through four cycles of 5 min of hindlimb ischemia (via a tourniquet), followed by 5 min reperfusion as previously described [13]; group 3 (capsaicin), 5 ml of 0.1% capsaicin topical analgesic cream (Capzasin-HP; Chattem, Inc.) was applied via syringe to a 15 cm by 4 cm rectangle of shaved abdomen, in keeping with the ratio of abdominal area to capsaicin cream area presented by Jones et al. [11], and rubbed into the skin. After 15 min, the cream was removed from the skin; group 4 (SNAP + capsaicin), animals were pretreated with a 10 min carotid artery infusion of 65 μg kg−1 min−1 S-nitroso-N-acetylpenicillamine (SNAP; NO donor; Sigma), immediately followed by capsaicin treatment; group 5 (DMSO + capsaicin), 3 ml of 80% dimethyl sulfoxide (DMSO, a sensory nerve blocker; Sigma; St. Louis, MO, USA) in 0.9% NaCl was applied to the shaved abdomen and left for 30 min. After wiping the abdomen clean, capsaicin treatment immediately followed; group 6 (DMSO alone), 3 ml of 80% dimethyl sulfoxide in 0.9% NaCl was applied to the shaved abdomen and left for 30 min; group 7 (FNS) the femoral nerve was exposed and directly stimulated using an electrode needle placed adjacent to the femoral nerve for four cycles each followed by a 5-min rest period. Stimulation of 500 μs pulse width, 3.1 Hz, 0.5–1.0 mA (Nicolet Endeavor CR, CareFusion, Madison, WI, USA) was applied; group 8 (SNAP + FNS) animals were pretreated with a 10-min intra-arterial (femoral artery) infusion of 65 μg kg−1 min−1 S-nitroso-N-acetylpenicillamine (SNAP; NO donor; Sigma), immediately followed by direct FNS; group 9 (SNAP alone), animals were pretreated with a 10-min intra-arterial (femoral artery) infusion of 65 μg kg−1 min−1 SNAP, and after 30 min blood was taken. Blood was taken for dialysate preparation after the end of each protocol (see below).
Schematic overview of experimental protocols applied to each of the groups. a Remote ischemic preconditioning (rIPC) [four cycles of transient ischemia (black box, 5 min) and reperfusion (5 min)] was performed; 5 ml of 0.1% capsaicin cream (C) applied to abdomen with or without prior injection SNAP (S) or DMSO (D). Femoral nerve stimulation (FNS) [using electrode needle directly stimulated adjacent to the femoral nerve for four cycles each followed by a 5-min rest period] was performed with or without prior injection of SNAP (S). Dialysate of blood from animals in each of the groups was perfused in naïve rabbit donor hearts on a Langendorff apparatus according to the protocol outlined graphically (b). See text (“Experimental design”) for additional details
Dialysate preparation
Both control and treated rabbits had a large bore cannula placed in the left carotid artery to rapidly draw blood at the end of the study protocol. The protocol for preparation of plasma dialysate was as previously described [25]. Approximately, 80 ml of blood was obtained from each animal at the end of treatment. Bleeding was limited to less than 2 min to minimize secondary hemodynamic effects. Blood gases and electrolyte measurements were taken at the end of the blood draw to confirm that each rabbit remained well oxygenated and neither acidotic nor hyperkalemic. Plasma was obtained by centrifugation (3,000 rpm for 20 min) of the whole blood. To prepare the dialysate, 50 ml of plasma was placed in dialysis tubing with a 12–14 kDa cutoff membrane (Spectra/Por; Spectrum Laboratories, Inc.; Rancho Dominguez, CA, USA) and dialyzed against a 20-fold volume of Krebs–Henseleit buffer (1,000 ml). Before perfusion of the donor hearts, d-glucose and NaHCO3 were added to the final concentration of 11 and 10 mmol/l, respectively, to the dialysate and then filtered through a 0.2-μm filter. The perfusate was equilibrated with 95% oxygen–5% CO2 and adjusted to a pH of 7.35–7.4.
Langendorff preparation
Once the dialysate was prepared, a heart from an untreated rabbit (male 2.7–3.5 kg body wt) was quickly excised, mounted on a Langendorff perfusion apparatus, and perfused under non-recirculating conditions at constant pressure (80 mmHg) at 37°C. Krebs–Henseleit buffer contained (in mM) 119 NaCl, 4.7 KCl, 1.2 MgSO4, 1.8 CaCl2, 1.2 KH2PO4, 25 NaHCO3, and 11 glucose, gassed with 95% oxygen–5% CO2. The solution was continuously oxygenated with 95% oxygen–5% CO2 to maintain a final pH of 7.4. The buffer was vacuum filtered with a 0.2-μm nitrocellulose filter to remove particulates. Once placed onto the perfusion apparatus, each heart was submerged in 37°C Krebs–Henseleit buffer for the duration of the experiment. A water-filled balloon, previously made with thin plastic Saran Wrap on PE-160 polyethylene tubing, was inserted into the left ventricle (LV) through the mitral valve and connected to a pressure transducer (ML844; AD Instruments; Colorado Springs, CO, USA). The balloon was inflated with water to adjust LV end-diastolic pressure (LVEDP) to 6–8 mmHg at the beginning of the experiment, and the volume was kept constant for the duration of the study.
Hemodynamic measurements, including heart rate, peak LV pressure, maximum rate of contraction (+dP/dt), maximum rate of relaxation (−dP/dt), and LVEDP; were recorded with a PowerLab data acquisition system (AD Instruments; Colorado Springs, CO, USA). The LV-developed pressure (LVDP) was calculated as the difference between the systolic and end-diastolic LV pressures. Coronary flow was measured continuously using a medical volume flow meter (H107, Transonic Systems, Inc; Ithaca, NY, USA), data were recorded with a PowerLab data acquisition system (AD Instruments), and an in-line flow meter probe was connected to the tubing close to the cannula. Each heart was allowed to stabilize for 20 min and then perfused with dialysate for 14–22 min, depending on the coronary flow of the dialysate. Then, Krebs buffer was added for the remaining 8–16 min for a total of 30 min of pretreatment after stabilization. The hearts were then subjected to 30 min of global ischemia (37°C) and 120 min of reperfusion (Fig. 1b). The following were excluded from subsequent analysis for the following predetermined reasons: (1) stabilized hearts that displayed poor contractile function (<100 mmHg developed pressure after stabilization), (2) bradycardic hearts (heart rate <140 beats/min) or unacceptably arrhythmic hearts during stabilization, and (3) hearts that sustained ventricular fibrillation (VF) during reperfusion.
Measurement of infarct size
At the end of the reperfusion period, the LV was removed and frozen at −80°C. The frozen heart was cut transversely into 2 mm-thick slices using a rabbit Heart Slicer Matrix (Zivic Instruments; Pittsburgh, PA, USA) and stained with 1.25% triphenyltetrazolium chloride (Sigma) in 200 mM Tris (pH 7.4) for 20 min in a water bath at 37°C. After fixation for 2 h in 10% neutral-buffered formaldehyde, each slice was photographed by electronic scanning (CanoScan 4400F). The viable myocardium stained brick red, and infarct tissues appeared pale. The infarct and LV areas were measured via automated planimetry using Adobe Photoshop CS2 software, with infarct size expressed as a percentage of the total LV area.
Quantification of capsaicin levels in plasma using LC–MS/MS
Capsaicin was extracted from rabbit plasma by mixing 50 μL of sample with 250 μL 50:50 methanol:acetone containing verapamil as internal standard (20 ng/mL). The sample was then vortexed vigorously and allowed to rest for 10 min at room temperature prior to centrifugation at 12,000g for 10 min. The supernatant (10 μL) was transferred into an injection vial for LC–MS/MS analysis
Capsaicin levels in plasma were quantified by LC/MS/MS analysis at the Advanced Instrumentation for Molecular (AIMS) Laboratory in the Department of Chemistry at the University of Toronto (Toronto, Canada). Extracted plasma samples from sham, capsaicin, and capsaicin + DMSO groups (n = 6 in each group) (10 μL) were applied via an Autosampler (CTC Analytics, HTS PAL) onto a Gemini-NX C18 50 mm × 4.6 mm, 3 μ column equipped with a C18 guard column (Opti-guard) connected to an HPLC (Agilent) equipped with a binary pump (Agilent 1100, G1312A) and degasser (Agilent 1100, G132A). Between each sample, the injection syringe and valves were washed three times with methanol followed by three washes with water. Mobile phases consisted of A: 0.1% formic acid in Milli-Q Water and B: methanol. Capsaicin was resolved using the following gradient 0–1 min: 20% B; 1–3 min 20–90% B; 3–4.5 min 90% B; 4.6 min 20% B. Material from the column (flow rate 600 μL/min) was injected onto a Applied Biosystems (MDS Sciex, Toronto, ON, Canada) API4000 triple–quadruple mass spectrometer operating in positive MRM mode, with current IS and temperature set to 4,500 and 350°C, respectively. Capsaicin levels were monitored using parent to product ion transitions of m/z 306.0 → 137.0 or alternatively 306.0 → 182.1 with DP and CE set to 41 and 24 or 40 and 16, respectively. Verapamil (internal standard) was monitored using transitions of m/z 455.2 → 165.1 or alternatively 455.2 → 150.1 with DP and CE set to 72 and 40 V or 79 and 55 V. Retention times for verapamil and capsaicin were 3.3 and 4.3 min, respectively. Dwell time for each compound was set to 100 ms. For each batch of unknown samples, a standard curve for capsaicin spanning the range 3.3–363 nM were generated with an LOQ of 3.3 nM (average accuracy 99.9%, range 94.0 –104%).
Total nitric oxide and nitrite/nitrate assay
Total nitrite/nitrate levels in dialysates were measured using a colorimetric assay kit (R&D Systems, Minneapolis, MN, USA) that involves the Griess reaction after the conversion of nitrates to nitrites by the enzyme nitrate reductase (sensitivity of 0.22 kmol/L for nitrites and 0.54 kmol/L for nitrates, with a variability coefficient of 0.8–5.1%).
Statistical analysis
Data are reported as mean ± standard error (SE). For each individual outcome, a linear regression model, with maximum likelihood algorithm for parameter determination, was created (PROC GENMOD with the SAS system, using standard settings, normal distribution, and identity link function). To eliminate the issue of multiple comparisons, binary dummy variables were created for each study group (mutually exclusive binary strings with 7 values each representing each study group) and all seven dummy variables were included in the same regression model (using the SHAM group as the reference category, against which all other groups were compared). This allowed between-group comparisons from the regression model parameter estimates without performing multiple statistical tests. Statistical significance of each between-group comparison was based on the likelihood ratio test, based on the ratio between the difference in parameter estimate of the experimental group versus the reference group and the standard error of that difference. This single-model approach allowed for better handling of the small sample sizes than a standard ANOVA, and eliminated the need for post hoc testing. The level of statistical significance was set as p < 0.05 for all analyses. All statistical analyses were performed using SAS v. 9.2 (SAS Institute; Cary, NC, USA).
Results
A total of 62 animals were used in this study. The data from five hearts were excluded at the time of Langendorff preparation due to VF during stabilization in one heart (sham), due to contractile failure during stabilization in one heart (capsaicin + SNAP group), and due to VF during reperfusion in three hearts (1, capsaicin + SNAP group; 1, capsaicin + DMSO; 1, FNS). There were no differences in the number of hearts excluded between the groups. The baseline function results of all groups are illustrated in Table 1.
Infarct size
Perfusion with rIPC dialysate significantly decreased the infarct size in comparison to the sham dialysate (28.1 ± 5.3% in rIPC vs. 45.4 ± 3.4% in sham, p < 0.01). Pretreatment with capsaicin also considerably reduced the infarct size when compared with the sham dialysate (26.7 ± 5.5% in capsaicin vs. 45.4 ± 3.4% in sham, p < 0.01). The cardioprotective effect of capsaicin was abolished with pretreatment with SNAP and DMSO (41.0 ± 5.5 and 41.7 ± 3.0% in SNAP and DMSO, p = ns compared to sham, respectively) (Fig. 2). Control dialysate obtained after DMSO or SNAP treatment alone did not affect infarct size (Fig. 2). Dialysate obtained after FNS also reduced the infarct size when compared with the sham (24.4 ± 2.8% in FNS vs. 45.4 ± 3.4% in sham, p < 0.01). The infarct size reduction by FNS was abolished by pretreatment with SNAP (SNAP + FNS, 46.6 ± 7.0%, p = ns vs. sham) (Fig. 3).
Bar graphs illustrating left ventricular infarct size (as a percentage of area at risk) following treatment with topical capsaicin, and capsaicin with pretreatment with intra-carotid arterial SNAP and topical DMSO, and treatment with topical DMSO alone as control group. *p < 0.01 versus sham. The cardioprotective effect of dialysate obtained from animals treated with topical capsaicin is abrogated by pretreatment with intra-arterial SNAP and pretreatment with topical DMSO. Representative images of TTC staining from each of the treatment groups are shown beneath each bar
Bar graphs illustrating left ventricular infarct size (as a percentage of area at risk) in FNS and FNS + SNAP groups. *p < 0.01 versus sham. The cardioprotective effect of dialysate obtained from animals after femoral nerve stimulation is abrogated by pretreatment with intra-arterial SNAP. Representative images of TTC staining from each of the groups shown below each bar
Functional hemodynamic data
Perfusion with the rIPC dialysate significantly improved the post-ischemic cardiac performance in isolated perfused hearts at 120 min of reperfusion (Table 1). The recovery of LVDP was significantly greater in hearts perfused with rIPC dialysate than in hearts perfused with the sham dialysate (p < 0.05). Both +dP/dt and −dP/dt trended toward being improved with rIPC compared to sham (p < 0.07, and 0.11, respectively). Additionally, there was a trend toward rIPC improving the diastolic recovery, with the LVEDP lower compared to the sham group (24.9 ± 4.4% in rIPC vs. 37.4 ± 6.8% in sham, p = 0.07, ns).
Likewise, perfusion with the dialysate obtained from capsaicin-treated rabbits significantly improved the post-ischemic cardiac performance in isolated perfused hearts at 120 min of reperfusion. The recovery of LVDP was greater in hearts perfused with dialysate obtained from capsaicin-treated rabbits than in hearts perfused with the sham dialysate (p < 0.0001). Both +dP/dt and −dP/dt were improved with capsaicin compared to sham (+dP/dt, p < 0.0001; and −dP/dt, p = 0.009). Additionally, capsaicin improved the diastolic recovery with the LVEDP appreciably lower compared to sham (23.9 ± 4.8% in capsaicin vs. 37.4 ± 6.8% in sham, p < 0.001). The addition of SNAP to capsaicin abolished the improvement in LVDP seen in hearts perfused with capsaicin dialysate alone (Table 1). There was no difference in post-ischemic cardiac performance at 120 min of reperfusion between sham and DMSO or SNAP dialysate perfusion (Table 1).
Functional recovery was also improved with dialysate produced from animals after direct FNS (LVDP, p < 0.0001; +dP/dt, p < 0.0001; and −dP/dt, p = 0.004). Additionally, FNS improved diastolic recovery with the LVEDP significantly lower compared to sham (p = 0.019). Pretreatment with SNAP abolished all improvements in the hemodynamics of FNS alone (Table 1).
Plasma capsaicin level
Plasma levels of capsaicin in capsaicin and capsaicin + DMSO groups were determined to be 2.3 ± 0.4 and 14 ± 0.4 nM, respectively (Fig. 4). These plasma concentration values were more than 20-fold lower than the referenced EC50 value of 300 nM for the agonist activity of capsaicin against TRPV1 receptors for releasing of CGRP from dorsal root ganglia [29]. Taking into consideration the volume of plasma dialyzed against buffer, capsaicin levels in dialysate derived from the capsaicin or capsaicin groups would be 20-fold lower.
Bar graphs showing plasma capsaicin levels in control and capsaicin-treated groups. All levels fall well below previously published EC50 data [29]
The nitrite/nitrate level in the dialysate
The question arises with the application of SNAP in the donor animal, whether the excess generation of NO might impact on the concentration of NO-related reaction products (e.g., biologically active nitrite, nitrate) in the donor animal’s circulation and the subsequent dialysate (Fig. 5). In SNAP dialysate, there was no significant difference in nitrate level compared to the sham dialysate (4.6 ± 0.4 in sham dialysate, 3.4 ± 0.2 in SNAP dialysate, respectively).
Discussion
We have shown for the first time that topical capsaicin and direct FNS induce cardioprotection by evoking dialyzable preconditioning factors into the bloodstream. As such, these methods share similar characteristics to the signal transduction previously demonstrated by us for rIPC induced through transient limb ischemia [25]. In that study, we showed that rIPC using the limb ischemia stimulus was associated with the release of a small (<15 kDa), hydrophobic factor(s) that was dialyzable from plasma obtained from preconditioned rabbits or humans and was protective in both a Langendorff model of regional IR injury, as well as in isolated cardiomyocyte preparations. The humoral nature of this type of rIPC was also supported by the findings of recent experiments by Lim et al. [19], whereby occlusion of the femoral vein draining the leg undergoing the rIPC stimulus completely abolished cardioprotection.
We have also recently shown that the release of circulating cardioprotective factors by rIPC induced by transient limb ischemia or intra-arterial adenosine is abrogated by prior femoral nerve transection and by prior treatment with the NO donor SNAP, but is not affected by pretreatment with the nitric oxide synthase antagonist l-NAME [26]. Not only were these data interesting mechanistically—suggesting an important role of NO-sensitive nerves (such as sensory C-fibers) in the local signal transduction of rIPC—but also highlight the need to dissect out each of the elements of the preconditioning stimulus to adequately understand its biology. The intracellular signaling associated with cardioprotection was recently reviewed by Heusch et al. [9], and the pivotal role of nitric oxide bioavailability and its manipulation emphasized. By using a Langendorff preparation, with an untreated naïve heart, to assess the presence of circulating cardioprotective factors in the dialysate from preconditioned animals, we were able to show the counter-intuitive effects of NO signaling in the limb subjected to rIPC. In the intact animal, increased NO bioavailability is integral to myocardial preconditioning induced by nitrates and sildenafil for example [22], and l-NAME inhibits local myocardial preconditioning [26]. Extending these previous observations, we speculated that if prior femoral nerve transection or other forms of neuro-inhibition abolishes rIPC, then direct stimulation of the femoral nerve may itself induce rIPC. Our data confirm this to be the case: dialysate after FNS is equally cardioprotective as dialysate from animals subjected to rIPC by transient limb ischemia. Furthermore, pretreatment with SNAP abrogated the cardioprotective effect of the dialysate after FNS, in the same way as we demonstrated for rIPC by transient limb ischemia [26].
Peripheral neural stimulation is de facto the mechanism of action of cardioprotection by nociceptive stimulation. In 2009, Jones and colleagues investigated nociceptive cardioprotection in an in vivo mouse model of LAD ischemia. Prior treatment by surgical incision over the abdomen or stimulation of C-fibers using topical capsaicin reduced infarct size by approximately 80%, and was associated with changes in kinase signaling typical of that seen with local and remote ischemic preconditioning [11]. They showed abrogation of cardioprotection by pretreatment with the ganglion blocker hexamethonium (as has been shown for other stimuli of remote ischemic preconditioning) [8], and after spinal transection at T7 but not C7, leading them to suggest that direct stimulation of cardiac nerves, via dorsal root reflexes, was responsible for transferring the signal from the periphery to the heart. They also suggested that this excluded the possibility of release of a humoral, blood-borne, factor. Our data show that this is not the case. While we cannot exclude that a direct neural connection is also involved, it is clear that release of a blood-borne factor(s) is integral to the cardioprotection induced by capsaicin stimulation of peripheral C-fibers. Furthermore, blockade of capsaicin-induced C-fiber stimulation using pretreatment with topical 80% DMSO (previously shown to be a relatively selective C-fiber blocker [6] abrogated the cardioprotective effect of dialysate. This appears to be a local effect within the skin, as plasma levels of capsaicin in the treated animals (albeit slightly higher in those with concomitant DMSO) were orders of magnitude below previously published EC50 data [29], and given that the concentration in dialysate will be some 20-fold lower, we can effectively exclude a transferred effect of capsaicin within the dialysate to the isolated heart receiving the treated dialysate. The effect of capsaicin was also abrogated by systemic pretreatment with the NO donor SNAP, as was the cardioprotective effect of FNS. Nitric oxide is known to be centrally [30] and peripherally neuroinhibitory and, for example, reduces the activity of non-adrenergic non-cholinergic nerves of the gut [28], modulates afferent nerve activity in the kidney [20], and modulates cardiovascular reflexes via inhibition of cardio-pulmonary C-fiber reflex-induced excitation [15]. We speculated, in our prior study, that release of the circulating cardioprotective factor(s) by rIPC induced by transient limb ischemia and intra-arterial adenosine was inhibited by nitric oxide via modulation of peripheral sensory nerve activity [26], and the current data suggest that a similar mechanism might exist with both FNS and capsaicin-mediated release of humoral cardioprotection. These observations may be important when designing clinical trials. For example, co-administration of NO donors (e.g., nitrates) or agents that increase NO bioavailability within cells (e.g., propofol) during RIPC may be inhibitory to the rIPC stimulus. While the majority of clinical trials so far published have shown cardioprotection, others have been neutral, with no obvious explanation for the lack of benefit of rIPC [12, 23]. The recent clinical data by Kottenberg et al. [17] are interesting in this regard. In a study of patients undergoing coronary bypass surgery, rIPC induced by transient limb ischemia was effective in those receiving isoflurane anesthesia, but was abrogated in those receiving propofol anesthesia.
Study limitations
While we have demonstrated that capsaicin induces the release of a blood-borne, dialyzable cardioprotective factor(s), we cannot exclude entirely the possibility of additional neurogenic mechanisms requiring direct linkage with, or stimulation of, cardiac nerves, as postulated by Jones et al. [11]. However, our previous data showing reduced ischemia–reperfusion injury in the denervated transplanted heart after rIPC in the recipient animal show that such an additional connection is not necessary for cardioprotection by rIPC induced by limb ischemia in vivo [16], and in vitro protection by dialysate not only is effective in the isolated heart in Langendorff preparation, but also in isolated fresh cardiomyocytes completely devoid of neural cells [25].
In summary, we have shown that direct FNS and C-fiber stimulation by capsaicin are associated with release of a blood-borne cardioprotective factor(s) similar to that associated with rIPC induced by transient limb ischemia.
References
Birnbaum Y, Hale SL, Kloner RA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96:1641–1646. doi:10.1161/01.CIR.96.5.1641
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. doi:10.1016/S0140-6736(09)62001-8
Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK (2011) Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol 106:135–145. doi:10.1007/s00395-010-0133-0
Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282. doi:10.1016/j.jacc.2006.01.066
Dong JH, Liu YX, Ji ES, He RR (2004) Limb ischemic preconditioning reduces infarct size following myocardial ischemia–reperfusion in rats. Sheng Li Xue Bao 56:41–46
Evans MS, Reid KH, Sharp JB Jr (1993) Dimethylsulfoxide (DMSO) blocks conduction in peripheral nerve C fibers: a possible mechanism of analgesia. Neurosci Lett 150:145–148
Gao J, Fu W, Jin Z, Yu X (2006) A preliminary study on the cardioprotection of acupuncture pretreatment in rats with ischemia and reperfusion: involvement of cardiac beta-adrenoceptors. J Physiol Sci 56:275–279. doi:10.2170/physiolsci.RP006606
Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD (1996) Myocardial protection by brief ischemia in noncardiac tissue. Circulation 94:2193–2200. doi:10.1161/01.CIR.94.9.2193
Heusch G, Boengler K, Schulz R (2008) Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118:1915–1919. doi:10.1161/CIRCULATIONAHA.108.805242
Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M (2012) STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res 110. doi:10.1161/CIRCRESAHA.111.259556
Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X (2009) Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 120:S1–S9. doi:10.1161/CIRCULATIONAHA.108.843938
Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, John L, Shah AM, Marber MS, Kunst G (2011) Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation? Basic Res Cardiol 106:511–519. doi:10.1007/s00395-011-0185-9
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883. doi:10.1161/01.CIR.0000043806.51912.9B
Kharbanda RK, Nielsen TT, Redington AN (2009) Translation of remote ischaemic preconditioning into clinical practice. Lancet 374:1557–1565. doi:10.1016/S0140-6736(09)61421-5
Kong SZ, Fan MX, Zhang BH, Wang ZY, Wang Y (2009) Nitric oxide inhibits excitatory vagal afferent input to nucleus tractus solitarius neurons in anaesthetized rats. Neurosci Bull 25:325–334. doi:10.1007/S12264-009-0624-x
Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN (2005) Remote ischemic preconditioning of the recipient reduces myocardial ischemia–reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation 79:1691–1695. doi:10.1097/01.TP.0000159137.76400.5D
Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J (2012) Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand 56:30–38. doi:10.1111/j.1399-6576.2011.02585.x
Li J, Xuan W, Yan R, Tropak MB, Jean-St-Michel E, Liang W, Gladstone R, Backx PH, Kharbanda RK, Redington AN (2011) Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of beta-catenin. Clin Sci (Lond) 120:451–462. doi:10.1042/CS20100466
Lim SY, Yellon DM, Hausenloy DJ (2010) The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol 105:651–655. doi:10.1007/s00395-010-0099-y
Ma HJ, Liu YX, Wu YM, He RR (2003) Intrarenal artery injection of l-arginine inhibits spontaneous activity of renal afferent nerve fibers. Sheng Li Xue Bao 55:225–231
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136. doi:10.1161/01.CIR.74.5.1124
Ockaili R, Salloum F, Hawkins J, Kukreja RC (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol 283:H1263–H1269. doi:10.1152/ajpheart.00324.2002
Peters J (2011) Remote ischaemic preconditioning of the heart: remote questions, remote importance, or remote preconditions? Basic Res Cardiol 106:507–509. doi:10.1007/s00395-011-0187-7
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899. doi:10.1161/01.CIR.87.3.893
Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN (2009) Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci 117:191–200. doi:10.1042/CS20080523
Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A (2010) Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol 299:H1598–H1603. doi:10.1152/ajpheart.00396.2010
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105:657–664. doi:10.1007/s00395-010-0104-5
Todorov S, Pozzoli C, Zamfirova R, Poli E (2003) Prejunctional modulation of non-adrenergic non-cholinergic (NANC) inhibitory responses in the isolated guinea-pig gastric fundus. Neurogastroenterol Motil 15:299–306. doi:10.1124/pr.57.3.4
Wardle KA, Ranson J, Sanger GJ (1997) Pharmacological characterization of the vanilloid receptor in the rat dorsal spinal cord. Br J Pharmacol 121:1012–1016. doi:10.1038/sj.bjp.0701199
Zhu GQ, Gao XY, Zhang F, Wang W (2004) Reduced nitric oxide in the rostral ventrolateral medulla enhances cardiac sympathetic afferent reflex in rats with chronic heart failure. Sheng Li Xue Bao 56:47–53
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research and a Transatlantic Network of Research Excellence grant from the Leducq Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Redington, K.L., Disenhouse, T., Strantzas, S.C. et al. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res Cardiol 107, 241 (2012). https://doi.org/10.1007/s00395-011-0241-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-011-0241-5