Abstract
Volatile organic compounds play an important role in the communication between plants and other organisms. The rhizosphere contains a large and diverse microbial community whose members use similar volatiles for intra- and interspecific communication. However, the analysis of volatiles produced in the rhizosphere and their ecological functions have been little explored so far. In this chapter, we outline what is known about the classes of volatiles that are emitted into the rhizosphere by roots and soil microbes, and the effect they have on different interactors in the soil. Additionally, we review current approaches to sample volatiles in mesocosms and field soils. We conclude that to better understand the production and functions of volatiles in the rhizosphere, it is of critical importance to design set-ups that account for the natural complexity of soils. This will help to apply this knowledge for sustainable agriculture and the identification of novel agrochemicals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The essential role of plant volatiles for communication with other organisms, or in other words as ‘infochemicals’, has been acknowledged for over 25 years (Dicke and Sabelis 1988). Nevertheless, their ecological functions have been mainly studied for aboveground interactions (e.g. Dicke and Baldwin 2010). However, it is well known that plant roots contain and produce similar classes of volatiles as aboveground organs. These volatiles are emitted especially in the rhizosphere. The rhizosphere, defined by Lorenz Hiltnet as the narrow zone surrounding and influenced by plant roots, is a hot spot for numerous organisms and is considered one of the most complex ecosystems on Earth. Organisms found in the rhizosphere include bacteria, fungi, oomycetes, nematodes, protozoa, algae, viruses, archaea, annelids and arthropods (Bonkowski et al. 2009; Buee et al. 2009; Raaijmakers et al. 2009).
Most members of the rhizosphere community are part of a complex food web that utilises the large amount of nutrients released by the plant. Rhizosphere organisms that have been well studied for their beneficial effects on plant growth and health include the nitrogen-fixing bacteria, mycorrhizal fungi, plant growth-promoting rhizobacteria (PGPR), mycoparasitic fungi and protozoa. For example, 80 % of terrestrial plant species actively associate with mycorrhizal fungi that may help the plant to overcome nutrient limitations in exchange for carbon resources (van der Heijden et al. 2015). In recent years, primarily driven by efforts towards sustainable intensification in agriculture, there has been an increased interest in PGPR. The main benefit of PGPR can be ascribed to direct growth promotion or to indirect effects via the protection of plants against (a) biotic stresses (Bulgarelli et al. 2012). Decomposers in particular, ranging from small organisms such as bacteria, fungi or nematodes to large macrofaunal organisms such as earthworms and dung beetles, are essential elements of the soil food web. They ensure that dead plant materials re-enter the soil nutrient cycle, thereby increasing plant growth (Kulmatiski et al. 2014).
Even though decomposers certainly may have an effect on, or be affected by, plant volatiles in the rhizosphere, in this chapter, we will focus mainly on the role of volatiles in communication between plants and rhizosphere organisms interacting with living roots. This also includes communication with organisms functioning at higher trophic levels, such as parasitoids or pathogens of root-feeding organisms. Rhizosphere organisms that are deleterious to plant growth and health include pathogenic fungi, oomycetes, bacteria, nematodes and insect herbivores (Blossey and Hunt-Joshi 2003; Bonkowski et al. 2009; Kulmatiski et al. 2014; Mendes et al. 2013). Despite their small size, soil pathogens can cause substantial agricultural losses and are also involved in large-scale ecosystem processes such as succession (de Deyn et al. 2003).
For each organism on earth, it is important to obtain information on the quality of its environment in order to assess opportunities and dangers. Aboveground, vision and light sensing play an important role for both autotrophic as well as heterotrophic organisms (Döring 2014; Kegge et al. 2015). However, belowground, this option is lacking due to the absence of sunlight, which makes chemical communication the more likely way for interaction partners to localise and recognise each other (van Dam 2009). For example, to establish their intimate relationship, host plants and mycorrhizal fungi exchange elaborate chemical communication involving non-volatile strigolactones produced by plant roots and lipochitooligosaccharides produced by arbuscular mycorrhizal fungi (reviewed in van der Heijden et al. 2015). Similarly, rhizosphere bacteria communicate with each other using, e.g. N-acyl homoserine lactones (AHL) in a process called ‘quorum sensing’ to assess if there is enough critical mass to colonise a plant or to form a biofilm (Bakker et al. 2013). This type of communication between collaborating partners can be tapped into by others. For example, parasitic plants use strigolactones to locate their host plant and optimise their timing of germination (Cardoso et al. 2011). Plants that perceive the increase in AHL in their rhizosphere may interfere with this bacterial communication by producing AHL mimics (Teplitski et al. 2000).
Since the early years of the twenty-first century, it has become increasingly clear that plant-produced volatile organic compounds such as terpenoids are also actively involved in rhizosphere communication (Rasmann et al. 2005; van Tol et al. 2001). Initially, these findings were met with scepticism; non-polar volatiles such as terpenoids were not considered to have the optimal chemical properties for travelling in a humid and dense medium such as the soil. This scepticism was experimentally refuted by the fact that the sesquiterpene (E)-β-caryophyllene, which is emitted by maize roots, diffuses best in the gaseous phase of humid soils (Hiltpold and Turlings 2008). At the same time, volatiles may be more stable and reliable cues for communication belowground than in the air, because of the lack of UV-light and the relatively constant temperature in the soil. Hence volatiles may be excellent vehicles to communicate between organisms in the rhizosphere.
In this chapter, we first outline which classes of volatiles are produced by the different organisms in the rhizosphere. Here we will focus on the production of volatiles by roots and microorganisms. It is very possible that other soil-dwelling organisms, such as insects and nematodes, also produce volatiles, but evidence to support this is currently lacking. Second we will outline what is known about the ecological roles of the different volatiles produced by plants and microbes in communication between different members of the soil community. Then we will review the various approaches that are currently used to sample and analyse rhizosphere and root-emitted volatiles. In our conclusion, we discuss the potential of certain volatiles to be the ‘lingua franca’ for communication between different taxa whose members interact in the rhizosphere. Moreover, we will discuss how the distinct roles of specific volatiles can be assessed experimentally and how we can explore their effect in belowground interactions.
2 Production of Volatiles in the Rhizosphere
2.1 Microbes in the Rhizosphere
Plant scientists frequently perceive plants as relatively independent organisms that rely on soil mineral nutrients, water and sunlight, while the role of microbes in plant life is restricted to that of pathogenic microbes or a few well-characterised symbionts, such as nitrogen-fixing bacteria. However, plants are colonised by an astonishing number of microorganisms, whose numbers supersede the number of plant cells. Moreover, the number of microbial genes in the plant rhizosphere by far outnumbers that of plant genes (Mendes et al. 2013). Most studies to date have mainly focused on the number and diversity of bacterial taxa in the rhizosphere, and depending on the sequencing techniques used, the reported numbers range from <100 to more than 55,000 operational taxonomic units (OTUs). Most rhizospheres are dominated by Proteobacteria, Bacteroides, Acidobacteria, Actinobacteria, Verrucomicrobia or Firmicutes (Badri et al. 2009; Berendsen et al. 2012; Bulgarelli et al. 2012; Mendes et al. 2013). Within the group of Proteobacteria, in addition to well-studied and described Gamma-Proteobacteria (Pseudomonas) and Alpha-Proteobacteria (Rhizobia), the importance of Beta-Proteobacteria (Oxalobacteraceae or Burkholderia) is increasingly recognised, due to their high relative observed abundance in rhizosphere metagenome surveys.
For a long time, it has been assumed that the rhizosphere is mainly dominated by bacteria, as fungi are mostly known to be involved in the decomposition of recalcitrant soil organic matter (de Boer et al. 2006). However, recent studies revealed significant utilisation of root exudates by saprotrophic fungi (Buee et al. 2009). These can either be fungi that can co-metabolise root exudates while decomposing recalcitrant organic matter or fungi that are specialised to decompose simple metabolites such as mono- and disaccharides, the so-called ‘sugar fungi’ (Buee et al. 2009). In addition, pre-infective growth of plant pathogenic soil fungi is also dependent on the availability of root exudates (Njoroge et al. 2008). Microorganisms living in the rhizosphere interact with plants in many ways and can have profound effects on plant growth and development by different plant growth-promoting mechanisms such as nitrogen fixation, phytohormone production, induction of systemic resistance or inhibition of phytopathogenic fungi (Berendsen et al. 2012; Lugtenberg et al. 2001; Mendes et al. 2013). Recent studies have revealed that the production of volatile organic compounds by plant-associated microorganisms can play a major role in long-distance plant–microbe interactions.
2.2 Volatiles Produced by Microbes
Microbial volatile compounds are produced by a wide array of microorganisms including bacteria and fungi. Most microbial volatiles are considered as by-products of primary and secondary metabolism. They are formed mainly by oxidation of glucose from various intermediates (Korpi et al. 2009). The underlying biosynthetic pathways are aerobic metabolism, heterotrophic carbon metabolism, fermentation, amino acid catabolism, terpenoid biosynthesis, fatty acid degradation and sulphur reduction (Peñuelas et al. 2014a). Recently, a microbial volatile organic compounds database, mVOC (http://bioinformatics.charite.de/mvoc), was developed where all microbial volatiles reported to date are compiled. This database reveals that bacterial volatiles are dominated by (in descending order) alkenes, alcohols, ketones, terpenes, benzenoids, pyrazines, acids and esters, whereas fungal volatile profiles are dominated by alcohols, benzenoids, aldehydes, alkenes, acids, esters and ketones. Below, we briefly review the biosynthesis of the most prominent volatile classes produced by microbes, which will later be compared with volatile production in plants.
Aromatic compounds are generated in bacteria and fungi via the shikimic acid pathway. 2-Phenylethanol, which is one of the most commonly emitted volatile aromatic compounds, is synthesised by using l-phenylalanine as a precursor. An aminotransferase catalyses the transamination to phenylpyruvate, followed by an oxidative decarboxylation to phenyl-acetaldehyde and an NADH-dependent reduction to the corresponding alcohol (Hazelwood et al. 2008; Kim et al. 2014).
Many bacterial and fungal volatile blends contain aliphatic hydrocarbons, mainly alkenes, alcohols and ketones. These compounds are typically derived from fatty acids, which are synthesised from acetyl-CoA via conversion into malonyl-CoA (Jenni et al. 2007; Schulz and Dickschat 2007).
Terpenoids represent one of the largest classes of volatiles with over 50,000 known members. Although they are mostly known as plant metabolites, it recently has become clear that microorganisms are a rich source of terpenes (Dickschat et al. 2014). An increasing number of terpenes has been reported for several soil-derived fungi, most of them being sesquiterpenes (Collado et al. 2007; Ebel 2010; Singh et al. 2011). One of the most well-known microbial volatiles is geosmin, a sesquiterpenoid responsible for the characteristic earthy odour of moist soil. Despite their remarkable chemical and functional diversity, the biosynthesis of all terpenoids starts from just a few acyclic precursors, including prenyldiphosphate, geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15) and geranylgeranyl diphosphate (GGPP, C20) (Dickschat et al. 2014). Terpene synthases are the primary enzymes responsible for catalysing the formation of hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15) or diterpenes (C20) from the substrates DMAPP, GPP, FPP or GGPP, respectively (Tholl 2006). The recently increased knowledge about bacterial genomes revealed many distinct terpene synthase genes widely distributed in bacteria, indicating that bacteria can be a rich source of terpenes (Cane and Ikeda 2012; Yamada et al. 2012, 2015). Many soil- and plant-associated bacteria harbour genes encoding such terpene synthases. However, most of these genes are silent in the parent microorganisms under laboratory culture conditions and only for few bacterial strains have the terpene synthases been chemically characterised. Although the principal processes of terpene biochemistry are well understood, it is difficult to predict terpene structures from the amino acid sequence of terpene synthases. To date, studies on bacterial terpenes were done mostly on Streptomyces spp. and only one terpene cyclase from Proteobacteria has been functionally characterised, the 2-methylenebornane synthase from Pseudomonas fluorescens Pf0-1 (Chou et al. 2011).
Recently, it was found that Collimonas strains (belonging to the class of Beta- Proteobacteria) harbour terpene synthase genes (CPter91_2617 and CPter291_2730; Song et al., 2015). When compared to other functionally characterised terpene cyclases, the Collimonas protein sequences showed maximally 23 % aa-identity to any previously characterised bacterial terpene cyclase. As the product specificity of mono- and sesquiterpene cyclases cannot be predicted from their primary biochemical characterisation, CPter91_2617 and CPter291_2730 genes were expressed in E. coli and tested for cyclization reactions using FPP, GPP or GGPP as substrates. When produced terpenes were analysed by gas chromatography–mass spectrometry (GC-MS), both Collimonas enzymes converted FPP to a mix of sesquiterpenes and sesquiterpene alcohols. The major peak was putatively identified as germacrene d-4-ol by comparison of the mass spectrum to a spectral library, as well as several minor sesquiterpene peaks which included δ-cadinene. When GPP was applied as a substrate, the production of two monoterpenes identified as β-pinene and β-linalool was observed (Song et al., 2015). The sesquiterpene products suggest that they are functionally related to plant and fungal cadinene/cadinol and germacrene d-4-ol synthases (Lauchli et al. 2014; Yoshikuni et al. 2006).
Volatile sulphur compounds play central roles in global sulphur biogeochemical cycles (Naeem 1998). The structural diversity of these compounds is large, ranging from relatively small compounds such as methanethiol, dimethyl sulphide (DMS), dimethyl disulphide (DMDS) and dimethyl trisulphide (DMTS) to more complex volatiles, such as 2-methyl-4,5-dihydrothiophene (Effmert et al. 2012; Splivallo et al. 2011). Two main biosynthetic pathways, both relying on l-methionine catabolism, have been described: the one-step conversion of l-methionine to methanethiol by methionine c-lyase or by other C-S lyases (e.g. cystathionine c-lyase) and a two-step pathway, initiated by l-methionine transamination to 4-methylthio- 2-oxobutyric acid, which is then converted to 3-(methylthio)propanal via decarboxylation. Alternatively, l-methionine is reduced to 4-methylthio-2-hydroxybutyric acid which ultimately results in the formation of methanethiol (Splivallo et al. 2011). DMS emission requires the gene dddD which was predicted to add CoA to dimethylsulphoniopropionate (DMSP), a key step preceding subsequent cleavage and release of DMS (Todd et al. (2007). DMS is mostly made via bacterial catabolism of dimethylsulphoniopropionate (DMDP). This so-called Ddd+ trait is found in several genera belonging to the phylum Proteobacteria (Peng et al. 2012; Todd et al. 2011, 2012). Microbial sulphur volatile compounds such as DMS, DMDS and DMTS play important roles in plant–microbe and interspecific fungal–bacterial and bacterial–bacterial interactions (see below).
2.3 Volatiles Emitted by Plant Roots
Chemical analyses of essential oil extracts show that roots are a rich source of plant volatiles. For example, vetiver grass (Chrysopogon zizanioides) root extracts, which are traditionally used in the perfume industry, may contain up to 300 different volatile compounds (Belhassen et al. 2015). However, whether these volatiles are emitted in the rhizosphere in vivo and in the same ratios as they are present in the root is as yet unknown (Peñuelas et al. 2014a, but see Jassbi et al. 2010). Thus instead of listing all possible volatile compounds that have been identified in roots and root extracts, we mainly focus on volatiles that have been shown to be emitted by roots into the rhizosphere or the root headspace as measured by non-destructive sampling techniques.
Small Organic Volatiles
Carbon dioxide (CO2) is one of the smallest volatiles that roots excrete as a result of their own respiration (Ghashghaie and Badeck 2014). In addition, plant roots may emit various alcohols, ketones and esters, such as methanol, acetone and ethyl acetate (Danner et al. 2015; Steeghs et al. 2004). These small organic volatiles are considered to be by-products of the plant’s primary processes. For example, the production of methanol is correlated with the activity of methylesterases involved in the loosening of cell walls which allows root growth and the release of root border cells (Driouich et al. 2013). Furthermore, aldehydes and short-chain fatty acid-derived C6 volatiles, such as hexanal and hex-2-en-1-ol, have also been detected in the root headspace (Peñuelas et al. 2014a; Steeghs et al. 2004). These compounds are produced from fatty acids such as linoleic or linolenic acid, which serve as substrates to 13-lipoxygenases (LOX). Plants contain several different LOX enzymes allocated to different plant organs including the roots, and with different functions in the response to abiotic and biotic stress signalling (Allmann et al. 2010; Grebner et al. 2013).
Terpenoids
Similar to their biosynthesis by microbes, the synthesis of terpenoids in plants may take place via the precursors DMAPP, GPP, FPP or GGPP. In plants, however, terpenoid synthesis can either take place via the mevalonic acid (MVA) or the methylerythritol 4-phosphate (MEP) pathways. In plant cells, these two pathways are compartmentalised; the MEP pathway is localised in the plastids, whereas the enzymes of the MVA pathway are localised in the cytosol (Gutensohn et al. 2013). Interestingly, this separation may be a remnant of evolution past when ancient eukaryotes engulfed cyanobacteria to form a symbiotic complex that evolved into higher plants (Wiesner et al. 2013). It is known that there is some cross-talk between the two biosynthetic pathways, but it is still generally assumed that monoterpenes (C10), diterpenes (C20) and more complex terpenoids, such as gibberellins and chlorophylls, are mainly produced in the plastid via the MEP pathway. Sesquiterpenes (C15), sterols and triterpenes (C30) are mainly produced in the cytosol via the MVA pathway (Gutensohn et al. 2013; Harrison et al. 2013; Peñuelas and Munne-Bosch 2005). Even though this knowledge is mainly based on studies analysing the biosynthesis of flower and leaf terpenoids, it seems that the subcellular localisation of the MEP pathway in root cells is similarly arranged. Genes involved in root-specific mono- and diterpene synthesis in the model plant Arabidopsis thaliana were found to have motifs that predestine them for plastid targeting (Chen et al. 2004; Vaughan et al. 2013). In maize, the root-specific gene farnesyl diphosphate synthase (fpps1) involved in herbivore-induced synthesis of the sesquiterpene (E)-β-caryophyllene indeed appeared to be located in the cytosol (Richter et al. 2015). Emissions of terpenes from the roots can strongly increase upon damage by insect herbivores. This is not only due to passive release of terpenoids from the wounds but involves active expression of terpene synthases (TPS) in the root tissue as well as de novo synthesis of terpenoids (Chen et al. 2004; Rasmann et al. 2005; Richter et al. 2015).
Sulphur- and Nitrogen-Containing Compounds
As well as C-based terpenoids, plants may produce a range of sulphur- and/or nitrogen-containing volatiles. Some of these volatiles are produced by special prefabricated two-component systems consisting of a glycosylated precursor compound and a β-glucosidase. Enzymes belonging to this class catalyse the hydrolysis of a β-glucosidic bond between two carbon moieties or between a carbohydrate and an aglucone moiety (Morant et al. 2008). This reaction results in the release of an aglucone, which may be further converted in bioactive volatiles, especially in the case of cyanogenic glycosides and glucosinolates (Kissen et al. 2009). In cassava roots, for example, cyanogenic glycosides stored in the vacuole react with β-glucosidases upon tissue rupture. This leads to the production of an unstable aglucone, which spontaneously degrades into the highly toxic volatile HCN. Cyanogenesis is a widespread trait and has been found to occur in more than 2600 plant species ranging from gymnosperms to mono- and dicotyledonous species (Morant et al. 2008).
A similar two-component system yielding sulphur- and nitrogen-containing volatiles is found in Brassicaceae. Members of this plant family contain sulphur-containing defence compounds, called glucosinolates. Upon tissue rupture, the glucosinolates in the vacuoles come into contact with myrosinase, a glucosidase that is stored in specialised cells (Bones and Rossiter 2006). As a consequence, sulphur- and/or nitrogen-containing volatiles, such as isothiocyanates (ITCs) and nitriles, are formed. These sulphur- and nitrogen-containing volatiles may serve different functions, among others as defences against insect herbivores, nematodes and (soil) pathogens (Brown and Morra 1997; Caboni et al. 2012; Hopkins et al. 2009). Overall, more than 130 structurally different glucosinolates have been identified to date (Agerbirk and Olsen 2012), and their chemical structure, together with the presence or absence of nitrile-specifier enzymes and the pH at the site of the reaction, greatly determines the types of volatiles that are formed (Halkier and Gershenzon 2006). Interestingly, overall root glucosinolate concentrations are higher than those in shoots, and specific glucosinolates, such as 2-phenylethyl glucosinolate or 1-methoxy-indol-3-ylmethyl glucosinolate (neoglucobrassicin), are more prominent in belowground organs (van Dam et al. 2009). This suggests a specific role for the volatile products that are formed in the rhizosphere. Indeed, 2-phenylethyl ITC was shown to confer resistance to root-feeding nematodes and soilborne pathogens (Potter et al. 1998; Sarwar et al. 1998).
In addition to ITCs, roots of Brassica species may produce a range of other sulphur-containing volatile compounds such as methanethiol, DMS, DMDS and DMTS (Crespo et al. 2012). Depending on the species, their emissions may increase upon root herbivory or mechanical wounding (van Dam et al. 2012). In plants, these sulphides may either result from thiol methyltransferases involved in the catabolism of glucosinolate conversion products, possibly to avoid autotoxicity (Attieh et al. 2000), or a combination of cysteine-sulphoxide lyases involved in the final degradation steps of the nonprotein amino acid S-methyl-l-cysteine (Chin and Lindsay 1994). Both ITC and sulphides from Brassica roots are found to be emitted constitutively at low levels, probably due to some spontaneous or chemically driven degradation of the precursor glucosinolate or to the continuous turnover of root tips (Bones and Rossiter 2006).
Another class of well-studied sulphur-containing rhizosphere volatiles produced by plant roots are thiophenes. These compounds are produced in the roots of Asteraceae, especially species of the genus Tagetes (Marigolds) (Croes et al. 1989; Jacobs et al. 1994). Thiophenes are well known for their nematicidal, antimicrobial and insecticidal effects, though soil microbial communities as a whole do not seem to be affected by marigold cultures (Caboni et al. 2012; Leger and Riga 2009). In situ analyses using passive sampling approaches combined with GC-MS analyses have shown that thiophenes are constitutively emitted into the rhizosphere by Tagetes roots (Mohney et al. 2009; Tang et al. 1987). Tagetes roots contain specialised structures, such as secretory channels in the root endodermis, which would allow a constant emission of thiophenes into the rhizosphere (Sacchetti et al. 2001).
Volatile Phytohormones
Several volatile signalling hormones are emitted by roots. Ethylene is by far the most studied volatile plant hormone. 1-Aminocyclopropane–carboxylic acid (ACC) is the direct precursor of ethylene and is synthesised from methionine. The enzyme ACC oxidase catalyses the final step in the synthesis of ethylene (Gepstein and Kieber 2010). It serves as a signalling hormone involved in biotic and abiotic stress responses, including shade avoidance, leaf senescence and the formation of root hairs (Gepstein and Kieber 2010; Pierik et al. 2006). Maize root systems constitutively emit ethylene, which is reduced when the plants are infested either aboveground or belowground by herbivores (Robert et al. 2012). Abiotic stresses, such as waterlogging, may enhance ethylene emissions by roots. For example, ethylene accumulates in Solanum dulcamara plants subjected to water logging, leading to the formation of aerenchymous adventitious roots that facilitate gas exchange underwater (Dawood et al. 2014).
Methyl salicylate (MeSA) is the volatile methylated form of the phytohormone salicylic acid (SA), which is produced via the shikimic acid pathway. The enzyme S-adenosyl-l-methionine:salicylic acid carboxyl methyltransferase (SAMT) converts SA into volatile MeSA (Dudareva et al. 2004). In aboveground plant organs, MeSA is involved in responses to biotrophic pathogens and piercing–sucking herbivores (De Vos et al. 2005). Aboveground MeSA is often induced by herbivore feeding and consequently is used by natural enemies as a cue to localise their host (De Boer et al. 2004; Kpoviessi et al. 2011). There is only indirect evidence that MeSA may play a similar role belowground. Roots of poplar trees, for example, contain a methyltransferase with high homology to SAMT that is able to convert SA into MeSA (Zhao et al. 2009). In leaves, the expression of this methyltransferase was strongly induced by wounding, SA and methyl jasmonate (MeJA) application; however, this was not tested for expression in roots (Zhao et al. 2009). In hairy root cultures of Atropa belladonna, SAMT activity was increased when the cultures were induced with SA (Fukami et al. 2002).
Taken together, these results suggest that methyltransferase gene activity and the production of MeSA by roots may indeed play a role in rhizosphere communication (Fukami et al. 2002; Loreto and Schnitzler 2010; Zhao et al. 2009). However, direct evidence that MeSA is emitted in the rhizosphere is lacking. Similarly, reports on the emission of MeJA in the headspace are scarce. Artemisia tridentata roots were found not to emit MeJA, even though it is one of the most prominent volatiles produced by the shoots (Jassbi et al. 2010). According to a recent review, the necessary enzymes are not found in roots, despite the presence of the LOX pathway and the fact that roots respond well to MeJA treatments (Peñuelas et al. 2014a).
3 The Ecological Role of Volatiles in the Rhizosphere
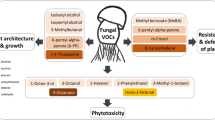
Volatiles play a versatile role in communication between the various members of the soil community. The interactions they are involved in and the volatiles that have been identified as critical actors are summarised in Fig. 8.1 and discussed in the following sections.
Schematic overview illustrating the versatile roles of soilborne volatiles as currently documented in the scientific literature. Volatiles are released by the different groups of organisms in the rhizosphere, i.e. plant roots, bacteria and fungi. The direction of the volatile emission is indicated by arrows that point towards the receivers, which also include predators, insects and nematodes. Next to the arrow, the effect on the receiver is stated. The compound classes reported in the literature to mediate these effects are specified. Abbreviations: DMDS dimethyl disulphide, HCN hydrogen cyanide, ITCs isothiocyanates. Design: Alexander Weinhold, iDiv and Kimberly Falk (moveslikenature.com)
3.1 Microbial Volatiles and Their Effect on Fungi and Oomycetes
Although the importance of volatiles as major fungistatic compounds has long been recognised (Hora and Baker 1970, 1972), this topic has received more extensive research attention in recent years. Surveys of soil bacteria have reported that 30–60 % of soil isolates can produce fungus-inhibiting volatiles (Wheatley 2002; Zou et al. 2007), and that these organisms span a wide phylogenetic spectrum, including members of the Alcaligenaceae, Bacillales, Burkholderia, Collimonas, Micrococcaceae, Pseudomonas, Rhizobiaceae, Serratia, Xanthomonadaceae and many others (Blom et al. 2011a; Effmert et al. 2012; Kai et al. 2007; Zou et al. 2007). Compared to the plant response to bacterial volatiles, which has almost exclusively been restricted to Arabidopsis, assays investigating the response of fungi to bacterial volatiles have tested a broad range of phytopathogenic fungi and oomycetes (Effmert et al. 2012; Garbeva et al. 2014b; Kai et al. 2007). Germination of fungal spores and hyphal growth can be strongly inhibited by bacterial volatiles. Furthermore exposure to bacterial volatiles has been reported to change fungal morphology, enzyme activity and gene expression (Garbeva et al. 2014b; Kai et al. 2008; Vespermann et al. 2007). Most work on microbial volatiles to date is done in vitro under nutrient-rich conditions (Kai et al. 2010; Weise et al. 2012) and may not be representative for the conditions that prevail in the natural environment. Recently Garbeva et al. (2014b) revealed that volatile production by Collimonas sp. in sand containing artificial root exudates differs from that on 1/10 TSBA agar plates. More than 45 % of the volatiles released by Collimonas on 1/10 TSBA were sulphur-containing volatiles, whereas the majority of volatiles released from the root exudate containing sand were ketones, aromatic volatiles and esters (Garbeva et al. 2014b).
Studies have tested many compounds individually over very different concentration ranges and with varying application modes. Some volatiles repeatedly showed inhibitory effects, including hydrogen cyanide (HCN), DMDS, DMTS, benzothiazole, benzaldehyde, benzonitrile and 2-undecanone (Fig. 8.1; Effmert et al. 2012; Garbeva et al. 2014b; Weisskopf et al. 2011). Hydrogen cyanide produced by some Pseudomonas species (such as P. fluorescens CHA0) was confirmed to be directly involved in the biocontrol of Thielaviopis-induced root rot of tobacco (Voisard et al. 1989). Application of DMDS produced by Bacillus cereus strains significantly protected tobacco plants against Botrytis cinerea and maize against Cochliobolus heterostrophus (Huang et al. 2012). Fungal volatiles can also have inhibitory effects on other fungi. For example, the endophytic fungi Muscodor albus and Oxyporus latemarginatus strongly inhibited growth of several plant pathogenic fungi including Botrytis cinerea and Rhizoctonia solani (Strobel et al. 2001).
Several independent studies have observed high variation in fungal sensitivity to bacterial volatiles (Effmert et al. 2012; Garbeva et al. 2014b; Kai et al. 2007, 2008; Weisskopf et al. 2011). For example, testing a range of saprotrophic and plant pathogenic fungi, it was revealed that Rhizoctonia solani, Fusarium culmorum and Pythium ultimum were the most sensitive, while the saprotrophic fungi Chaetomium sp., Mucor hiemalis and Trichoderma harzianum were the most resistant (Garbeva et al. 2014b). This confirms previous reports on difference in fungistasis sensitivity between pathogenic and saprotrophic fungi (Garbeva et al. 2011).
3.2 Microbial Volatiles Produced as a Result of Interactions with Other Microbes
Recently, several independent studies have reported that the production of specialised metabolites by soil bacteria is the direct result of interactions with other microorganisms in their immediate vicinity (Traxler et al. 2013; Tyc et al. 2014). This explains the fact that the genomes of soil and rhizosphere bacteria contain numerous cryptic gene clusters encoding genes involved in the production of secondary metabolites that are not expressed during growth under typical laboratory conditions.
Some volatiles appear to be emitted as result of microbial interactions. Recently, Minerdi et al. (2008) observed that antagonistic interactions between two Fusarium oxysporum strains were related to volatile production. Interestingly, the inhibiting volatiles were only produced when the antagonistic strain was associated with a consortium of bacterial species. The origin of the volatiles, i.e. bacterial or fungal, remained unclear, but the need for the bacterial–fungal association was evident (Minerdi et al. 2008). Furthermore, the composition of volatiles produced by a mixture of bacterial species can differ from those produced by each bacterial monoculture (Garbeva et al. 2014a). Recently Hol et al. (2015) revealed that less abundant (so-called ‘rare’) bacterial species play an important role in antifungal volatile production. The loss of rare soil bacteria affected the production of antifungal volatiles, an important factor in the natural control of soilborne pathogenic fungi (Hol et al. 2015). Furthermore, small shifts in soil microbial community composition can lead to significant shifts in volatile compositions (Schulz-Bohm et al. 2015). Microbial volatiles play important roles in the rhizosphere as infochemicals affecting the behaviour, populations and gene expression of responding organisms. For example, bacterial volatiles play an important regulatory role in mycorrhizal network establishment (Bonfante and Anca 2009) and volatiles from mycorrhiza helper bacteria (MHB) can promote the growth of ectomycorrhizal fungi (Schrey et al. 2005).
To obtain insight into the importance of interspecific volatile interactions between soil bacteria, Garbeva et al. (2014a) performed several microcosm experiments mimicking the natural nutritional heterogeneity in soil in which the model bacteria P. fluorescens grown on nutrient-limited agar was exposed to volatiles produced by four phylogenetically different soil bacteria growing in sand containing artificial root exudates. The main research questions addressed were: (1) Do rhizobacteria protect their ‘territory’ from potential rhizosphere invaders by producing volatiles that suppress bacteria outside the rhizosphere? (2) Can bacteria outside the rhizosphere profit from the volatiles produced by rhizosphere-inhabiting bacteria? Their results revealed that bacterial volatiles stimulated rather than inhibited the growth of P. fluorescens. A genome-wide microarray-based analysis revealed that exposure to bacterial volatiles had clear effects on gene expression in P. fluorescens and that the change in gene expression differed among the different volatile-producing bacterial species. Besides other transcriptional changes, such as those assigned to energy production and conversion, bacterial volatiles appeared to induce a chemotactic motility response in P. fluorescens but also an oxidative stress response. A more detailed study revealed that some of the volatile-producing bacteria triggered antimicrobial secondary metabolite production in P. fluorescens (Garbeva et al. 2014b). The volatile-triggered antibiotic production in P. fluorescens pointed to a strategy to combine movement (chemotaxis and motility genes) with increasing competitive strength (antibiotics) to invade into the nutrient-providing rhizosphere zone.
Volatiles may also be involved in tritrophic interactions involving bacteria, fungi and nematodes as shown by the work of Son et al. (2009). Paenibacillus polymyxa and P. lentimorbus exhibited strong antifungal activities, interfering with interactions between the nematode Meloidogyne incognita and the fungus Fusarium oxysporum which significantly reduced nematode infestation of tomato plants (Son et al. 2009). Recently it was reported that bacterial volatiles may interfere with the quorum sensing of other phylogenetically different bacteria due to suppression of the transcription of AHL synthase genes (Chernin et al. 2011). DMDS was identified as one such quorum sensing inhibiting compound (Fig. 8.1; Chernin et al. 2011). The same compound was reported to stimulate bacterial growth, whereas it completely inhibits fungal growth (Garbeva et al. 2014a, b; Kai et al. 2007).
3.3 Effect of Microbial Volatiles on Plants
Over the last decade, evidence has accumulated that plants respond strongly to volatiles produced by microorganisms. Most of the research carried out so far has investigated the impact of bacterial volatiles on the model plant A. thaliana. This has revealed that, without physical contact, bacteria are able to drastically alter the plant’s root system development and biomass production, ranging from plant death to a sixfold increase in biomass compared with nonexposed plants. Significant growth promotion of A. thaliana after exposure to complex blends of volatiles emitted by a range of PGPR was reported by Ryu et al. (2003). Using two-compartment Petri dishes where only volatiles can be exchanged between the plant and bacteria, the authors obtained a fourfold growth promotion with two Bacillus strains. The growth promotion effect was ascribed to 2,3-butanediol and acetone, based on the application of pure compounds and based on lack of plant growth promotion after exposure to the volatiles emitted by a strain mutated in the butanediol fermentation pathway (Fig. 8.1; Ryu et al. 2003).
The opposite effect of bacterial volatiles on A. thaliana was reported by Vespermann et al. (2007), where plants exposed to volatiles emitted by Serratia strains were killed within a very short time. The effect was alleviated by addition of charcoal, demonstrating that the killing effects were indeed caused by the emitted bacterial volatiles (Vespermann et al. 2007). More recently Blom and coworkers assessed 42 bacterial strains originating from the soil and rhizosphere for emission of plant growth-modulating volatiles (Blom et al. 2011a, b). All strains were found to emit plant growth-modulating volatiles but with contrasting effects that strongly depended on the growth conditions. Dose-dependent plant growth-promoting effects were observed for several compounds including indole, 1-hexanole and pentadecane. For example, indole was active when applied in very low amounts and toxic when plants were exposed to higher amounts (e.g. 10 μg), while pentadecane was active when applied in high amounts (1 mg).
To understand plant physiological changes caused by exposure to bacterial volatiles, Zhang et al. (2007) applied a microarray approach to analyse genes expressed upon exposure to volatiles emitted by Bacillus subtilis GB03. The transcriptomic analysis revealed differential expression in about 600 genes, with auxin-related genes being particularly affected. Auxin synthesis appeared to be specifically increased in the aerial parts of the plants, but the auxins were actively transported as evidenced by a shift in auxin distribution from the shoots to the roots in response to volatile exposure (Zhang et al. 2007). Genes upregulated by exposure to volatiles emitted by Bacillus subtilis GB03 included ethylene biosynthesis and ethylene response genes, which were further confirmed at the proteome level by Kwon et al. (2010). Plant iron uptake can be increased by exposure to bacterial volatiles. This is linked to the acidifying potential of the produced volatiles, leading to better solubilisation and uptake of iron (Zhang et al. 2009).
One of the few identified volatile compounds showing growth promotion in A. thaliana is indole (Fig. 8.1; Blom et al. 2011a, b). Indole, a hetero-aromatic compound derived from l-tryptophan is emitted by a range of bacteria including PGPR Pseudomonas and Burkholderia (Audrain et al. 2015; Blom et al. 2011a, b; Zamioudis et al. 2013). Indole-producing bacteria were shown to significantly increase lateral root formation and this effect was lost when plants were exposed to indole-deficient bacterial mutants (Bailly et al. 2014). Another common volatile emitted by microorganisms is DMDS (Blom et al. 2011a; Garbeva et al. 2014b; Groenhagen et al. 2014). DMDS is reported to significantly promote plant growth and increase the number of lateral roots and root hairs even at very low concentrations (Meldau et al. 2013). The mechanism of plant growth promotion by DMDS was related to direct increase of sulphur supply. Furthermore, DMDS supplementation significantly reduced the expression of sulphur-assimilation genes as well as methionine biosynthesis and recycling in tobacco plants (Meldau et al. 2013). In contrast to indole and DMDS, some microbial volatiles like hydrogen cyanide and ammonia were determined to be deleterious (Blom et al. 2011a; Kai et al. 2010; Wenke et al. 2010).
The effect of microbial volatiles may be strongly dependent on the ontogenetic stage of the plant. When A. thaliana seeds were exposed to volatiles emitted by fungal isolate Trichoderma atroviride for 14 days, reduction in plant size, formation of necrotic lesions and loss of chlorophyll was observed (Lee et al. 2015). However, when A. thaliana seedlings were exposed to volatiles produced by the same fungus under the same conditions, they exhibited significant increases in growth and chlorophyll production. Similarly, volatile mixtures emitted from the biocontrol fungus Trichoderma viride enhanced growth of A. thaliana (Hung et al. 2013) and volatiles emitted by Cladosporium cladosporioides enhanced growth of tobacco plants (Paul and Park 2013). Overall, the plant’s response to growth-promoting volatiles seems to be mediated by auxin, in part due to better iron acquisition and photosynthesis. Furthermore, increased resistance to pathogens can be conferred by exposure to bacterial volatiles, through induction of ISR (induced systemic resistance), and the growth of phytopathogenic fungi can be reduced by exposure to microbial volatiles (see Sect. 8.3.1). Most bacteria activate ISR in plants via a SA-independent pathway involving JA and ethylene signalling. Volatiles produced by Bacillus amyloliquefaciens triggered ISR through an ethylene-independent signalling pathway, whereas volatiles produced by Bacillus subtilis appear to do this via an ethylene-dependent pathway, albeit independent of the SA or JA signalling pathways (Ryu et al. 2004).
In general, studying volatile-mediated interactions between plants and microorganisms is challenging because of the variation in volatile emission dependent on the physiological state of the producing microorganism and environmental conditions. Additionally, the methods used to study volatile-mediated plant–microbe interactions can lead to different responses in plants and contrasting results, as recently indicated by Lee et al. (2015). Furthermore, plant-associated microorganisms can affect the blend of volatiles released by plants. For example, tomato plants inoculated with the fungal endophyte Acremonium strictum emitted diverse terpenes and sesquiterpenes in significantly lower amounts than endophyte-free plants (Jallow et al. 2008). Additionally, endophytic fungi that live within plants can produce many metabolites including volatiles that benefit the host plant. For example Phoma spp. isolated from creosote bush produce volatiles that help the shrub to survive harsh desert habitats (Strobel et al. 2011). In a recent study, Peñuelas et al. (2014b) revealed that phyllosphere microbiota can significantly influence plant terpene emissions. Removing floral microbiota of Sambucus nigra L. affected both the quality and quantity floral terpene emission (Peñuelas et al. 2014b). Similar studies on the effect of the rhizosphere microbiome as a whole on root volatile production are missing.
3.4 Effect of Plant Volatiles on Bacteria
Volatiles produced by plant roots may exert short (μm)- and long (mm)-distance effects on microbes in the rhizosphere. As mentioned above, only a few studies have shown that volatiles produced by roots are also emitted in the rhizosphere (Cecchini et al. 2010; Del Giudice et al. 2008; Kpoviessi et al. 2011; Steeghs et al. 2004; Yeo et al. 2013). Based on in vitro assays, the bioactivities of root-specific volatile terpenoids and phenolic compounds have been associated primarily with growth-inhibiting effects (Wenke et al. 2010). Terpenes and other root-derived VOCs most likely serve multiple roles as C-sources, defence metabolites and chemoattractants. Degradation of plant monoterpenes such as geraniol by soil microbial activity has been demonstrated (Owen et al. 2007), and rhizobacteria such as Pseudomonas fluorescens and Alcaligenes xylosoxidans have been shown to metabolise α-pinene as their sole carbon source (Kleinheinz et al. 1999). Del Giudice et al. (2008) also reported that bacteria associated with the roots of vetiver grass (V. zizanioides) use sesquiterpenes as a carbon source. Many bacterial species use quorum sensing to coordinate gene expression according to the density of their local population. Some plant volatiles may interfere with bacterial quorum sensing (QS) and this can be in both directions. For example, plant volatiles like (+)-enantiomers of carvone, limonene and borneol stimulated bacterial QS, while compounds like α-terpineol and cis-3-nonen-1-ol completely inhibited bacterial QS (Ahmad et al. 2015).
3.5 Plant Volatiles in Belowground Plant–Herbivore Interactions
As for aboveground produced volatiles, root volatiles may serve as cues for herbivores to locate their host plant. Belowground herbivores may use CO2 gradients in the soil to locate roots (Johnson and Nielsen 2012); however, there is some debate on the reliability of such a generic cue. It does not allow herbivores to discriminate between hosts and (toxic) non-hosts, and many other nontarget organisms in the soil produce CO2 (Erb et al. 2013). Therefore more specific plant volatiles may be better cues for herbivores searching for a suitable host plant. At the same time, these more specific plant volatiles may serve as direct or indirect defences. In particular, volatile products resulting from glucosinolate or cyanogenic glycoside conversion, i.e. cyanides and isothiocyanates, may serve as direct plant defences. They have been found to be toxic or noxious to a wide range of belowground herbivores and pathogens (Hopkins et al. 2009; Kissen et al. 2009; Potter et al. 1998), though specialist herbivores possessing mechanisms to overcome the toxicity of these compounds may use them to locate their host plant. For example, larvae of cabbage white butterflies (Pieris spp.) possess specific enzymes to interfere with the formation of ITC which renders the plant less toxic (Wittstock et al. 2003). The adults indeed use ITCs typically produced by cabbages and mustards to locate host plants for oviposition (Hopkins et al. 2009). Similarly, root-feeding herbivores specialised on Brassica species, such as the larvae of the cabbage root fly (Delia spp.), use ITC to orient towards their food plant in the soil (Fig. 8.1; Kostal 1992). These larvae do not have their own detoxification mechanism but rely on gut microbes to detoxify 2-phenylethyl ITC which is produced upon larval damage (Crespo et al. 2012; Welte et al. 2015).
Plant volatiles are more often studied in their role as indirect plant defences, i.e. to attract natural enemies or predators of herbivores. The evolutionary-ecological framework of indirect defences against arthropod herbivores and the role of plant volatiles therein have been elucidated for aboveground tritrophic interactions since the late 1980s (Dicke and Sabelis 1988; Vet et al. 1991). In one of the first studies showing that indirect defences via volatile emissions function belowground as well, van Tol et al. (2001) reported that entomopathogenic nematodes (EPNs) were attracted to the roots of Thuja occidentalis damaged by larvae when given a choice in a Y-tube olfactometer filled with sand. At the time, no specific volatiles were identified. A few years later, it was found in various other plant species that roots damaged by herbivores emit specific mono- and sesquiterpenes (Ali et al. 2010; Rasmann et al. 2005; Steeghs et al. 2004). For example, when damaged by the corn rootworm Diabrotica virgifera virgifera, maize roots emit the sesquiterpene (E)-β-caryophyllene which attracts EPN that infest and kill the root-feeding larvae (Fig. 8.1; Rasmann et al. 2005). Interestingly, commercial cultivars from the USA have lost the ability to produce this compound, suggesting that the ability to attract natural enemies to the rhizosphere can be selected for (Degenhardt et al. 2009). Restoring the ability to produce (E)-β-caryophyllene in one of these varieties, however, also increased its susceptibility to a fungal disease (Fantaye et al. 2015), underscoring the multifaceted function of each volatile compound. Similarly, citrus roots infested by root-feeding herbivores recruit EPN via the emission of several mono- and sesquiterpenes detected in the rhizosphere (Ali et al. 2010). Interestingly, the response of other organisms in the rhizosphere did not always follow this pattern; bacterivorous nematodes that feed on the cadavers of EPN infested larvae displayed similar behaviours as EPN, whereas nematopathogenic fungi did not seem to respond to these cues (Ali et al. 2013).
Another well-studied plant–herbivore system is the interaction of milkweeds (Asclepias spp.) with their specialist root herbivores. Apart from the production of latex containing toxic cardenolides, the roots of these plants also produce various volatiles upon induction by root feeders (Rasmann et al. 2011). These volatiles attract EPN that reduce the impact of the herbivores on plant performance, showing that these rhizosphere volatiles serve as true sensu stricto defences (Karban and Baldwin 1997). In addition, there have been several studies showing that aboveground predators or parasitoids of root herbivores are attracted to infested plants via root-emitted volatiles. For example, ground-dwelling Aleochara beetles predating on eggs and larvae of D. radicum are attracted by DMDS, a volatile organic compound specifically emitted at high levels by root fly-infested Brassica roots (Fig. 8.1; Crespo et al. 2012; Ferry et al. 2007; van Dam et al. 2012). Such volatile cues emitted by herbivore-infested roots could potentially be used by other ground-dwelling or belowground predators such as ants, predatory mites, spiders and even mammals such as moles and rodents (Johnson and Rasmann 2015; van Dam 2009). However, experimental data to support this hypothesis are still lacking.
3.6 Plant–Plant Communication
Plants are able to respond to their neighbours in order to avoid competition for light and nutrients. Aboveground, this process is often associated with light perception and involves the emission and perception of ethylene (Kegge et al. 2015; Pierik et al. 2006). In the rhizosphere, root exudates and compounds therein play an important role. One well-studied mechanism for plant–plant communication in the rhizosphere is allelopathy. Allelopathy is a chemical–ecological process in which the secretions or emissions of one plant reduce growth or even kill another plant (Inderjit et al. 2011). Several plant volatiles, including mono- and sesquiterpenes, thiophenes and ITC, have been shown to possess allelopathic properties (Fig. 8.1). In fact, several Brassica species are commonly used for biofumigation purposes as the ITCs that are formed upon ploughing reduces weed germination (Vaughn and Boydston 1997). Moreover, it has been shown that sagebrush plants emit various volatiles from the roots that may have an allelopathic effect. MeJA was not among them, even though it has a strong inhibitory effect on the germination of other species (Jassbi et al. 2010). Interestingly, the zone of influence of the allelopathic compounds may be increased by mycorrhizal associations. In an experimental set-up using Tagetes tenuifolia plants in a mesocosm, it was shown that common mycorrhizal networks connecting plants may enhance thiophene accumulation away from the rhizosphere of the plant (Barto et al. 2011). In contrast to most studies assessing allelopathic effects of root volatiles, Barto et al. (2011) used a ‘phytometer’ approach to show in vivo that competing plants suffer biomass reductions when growing in soils with higher thiophene accumulations.
Another interesting aspect related to plant–plant communication is the plasticity in root placement. Plants growing next to each other may adapt their root allocation patterns according to their neighbour’s identity and even the level of relatedness (Depuydt 2014; Semchenko et al. 2014). It has been experimentally assessed that root exudates can affect the placement of roots away from competitors or kin (Schmid et al. 2013; Semchenko et al. 2014). In recent reviews, most compounds that are listed as being important in such root allocation processes are water soluble and non-volatile (Biedrzycki and Bais 2010; Depuydt 2014). However, based on what is known about the role of volatiles in aboveground plant–plant communication and self-recognition (Heil and Land 2014; Karban et al. 2014a, b), a call for more research on the role of plant volatiles in belowground plant–plant interactions seems reasonable (Biedrzycki and Bais 2010).
4 How to Measure Volatiles in the Soil?
Studying the volatiles emitted in the rhizosphere is a challenging task for several reasons. Compared to the aerial headspace of plants, the soil is a dense and heterogeneous matrix, so sampling of rhizosphere volatiles requires more preparation. The first point to consider is the composition of the substrate. For example, the adsorption capacity and smaller grain size of clay will influence the distribution and diffusion of volatile compounds (Barnett and Johnson 2013). In addition, the capacity of the soil to bind water will influence the result of soil volatile trapping experiments, as soil humidity affects the diffusion and distribution of volatiles in the rhizosphere (Hiltpold and Turlings 2008). In a later phase, water in the traps may interfere with chemical analysis by gas chromatography. Those factors may be partially controlled in a greenhouse experiment but not in more realistic field experiments.
Moreover, the properties of the biological system as a whole are of importance for the sampling strategy. In a single species experiment, volatiles emerging from the plant roots and those emerging from the soil can be easily separated by including ‘soil blank’ samples. When it comes to identifying the volatile profiles of roots growing in a plant community, it gets more difficult. The first question would be how to separate the volatiles of different plant species, especially when the roots are intertwined. Another challenge is to collect plant volatiles in vivo. There are several approaches described in the literature, e.g. dynamic and static headspace sampling of roots in mesocosms, but most of these can only be performed under laboratory conditions. As for every experiment dealing with living organisms, the biggest challenge might be to do the analysis in a non-invasive manner. This is particularly difficult since most of the existing volatile trapping methods rely on inserting sampling devices in the soil next to the root, thereby possibly damaging the root tissues. In the next sections, we discuss different sampling approaches that have been used to sample root or rhizosphere volatiles and evaluate their suitability for root samplings based on published experiments (see also Table 8.1).
4.1 Solid-Phase Micro-Extraction (SPME)
Solid-phase micro-extraction (SPME) is widely used for the trapping of aboveground plant volatiles (Yang et al. 2013). The advantage of SPME is that the volatiles are enriched on the fibres, which allows the analysis of trace compounds. A large number of fibres with different adsorptive properties are commercially available. In principle, SPME fibres are easy to handle and can be easily inserted in preformed slots in the rhizosphere. SPME is well suited for determining the spatial distribution of soilborne volatiles around a plant, e.g. by sampling at defined depths or distances from the plant. For example, SPME was used to show that (E)-β-caryophyllene added to sand diffuses over a distance of 10 cm within half an hour (Rasmann et al. 2005). A drawback is that SPME is more expensive, less useful for high-throughput analysis and less suitable for exactly quantifying volatile emissions (Table 8.1). Using SPME, Rasmann et al. (2011) performed a dynamic headspace sampling of root volatiles of milkweed (Asclepias syriaca) released after attack by a root-boring beetle Tetraopes tetrophthalmus and studied their attractiveness to EPN (Rasmann et al. 2011). Prior to trapping the volatiles on SPME fibres, plants were removed from the soil and the roots were washed with tap water. The results showed that a mixture of 15 root volatiles was significantly increased after 4 days of root herbivory. In this study, SPME was performed only on ground root material to analyse the total pool of root volatiles and showed that inducibility of volatiles is negatively correlated to the constitutive levels (Rasmann et al. 2011). SPME was also used to analyse root volatiles of maize, cotton and cowpea (Rasmann and Turlings 2008; Robert et al. 2012). In these studies, however, roots were harvested and ground before analysis.
Weissteiner et al. (2012) used SPME to measure volatiles emitted from oak trees infested with cockchafer larvae Melolontha hippocastani. In addition to SPME sampling, they also used dynamic headspace with thermal desorption tubes. The root volatiles collected by SPME were later used to estimate the concentration for choice assays (Weissteiner et al. 2012). Gfeller et al. (2013) studied the emission of volatiles from barley roots and their effect on wireworms. In contrast to previous studies, roots were left intact even though they were separated from the shoots. Thus, 29 root volatiles could be identified and the authors were able to show that detection was dependent on the cultivation medium (Gfeller et al. 2013). Taken together, these studies illustrate that SPME can be a powerful tool to sample rhizosphere volatiles, especially for trace analysis. The ease of use also makes SPME suitable for field sampling; however, to our knowledge, no study has been published that applies SPME in field experiments.
4.2 Direct Thermal Desorption
In general, direct thermal desorption (TD) is a robust technique to collect plant volatiles. In contrast to SPME, the volatile compounds are adsorbed on trapping material packed in a glass or metal tube. Like the SPME fibres, the adsorbent material can have various compositions depending on the target analytes (Harper 2000). One tube can contain different types of trapping materials (mixed bedding), which increases the range of volatiles that can be trapped (e.g. van Dam et al. 2010). The advantage is that the volatile sample can be analysed as emitted in the field or greenhouse without solvent elution. Another advantage of TD is that samples can be stored over a longer time in capped and cooled tubes. Moreover, recently developed TD instruments allow for sample recollection and enable researchers to perform repeated injections, for instance with different GC columns. Another difference to SPME is that TD tubes have to be used in a dynamic sampling system. Plants are enclosed in glass containers or inert plastic bags to which the trap is attached (Stewart-Jones and Poppy 2006), after which a gas flow is applied by either pushing or pulling air through the tube. A push–pull system is the best option, but this may not be feasible in the field. When working with a dynamic sampling system, the applied flow rates and sampling time are important since they determine the amount of compounds adsorbed. Because TD sampling is often used for assessing environmental air quality, national and international agencies have developed standard methods for sampling procedures. The EPA compendium method TO-17Footnote 1 and ISO 16017Footnote 2 provide a detailed description of the methodologies used for TD volatile trapping.
Disadvantages of TD are the relatively high cost of the equipment and the trapping tubes, even though they can be reused multiple times. TD tubes were used to identify glucosinolate breakdown products in the headspace of Brassica nigra roots infested with cabbage root fly larvae (Crespo et al. 2012). In this study, cooking bags prepared according to Stewart-Jones and Poppy (2006) were used to enclose the root headspace of a potted plant, and TD tubes with mixed Carbopack-Tenax bedding were inserted in the bags (Fig. 8.2). TD tubes can also be used with other sampling materials. In a study on root volatiles of dandelion (Taraxacum ruderalia), laboratory silicone tubing (PDMS) was used to collect root volatiles in specially designed mesocosms and inserted in empty TD tubes before desorption (Eilers et al. 2015). Fifteen volatiles could be extracted from the rhizosphere and identified by GC-MS. This is one of the few studies where volatiles from the rhizosphere were trapped in situ. However, this method, like SPME, is a ‘single-shot’ analysis, where volatiles cannot be resampled.
Overview (a) and a detail (b) of a root headspace sampling set-up using direct thermal desorption tubes (indicated in by the yellow arrow in b). The blue arrows indicate the direction of the airflow. Labels: VP vacuum pump, FC flow controllers, AT air tube. The volume of the root headspace is restricted by mounting a pretreated frying bag around the base of the stem. The tubes are inserted into the bags via slit. Air is pulled over the trap via vacuum pump (‘pull’ system). For more details, see Crespo et al. (2012). Photographs: Nicole M. van Dam
4.3 Volatile Trapping with Subsequent Elution
Dynamic headspace sampling can also be combined with conventional solvent-elution traps. Similar to TD, volatiles from the rhizosphere are directed through a glass or metal tube filled with an adsorbent by a push, a pull or a push–pull system. After trapping, the volatiles are eluted from the trap with a defined amount of organic solvent and analysed on a GC. The advantage of this approach is that at this point standard compounds can be added to the solvent, which allows for the normalisation and exact quantification of the data. Another advantage is that the liquid sample can be stored and injected multiple times. Disadvantages are that it is more labour-intensive, less sensitive due to losses during elution and prone to contaminants and spontaneous conversions in the elution solvent (Table 8.1). Nevertheless, this technique is widely used as no specific TD equipment is needed. For example, solvent-eluted traps were used in a push–pull system to study the emission of citrus root volatiles and their effect on the behaviour of different nematode species (Ali et al. 2011). The authors used a volatile collection apparatus to simultaneously trap below- and aboveground volatiles, allowing a direct comparison of the relationship of both volatile profiles. In a similar study, four major terpenes that were only produced by infested roots were identified (Ali et al. 2010). Finally the same authors conducted a study where they used a soil probe to collect volatiles from infested roots in the field (Ali et al. 2012), a rare example of root volatile trapping outside the laboratory.
4.4 Non-invasive Time-Resolved Measurements
All of the above mentioned methods lack temporal resolution, which is an important factor for the understanding of volatile function in an ecological context. SPME, TD and conventional solvent-elution traps mirror only the time interval of the volatile trapping. Dynamic changes in the volatile bouquet within the trapping interval cannot be resolved. Proton-transfer reaction MS (PTR-MS) overcomes this constraint and allows the measurement of plant and root volatiles in real time, which reveals how volatile emissions change over an ecologically relevant timescale (Danner et al. 2012; Steeghs et al. 2004). Besides this great advantage, there are several drawbacks (Table 8.1). After proper calibration and optimisation of the system has been achieved (Samudrala et al. 2015), PTR-MS can be successfully used to analyse particular groups of low molecular weight volatiles. This is illustrated by studies analysing the volatile emissions of Arabidopsis or Brassica plants both in vitro and in vivo. PTR-MS was successfully applied to follow the volatile emission dynamics of an A. thaliana root culture (Steeghs et al. 2004). Interestingly, when these roots were challenged by a root pathogen or a root-feeding aphid, emissions of the monoterpene 1,8-cineole increased. PTR-MS was also used to analyse the root volatiles of weeds, how they are influenced by an endophytic fungus and the response of root herbivores to the changes in root volatiles. In this study, roots were removed from the soil before analysis (Rostás et al. 2015). PTR-MS in situ analyses of volatiles emitted in the root headspace of various Brassica species subjected to artificial damage or infested with cabbage root fly larvae revealed that various sulphur-containing compounds show specific dynamic patterns depending on the larval instar of the root herbivore (Crespo et al. 2012) or the Brassica species used (van Dam et al. 2012). More recently, using separate cuvettes for sampling roots and shoots, it was shown with PTR-MS that shoot feeders can also significantly enhance DMDS emissions into the root headspace, though not as strongly as local infestation by root-feeding herbivores (Danner et al. 2015). Taken together, these studies illustrate how PTR-MS can acquire time-resolved data on intact plants, even though they were not directly performed in the rhizosphere.
5 Discussion
Understanding complex volatile-mediated interactions belowground is a large and intricate puzzle and any attempt to cover this broad topic will remain incomplete. From the evidence in the current scientific literature, it is clear that the two main producers of volatiles belowground are plants and microorganisms. It should, however, be noted that the current lack of knowledge on the emission of volatiles by other rhizosphere organisms, such as nematodes or earthworms, does not mean that they do not produce volatiles that may be relevant for rhizosphere communication. It rather indicates that these groups are currently understudied with regards to this aspect.
Without doubt, plants are involved in intimate interactions with microorganism during their entire life, starting from its infancy as a seed. Investigation of surface-sterilised seeds revealed that the majority of plant species seeds were colonised by bacteria (Cankar et al. 2005; Compant et al. 2005; Graner et al. 2003; Mundt and Hinkle 1976). Molecular methods for detecting seed endophytes (Johnston-Monje and Raizada 2011) revealed distinct community structure between plants as well as between different geographic locations. The zone of influence of the germinating seed has been named the ‘spermosphere’ (Nelson 2004), and the interactions in the spermosphere can be important first steps of the association between bacteria and plant. However, the role of volatiles in spermosphere interactions has not been studied so far.
As indicated in this chapter, several volatiles such as terpenoids and sulphur compounds are commonly produced and emitted by both plant roots and microorganisms. Genomic studies reveal that both groups of organisms carry many genes responsible for the synthesis of such volatiles, possibly with a common evolutionary origin. Based on these commonalities, it is possible that terpenoids and volatile sulphur compounds are a ‘lingua franca’ for inter-kingdom communication between plants, bacteria and fungi.
However, there are several open questions regarding this hypothesis. First, how can an organism distinguish the source of such a common signal? Possibly, this can be achieved by sensing the concentration of the signal similar to what has been reported for quorum sensing. Additionally, the chemical background of other volatiles and non-volatile compounds present in the environment may be important. In aboveground tritrophic interactions, it has indeed been shown that the composition of the background volatile profile is important for the attractiveness of a single volatile to an egg parasitoid (Mumm and Hilker 2005). Finally, for most volatiles, it is as yet unknown exactly how they are perceived by plants. Whereas there is an extensive body of literature on olfactory receptors in insects and mammals, molecular receptors for the perception of terpenoids, for example, have not yet been identified in plants, even though the roles of volatiles in plant–plant interactions were one of the first to be recognised (Heil 2014). The current view is that due to their lipophilic nature, volatiles such as mono- and sesquiterpenes may interfere with membrane structures, thereby causing depolarization of the membranes and triggering Ca2+ signalling in plants (Heil 2014; Chap. 12). However, this constitutes a very unspecific mechanism, which raises the question whether such volatiles per se may serve as reliable infochemicals at all (Dicke and Sabelis 1988). It is thus not surprising that the search for volatile receptors in plants was recently coined as one of the ‘hot topics’ in the field (Heil 2014). For microbes, it may be easier to elucidate how volatiles are perceived, as they are more easily transformed and screened for mutations in a high-throughput manner. This facilitates the generation of transformants overexpressing certain volatile production genes, mutants lacking a response to certain volatiles or the use of genetic markers, such as green fluorescent protein (GFP), which may reveal genes that are activated during volatile communication. In such experiments, it will be of utmost importance to mimic common natural conditions, especially with regards to the nutrient level. It was found that the emission of a certain volatile is conditional and may not occur under the nutrient-rich conditions (Garbeva et al. 2014a, b).
A further major challenge is to correctly identify the origin of any particular volatile belowground, especially since many volatile compounds are produced only as a result of interactions. For plant–insect interactions it, has been known for decades that specific volatiles are only produced by a plant when attacked by an herbivore (Heil 2014). The same seems to be true for soil-dwelling bacteria and fungi that respond to each other’s presence by producing (antibiotic) volatiles (Garbeva et al. 2014b; Kai et al. 2007). It is one thing to sample and detect such compounds when both interaction partners are growing as isolates in a Petri dish on different sides of a divider, but it will be quite another to identify individual compounds in a fully populated rhizosphere where the organisms of interest may not be the most abundant and many other organisms may interfere with the communication. The latter may apply when other bacterial species in the rhizosphere consume or convert the volatile signal before it has reached the receiver. The same may be true for plant-emitted compounds. Thus, when sampling living soils for volatiles, the volatile profile that is found is mostly a mix of originally emitted compounds and catabolic products thereof.
One approach to distinguish the originals might be to first extract roots destructively or to measure emissions from a sterile plant. However, the medium in which the plants are grown strongly affects the volatile profiles (Jassbi et al. 2010). In that sense, labelling organisms with stable isotopes may be a better approach to follow the fate of volatiles in the rhizosphere. Moreover, the conversion of the original signal by a third-party organism does not necessarily lead to distorted communication, as it may provide additional information on potential competitors. Such studies should preferably be conducted in soil mesocosms where the number of interactors, the conditions and the substrate can be somewhat controlled. It should also be considered that prefabricated slits or tubes for inserting sampling devices into the soil mesocosms would be ideal to prevent root damage.
Currently there is an increased interest in using PGPR and other beneficial microbes such as Trichoderma and mycorrhizal isolates for sustainable agriculture (Mendes et al. 2013; Raaijmakers et al. 2009). However, experimental additions of beneficial microbes to existing soil communities often fail. Apparently, it is difficult for the microbes that are added to establish in the standing soil communities, which prevents farmers from reaping the full benefits. A greater understanding of the role of volatile communication in rhizosphere processes may help to increase the efficacy of such novel approaches. All in all, it is due time to open the black box of the soil a bit further and stick our noses in it to ‘sniff out’ the compounds that mediate the many interactions belowground. This may not only lead to a better understanding of the role of volatiles for belowground communication but also increase the potential to find sustainable solutions for agriculture and novel agrochemicals.
Notes
- 1.
EPA compendium TO-17 (version 1999), see http://www.epa.gov/ttnamti1/files/ambient/airtox/to-17r.pdf.
- 2.
ISO 16017–1:2000, see http://www.iso.org/iso/catalogue_detail.htm?csnumber=29194; for ISO 16017–2:2003, see http://www.iso.org/iso/catalogue_detail.htm?csnumber=29195.
References
Agerbirk N, Olsen CE (2012) Glucosinolate structures in evolution. Phytochemistry 77:16–45. doi:10.1016/j.phytochem.2012.02.005
Ahmad A, Viljoen AM, Chenia HY (2015) The impact of plant volatiles on bacterial quorum sensing. Lett Appl Microbiol 60:8–19. doi:10.1111/lam.12343
Ali JG, Alborn HT, Stelinski LL (2010) Subterranean herbivore-induced volatiles released by Citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. J Chem Ecol 36:361–368. doi:10.1007/s10886-010-9773-7
Ali JG, Alborn HT, Stelinski LL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol 99:26–35. doi:10.1111/j.1365-2745.2010.01758.x
Ali JG, Alborn HT, Campos-Herrera R, Kaplan F, Duncan LW, Rodriguez-Saona C, Koppenhoefer AM, Stelinski LL (2012) Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS One 7. doi:10.1371/journal.pone.0038146
Ali JG, Campos-Herrera R, Alborn HT, Duncan LW, Stelinski LL (2013) Sending mixed messages: a trophic cascade produced by a belowground herbivore-induced cue. J Chem Ecol 39:1140–1147. doi:10.1007/s10886-013-0332-x
Allmann S, Halitschke R, Schuurink RC, Baldwin IT (2010) Oxylipin channelling in Nicotiana attenuata: lipoxygenase 2 supplies substrates for green leaf volatile production. Plant Cell Environ 33:2028–2040. doi:10.1111/j.1365-3040.2010.02203.x
Attieh J, Kleppinger-Sparace KF, Nunes C, Sparace SA, Saini HS (2000) Evidence implicating a novel thiol methyltransferase in the detoxification of glucosinolate hydrolysis products in Brassica oleracea L. Plant Cell Environ 23:165–174
Audrain B, Farag MA, Ryu C-M, Ghigo J-M (2015) Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol Rev 39:222–233. doi:10.1093/femsre/fuu013
Badri DV, Weir TL, van der Lelie D, Vivanco JM (2009) Rhizosphere chemical dialogues: plant–microbe interactions. Curr Opin Biotechnol 20:642–650. doi:10.1016/j.copbio.2009.09.014
Bailly A, Groenhagen U, Schulz S, Geisler M, Eberl L, Weisskopf L (2014) The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J 80:758–771. doi:10.1111/tpj.12666
Bakker PAHM, Berendsen RL, Doornbos RF, Wintermans PCA, Pieterse CMJ (2013) The rhizosphere revisited: root microbiomics. Front Plant Sci 4:7. doi:10.3389/fpls.2013.00165
Barnett K, Johnson SN (2013) Living in the soil matrix: abiotic factors affecting root herbivores. Adv Insect Physiol 45:1–52. doi:10.1016/b978-0-12-417165-7.00001-5
Barto EK, Hilker M, Muller F, Mohney BK, Weidenhamer JD, Rillig MC (2011) The fungal fast lane: common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS One 6:7. doi:10.1371/journal.pone.0027195
Belhassen E, Filippi JJ, Brevard H, Joulain D, Baldovini N (2015) Volatile constituents of vetiver: a review. Flavour Fragance J 30:26–82. doi:10.1002/ffj.3227
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486. doi:10.1016/j.tplants.2012.04.001
Biedrzycki ML, Bais HP (2010) Kin recognition in plants: a mysterious behaviour unsolved. J Exp Bot 61:4123–4128. doi:10.1093/jxb/erq250
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011a) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058. doi:10.1111/j.1462-2920.2011.02582.x
Blom D, Fabbri C, Eberl L, Weisskopf L (2011b) Volatile-mediated killing of Arabidopsis thaliana by bacteria is mainly due to hydrogen cyanide. Appl Environ Microbiol 77:1000–1008. doi:10.1128/aem.01968-10
Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48:521–547
Bones AM, Rossiter JT (2006) The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 67:1053–1067
Bonfante P, Anca IA (2009) Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol 63:363–383. doi:10.1146/annurev.micro.091208.073504
Bonkowski M, Villenave C, Griffiths B (2009) Rhizosphere fauna: the functional and structural diversity of intimate interactions of soil fauna with plant roots. Plant Soil 321:213–233. doi:10.1007/s11104-009-0013-2
Brown PD, Morra MJ (1997) Control of soil-borne plant pests using glucosinolate-containing plants. Adv Agron 61:167–231
Buee M, De Boer W, Martin F, van Overbeek L, Jurkevitch E (2009) The rhizosphere zoo: an overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 321:189–212. doi:10.1007/s11104-009-9991-3
Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, Rauf P, Huettel B, Reinhardt R, Schmelzer E, Peplies J, Gloeckner FO, Amann R, Eickhorst T, Schulze-Lefert P (2012) Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 488:91–95. doi:10.1038/nature11336
Caboni P, Sarais G, Aissani N, Tocco G, Sasanelli N, Liori B, Carta A, Angioni A (2012) Nematicidal activity of 2-thiophenecarboxaldehyde and methylisothiocyanate from Caper (Capparis spinosa) against Meloidogyne incognita. J Agric Food Chem 60:7345–7351. doi:10.1021/jf302075w
Cane DE, Ikeda H (2012) Exploration and mining of the bacterial terpenome. Acc Chem Res 45:463–472. doi:10.1021/ar200198d
Cankar K, Kraigher H, Ravnikar M, Rupnik M (2005) Bacterial endophytes from seeds of Norway spruce (Picea abies L. Karst). FEMS Microbiol Lett 244:341–345. doi:10.1016/j.femsle.2005.02.008
Cardoso C, Ruyter-Spira C, Bouwmeester HJ (2011) Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp. Plant Sci 180:414–420. doi:10.1016/j.plantsci.2010.11.007
Cecchini C, Coman MM, Cresci A, Tirillini B, Cristalli G, Papa F, Sagratini G, Vittori S, Maggi F (2010) Essential oil from fruits and roots of Ferulago campestris (Besser) Grecescu (Apiaceae): composition and antioxidant and anti-Candida activity. Flavour Fragance J 25:493–502. doi:10.1002/ffj.2010
Chen F, Ro DK, Petri J, Gershenzon J, Bohlmann J, Pichersky E, Tholl D (2004) Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol 135:1956–1966. doi:10.1104/pp.104.044388
Chernin L, Toklikishvili N, Ovadis M, Kim S, Ben-Ari J, Khmel I, Vainstein A (2011) Quorum-sensing quenching by rhizobacterial volatiles. Environ Microbiol Rep 3:698–704. doi:10.1111/j.1758-2229.2011.00284.x
Chin H-W, Lindsay RC (1994) Mechanisms of formation of volatile sulfur compounds following the action of cysteine sulfoxide lyases. J Agric Food Chem 42:1529–1536. doi:10.1021/jf00043a026
Chou WKW, Ikeda H, Cane DE (2011) Cloning and characterization of Pfl_1841, a 2-methylenebornane synthase in Pseudomonas fluorescens PfO-1. Tetrahedron 67:6627–6632. doi:10.1016/j.tet.2011.05.084
Collado IG, Sanchez AJ, Hanson JR (2007) Fungal terpene metabolites: biosynthetic relationships and the control of the phytopathogenic fungus Botrytis cinerea. Nat Prod Rep 24:674–686. doi:10.1039/b603085h
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Barka EA (2005) Endophytic colonization of Vitis vinifera L. by plant growth promoting bacterium Burkholderia sp strain PsJN. Appl Environ Microbiol 71:1685–1693. doi:10.1128/aem.71.4.1685-1693.2005
Crespo E, Hordijk CA, de Graaf RM, Samudrala D, Cristescu SM, Harren FJM, van Dam NM (2012) On-line detection of root-induced volatiles in Brassica nigra plants infested with Delia radicum L. root fly larvae. Phytochemistry 84:68–77. doi:10.1016/j.phytochem.2012.08.013
Croes AF, Vandenberg AJR, Bosveld M, Breteler H, Wullems GJ (1989) Thiophene accumulation in relation to morphology in roots of Tagetes patula—effects of auxin and transformation by Agrobacterium. Planta 179:43–50. doi:10.1007/bf00395769
Danner H, Samudrala D, Cristescu SM, Van Dam NM (2012) Tracing hidden herbivores: time-resolved non-invasive analysis of belowground volatiles by proton-transfer-reaction mass spectrometry (PTR-MS). J Chem Ecol 38:785–794. doi:10.1007/s10886-012-0129-3
Danner H, Brown P, Cator E, Harren FM, van Dam N, Cristescu S (2015) Aboveground and belowground herbivores synergistically induce volatile organic sulfur compound emissions from shoots but not from roots. J Chem Ecol 41:631–640. doi:10.1007/s10886-015-0601-y
Dawood T, Rieu I, Wolters-Arts M, Derksen EB, Mariani C, Visser EJW (2014) Rapid flooding-induced adventitious root development from preformed primordia in Solanum dulcamara. AoB Plants 6:13. doi:10.1093/aobpla/plt058
De Boer JG, Posthumus MA, Dicke M (2004) Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 30:2215–2230
de Boer W, Kowalchuk GA, van Veen JA (2006) ‘Root-food’ and the rhizosphere microbial community composition. New Phytol 170:3–6. doi:10.1111/j.1469-8137.2006.01674.x
de Deyn GB, Raaijmakers CE, Zoomer HR, Berg MP, De Ruiter PC, Verhoeff HA, Bezemer TM, Van der Putten WH (2003) Soil invertebrate fauna enhances grassland succession and diversity. Nature 422:711–713
De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Metraux JP, Van Loon LC, Dicke M, Pieterse CMJ (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact 18:923–937
Degenhardt J, Hiltpold I, Kollner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ (2009) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106:13213–13218. doi:10.1073/pnas.0906365106
Del Giudice L, Massardo DR, Pontieri P, Bertea CM, Mombello D, Carata E, Tredici SM, Tala A, Mucciarelli M, Groudeva VI, De Stefano M, Vigliotta G, Maffei ME, Alifano P (2008) The microbial community of Vetiver root and its involvement into essential oil biogenesis. Environ Microbiol 10:2824–2841. doi:10.1111/j.1462-2920.2008.01703.x
Depuydt S (2014) Arguments for and against self and non-self root recognition in plants. Front Plant Sci 5:7. doi:10.3389/fpls.2014.00614
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175. doi:10.1016/j.tplants.2009.12.002
Dicke M, Sabelis MW (1988) Infochemical terminology: based on cost–benefit analysis rather than origin of compounds? Funct Ecol 2:131–139
Dickschat JS, Pahirulzaman KA, Rabe P, Klapschinski TA (2014) An improved technique for the rapid chemical characterisation of bacterial terpene cyclases. Chembiochem 15:810–814. doi:10.1002/cbic.201300763
Döring TF (2014) How aphids find their host plants, and how they don’t. Ann Appl Biol 165:3–26. doi:10.1111/aab.12142
Driouich A, Follet-Gueye ML, Vicre-Gibouin M, Hawes M (2013) Root border cells and secretions as critical elements in plant host defense. Curr Opin Plant Biol 16:489–495. doi:10.1016/j.pbi.2013.06.010
Dudareva N, Pichersky E, Gershenzon J (2004) Biochemistry of plant volatiles. Plant Physiol 135:1893–1902
Ebel R (2010) Terpenes from marine-derived fungi. Mar Drugs 8:2340–2368. doi:10.3390/md8082340
Effmert U, Kalderas J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38:665–703. doi:10.1007/s10886-012-0135-5
Eilers EJ, Pauls G, Rillig MC, Hansson BS, Hilker M, Reinecke A (2015) Novel set-up for low-disturbance sampling of volatile and non-volatile compounds from plant roots. J Chem Ecol. doi:10.1007/s10886-015-0559-9
Erb M, Huber M, Robert CAM, Ferrieri AP, Machado RAR, Arce CCM (2013) The role of plant primary and secondary metabolites in root-herbivore behaviour, nutrition and physiology. In: Johnson SN, Hiltpold I, Turlings TCJ (eds) Behaviour and physiology of root herbivores, vol 45, 1st edn. Academic Press, Oxford, pp 53–95
Fantaye CA, Kopke D, Gershenzon J, Degenhardt J (2015) Restoring (E)-beta-caryophyllene production in a non-producing Maize line compromises its resistance against the fungus Colletotrichum graminicola. J Chem Ecol 41:213–223. doi:10.1007/s10886-015-0556-z
Ferry A, Dugravot S, Delattre T, Christides JP, Auger J, Bagneres AG, Poinsot D, Cortesero AM (2007) Identification of a widespread monomolecular odor differentially attractive to several Delia radicum ground-dwelling predators in the field. J Chem Ecol 33:2064–2077. doi:10.1007/s10886-007-9373-3
Fukami H, Asakura T, Hirano H, Abe K, Shimomura K, Yamakawa T (2002) Salicylic acid carboxyl methyltransferase induced in hairy root cultures of Atropa belladonna after treatment with exogeneously added salicylic acid. Plant Cell Physiol 43:1054–1058. doi:10.1093/pcp/pcf119
Garbeva P, Hol WHG, Termorshuizen AJ, Kowalchuk GA, de Boer W (2011) Fungistasis and general soil biostasis—a new synthesis. Soil Biol Biochem 43:469–477. doi:10.1016/j.soilbio.2010.11.020
Garbeva P, Hordijk C, Gerards S, de Boer W (2014a) Volatile-mediated interactions between phylogenetically different soil bacteria. Front Microbiol 5:9. doi:10.3389/fmicb.2014.00289
Garbeva P, Hordijk C, Gerards S, de Boer W (2014b) Volatiles produced by the mycophagous soil bacterium Collimonas. FEMS Microbiol Ecol 87:639–649. doi:10.1111/1574-6941.12252
Gepstein S, Kieber J (2010) Ethylene: the gaseous hormone. In: Taiz L, Zeiger E (eds) Plant physiology, 5th edn. Sinauer Associates, Sunderland, MA, pp 649–672
Gfeller A, Laloux M, Barsics F, Kati DE, Haubruge E, du Jardin P, Verheggen FJ, Lognay G, Wathelet JP, Fauconnier ML (2013) Characterization of volatile organic compounds emitted by barley (Hordeum vulgare L.) roots and their attractiveness to wireworms. J Chem Ecol 39:1129–1139. doi:10.1007/s10886-013-0302-3
Ghashghaie J, Badeck FW (2014) Opposite carbon isotope discrimination during dark respiration in leaves versus roots—a review. New Phytol 201:751–769. doi:10.1111/nph.12563
Graner G, Persson P, Meijer J, Alstrom S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224:269–276. doi:10.1016/s0378-1097(03)00449-x
Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S (2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161:2159–2170. doi:10.1104/pp.113.214544
Groenhagen U, Maczka M, Dickschat JS, Schulz S (2014) Streptopyridines, volatile pyridine alkaloids produced by Streptomyces sp. FORM5. Beilstein J Org Chem 10:1421–1432. doi:10.3762/bjoc.10.146
Gutensohn M, Nagegowda DA, Dudareva N (2013) Involvement of compartimentalisation in monoterpene and sesquipterpene biosynthesis in plants. In: Bach TJ, Rohmer M (eds) Isoprenoid synthesis in plants and microorganisms. New concepts and experimental approaches. Springer, New York, NY, pp 155–169
Halkier BA, Gershenzon J (2006) Biology and biochemistry of glucosinolates. Ann Rev Plant Biol 57:303–333
Harper M (2000) Sorbent trapping of volatile organic compounds from air. J Chromatogr A 885:129–151. doi:10.1016/s0021-9673(00)00363-0
Harrison SP, Morfopoulos C, Dani KGS, Prentice IC, Arneth A, Atwell BJ, Barkley MP, Leishman MR, Loreto F, Medlyn BE, Niinemets U, Possell M, Penuelas J, Wright IJ (2013) Volatile isoprenoid emissions from plastid to planet. New Phytol 197:49–57. doi:10.1111/nph.12021
Hazelwood LA, Daran JM, van Maris AJ, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74:2259–2266. doi:10.1128/aem.02625-07
Heil M (2014) Herbivore-induced plant volatiles: targets, perception and unanswered questions. New Phytol 204:297–306. doi:10.1111/nph.12977
Heil M, Land WG (2014) Danger signals—damaged-self recognition across the tree of life. Front Plant Sci 5:16. doi:10.3389/fpls.2014.00578
Hiltpold I, Turlings TCJ (2008) Belowground chemical signaling in maize: when simplicity rhymes with efficiency. J Chem Ecol 34:628–635. doi:10.1007/s10886-008-9467-6
Hol WHG, Garbeva P, Hordijk C, Hundscheid MPJ, Gunnewiek PJAK, van Agtmaal M, Kuramae EE, de Boer W (2015) Non-random species loss in bacterial communities reduces antifungal volatile production. Ecology 96:2042–2048. doi:10.1890/14-2359.1
Hopkins RJ, van Dam NM, van Loon JJA (2009) Role of glucosinolates in insect–plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83. doi:10.1146/annurev.ento.54.110807.090623
Hora TS, Baker R (1970) Volatile factors in soil fungistasis. Nature 225:1071–1072. doi:10.1038/2251071a0
Hora TS, Baker R (1972) Influence of volatile inhibitor from soil on seed germination. Phytopathol 62:765–765
Huang CJ, Tsay JF, Chang SY, Yang HP, Wu WS, Chen CY (2012) Dimethyl disulfide is an induced systemic resistance elicitor produced by Bacillus cereus C1L. Pest Manag Sci 68:1306–1310. doi:10.1002/ps.3301
Hung R, Lee S, Bennett JW (2013) Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol 6:19–26. doi:10.1016/j.funeco.2012.09.005
Inderjit, Wardle DA, Karban R, Callaway RM (2011) The ecosystem and evolutionary contexts of allelopathy. Trends Ecol Evol 26:655–662. doi:10.1016/j.tree.2011.08.003
Jacobs JJMR, Engelberts A, Croes AF, Wullems GJ (1994) Thiophene synthesis and distribution in young developing plants of Tagetes patula and Tagetes erecta. J Exp Bot 45:1459–1466. doi:10.1093/jxb/45.10.1459
Jallow MFA, Dugassa-Gobena D, Vidal S (2008) Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod Plant Interact 2:53–62. doi:10.1007/s11829-008-9033-8
Jassbi AR, Zamanizadehnajari S, Baldwin IT (2010) Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J Chem Ecol 36:1398–1407. doi:10.1007/s10886-010-9885-0
Jenni S, Leibundgut M, Boehringer D, Frick C, Mikolasek B, Ban N (2007) Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science 316:254–261. doi:10.1126/science.1138248
Johnson SN, Nielsen UN (2012) Foraging in the dark—chemically mediated host plant location by belowground insect herbivores. J Chem Ecol 38:604–614. doi:10.1007/s10886-012-0106-x
Johnson SN, Rasmann S (2015) Root-feeding insects and their interactions with organisms in the rhizosphere. Annu Rev Entomol 60:517–535. doi:10.1146/annurev-ento-010814-020608
Johnston-Monje D, Raizada MN (2011) Conservation and diversity of seed associated endophytes in Zea across boundaries of evolution, ethnography and coology. PLoS One 6. doi:10.1371/journal.pone.0020396
Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 187:351–360. doi:10.1007/s00203-006-0199-0
Kai M, Vespermann A, Piechulla B (2008) The growth of fungi and Arabidopsis thaliana is influenced by bacterial volatiles. Plant Signal Behav 3:482–484
Kai M, Crespo E, Cristescu SM, Harren FJM, Francke W, Piechulla B (2010) Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl Microbiol Biotechnol 88:965–976. doi:10.1007/s00253-010-2810-1
Karban R, Baldwin IT (1997) Induced responses to herbivory. University of Chicago Press, Chicago, IL
Karban R, Wetzel WC, Shiojiri K, Ishizaki S, Ramirez SR, Blande JD (2014a) Deciphering the language of plant communication: volatile chemotypes of sagebrush. New Phytol 204:380–385. doi:10.1111/nph.12887
Karban R, Yang LH, Edwards KF (2014b) Volatile communication between plants that affects herbivory: a meta-analysis. Ecol Lett 17:44–52. doi:10.1111/ele.12205
Kegge W, Ninkovic V, Glinwood R, Welschen RAM, Voesenek L, Pierik R (2015) Red:far-red light conditions affect the emission of volatile organic compounds from barley (Hordeum vulgare), leading to altered biomass allocation in neighbouring plants. Ann Bot 115:961–970. doi:10.1093/aob/mcv036
Kim T-Y, Lee S-W, Oh M-K (2014) Biosynthesis of 2-phenylethanol from glucose with genetically engineered Kluyveromyces marxianus. Enzyme Microb Technol 61–62:44–47. doi:10.1016/j.enzmictec.2014.04.011
Kissen R, Rossiter JT, Bones AM (2009) The ‘mustard oil bomb’: not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem Rev 8:69–86
Kleinheinz GT, Bagley ST, St John WP, Rughani JR, McGinnis GD (1999) Characterization of alpha-pinene-degrading microorganisms and application to a bench-scale biofiltration system for VOC degradation. Arch Environ Contam Toxicol 37:151–157
Korpi A, Jarnberg J, Pasanen AL (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39:139–193. doi:10.1080/10408440802291497
Kostal V (1992) Orientation behavior of newly hatched larvae of the cabbage maggot, Delia radicum (L.) (Diptera, Anthomyiidae) to volatile plant metabolites. J Insect Behav 5:61–70
Kpoviessi DSS, Gbaguidi FA, Kossouoh C, Agbani P, Yayi-Ladekan E, Sinsin B, Moudachirou M, Accrombessi GC, Quetin-Leclercq J (2011) Chemical composition and seasonal variation of essential oil of Sclerocarya birrea (A. Rich.) Hochst subsp birrea leaves from Benin. J Med Plants 5:4640–4646
Kulmatiski A, Anderson-Smith A, Beard KH, Doucette-Riise S, Mazzacavallo M, Nolan NE, Ramirez RA, Stevens JR (2014) Most soil trophic guilds increase plant growth: a meta-analytical review. Oikos 123:1409–1419. doi:10.1111/oik.01767
Kwon YS, Ryu CM, Lee S, Park HB, Han KS, Lee JH, Lee K, Chung WS, Jeong MJ, Kim HK, Bae DW (2010) Proteome analysis of Arabidopsis seedlings exposed to bacterial volatiles. Planta 232:1355–1370. doi:10.1007/s00425-010-1259-x
Lauchli R, Pitzer J, Kitto RZ, Kalbarczyk KZ, Rabe KS (2014) Improved selectivity of an engineered multi-product terpene synthase. Org Biomol Chem 12:4013–4020. doi:10.1039/C4ob00479e
Lee S, Hung R, Yap M, Bennett JW (2015) Age matters: the effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch Microbiol 197:723–727. doi:10.1007/s00203-015-1104-5
Leger C, Riga E (2009) Evaluation of marigolds and entomopathogenic nematodes for control of the cabbage maggot Delia radicum. J Sustain Agric 33:128–141. doi:10.1080/10440040802394992
Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15:154–166. doi:10.1016/j.tplants.2009.12.006
Lugtenberg BJJ, Dekkers L, Bloemberg GV (2001) Molecular determinants of rhizosphere colonization by Pseudomonas. Annu Rev Phytopathol 39:461–490. doi:10.1146/annurev.phyto.39.1.461
Meldau DG, Meldau S, Hoang LH, Underberg S, Wunsche H, Baldwin IT (2013) Dimethyl disulfide produced by the naturally associated bacterium Bacillus sp B55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 25:2731–2747. doi:10.1105/tpc.113.114744
Mendes R, Garbeva P, Raaijmakers JM (2013) The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37:634–663. doi:10.1111/1574-6976.12028
Minerdi D, Moretti M, Gilardi G, Barberio C, Gullino ML, Garibaldi A (2008) Bacterial ectosymbionts and virulence silencing in a Fusarium oxysporum strain. Environ Microbiol 10:1725–1741. doi:10.1111/j.1462-2920.2008.01594.x
Mohney BK, Matz T, LaMoreaux J, Wilcox DS, Gimsing AL, Mayer P, Weidenhamer JD (2009) In situ silicone tube microextraction: a new method for undisturbed sampling of root-exuded thiophenes from Marigold (Tagetes erecta L.) in soil. J Chem Ecol 35:1279–1287. doi:10.1007/s10886-009-9711-8
Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S (2008) ß-glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813
Mumm R, Hilker M (2005) The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem Senses 30:337–343
Mundt JO, Hinkle NF (1976) Bacteria within ovules and seeds. Appl Environ Microbiol 32:694–698
Naeem S (1998) Species redundancy and ecosystem reliability. Conserv Biol 12:39–45. doi:10.1111/j.1523-1739.1998.96379.x
Nelson EB (2004) Microbial dynamics and interactions in the spermosphere. Annu Rev Phytopathol 42:271–309. doi:10.1146/annurev.phyto.42.121603.131041
Njoroge SMC, Riley MB, Keinath AP (2008) Effect of incorporation of Brassica spp. residues on population densities of soilborne microorganisms and on damping-off and Fusarium wilt of watermelon. Plant Dis 92:287–294. doi:10.1094/pdis-92-2-0287
Owen SM, Clark S, Pompe M, Semple KT (2007) Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol Lett 268:34–39. doi:10.1111/j.1574-6968.2006.00602.x
Paul D, Park KS (2013) Identification of volatiles produced by Cladosporium cladosporioides CL-1, a fungal biocontrol agent that promotes plant growth. Sensors 13:13969–13977. doi:10.3390/s131013969
Peng M, Xie Q, Hu H, Hong K, Todd JD, Johnston AW, Li Y (2012) Phylogenetic diversity of the dddP gene for dimethylsulfoniopropionate-dependent dimethyl sulfide synthesis in mangrove soils. Can J Microbiol 58:523–530. doi:10.1139/w2012-019
Peñuelas J, Munne-Bosch S (2005) Isoprenoids: an evolutionary pool for photoprotection. Trends Plant Sci 10:166–169
Peñuelas J, Asensio D, Tholl D, Wenke K, Rosenkranz M, Piechulla B, Schnitzler JP (2014a) Biogenic volatile emissions from the soil. Plant Cell Environ 37:1866–1891. doi:10.1111/pce.12340
Peñuelas J, Farré-Armengol G, Llusia J, Gargallo-Garriga A, Rico L, Sardans J, Terradas J, Filella I (2014b) Removal of floral microbiota reduces floral terpene emissions. Sci Rep 4:4. doi:10.1038/srep06727
Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek L (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11:176–183. doi:10.1016/j.tplants.2006.02.006
Potter MJ, Davies K, Rathjen AJ (1998) Suppressive impact of glucosinolates in Brassica vegetative tissues on root lesion nematode Pratylenchus neglectus. J Chem Ecol 24:67–80
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361. doi:10.1007/s11104-008-9568-6
Rasmann S, Turlings TCJ (2008) First insights into specificity of belowground tritrophic interactions. Oikos 117:362–369. doi:10.1111/j.2007.0030-1299.16204.x
Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737. doi:10.1038/nature03451
Rasmann S, Erwin AC, Halitschke R, Agrawal AA (2011) Direct and indirect root defences of milkweed (Asclepias syriaca): trophic cascades, trade-offs and novel methods for studying subterranean herbivory. J Ecol 99:16–25. doi:10.1111/j.1365-2745.2010.01713.x
Richter A, Seidl-Adams I, Kollner TG, Schaff C, Tumlinson JH, Degenhardt J (2015) A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 241:1351–1361. doi:10.1007/s00425-015-2254-z
Robert CAM, Erb M, Duployer M, Zwahlen C, Doyen GR, Turlings TCJ (2012) Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol 194:1061–1069. doi:10.1111/j.1469-8137.2012.04127.x
Rostás M, Cripps MG, Silcock P (2015) Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 177:487–497. doi:10.1007/s00442-014-3104-6
Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100:4927–4932. doi:10.1073/pnas.0730845100
Ryu CM, Farag MA, Hu CH, Reddy MS, Kloepper JW, Pare PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026. doi:10.1104/pp.103.026583
Sacchetti G, Romagnoli C, Bruni A, Poli F (2001) Secretory tissue ultrastructure in Tagetes patula L. (Asteraceae) and thiophene localization through X-ray microanalysis. Phyton 41:35–47
Samudrala D, Brown PA, Mandon J, Cristescu SM, Harren FJM (2015) Optimization and sensitive detection of sulfur compounds emitted from plants using proton transfer reaction mass spectrometry. Int J Mass Spectrom 386:6–14. doi:10.1016/j.ijms.2015.05.013
Sarwar M, Kirkegaard JA, Wong PTW, Desmarchelier JM (1998) Biofumigation potential of brassicas—III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil 201:103–112
Schmid C, Bauer S, Muller B, Bartelheimer M (2013) Belowground neighbor perception in Arabidopsis thaliana studied by transcriptome analysis: roots of Hieracium pilosella cause biotic stress. Front Plant Sci 4:17. doi:10.3389/fpls.2013.00296
Schrey SD, Schellhammer M, Ecke M, Hampp R, Tarkka MT (2005) Mycorrhiza helper bacterium Streptomyces AcH 505 induces differential gene expression in the ectomycorrhizal fungus Amanita muscaria. New Phytol 168:205–216. doi:10.1111/j.1469-8137.2005.01518.x
Schulz S, Dickschat JS (2007) Bacterial volatiles: the smell of small organisms. Nat Prod Rep 24:814–842. doi:10.1039/b507392h
Schulz-Bohm K, Zweers H, de Boer W, Garbeva P (2015) A fragrant neighborhood: volatile mediated bacterial interactions in soil. Front Microbiol. doi:10.3389/fmicb.2015.01212
Semchenko M, Saar S, Lepik A (2014) Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol 204:631–637. doi:10.1111/nph.12930
Singh SK, Strobel GA, Knighton B, Geary B, Sears J, Ezra D (2011) An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb Ecol 61:729–739. doi:10.1007/s00248-011-9818-7
Son SH, Khan Z, Kim SG, Kim YH (2009) Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and fusarium wilt fungus. J Appl Microbiol 107:524–532. doi:10.1111/j.1365-2672.2009.04238.x
Song C, Schmidt RL, de Jager VCL, Krzyzanowska D, Jongedijk E, Cankar K, Beekwilder J, van Veen A, de Boer W, van Veen JA, Garbeva P (2015) Exploring the genomic traits of fungusfeeding bacterial genus Collimonas. BMC Genomics 16(1103). doi:10.1186/s12864-015-2289-3
Splivallo R, Ottonello S, Mello A, Karlovsky P (2011) Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol 189:688–699. doi:10.1111/j.1469-8137.2010.03523.x
Steeghs M, Bais HP, de Gouw J, Goldan P, Kuster W, Northway M, Fall R, Vivanco JM (2004) Proton-transfer-reaction mass spectrometry as a new tool for real time analysis of root-secreted volatile organic compounds in Arabidopsis. Plant Physiol 135:47–58. doi:10.1104/pp.104.038703
Stewart-Jones A, Poppy GM (2006) Comparison of glass vessels and plastic bags for enclosing living plant parts for headspace analysis. J Chem Ecol 32:845–864
Strobel GA, Dirkse E, Sears J, Markworth C (2001) Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943–2950
Strobel G, Singh SK, Riyaz-Ul-Hassan S, Mitchell AM, Geary B, Sears J (2011) An endophytic/pathogenic Phoma sp from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol Lett 320:87–94. doi:10.1111/j.1574-6968.2011.02297.x
Tang CS, Wat CK, Towers GHN (1987) Thiophenes and benzofurans in the undisturbed rhizosphere of Tagetes patula L. Plant Soil 98:93–97. doi:10.1007/bf02381730
Teplitski M, Robinson JB, Bauer WD (2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant Microbe Interact 13:637–648. doi:10.1094/mpmi.2000.13.6.637
Tholl D (2006) Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol 9:297–304. doi:10.1016/j.pbi.2006.03.014
Todd JD, Rogers R, Li YG, Wexler M, Bond PL, Sun L, Curson AR, Malin G, Steinke M, Johnston AW (2007) Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science 315:666–669. doi:10.1126/science.1135370
Todd JD, Curson AR, Kirkwood M, Sullivan MJ, Green RT, Johnston AW (2011) DddQ, a novel, cupin-containing, dimethylsulfoniopropionate lyase in marine roseobacters and in uncultured marine bacteria. Environ Microbiol 13:427–438. doi:10.1111/j.1462-2920.2010.02348.x
Todd JD, Kirkwood M, Newton-Payne S, Johnston AW (2012) DddW, a third DMSP lyase in a model Roseobacter marine bacterium, Ruegeria pomeroyi DSS-3. ISME J 6:223–226. doi:10.1038/ismej.2011.79
Traxler MF, Watrous JD, Alexandrov T, Dorrestein PC, Kolter R (2013) Interspecies interactions stimulate diversification of the Streptomyces coelicolor secreted metabolome. MBio 4. doi:10.1128/mBio.00459-13
Tyc O, van den Berg M, Gerards S, van Veen JA, Raaijmakers JM, de Boer W, Garbeva P (2014) Impact of interspecific interactions on antimicrobial activity among soil bacteria. Front Microbiol 5. doi:10.3389/fmicb.2014.00567
van Dam NM (2009) Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst 40:373–392
van Dam NM, Tytgat TOG, Kirkegaard JA (2009) Root and shoot glucosinolates: a comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem Rev 8:171–186
van Dam NM, Qiu BL, Hordijk CA, Vet LEM, Jansen JJ (2010) Identification of biologically relevant compounds in aboveground and belowground induced volatile blends. J Chem Ecol 36:1006–1016. doi:10.1007/s10886-010-9844-9
van Dam NM, Samudrala D, Harren FJM, Cristescu SM (2012) Real-time analysis of sulfur-containing volatiles in Brassica plants infested with root-feeding Delia radicum larvae using proton-transfer reaction mass spectrometry. AoB Plants. doi:10.1093/aobpla/pls1021
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. doi:10.1111/nph.13288
van Tol RHWM, van der Sommen ATC, Boff MIC, van Bezooijen J, Sabelis MW, Smits PH (2001) Plants protect their roots by alerting the enemies of grubs. Ecol Lett 4:292–294. doi:10.1046/j.1461-0248.2001.00227.x
Vaughan MM, Wang Q, Webster FX, Kiemle D, Hong YJ, Tantillo DJ, Coates RM, Wray AT, Askew W, O’Donnell C, Tokuhisa JG, Tholl D (2013) Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell 25:1108–1125. doi:10.1105/tpc.112.100057
Vaughn SF, Boydston RA (1997) Volatile allelochemicals released by crucifer green manures. J Chem Ecol 23:2107–2116
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73:5639–5641. doi:10.1128/aem.01078-07
Vet LEM, Wäckers FL, Dicke M (1991) How to hunt for hiding hosts—the reliability-detectability problem in foraging parasitoids. Neth J Zool 41:202–213
Voisard C, Keel C, Haas D, Defago G (1989) Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J 8:351–358
Weise T, Kai M, Gummesson A, Troeger A, von Reuss S, Piepenborn S, Kosterka F, Sklorz M, Zimmermann R, Francke W, Piechulla B (2012) Volatile organic compounds produced by the phytopathogenic bacterium Xanthomonas campestris pv. vesicatoria 85–10. Beilstein J Org Chem 8:579–596. doi:10.3762/bjoc.8.65
Weisskopf L, Heller S, Eberl L (2011) Burkholderia species are major inhabitants of white lupin cluster roots. Appl Environ Microbiol 77:7715–7720. doi:10.1128/aem.05845-11
Weissteiner S, Huetteroth W, Kollmann M, Weißbecker B, Romani R, Schachtner J et al (2012) Cockchafer larvae smell host root scents in soil. PLoS One 7(10):e45827. doi:10.1371/journal.pone.0045827
Welte C, De Graaf RM, van den Bosch TJM, Op den Camp HJM, van Dam NM, Jetten MJM (2015) Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyzes the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ Microbiol 8:1379-90. doi: 10.1111/1462-2920.12997
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506. doi:10.1007/s00425-009-1076-2
Wheatley RE (2002) The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek Int J Gen Mol Microbiol 81:357–364. doi:10.1023/a:1020592802234
Wiesner J, Reichenberg A, Hintz M, Ortmann R, Schlitzer M, Van Calenbergh S, Borrmann S, Lell B, Kremsner PG, Hutchinson D, Jomaa H (2013) Fosmidomycin as an antimalarial agent. In: Bach TJ, Rohmer M (eds) Isoprenoid synthesis in plants and microorganisms. New concepts and experimental approaches. Springer, New York, NY, pp 119–137
Wittstock U, Kliebenstein DJ, Lambrix V, Reichelt M, Gershenson J (2003) Glucosinolate hydrolysis and its impact on generalist and specialist herbivores. In: Romeo JT (ed) Integrative phytochemistry: from ethnobotany to molecular ecology, vol 37. Pergamon, Amsterdam
Yamada Y, Cane DE, Ikeda H (2012) Diversity and analysis of bacterial terpene synthases. Methods Enzymol 515:123–162
Yamada Y, Kuzuyama T, Komatsu M, Shin-Ya K, Omura S, Cane DE, Ikeda H (2015) Terpene synthases are widely distributed in bacteria. Proc Natl Acad Sci USA 112:857–862. doi:10.1073/pnas.1422108112
Yang C, Wang J, Li D (2013) Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: a review. Anal Chim Acta 799:8–22. doi:10.1016/j.aca.2013.07.069
Yeo H, Youn K, Kim M, Yun E-Y, Hwang J-S, Jeong W-S, Jun M (2013) Fatty acid composition and volatile constituents of Protaetia brevitarsis larvae. Prev Nutr Food Sci 18:150–156. doi:10.3746/pnf.2013.18.2.150
Yoshikuni Y, Martin VJ, Ferrin TE, Keasling JD (2006) Engineering cotton (+)-delta-cadinene synthase to an altered function: germacrene D-4-ol synthase. Chem Biol 13:91–98. doi:10.1016/j.chembiol.2005.10.016
Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse CMJ (2013) Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318. doi:10.1104/pp.112.212597
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Allen R, Melo IS, Pare PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226:839–851. doi:10.1007/s00425-007-0530-2
Zhang HM, Sun Y, Xie XT, Kim MS, Dowd SE, Pare PW (2009) A soil bacterium regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J 58:568–577. doi:10.1111/j.1365-313X.2009.03803.x
Zhao N, Guan J, Forouhar F, Tschaplinski TJ, Cheng ZM, Tong L, Chen F (2009) Two poplar methyl salicylate esterases display comparable biochemical properties but divergent expression patterns. Phytochemistry 70:32–39. doi:10.1016/j.phytochem.2008.11.014
Zou C-S, Mo M-H, Gu Y-Q, Zhou J-P, Zhang K-Q (2007) Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol Biochem 39:2371–2379. doi:10.1016/j.soilbio.2007.04.009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
van Dam, N.M., Weinhold, A., Garbeva, P. (2016). Calling in the Dark: The Role of Volatiles for Communication in the Rhizosphere. In: Blande, J., Glinwood, R. (eds) Deciphering Chemical Language of Plant Communication. Signaling and Communication in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-33498-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-33498-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33496-7
Online ISBN: 978-3-319-33498-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)