Abstract
The difficulties of monitoring allelochemical concentrations in soil and their dynamics over time have been a major barrier to testing hypotheses of allelopathic effects. Here, we evaluate three diffusive sampling strategies that employ polydimethylsiloxane (PDMS) sorbents to map the spatial distribution and temporal dynamics of root-exuded thiophenes from the African marigold, Tagetes erecta. Solid phase root zone extraction (SPRE) probes constructed by inserting stainless steel wire into PDMS tubing were used to monitor thiophene concentrations at various depths beneath marigolds growing in PVC pipes. PDMS sheets were used to map the distribution of thiophenes beneath marigolds grown in thin glass boxes. Concentrations of the two major marigold thiophenes measured by these two methods were extremely variable in both space and time. Dissection and analysis of roots indicated that distribution of thiophenes in marigold roots also was quite variable. A third approach used 1 m lengths of PDMS microtubing placed in marigold soil for repeated sampling of soil without disturbance of the roots. The two ends of the tubing remained out of the soil so that solvent could be washed through the tubing to collect samples for HPLC analysis. Unlike the other two methods, initial experiments with this approach show more uniformity of response, and suggest that soil concentrations of marigold thiophenes are affected greatly even by minimal disturbance of the soil. Silicone tube microextraction gave a linear response for α-terthienyl when maintained in soils spiked with 0–10 ppm of this thiophene. This method, which is experimentally simple and uses inexpensive materials, should be broadly applicable to the measurement of non-polar root exudates, and thus provides a means to test hypotheses about the role of root exudates in plant-plant and other interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Information on allelochemical dynamics in the rhizosphere is crucial for evaluating specific hypotheses of allelopathic effects, understanding allelopathic mechanisms, and assessing the importance of allelopathic processes in plant communities (Williamson and Weidenhamer 1990; Weidenhamer 1996, 2005). The lack of tools to obtain such information has been a major barrier to investigations of allelopathy.
The feasibility of using polydimethylsiloxane (PDMS) materials to trap rhizosphere allelochemicals was demonstrated in a greenhouse study with sorghum-sudangrass (Sorghum bicolor x S. sudanense) (Weidenhamer 2005). The materials tested in that study included stir bars coated with PDMS (stir bar sorptive extraction), technical grade optical fiber coated with a thin film of PDMS (matrix-solid phase microextraction), and PDMS tubing. The PDMS sorbents were placed in the pots at planting and removed at harvests 29, 55, and 88 days after planting. As analyzed by HPLC, the amount of sorgoleone recovered increased with time. Barto and Cipollini (2009) used 10 cm lengths of PDMS tubing (buried in soil, then recovered and extracted) in an attempt to measure allelochemicals beneath garlic mustard, Alliaria petiolata, and were able to detect low amounts of one flavonoid, isovitexin-6″-O-β-D-glucopyranoside. Weidenhamer et al. (2009) used solid phase root zone extraction (SPRE) probes constructed by inserting stainless steel wire into PDMS tubing to recover nano- to microgram quantities of thiophenes from soil beneath growing marigolds, using a 24 hour sampling time.
PDMS-based materials are used widely for trace analysis of anthropogenic contaminants. They are used as stationary phase in capillary columns for the analyte separation in gas chromatography, and as enrichment material in several analytical techniques that include solid phase microextraction (Arthur and Pawliszyn 1990), stir bar sorptive extraction (Baltussen et al. 1999), and thin-film microextraction (Bruheim et al. 2003). PDMS also has been applied for diffusive sampling in sediment and soil (Mayer et al. 2000; Weidenhamer 2005; Weidenhamer et al. 2009) and for passive sampling in the aquatic environment (e.g., Cornelissen et al. 2008). Recently developed techniques are based on the permeation of non-polar analytes through the wall of PDMS microtubing: Ooki and Yokouchi (2008) constructed a silicone membrane tube equilibrator from PDMS tubing for the sea water to gas equilibration of volatile organic compounds; Mayer et al. (2009) used 6 m PDMS microtubes as silicone membrane equilibrator to measure polycyclic aromatic hydrocarbons directly in tissue and oil samples. The tubing was immersed in the sample, and compounds were extracted by pushing a plug of methanol through the tubing and collecting it in an HPLC vial for analysis. In the present study we apply PDMS microtubing for the in situ sampling of root exudates and the subsequent elution into methanol while keeping the tubing in place.
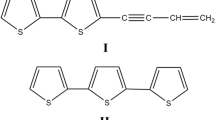
The objective of the experiments described in this paper was to compare the ability of three PDMS-based techniques to measure the spatial and temporal dynamics of thiophenes in the root zone of African marigold, Tagetes erecta L. Solid phase root zone extraction probes were used to monitor thiophene concentrations at different depths beneath marigolds grown in PVC pipes. Marigolds were grown between foil-lined glass plates, and thiophene distributions in the soil were measured by two techniques: (a) PDMS sheets were press-applied to the roots and analyzed after 24 h; and (b) PDMS microtubing (1 m lengths) was placed over the roots and sampled at 24 h intervals by flushing 95% methanol through the tubing. Because PDMS selectively sorbs lipophilic compounds (Baltussen et al. 1999), marigold was considered to be a good model species for these studies due to the highly lipophilic character of the thiophenes produced by marigold roots (Fig. 1, Bohlmann et al. 1973; Downum and Towers 1983). In addition, these thiophenes have high biological activity against a number of organisms (Bakker et al. 1979; Campbell et al. 1982), and have been reported to be released into the marigold root zone (Campbell et al. 1982; Tang et al. 1987; Martin and Weidenhamer 1995; Weidenhamer et al. 2009).
Methods and Materials
Growth Media and Plant Cultivation Conditions
Plants were grown in a greenhouse under natural sunlight during June – July 2009. In general, daytime temperatures ranged from 21–32o C and nighttime temperatures from 16–22o C. A 1:1 (v:v) mixture of sand and a peat/vermiculite-based growth medium (Redi-earth® plug and seedling mix) was used for all experiments. Plants (Tagetes erecta cv. ‘Crackerjack mix’ and Tagetes patula cv. ‘Janie primrose’) were watered as needed, and received weekly applications of a general purpose fertilizer solution (Peters Professional® all purpose plant food, 24-8-16 plus B, Cu, Fe, Mn, Mo, and Zn). Fertilization was not done immediately before or during sampling.
SPRE Probe Study
Marigolds (T. erecta cv. ‘Crackerjack mix’, thinned to 3 plants per pipe) were grown in four 7.5 cm ID, 44 cm tall PVC pipes. The pipes were mounted on a flat, circular base. A 10 cm layer of gravel at the base and three drainage holes drilled at the bottom kept the pots well-drained. Three evenly spaced holes (1.5 mm diam, 5 cm apart) were drilled at eight depths (2.5 cm intervals, from 2.5–20 cm below soil surface) to allow access for sampling. Pipes were wrapped with polyethylene film to prevent the drainage of water through the probe access holes.
SPRE probes were prepared as described in Weidenhamer et al. (2009). Stainless steel wire (22-gauge) was inserted into 5 cm lengths of hexane-swelled Silastic® tubing (0.64 mm ID × 1.19 mm OD, Fisher Scientific catalog no. 11-189-15B). The probes were sequentially cleaned with hexanes, dichloromethane, methanol, and water by soaking for 10 min in each, and then dried in an oven at 70°C. Once the probes were dried, they were stored in tightly capped vials until use.
At 3, 4, and 5 wk after planting, SPRE probes were inserted into the 96 probe access holes (3 access holes × 8 depths × 4 pipes) and left in place for 24 h. The 3 probes at each depth were pooled, and thiophenes were extracted from the probes by first rinsing with water to remove adhering soil particles, blotting dry, stripping the tubing from the wire support, and sonication in an amber autosampler vial with 700 µl of 95% methanol (methanol:water 95:5, v:v) for 12 min.
Glass Box PDMS Sheet Study
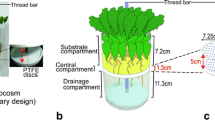
Glass boxes were constructed by using two 20x20 cm glass plates (0.3 cm thickness). Four bottle caps were glued in the corners of one plate to maintain a 1 cm spacing between the plates. Plates were covered with non-stick aluminum foil (to protect light-sensitive thiophenes from degradation), sealed with polyethylene film on three sides, and held together with clamps before filling with growth medium. Drainage holes allowed removal of excess water. Marigolds (T. erecta cv. ‘Crackerjack mix’) were thinned to two seedlings per box.
Three-five wk after planting, boxes were unclamped and a 17.5 × 20 cm sheet of clear PDMS (0.8 mm thickness, obtained from Specialty Silicone Products, Ballston Spa, NY, USA) was press-applied to the root zone. To insure that PDMS sheets were clean, they were soaked in methanol for at least 24 h and then dried at 70o C before application. The glass plate was replaced, the box clamped, and the sheet was left in place for 24 h. PDMS sheets were then washed to remove adhering soil and immediately cut into fifty-six 2.5 × 2.5 cm segments. Each segment was placed in a vial with 3 ml of 95% methanol and sonicated for 12 min to extract thiophenes.
Glass Box Microtubing Study
A glass box with established marigolds (T. erecta cv. ‘Crackerjack mix’) was sampled by using five replicate 1 m lengths of PDMS Silastic® microtubing (0.30 mm ID × 0.64 mm OD, Fisher Scientific catalog no. 11-189-14). Prior to use, microtubing was cleaned by soaking in methanol for 24 h and dried at 70o C. One piece of microtubing was coiled over a 4 cm width × 17.5 cm depth section of the root zone (a total of 5 pieces over the 20 cm wide box), with the ends of the tubing left exposed above the soil surface to allow repeated sampling (Fig. 2). Microtubing was sampled by pushing 500 µl of 95% methanol through each tube at a rate of 1 ml/min followed by a 500 μl bolus of air to extrude any residual solvent. The resulting extract was collected for HPLC analysis as it exited the tubing.
The operative range of the silicone tube microextraction method was tested by preparing 1:1 sand:growth medium mixtures spiked with known concentrations of α-Terthienyl (2,2′:5′,2″-terthiophene) (α-T) (Sigma Aldrich). α-Terthienyl was applied in hexane to sand, the hexane allowed to evaporate, and the spiked sand used to prepare sand:growth medium mixtures containing concentrations of 0–10 µg g−1. In a Petri dish, one microtube was coiled over 10 g sand:growth medium, covered, and a second microtube was coiled and then covered with sand:growth medium. A total of 43 g of 1:1 sand:growth medium was used per dish. Holes were drilled in the top of the Petri dish to provide access for sampling. Water was added to bring the dishes to 20% moisture content, and microtubing was sampled at 24, 48, and 72 h (using separate dishes for each sampling time).
Root Dissection
In order to gain insight into the possible reasons for the tremendous heterogeneity of measured thiophene concentrations seen in the SPRE probe and glass box PDMS sheet studies, roots of several T. erecta and T. patula seedlings were dissected and analyzed for thiophene content. Primary and secondary roots and root tips of several seedlings grown in a 1:1 mixture of sand and growth medium were analyzed by extraction in methanol. Two week old plants (5–6 cm in length from root cap to cotyledon) were separated from the soil by submersion in distilled water. Growth medium was carefully washed from the roots. Root hairs (secondary roots) were separated from the main root with a scalpel and forceps. Roots were transferred to a Petri dish that contained distilled water, blotted dry on KimWipes®, and weighed on a microbalance to obtain a fresh root mass.
Diffusion Studies
For the diffusion studies, SPRE probes loaded with known amounts of α-T were prepared by taking freshly prepared probes and placing them in sand spiked with a known amount of α-T (20–100 ppm) and moistened to 12.5% moisture content. Probes were incubated for 1, 2, 4, or 7 d, and 4 probes were analyzed at each time point to determine the amount of loading. The amount of α-T loaded on the probes increased with concentration and time (data not shown) up to a maximum of 29 µg after 7 d incubation in 100 ppm sand. In subsequent studies, a loading time of 4 d typically was used, and several probes from each batch were analyzed to verify the α-T content.
Loaded SPRE probes (containing 22 µg α-T) were used to measure the diffusion of α-T through sand and a 1:1 mixture of sand and growth medium. A loaded probe was placed in a Petri dish containing the sand or growth medium maintained at 12.5% moisture content, and a blank probe placed 1–5 cm away. After 7 d, both probes were extracted and analyzed for α-T content.
HPLC Methods
A standard sample of α-T was obtained from Sigma Aldrich. 5-(3-buten-1-ynyl)-2,2′-bithienyl (BBT) was obtained by bulk extraction of marigold roots and preparative TLC following methods of Downum and Towers (1983), and its identification confirmed by GC-MS (data not shown). The HPLC system was an Agilent 1100 Series equipped with a binary gradient pump, autosampler, and diode array detector. Separation was carried out on an Ultra C18 5 µm particle size column (150 × 4.6 mm), with detection at 360 nm. BBT and α-T were identified based on both retention time and UV spectra using the diode array detector. Analysis of unused PDMS materials verified that these were free from contaminants when extracted and analyzed by HPLC. BBT was quantified based on the ratio of the molar absorptivity of BBT to that of α-T at 360 nm. Detector response was linear for α-T over a concentration range of 0.05–50 mg L−1. The quantitation limit was below 2 ng thiophene injected. The mobile phase was 95:5 methanol:water (v:v), at a flow rate of 1 mL min−1. All PDMS extracts were analyzed within 24 h of extraction, and were stored in amber autosampler vials to prevent degradation from light exposure. PDMS samples that could not be analyzed immediately were stored in a freezer (−20o C) until extraction and analysis. All samples were syringe filtered (0.22 µm nylon filter) prior to analysis, and injected in duplicate with the two runs averaged. Typical run to run variation was less than 1%. The injection volume was 25 or 75 µL depending on sample size.
Results and Discussion
SPRE Probe Study
Results of analyses of SPRE probes placed at varying depths beneath marigolds grown in PVC pipes are shown in Tables 1 and 2. There was tremendous variability in the amounts of α-T and BBT recovered. Table 1 shows recovery as a function of depth at 3 weeks after planting, and it can be seen that the standard deviations approach or exceed the means for all values. High probe to probe variability also was seen in studies with Tagetes patula, in which SPRE probes were inserted into soil around marigolds growing in outdoor garden beds (Weidenhamer et al. 2009). This variability was reduced somewhat by pooling multiple probes to obtain a more integrated sample, but standard deviations in the previous study still were above 50% of the mean values measured even when three probes were combined (Weidenhamer et al. 2009).
When the total amounts of α-T and BBT recovered from each pipe at the three samplings are compared, high variability is also seen (Table 2). For example, at the first sampling 3 weeks after planting, the total amount of α-T recovered (from 24 SPRE probes) ranged from 280 to 3,170 ng, and the total amount of BBT recovered ranged from 870 to 8,150 ng. There also was variability from one sampling to another, as seen by the fact that pipe 1 yielded the most α-T at the first sampling, but the least α-T at the next sampling. While overall the average amount of BBT found was four times that of α-T, the amounts found in individual pipes at a given sampling sometimes deviated widely from that proportion, as seen with pipes 1 and 3 at the first sampling.
Glass Box PDMS Sheet Study
PDMS sheets successfully recovered both α-T and BBT, but the amounts found were highly variable as found in the PVC pipe study (Table 3). Two boxes were sampled once approximately 3 weeks after planting. Immediately after sampling, the soil in each box was sectioned into a grid corresponding to the 2.5 × 2.5 cm2 sections of the PDMS sheet, and analyzed for either total thiophene content (box 1) or total root mass (box 2). No correlation was found between either total thiophene content or root mass and the amounts of thiophenes measured per PDMS section (data not shown). A third box was sampled twice, at 4 and 5 weeks after planting. As can be seen from the results summarized in Table 3, very different overall results were obtained at each sampling. The maximum amount of α-T recovered on one of the 2.5 × 2.5 cm2 sections was 5,990 ng (bordered by sections all yielding less than 40 ng), while BBT concentrations were less extreme in their variation, with a maximum amount of 260 ng recovered (bordered by sections containing 85–216 ng).
Glass Box Microtubing Study
The final approach used to measure thiophenes in the marigold root zone was to place several 1 m long coiled PDMS microtubes over portions of the marigold root zone. The silicone tube microextraction approach allows repeated sampling of root exudates without further disturbance of the soil, and experimentally is quite simple, with samples collected directly for HPLC analysis by passing 95% methanol through the microtube. The results of our first applications of silicone tube microextraction to measure thiophenes were remarkably different from those seen with other sampling methods (Fig. 3). The amounts of both BBT and α-T recovered both were highest at the initial sampling, and BBT was higher in the middle and one side of the plate, while α-T recovery was uniform across the plate (Fig. 3). Recovery of BBT slowly dropped from days 2–6, while amounts of α-T recovered and stabilized quickly at approximately 11–14 ng. While the recovery of BBT remained fairly variable among replicate microtubing extractors, with relative standard deviations of 63–87% of the mean values, recovery of α-T with the microtubing was more uniform, with relative standard deviations of 4.0–8.4% of the mean values (Table 4).
Amounts of (a) 5-(3-buten-1-ynyl)-2,2′-bithienyl (BBT) and (b) α-terthienyl (2,2′:5′,2″-terthiophene) (α-T) recovered from five replicate 1 m lengths of PDMS microtubing coiled over successive 4 cm wide × 17.5 cm deep sections of marigold root zone across a 20 cm wide glass box. Samples were taken daily for six days. Means and standard deviations for each day are presented in Table 4
The operative range of the silicone tube microextraction was tested for α-T by spiking 1:1 sand:growth medium mixtures with known amounts of α-T and incubating microtubing in the spiked soil for 24 h. This yielded a highly linear response over the range of 0–10 ppm α-T (Fig. 4), and additional measurements (data not shown) show that response was linear to 800 ppm. These data imply that recoveries of 11–42 ng α-T from silicone tube microextractors in the marigold root zone correspond to soil concentrations well below 1 ppm α-T. Separate spiked Petri dishes sampled at 48 and 72 h also yielded linear responses, with slightly increased slopes for each 24 h period. However, repeat sampling of the 24 h dishes at 48 h yielded a lower slope, suggesting that the α-T removed by sampling at 24 h was not completely replaced by diffusion over the next 24 h. Given that sorption to different soil components will vary with time and affect the partitioning behavior of thiophenes, calibration to actual soil concentrations would be difficult. We envisage two strategies for the calibration of the silicone tube microextraction. (1) The silicone tube can be equilibrated following the principles of equilibrium sampling devices (Mayer et al. 2003), and will then yield measurements of freely dissolved concentrations, chemical activities, or fugacities (Reichenberg and Mayer 2006). This approach will require high surface to volume ratios of the microtubing, and it will be suited for analytes that can be equilibrated within minutes, hours, and possibly days, which appears difficult to achieve for the thiophenes of the present study. (2) Alternatively, the extraction can be operated in the kinetic mode, and it will then yield measurements of the diffusive flux into the silicone similar to the principles of Diffusive Gradients in Thin Films (DGT) that is well established for the sampling of cationic metals (Zhang et al. 1995, 2001).
In follow-up experiments, the effectiveness of the methanol extraction of the tubing was tested by pushing ten 500 µl portions of 95% methanol through tubing incubated in a spiked sand:growth medium mixture, and then extracting remaining α-T from the tubing by sonication in excess 95% methanol. The first wash recovered approximately 40% of the total α-T on the tube, and amounts recovered by subsequent washes followed an exponential decrease, with less than 1% recovered on the tenth wash. After ten washes, less than 5% of the total α-T remained on the tubing. Further work will be required to optimize the methanol extraction procedure and there again are two different strategies. (1) The methanol volume can be minimized to e.g., 100 µl in order to avoid analyte depletion of the silicone, and this will yield the highest possible analyte concentrations in the methanol (C MeOH = C PDMS *K MeOH,PDMS ) (Mayer et al. 2009). (2) Alternatively, the methanol volume can be increased to several ml aiming for a complete extraction of the PDMS. Optimization of both the sampling step (soil to silicone) and of the extraction step (silicone to methanol) should be feasible, and further studies aiming at a well defined calibration of silicone tube microextraction are underway.
Root Dissection and Diffusion Studies
Thiophene content of roots was variable, with no clear pattern in variation from primary to secondary roots (data not shown). Concentrations of BBT and α-T in T. patula roots ranged from 0.22–1.38 µmol g−1 fresh weight and 0.01–0.04 µmol g−1 fresh weight, respectively. Concentrations of BBT and α-T in T. erecta roots were lower, ranging from 0.09–0.17 µmol g−1 fresh weight and 0.01–0.02 µmol g−1 fresh weight, respectively. These data are similar in magnitude to literature reports of thiophene concentrations in marigold roots. Croes et al. (1989) reported total thiophene concentrations of 0.22–0.41 µmol g−1 in attached T. patula roots, while Croes et al. (1994) found 0.77 µmol thiophenes g−1 in roots of six-day-old seedlings of T. patula. Jacobs et al. (1994) found total thiophene concentrations in T. patula roots to be consistently higher than those of T. erecta, which they reported to range from 0.14–0.21 µmol g−1.
The amounts of BBT found in roots are in general much higher than α-T. For T. erecta, the ratio of BBT to α-T ranged from 3.7:1 to more than 20:1 depending on the sample, considerably higher than the typical ratio of BBT to α-T recovered with the various PDMS extraction methods (Tables 3 and 4), suggesting that α-T is either preferentially exuded by roots or is more stable in soil than BBT. Further study will be required to answer this question.
Over seven days, limited diffusion of α-T was observed in sand, with ng quantities of the thiophene detected up to 4 cm from the source probe (Fig. 5a). Slightly greater diffusion was observed in the sand:growth medium mixture, but was still in the ng range compared to the 22 µg loading on the probes (Fig. 5b). Such a result was expected for these highly lipophilic compounds. The limited ability of α-T to diffuse in soil implies that it will tend to stay where it is produced, and indicates that direct contact with marigold roots or rhizosphere soil will be necessary for thiophenes to be available to target plants or organisms.
Taken together, our results provide some evidence for continued release of thiophenes from marigold roots. The limited ability of α-T to diffuse in soil, combined with the decreased amount of α-T recovered when spiked soils were resampled in the microtubing calibration study, implies that the stable concentrations of α-T measured over days 2–6 with the microtubing reflect continued release of α-T into the root zone. However, further studies will be required to verify this.
Comparison of PDMS Sampling Methods
The three PDMS-based methods gave markedly divergent results, with much lower variability in amounts recovered seen with the microtubing, especially for α-T. The three techniques are not equivalent in their potential for measuring thiophene distributions in the marigold root zone. The SPRE probes (5 cm length, 1.19 mm outer diam) have an exposed surface area of approximately 1.9 cm2, and gather data for specific, separate regions of the root zone. The PDMS sheet provides data on a vertical slice of the root zone, providing an average value over each 2.5 cm square (= 6.25 cm2) segment. The PDMS microtubing (1 m length, 0.64 mm outer diam) has an exposed surface area of approximately 19 cm2 (accounting for 2 cm at each end of the tube not in contact with soil), and thus averages thiophene concentrations over a larger region of soil than the other two methods. The question of whether the heterogeneity of thiophene distributions observed with the SPRE probe and PDMS sheet methods is real or an artifact of the sampling methods is crucial, given that such variation in the soil could have profound impacts on biological activities of these compounds.
We interpret our data to support the conclusion that the extreme heterogeneity of thiophene concentrations observed with the SPRE probes and PDMS sheets is an artifact of the sampling method and probably due to root injury and/or disturbance by these methods. We base this conclusion on several observations: (a) Variability of the SPRE probe method was extremely high even if the amount of thiophenes found on all 24 probes per sampling was summed (Table 2). The total exposed surface area of 24 SPRE probes is 45.6 cm2, more than double that of the 1 m microtubes, yet variability was very high for the SPRE method in comparison to the microtubes; (b) Heterogeneity of soil concentrations could be due to localized interactions of roots with soil microbes and microfauna, but as noted above there was no correlation between thiophene recovery and marigold root mass in the PDMS sheet study; (c) The higher recovery of thiophenes on the initial day following application of the microtubing, which was less disruptive than the press-application of PDMS sheets, also seems to underscore the importance of minimizing soil disturbance during sampling, at least for this system. A crucial requirement for obtaining accurate data on allelochemical concentrations in soil is that those dynamics not be altered by the attempt to measure them.
Finally, while PDMS in the form of SPRE probes may not be useful as originally envisioned for measurement of allelochemical dynamics (Weidenhamer et al. 2009), loaded probes may prove useful as a means to deliver known amounts of lipophilic compounds for bioassay (e.g., Mayer and Holmstrup 2008; Kwon et al. 2009).
Advantages and Potential Applications of the Silicone Tube Microextraction Method
The silicone tube microextraction method is a further development of the silicone membrane equilibrator technique, which was developed and applied for measurements of volatile organic compounds in seawater (Ooki and Yokouchi 2008) and of polycyclic aromatic hydrocarbons in vegetable and fish oils and mussel tissue (Mayer et al. 2009). In this paper, we call this method silicone tube microextraction as a more descriptive name for placing silicone microtubing for sampling in the root zone. This method has several key advantages for studies of root exudates: (a) Once microtubing is in place, the root zone can be repeatedly sampled without further disturbance; (b) the sampling method is quite simple, and the materials are inexpensive; (c) the method is sensitive to daily and spatial variations in allelochemical content; and (d) calibration of results to available analyte concentrations seems feasible, though this requires further research. The technique should be broadly applicable to the measurement of non-polar root exudates, providing a means to test hypotheses about the role of root exudates in plant-plant and other interactions. It should be possible to place microtubing in pots prior to seeding plants to monitor allelochemical dynamics over time or plant response to treatments such as herbivory, nutrient stress, or competition. Applications of microtubing for field studies also should be possible. Further explorations of the potential of this method for allelochemical analysis in soil are underway.
References
Arthur, C., and Pawliszyn, J. 1990. Solid-phase microextraction with thermal desorption using fused-silica optical fibers. Anal. Chem. 62:2145–2148.
Bakker, J., Gommers, F., Nieuwenhuis, I., and Wynberg, H. 1979. Photoactivation of the nematicidal compound α-terthienyl from roots of marigolds (Tagetes spp.). J. Biol. Chem. 254:1841–1844.
Baltussen, E., Sandra, P., David, F., and Cramers, C. 1999. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: theory and principles. J. Microcolumn Sep. 11:737-747.
Barto, K. and Cipollini, D. 2009. Half-lives and field soil concentrations of Alliaria petiolata secondary metabolites. Chemosphere 76:71–75.
Bohlmann, F., Burkhardt, T., and Zdero, C. 1973. Naturally occurring acetylenes. Academic, London.
Bruheim, I., Liu, X., and Pawliszyn, J., 2003. Thin-film microextraction. Anal. Chem. 75:1002–1010.
Campbell, G., Lambert, J., Arnason, T., and Towers, G. 1982. Allelopathic properties of α-terthienyl and phenylheptatriyne, naturally occurring compounds from species of Asteraceae. J. Chem. Ecol. 8:961–972.
Cornelissen, G., Pettersen, A., Broman, D., Mayer, P., and Breedveld, G. 2008. Field testing of equilibrium passive samplers to determine freely dissolved native polycyclic aromatic hydrocarbon concentrations. Environ. Toxicol. Chem. 27:499–508.
Croes, A., Van Den Berg, A., Bosveld, M., Breteler, H., and Wullems, G. 1989. Thiophene accumulation in relation to morphology in roots of Tagetes patula. Planta 179:43–50.
Croes, A., Jacobs, J., Arroo, R. and Wullems, G. 1994. Thiophene biosynthesis in Tagetes roots: Molecular vs. metabolic regulation. Plant Cell Tiss. Org. 38:159–165.
Downum, K., and Towers, G. 1983. Analysis of thiophenes in the Tageteae (Asteraceae) by HPLC. J. Nat. Prod. 46:98–103.
Jacobs, J., Croes, A. Engelberts, A. and Wullems G. 1994. Thiophene biosynthesis and distribution in young developing plants of Tagetes patula and T. erecta. J. Exp. Bot. 45:1459–1466.
Kwon, J. -H., Wuethrich, T., Mayer, P. and ESCHER., B. 2009. Development of a dynamic delivery method for in vitro bioassays. Chemosphere 76:83–90.
Martin, J., and Weidenhamer, J. 1995. Potassium deficiency increases thiophene production in Tagetes erecta. Curr. Top. Plant Physiol. 15:277–279.
Mayer, P., and Holmstrup, M. 2008. Passive dosing of soil invertebrates with polycyclic aromatic hydrocarbons: Limited chemical activity explains toxicity cutoff. Environ. Sci. Technol. 42:7516–7521.
Mayer, P., Vaes, W., Wijnker, F., LeGierse, K., Kraaij, R., Tolls, J., and Hermens, J. 2000. Sensing dissolved sediment porewater concentrations of persistent and bioaccumulative pollutants using disposable solid-phase microextraction fibers. Environ. Sci. Technol. 34:5177–5183.
Mayer, P., Tolls, J., Hermens, J., and Mackay, D. 2003. Equilibrium sampling devices. Environ. Sci. Technol. 37:184A–191A.
Mayer, P., Toräng, L., Glæsner, N., and Jönsson, J. 2009. Silicone membrane equilibrator: Measuring chemical activity of nonpolar chemicals with poly(dimethylsiloxane) microtubes immersed directly in tissue and lipids. Anal. Chem. 81:1536–1542.
Ooki, A., and Yokouchi, Y. 2008. Development of a silicone membrane equilibrator for measuring partial pressures of volatile organic compounds in natural water. Environ. Sci. Technol. 42:5706–5711.
Reichenberg, F. and Mayer, P. 2006. Two complementary sides of bioavailability: Accessibility and chemical activity of organic contaminants in sediments and soils. Environ. Toxicol. Chem. 25:1239–1245.
Tang, C., Wat, C., and Towers, G. 1987. Thiophenes and benzofurans in the undisturbed rhizosphere of Tagetes patula L. Plant and Soil 98:93–97.
Weidenhamer, J. 1996. Distinguishing resource competition and chemical interference: Overcoming the methodological impasse. Agron. J. 88:866–875.
Weidenhamer, J. 2005. Biomimetic measurement of allelochemical dynamics in the rhizosphere. J. Chem. Ecol. 31:221–236.
Weidenhamer, J., Boes, P., and Wilcox, D. 2009. Solid-phase root zone extraction (SPRE): A new methodology for measurement of allelochemical dynamics in soil. Plant and Soil 322:177–186. doi: 10.1007/s11104-009-9905-4.
Williamson, G. B., and Weidenhamer, J. D. 1990. Bacterial degradation of juglone: Evidence against allelopathy? J. Chem. Ecol. 16: 1739–1742.
Zhang, H., Davison, W., Miller, S., and Tych, W. 1995. In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochim. Cosmochim. Acta 59:4181–4192.
Zhang, H., Zhao, F. -J., Sun, B., Davison, W., and McGrath, S. 2001. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ. Sci. Technol. 35: 2602–2607.
Acknowledgments
This research was supported by a grant from the National Science Foundation (DEB 0515826) and from the European Commission (MODELPROBE, no. 213161). Philip Boes assisted with isolation and characterization of BBT. Udo Blum and two anonymous reviewers provided helpful comments on the manuscript. Kelly Harrison assisted with greenhouse cultivation of plants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohney, B.K., Matz, T., LaMoreaux, J. et al. In Situ Silicone Tube Microextraction: A New Method for Undisturbed Sampling of Root-exuded Thiophenes from Marigold (Tagetes erecta L.) in Soil. J Chem Ecol 35, 1279–1287 (2009). https://doi.org/10.1007/s10886-009-9711-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9711-8