Abstract
Root volatile organic compounds (VOCs), their chemistry and ecological functions have garnered less attention than aboveground emitted plant VOCs. We report here on the identification of VOCs emitted by barley roots (Hordeum vulgare L.). Twenty nine VOCs were identified from isolated 21-d-old roots. The detection was dependent on the medium used for root cultivation. We identified 24 VOCs from 7-d-old roots when plants were cultivated on sterile Hoagland gelified medium, 33 when grown on sterile vermiculite, and 34 on non-sterile vermiculite. The major VOCs were fatty acid derived compounds, including hexanal, methyl hexanoate, (E)-hex-2-enal, 2-pentylfuran, pentan-1-ol, (Z)-2-(pentenyl)-furan, (Z)-pent-2-en-1-ol, hexan-1-ol, (Z)-hex-3-en-1-ol, (E)-hex-2-en-1-ol, oct-1-en-3-ol, 2-ethylhexan-1-ol (likely a contaminant), (E)-non-2-enal, octan-1-ol, (2E,6Z)-nona-2,6-dienal, methyl (E)-non-2-enoate, nonan-1-ol, (Z)-non-3-en-1-ol, (E)-non-2-en-1-ol, nona-3,6-dien-1-ol, and nona-2,6-dien-1-ol. In an olfactometer assay, wireworms (larvae of Agriotes sordidus Illiger, Coleoptera: Elateridae) were attracted to cues emanating from barley seedlings. We discuss the role of individual root volatiles or a blend of the root volatiles detected here and their interaction with CO2 for wireworm attraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A complex blend of volatile organic compounds (VOCs) is emitted by plants, ranging from fatty acid derivatives, terpenoids, and sulfur compounds to phenylpropanoids (Qualley and Dudareva 2009). The emission can be constitutive and/or induced by environmental or physiological stresses (Maffei 2010). Depending on the stress type (wounding, herbivory, pathogen attack, dehydration, (UV) light, heat, etc.), the composition and amounts of released VOCs can vary (Ferry et al. 2004; Filella et al. 2009; Jansen et al. 2011; Kuhn et al. 2004).
Plant VOCs are emitted by various organs (seeds, flowers, leaves, stems, and roots). The rhizosphere provides a nutrient-rich environment for many organisms, as up to 20 % of the photosynthetically fixed carbon is released by roots (Barber and Martin 1976). Vertebrates, invertebrates, plants, fungi, and bacteria all share the same underground space in which VOC-mediated interactions can take place and even affect aboveground plant insect interactions (Effmert et al. 2012; Johnson et al. 2009; Soler et al. 2012; Wenke et al. 2010); however, the belowground VOCs potentially responsible for such interactions have to date been partially neglected, due to technical limitations. The release of root VOCs can mediate various interactions: direct or indirect defense of roots against herbivores (Ali et al. 2011; Rasmann et al. 2005, 2012a), plant – plant competition (Ens et al. 2009; Jassbi et al. 2010; Viles and Reese 1996), resistance of roots against pathogens (Cobb et al. 1968; Kalemba et al. 2002; Vilela et al. 2009), and symbiotic interactions (Asensio et al. 2012; Paavolainen et al. 1998). Root derived compounds can also attract herbivores (Wenke et al. 2010), and their emission tends to decrease in unattacked conditions (Piesik et al. 2011b), thus limiting the energy costs incurred by their synthesis (Herms and Mattson 1992; Rasmann et al. 2012a, b). Carbon dioxide gradients are an ubiquitous belowground herbivore attractant, but other volatile and non-volatile semiochemicals also are involved in directing herbivores towards roots (Johnson and Nielsen 2012; Reinecke et al. 2008; Weissteiner et al. 2012). Several studies have shown the attractive role of root-emitted VOCs towards arthropods. For example, di- and trisulfides produced by Allium cepa are potent attractants of the larvae of the fly Delia antiqua (Matsumoto 1970); VOCs released by damaged oak roots are perceived by the larvae of the forest cockchafer Melalontha hippocastani and attract the larvae in natural soil (Weissteiner et al. 2012); volatiles of ryegrass roots attracted the larvae of Costelytra zealandica (Sutherland and Hillier 1972). Arthropods can differentiate between root VOCs released from plants that differ with respect to physical or physiological traits (Aratchige et al. 2004; Tapia et al. 2007; Witcosky et al. 1987); they also can differentiate between root VOCs released by different varieties of plants (Guerin and Ryan 1984).
In this study, we characterized VOCs emitted by isolated barley roots. Root emissions from barley cultivated under sterile and non-sterile conditions were compared in order to characterize root emission in the absence of microorganisms. Moreover, the development of an orientation test with the larvae of the click beetle Agriotes sordidus Illiger (Coleoptera: Elateridae) led us to investigate the potential semiochemical role of the VOC blend emitted by isolated barley roots. The general working questions were: (i) How complex is the volatile blend of isolated barley roots? (ii) What is the impact of microorganisms on the volatile blend released by barley roots? (iii) How attractive is this volatile blend of barley roots to wireworms?
Larvae of Agriotes are polyphagous wireworms that feed on the roots of a variety of crops, including cereals (Johnson et al. 2009; van Herk and Vernon 2013). Baits based on germinating wheat and barley seeds have been proven to be efficient (Parker 1996). However, few studies of wireworm - barley interactions are available, although barley is the second most important crop in Europe (production, 2010, FAOstat). As for most of the root herbivores, the CO2 gradient is the general search trigger for wireworms. (Doane et al. 1975; Johnson and Nielsen 2012). Plant-derived VOCs can affect the behavior of root-feeding insects, while their identity and role in wireworms’ chemical ecology still has to be revealed (Barsics et al. 2013; Johnson and Nielsen 2012). Since such signals could be potent wireworm attractants or repellents, we focused on the release of VOCs from barley roots and tested the attraction of wireworms to barley roots.
Methods and Materials
Plant Material
Growth Conditions
Barley plants (var. Quench, Jorion, Belgium) were grown at 22 °C under LED light (95 μmol m−2 sec−1) with a 20/4 h L/D photoperiod and 65 % RH.
Cultivation of 21-d-Old Plants
Caryopses were sown at a density of 10 plants per pot (7 l) in vermiculite (Sibli, Belgium). Plants were watered daily and fertilized × 3 per week with aqueous Hoagland’s solution (Hoagland’s NO.2 basal salt mixture, Sigma, Belgium).
Aseptical Cultivation of 7-d-Old Plants
Barley caryopses (28 g) were sterilized as described by Lanoue et al. (2010). Briefly, caryopses were incubated in 50 ml H2SO4 (50 % v/v) for 1 h and washed x 5 in 150 ml sterile bidistilled water. Caryopses then were shaken for 20 min in 80 ml AgNO3 (1 % w/v) and washed successively with 150 ml sterile NaCl (1 %, w/v), 150 ml sterile bidistilled water, 150 ml sterile NaCl (1 %, w/v) and × 5 with 150 ml sterile bidistilled water, before sowing: (a) on 124 cm2 Petri dishes filled with Hoagland medium (Hoagland’s NO.2 basal salt mixture, Sigma, Belgium), solidified with 0.8 % agar (w/v; Plant agar, Duchefa Biochemie, Belgium) or (b) on vermiculite with Hoagland solution (Hoagland’s NO.2 basal salt mixture, Sigma, Belgium).
(a) Sterile caryopses were placed on Hoagland’s medium with the ventral furrow underneath and left to grow for 7 d vertically in a growth chamber. (b) Sterile caryopses were sown aseptically in 2 l jars (le Parfait, Villeurbanne, France), filled with 600 ml sterile vermiculite humidified with 300 ml sterile Hoagland solution. Jars were closed and sealed with plastic film and left for 7 d in a growth chamber. On the sampling day, vermiculite isolated in the vicinity of the roots was incubated on tryptic soy agar (Fluka, Belgium) for 1 wk at 37 °C to check sterility. All glass, media and jars were sterilized.
As controls, non-sterile plants were grown for 7 d in 600 ml vermiculite humidified with 300 ml Hoagland solution in 2 l open jars.
Analyses of Volatile Organic Compounds
Head-Space Solid-Phase-Microextraction (HS-SPME)
Roots were isolated from the substrate by shaking the plant gently and were separated from the upper part of the plant by cutting just below the caryopsis. Then 3 g ± 0.1 g of fresh entire roots were placed in 20 ml SPME vials (Filter Service, Belgium) fitted with a sealed cap (white silicone/blue PTFE, Filter Service). Roots were not cut into pieces, and the sampling conditions were the same for all samples. An internal standard (1 μl of a methanolic solution of butyl benzene (≥ 99 %, Sigma-Aldrich (S.-A.), Belgium) at 0.86 mgl−1) was added on the surface of the vial without touching the roots. The fiber (divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS (50/30 μm); S.-A.) was the same for all repetitions of the same experiment. The fiber was conditioned before first use at 270 °C for 1 h. After equilibration of the vial for 15 min at 30 °C, the fibre was inserted into the headspace for 30 min at the same temperature.
Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
After extraction, the volatile compounds were desorbed in pulsed splitless mode for 10 min at 250 °C. GC-MS analyses were performed on an Agilent Technologies 7890A GC System coupled to an Agilent Technologies 5975C Mass Spectrometer equipped with Wax factor four (Agilent technologies USA; 30 m x 0.250 mm I.D, 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1.5 ml/min. The inlet temperature was 250 °C. Pulsed splitless injection mode was used in a 1.5 mm HS-liner (injection pulse pressure of 30 psi for 1 min). The following temperature program was used: 40 °C for 4 min; 15 °C/min to 160 °C; 20 °C/min to 250 °C; and 250 °C for 5 min; 30 °C/min to 300 °C; and final hold at 300 °C for 15 min.
The MS was carried out in EI mode at 70 eV; source temperature, 230 °C; quadrupole temperature, 150 °C; scanned mass range: from 20 to 350 amu, threshold of 150 amu; scan speed, 4.27 scans/s.
Chemical Identification
Components were identified by comparing recorded mass spectra with the NIST and Wiley spectral databases. Further identification was carried out by calculating non-isothermal Kovats retention indices by injecting saturated n-alkane standard solution (C7-C30 1,000 μg/ml in hexane, Supelco, Belgium) under the same chromatographic conditions, using the definition of Van den Dool and Kratz (1963).
Whenever possible, identifications were confirmed by injection of available commercial standards. References of commercial standards are listed in the supplemental text. As the same chromatographic conditions with the same column were used for the analyses of the standards, identification of the detected compounds in the headspace of barley roots was confirmed by comparing their retention data and mass spectra with those of the commercially available reference compounds.
Peaks which showed a signal/noise ratio of three compared to the blank controls were identified and integrated manually with the Agilent MSD Chemstation. The relative area of a target compound was calculated by dividing the peak area of this compound by the total peak area of the sample. Statistical analysis was performed on the relative area with a two-tailed paired t-test after having checked that the data were normally distributed with Kolmogorov-Smirnov test.
Concentration Estimation
Samples were extracted using an autosampler (MPS2, Gerstel) equipped with a sample tray holder and a needle heater for heating the vials. Gerstel Maestro software was used for autosampler control. Standards and dilutions were always handled with a Hamilton syringe with a volume of 1 μl. The molecules identified by GC-MS were grouped into the following classes: alkanes, aldehydes, alcohols, esters, sulfur, and furan compounds. In each class, a representative compound was selected as the basis of the calibration curves: n-tetradecane (S.-A.; 99 %), (E)-non-2-enal (SAFC; ≥ 93 %), (E)-non-2-en-1-ol (SAFC; ≥ 96 %), methyl benzoate (Fluka; ≥ 99.5 %), dimethyl sulfoxide (S.-A.; ≥ 99,9 %), and 2-pentylfuran (SAFC; 97 %). The calibration curves included at least four points and were performed in triplicate (Supplemental Table S1). For each measurement, the experiment was carried out according to the following process. A stock solution was diluted in methanol by using volumetric glassware. One microliter of each dilution then was placed in a vial (20 ml) with 1 μl of the internal standard (butylbenzene 0.86mgl−1 in methanol). After an equilibration period of 15 min at 30 °C, the fiber was exposed for 30 min (at 30 °C) before analysis as described above.
Wireworms Olfactory Orientation Bioassay
Wireworms were collected in November 2011 in Montardon (Pau, France), from the soil of grass edging a fallow plot and an untreated wheat field. Morphological criteria described in the keys of Cocquempot et al. (1999) and Pic et al. (2008) allowed Agriotes sordidus individuals to be identified. Each larva was kept individually in an 80 ml capped vial, with a mix of leaf mold and vermiculite (1/1 v/v, 16.5 % water) and a mix of meadow seeds (0.130–0.160 g, Prelac Bio, SCAR, Belgium). All vials were kept in the dark at 21.2 ± 0.7 °C. Seven days before being tested, wireworms greater than 10 mm in length were individually isolated in vermiculite (16.5 % water). Wireworms were selected for testing from the isolated individuals according to their apparent activity: those visibly in the pre-molting or post-molting phases were excluded from the tests. In total, 60 larvae were submitted to the olfactometry bioassay.
The bioassay set up (Fig. S1) consisted of a glass pipe (32 cm long, 3.6 cm internal diameter), with both extremities closed with GL45 caps (Duran, Belgium), which allowed the set-up to be filled and emptied with substrate. Two GL14 holes (Duran, Belgium) were present at a distance of 3 cm from both extremities to allow the introduction of stimuli from both sides. The entry point for the larvae was provided by a third lateral hole in the middle of the pipe, diametrically opposite to both lateral connections.
The set-up was filled with wet vermiculite, which was removed to a depth of 4 cm at the two ends of the equipment to leave room for the bait and control compartments (final vermiculite content: 64.4 ± 0.9 g, 53.0 ± 0.4%water). In each of the pipes, the bait consisted of the roots of 10 developed barley seedlings gently removed from growth medium before they were introduced into the bait zone. The roots were positioned through one of the lateral tubular perforations at the distal end of the olfactometer. Plants were held in position with both aluminum foil and PTFE tape (EGEDA, Belgium). Thus, roots were the only plant material exposed to wireworms, as the rest of the plant was isolated out of the bioassay. Blank culture medium (240 mg – the average amount of medium still upon the roots after extraction from the medium) was introduced into the opposite side of the bait compartment. To prevent any contact between a wireworm and the roots, a gauze (3.6 cm diam., stainless steel; width: 0.042 mm; mesh: 0.036 mm; Haver, Belgium) was used to separate the substrate from the bait and control compartments. Tests were performed in batches of 10 olfactometers at a room temperature of 21.9 ± 0.5 °C.
Bioassays were performed in the dark. Each wireworm was introduced individually 40 min after the bait was set in the system, and a red plastic sheet was placed on each bioassay during the test in order to suppress light biases. The position (left or right) of the baits on the laboratory bench was randomly assigned and noted, as was the position of the baits with respect to the side by which the olfactometers had been filled with substrate.
The position of the wireworms was recorded after 60 min. Any wireworm located within a distance of 3 cm from the entry point was considered as non-responding. We performed replicates until 50 responses were recorded, which took a total of 60 tested individuals. All material was cleaned with water and norvanol (VWR, Belgium) between each test. Observed frequencies relating to the choice of wireworm larvae in dual choice bioassays were compared to corresponding theoretical frequencies by applying a χ 2 goodness-of-fit test, using Minitab® release 14.2. Potential sources of bias (bait position on the laboratory bench and bait position with regard to the entry side of the substrate) were tested with Fisher’s exact test for count data (one factor, four modalities) using R software, version 2.14.1 (2011-12-22; Development Core Team 2008). The orientation bioassay also was performed ‘blank-to-blank’, i.e. without baits and controls, in order to assess the nature of wireworm behavior in an odor-free testing environment. The results were treated with a χ 2 goodness-of-fit test with regard to wireworm position. The potential impact of the entry side of the substrate on the results was tested with a χ 2 test for independence, using Minitab® release 14.2.
Results
Identification of Volatile Organic Compounds in 21-d-Old Barley Roots
We detected 29 volatile compounds in the headspace of isolated 21-d-old barley roots (Table 1, N = 5 replicates). Confirmation of the occurrence of 24 VOCs was possible by comparison with the NIST 08 and Wiley 275 k databases, the library retention index, and standards. The retention index (RI) of dimethyl sulfide could not be precisely calculated as it was eluted in the very early phase of the chromatogram. Relative experimental RI deviations from the database’s RI ranked from −1.4 to 1.7 %. Relative quantities were determined by determining the area of the compound peak relative to the total peaks area. In order to estimate the amounts of the 29 compounds listed in (Supplemental Table S1), response curves were calculated for major chemical families present in the list by using one representative compound of each family. This approach involved performing six linear calibration curves linear in the concentration ranges tested, as evidenced by the values of correlation coefficients, always > 0.99 (Table 1).
In Figure S2 and the supplemental text, the development of the SPME-GC-MS method is described. An optimized protocol for SPME analysis of root volatiles was developed. An important point was the use of the DVB/CAR/PDMS fiber with a 50/30 μm coating, and a fixed equilibration and sampling time. Exactly 3 g root material were used, and a wax column proved to be suitable for separation of the volatiles. Figure S3 shows a total ion current chromatogram of a headspace sample of 21-d-old isolated barley roots.
Barley Root VOCs After Seven Days of Culture on Hoagland Gelified Medium
Table 2 provides a list of the compounds that were emitted by 7-d-old barley roots cultivated on Hoagland gelified medium. Confirmation of the occurrence of 16 VOCs was possible by the injection of a standard, and 4 VOCs could be tentatively identified by their theoretical RI. Estimation of the amount of VOCs released was performed in the same way as described for the data shown in Table 1.
Barley Root VOCs After Seven Days of Culture in Sterile/Non-Sterile Conditions
The potential contribution of microorganisms present in the environment of the roots in the degradation or emission of the VOCs was assayed by cultivating plants for 7 d in sterile vermiculite (ST) and in non-aseptic (NS) vermiculite fertilized with Hoagland solution. Thirty-three VOCs were identified from the roots of ST plants, 34 were detected as emitted from the roots of NS plants (Table 3, Fig. S4).
Statistical analysis of the relative area of VOCs emitted by ST and NS roots showed that six VOCs (pentan-3-one, pent-1-en-3-ol, 2-ethylhexan-1-ol, dodecan-1-ol, dihydro-5-pentyl-2(3H)-furanone, hexadecanal) were differentially detected (two-tailed paired t-test; P < 0.05) in NS compared to ST conditions (Table 3), whereas (E)-pent-2-en-1-ol and methyl-dodecanoate were found only in the headspace of roots of NS plants.
Wireworms Olfactory Orientation Bioassay
Seven-day-old barley roots grown in axenic conditions were used as volatile-emitting sources (10 plantlets/olfactometric test) for an orientation bioassay of the belowground pest insect Agriotes sordidus. We tested N = 60 larvae; 10 larvae did not respond (16.5 %), 35 oriented towards barley roots, and 15 towards the control (χ 2 = 8; P = 0.005).
The bait position with regard to both the substrate entry side and the left or right position of the roots on the laboratory bench did not significantly affect the response of the wireworms (Fisher’s test of exact count, (P = 0.861). The blank-to-blank experimentation confirmed the absence of biases in the experimental set-up. Fifteen wireworms orientated to the left, eleven to the right (χ 2 = 0.615; P = 0.433), and nine did not respond (26 % of the tested individuals). The χ 2 test for independence showed that the entry side of the substrate had no impact on the results (χ 2 = 1.418; P = 0.214).
Discussion
We developed an SPME method that enabled us to detect a wide range of VOCs released by barley roots. Furthermore, our method allowed the estimation of the amounts of VOCs emitted from roots. The physicochemical properties of a VOC depend significantly on its chemical family. The sorption of a volatile compound on the SPME fiber depends on the functional groups, vapor pressure, and constitution of the headspace of the sample. This needs to be taken into account when considering the quantitative values presented in Tables 1, 2, and 3 (columns B). These quantitative values are just estimations based on calibration curves obtained from the analyses of compounds that are considered representative for the respective detected root volatile. Exact quantification of VOCs in a mixture of approximately thirty compounds is quite difficult as many VOCs have to be quantified simultaneously in the volatile blend.
The major volatiles emitted by barley aerial parts were described by Bukovinszky et al. (2005), Piesik et al. (2010, 2011a), and Wenda-Piesik et al. (2010), whereas VOCs emitted by barley roots have not been reported previously in the literature, nor has any description of their biological activity been published. In our research reported herein, a total of 29 compounds were identified from excised roots after 21 days of growth. Estimation of the concentration of barley VOCs on a fresh weight basis revealed a similar range of emission when compared to that reported for barley leaves under controlled conditions or for β-caryophyllene in maize roots (Hiltpold et al. 2011; Piesik et al. 2010). In our study, VOC profiling was performed on roots separated from the aerial parts of the plant. In order to validate the working conditions, roots were wounded manually and changes in the profile of VOCs were analyzed. This additional wounding of roots resulted in a dramatic increase in the amount of VOCs emitted with no major changes in the qualitative VOC profile (data not shown).
VOCs derived from polyunsaturated fatty acids constituted the largest number of VOCs of the barley root blend. These comprise hexanal, methyl hexanoate, (E)-hex-2-enal, 2-pentylfuran, pentan-1-ol, (Z)-2-(pentenyl)-furan, (Z)-pent-2-en-1-ol, hexan-1-ol, (Z)-hex-3-en-1-ol, (E)-hex-2-en-1-ol, oct-1-en-3-ol, 2-ethylhexan-1-ol, (E)-non-2-enal, octan-1-ol, (2E,6Z)-nona-2,6-dien-al, methyl (E)-non-2-enoate, nonan-1-ol, (Z)-non-3-en-1-ol, (E)-non-2-en-1-ol, nona-3,6-dien-1-ol, nona-2,6-dien-1-ol, and dihydro-5-pentyl-2(3H)-furanone (Min et al. 2003; Shiojiri et al. 2006). Most of these compounds have been largely described in leaves as direct or indirect defense molecules, produced in response to herbivory or wounding (Arimura et al. 2000). Similarly to our study, barley aerial volatiles emitted under unwounded conditions were mainly C18 fatty acid derived volatile compounds, such as (E)-2-hexenal and (Z)-hex-3-en-1-ol (Piesik et al. 2010; Wenda-Piesik et al. 2010). Fatty acid derived VOCs might have a basal level of emission under unattacked conditions. With respect to root VOCs, quite similar compounds (hexanal, (E)-2-hexenal, 2-pentylfuran, 2-ethylhexan-1-ol, octan-1-ol, (E)-non-2-enal) were identified in grapevine ground roots (Lawo et al. 2011). As these VOCs have been described in the wound response, they are interesting candidates in the study of root - wireworms interactions. As 2-ethylhexan-1-ol has never been clearly demonstrated to be of plant origin, this compound might be regarded as a plastifying contaminant (Yi et al. 2009).
Apart from fatty acid derived VOCs, two sulfur-containing volatile molecules (dimethyl sulfide and dimethyl sulfoxide) were constituents of the volatile blend. These two compounds have not previously been described as emitted by barley. Dimethyl sulfide is released from wounded citrus and guava leaves (Rouseff et al. 2008). Sulfur compounds have been shown to be attractants of the fly Delia antiqua (Matsumoto 1970).
Surprisingly, we did not identify any terpenes, such as β-caryophyllene. Nevertheless, we detected exogenously applied monoterpenes and sesquiterpenes when using our method (data not shown). This means either that barley roots do not emit terpenes or emit them below detection limits. This result is in agreement with the measurement of VOCs emitted by barley leaves; no terpene was detected in the headspace of non-wounded barley leaves; however, terpenes were detected after wounding (Piesik et al. 2010; Wenda-Piesik et al. 2010).
The impact of microorganisms in the measured volatile blend of barley roots was low since 32 VOCs out 34 were the same between the NS and ST roots (Table 3). Similarly, 28 VOCs were present in similar amounts in the headspace of roots kept under the two conditions.
The orientation bioassay showed that wireworms exploit the emission of VOCs from 7-d-old barley roots and use it for location of host roots. The percentages of non-responding individuals in the blank-to-blank bioassay and in the root-baited bioassay tended to show a slightly increased activity of wireworms in the presence of a stimulus. As roots were still respiring, CO2 obviously formed part of the blend. Its involvement in the attraction of Ctenicera destructor (Brown) and other wireworms has been demonstrated (Doane et al. 1975). However, CO2 emission from roots is probably not a reliable cue for host root location by rhizophagous insects, since CO2 is emitted from numerous sources in the soil and thus, lacks specificity. Nevertheless, it probably acts as a general signal or a search trigger. Moreover, chemically-mediated orientation due to volatile or non-volatile compounds of the rhizosphere often is proposed whenever root location by subterranean insects is investigated (Johnson and Gregory 2006; Reinecke et al. 2008;,Weissteiner et al. 2012; Wenke et al. 2010). Such cues could be considered within an integrated management perspective, which will never be the case for CO2 which is present in all soils.
Future studies need to elucidate whether each of the barley root volatiles detected in our study serves as an attractant to a rhizophagous insect. The VOCs identified using the protocol described for 7-d-old sterile roots (Table 2) need to be tested alone and in combination in the olfactometers, in order to assess both their potential attractive properties and their possible synergistic interactions with each other and with CO2. This can be achieved by using slow release systems such as alginate beads (Heuskin et al. 2011), with the advantage of suppressing carbon dioxide gradients.
The assessment of the role of volatile compounds in the chemical ecology of wireworms is promising, especially regarding the developed bioassay. Further experiments involving the natural enemies of wireworms, such as entomopathogenic nematodes, could lead to a higher trophic level and could also provide useful information. Such tri-trophic interactions have already been studied between the root pest Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), maize (Zea mays, L. Poaceae), and the entomopathogenic nematode Heterorhabditis megidis (Hiltpold et al. 2011).
References
Ali JG, Alborn HT, Stelinski LL (2011) Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J Ecol 99:26–35
Aratchige NS, Lesna I, Sabelis MW (2004) Below-ground plant parts emit herbivore-induced volatiles: Olfactory responses of a predatory mite to tulip bulbs infested by rust mites. Exp Appl Acarol 33:21–30
Arimura G, Ozawa R, Shimoda T, Nishloka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515
Asensio D, Rapparini F, Peñuelas J (2012) AM fungi root colonization increases the production of essential isoprenoids vs. nonessential isoprenoids especially under drought stress conditions or after jasmonic acid application. Phytochemistry. 77:149–161
Barber DA, Martin JK (1976) The release of organic substances by cereal roots into soil. New Phytol 76:69–80
Barsics F, Haubruge E, Verheggen FJ (2013) Wireworms’ management: an overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 4:117–152
Binder RG, Turner CE, Flath RA (1990) Volatile components of purple starthistle. J Agric Food Chem 38:1053–10556
Bukovinszky T, Gols R, Posthumus MA, Vet LEM, Van Lenteren JC (2005) Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellén). J Chem Ecol 31:461–480
Chisholm MG, Wilson MA, Gaskey GM (2003) Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle). Flavour Frag J 18:106–115
Choi H-S (2003) Character impact odorants of citrus hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. J Agric Food Chem 51:2687–2692
Chung TY, Eiserich JP, Shibamoto T (1993) Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb). J Agric Food Chem 41:1693–1697
Cobb FW Jr, Krstic M, Zavarin E, Barber HW Jr (1968) Inhibitory effects of volatile oleoresin components on Fomes annosus and four Ceratocystis species. Phytopathology 58:1327–1335
Cocquempot C, Martinez M, Courbon R, Blanchet A, Caruhel P (1999) Nouvelles données sur l’identification des larves de taupins (Coleoptera: Elateridae): une aide à la connaissance biologique et à la cartographie des espèces nuisibles. In: ANPP - 5ème conférence internationale sur les ravageurs en agriculture, Montpellier, 7-8-9 décembre 1999, Paris: ANPP, 477–486
De Marques FA, Mcelfresh JS, Millar JG (2000) Kováts retention indexes of monounsaturated C12, C14, and C16 alcohols, acetates and aldehydes commonly found in lepidopteran pheromone blends. J Braz Chem Soc 11:592–599
Doane JF, Lee YW, Klingler J, Westcott ND (1975) Orientation response of Ctenicera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and to carbon dioxide. Can Entomol 107:1233–1252
Effmert U, Kalderás J, Warnke R, Piechulla B (2012) Volatile mediated interactions between bacteria and fungi in the soil. J Chem Ecol 38:665–703
Ens EJ, Bremner JB, French K, Korth J (2009) Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biol Invasions 11:275–287
FAOstat, Food and Agriculture Organization of the United Nations, http://faostat.fao.org/
Ferreira V, Aznar M, Lopez R, Cacho J (2001) Quantitative gas chromatography-olfactometry carried out at different dilutions of an extract. Key differences in the odor profiles of four high-quality Spanish aged red wines. J Agric Food Chem 49:4818–4824
Ferry N, Edwards MG, Gatehouse JA, Gatehouse AMR (2004) Plant-insect interactions: Molecular approaches to insect resistance. Curr Opin Biotechnol 15:155–161
Filella I, Peñuelas J, Seco R (2009) Short-chained oxygenated VOC emissions in Pinus halepensis in response to changes in water availability. Acta Physiol Plant 31:311–318
Guerin PM, Ryan MF (1984) Relationship between root volatiles of some carrot cultivars and their resistance to the carrot fly, Psila rosae. Entomol Exp Appl 36:217–224
Hayata Y, Sakamoto T, Maneerat C, Li X, Kozuka H, Sakamoto K (2003) Evaluation of aroma compounds contributing to muskmelon flavor in Porapak Q extracts by aroma extract dilution analysis. J Agric Food Chem 51:3415–3418
Herms DA, Mattson WJ (1992) The dilemma of plants - to grow or defend. Q Rev Biol 67:283–335
Heuskin S, Verheggen FJ, Haubruge E, Wathelet JP, Lognay G (2011) The use of semiochemical slow-release devices in integrated pest management strategies. Biotechnol Agron Soc Environ 15:459–470
Hiltpold I, Erb M, Robert CAM, Turlings TCJ (2011) Systemic root signalling in a belowground, volatile-mediated tritrophic interaction. Plant Cell Environ 34:1267–1275
Jansen RMC, Wildt J, Kappers IF, Bouwmeester HJ, Hofstee JW, Van Henten EJ (2011) Detection of diseased plants by analysis of volatile organic compound emission. Annu Rev Phytopathol 49:157–174
Jassbi AR, Zamanizadehnajari S, Baldwin IT (2010) Phytotoxic volatiles in the roots and shoots of Artemisia tridentata as detected by headspace solid-phase microextraction and gas chromatographic-mass spectrometry analysis. J Chem Ecol 36:1398–1407
Jennings W, Shibamoto T (1980) Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. Academic, New York
Johnson SN, Nielsen UN (2012) Foraging in the dark – chemically mediated host plant location by belowground insect herbivores. J Chem Ecol 38:604–614
Johnson SN, Hawes C, Karley AJ (2009) Reappraising the role of plant nutrients as mediators of interactions between root- and foliar-feeding insects interactions between root- and foliar-feeding insects. Funct Ecol 23:699–706
Kalemba D, Kusewicz D, Swider K (2002) Antimicrobial properties of the essential oil of Artemisia asiatica Nakai. Phytother Res 16:288–291
Kohara K, Kadomoto R, Kozuka H, Sakamoto K, Hayata Y (2006) Deodorizing effect of coriander on the offensive odor of the porcine large intestine. Food Sci Technol Res 12:38–42
Kuhn U, Rottenberger S, Biesenthal T, Wolf A, Schebeske G, Ciccioli P, Brancaleoni E, Frattoni M, Tavares TM, Kesselmeier J (2004) Seasonal differences in isoprene and light-dependent monoterpene emission by amazonian tree species. Global Change Biol 10:663–682
Lanoue A, Burlat V, Schurr U, Röse USR (2010) Induced root-secreted phenolic compounds as a belowground plant defense. Plant Signal Behav 5:1037–1038
Lawo NC, Weingart GJF, Schuhmacher R, Forneck A (2011) The volatile metabolome of grapevine roots: First insights into the metabolic response upon Phylloxera attack. Plant Physiol Biochem 49:1059–1063
Maffei M (2010) The plant volatilome, essay 13.7 in L. Taiz and E. Zeiger. Plant physiology, fifth edition online. Sinauer associates, Sunderland, Massachusetts. http://5e.plantphys.net/article.php?ch=e&id=520
Matsumoto Y (1970) Volatile organic sulfur compounds as insect attractants with special reference to host selection. In: Wood DL, Silverstein RM, Nakajima M (eds) Control of insect behavior by natural products. Academic, New York, pp 133–160
Min DB, Callison AL, Lee HO (2003) Singlet oxygen oxidation for 2-pentylfuran and 2-pentenyfuran formation in soybean oil. J Food Sci 68:1175–1178
Paavolainen L, Kitunen V, Smolander A (1998) Inhibition of nitrification in forest soil Fby monoterpenes. Plant Soil 205:147–154
Parker WE (1996) The development of baiting techniques to detect wireworms (Agriotes spp., Coleoptera: Elateridae) in the field, and the relationship between bait-trap catches and wireworm damage to potato. Crop Prot 15:521–527
Pic M, Pierre E, Martinez M, Genson G, Rasplus JY, Albert H (2008) Les taupins du genre Agriotes démasqués par leurs empreintes génétiques. AFPP - 8ème conférence internationale sur les ravageurs en agriculture. Montpellier 22–23 Octobre 2008, Paris: ANPP, 23–32
Piesik D, Łyszczarz A, Tabaka P, Lamparski R, Bocianowski J, Delaney KJ (2010) Volatile induction of three cereals: Influence of mechanical injury and insect herbivory on injured plants and neighbouring uninjured plants. Ann Appl Biol 157:425–434
Piesik D, Panka D, Delaney KJ, Skoczek A, Lamparski R, Weaver DK (2011a) Cereal crop volatile organic compound induction after mechanical injury, beetle herbivory (Oulema spp.), or fungal infection (Fusarium spp.). J. Plant Physiol 168:878–886
Piesik D, Wenda-Piesik A, Kotwica K, Lyszczarz A, Delaney KJ (2011b) Gastrophysa polygoni herbivory on rumex confertus: Single leaf VOC induction and dose dependent herbivore attraction/repellence to individual compounds. J Plant Physiol 168:2134–2138
Qualley AV, Dudareva N (2009) Metabolomics of plant volatiles. Methods Mol Biol 553:329–343
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737
Rasmann S, Ali JG, Helder J, van der Putten WH (2012a) Ecology and evolution of soil nematode chemotaxis. J Chem Ecol 38:615–628
Rasmann S, Hiltpold I, Ali J (2012b) The role of root-produced volatile secondary metabolites in mediating soil interactions. In: Advances in selected plant physiology aspects. Tech Open Access Publisher, Croatia, pp 269–290
Reinecke A, Müller F, Hilker M (2008) Attractiveness of CO2 released by root respiration fades on the background of root exudates. Basic Appl Ecol 9:568–576
Rouseff RL, Onagbola EO, Smoot JM, Stelinski LL (2008) Sulfur volatiles in guava (psidium guajava L.) leaves: Possible defense mechanism. J Agric Food Chem 56:8905–8910
Ruther J (2000) Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography–mass spectrometry. J Chromatogr A 890:313–319
Sanchez-Ortiz A, Romero-Segura C, Sanz C, Perez AG (2012) Synthesis of volatile compounds of virgin olive oil is limited by the lipoxygenase activity load during the oil extraction process. J Agric Food Chem 60:812–822
Sanz C, Ansorena D, Bello J, Cid CC (2001) Optimizing headspace temperature and time sampling for identification of volatile compounds in ground roasted Arabica coffee. J Agric Food Chem 49:1364–1369
Shiojiri K, Ozawa R, Matsui K, Kishimoto K, Kugimiya S, Takabayashi J (2006) Role of the lipoxygenase/lyase pathway of host-food plants in the host searching behavior of two parasitoid species, Cotesia glomerata and Cotesia plutellae. J Chem Ecol 32:969–979
Soler R, Van der Putten WH, Harvey JA, Vet LE, Dicke M, Bezemer TM (2012) Root herbivore effects on aboveground multitrophic interactions: patterns, process and mechanisms. J Chem Ecol 38:755–767
Sutherland ORW, Hillier JR (1972) Olfactory responses of Costelytra zealandica (Coleoptera: Melolonthinae) larvae to grass root odours. N Z J Sci 15:165–172
Tapia T, Perich F, Pardo F, Palma G, Quiroz A (2007) Identification of volatiles from differently aged red clover (Trifolium pratense) root extracts and behavioural responses of clover root borer (Hylastinus obscurus) (Marsham) (Coleoptera: Scolytidae) to them. Biochem Syst Ecol 35:61–67
Tressl R, Friese L, Fendesack F, Koppler H (1978) Studies of the volatile composition of hops during storage. J Agric Food Chem 26:1426–1430
Umano RP, Hagi Y, Shibamoto T (2002) Volatile chemicals identified in extracts from newly hybrid citrus, Dekopon (Shiranuhi mandarin Suppl. J.). J Agric Food Chem 50:5355–5359
Valim MF, Rouseff RL, Lin J (2003) Gas chromatographic-olfactometric characterization of aroma compounds in two types of cashew apple nectar. J Agric Food Chem 51:1010–1015
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr 11:463–471
van Herk W, Vernon R, (2013) Wireworm damage to wheat seedlings: effect of temperature and wireworm state. J Pest Sci 86:63–75
Varming C, Petersen MA, Poll L (2004) Comparison of isolation methods for the determination of important aroma compounds in blackcurrant (Ribes nigrum L.) juice, using nasal impact frequency profiling. J Agric Food Chem 52:1647–1652
Vilela GR, de Almeida GS, D’Arce MABR, Moraes MHD, Brito JO, da Silva MFGF, Silva SC, de Stefano Piedade SM, Calori-Domingues MA, da Gloria EM (2009) Activity of essential oil and its major compound 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus Xavus Link and Aspergillus parasiticus Speare. J Stored Prod Res 45:108–111
Viles AL, Reese RN (1996) Allelopathic potential of Echinacea angustifolia D.C. Environ Exp Bot 36:39–43
Weckerle B, Bastl-Borrmann R, Richling E, Hör K, Ruff C, Schreier P (2001) Cactus pear (Opuntia ficus indica) flavour constituents - chiral evaluation (MDGC–MS) and isotope ratio (HRGC–IRMS) analysis. Flavour Frag J 16:360–363
Wei A, Mura K, Shibamoto T (2001) Antioxidative activity of volatile chemicals extracted from beer. J Agric Food Chem 49:4097–4101
Weingart G, Kluger B, Forneck A, Krska R, Schumacher R (2011) Establishment and application of a metabolomics workflow for identification and profiling of volatiles from leaves of Vitis vinifera by HS-SPME-GC-MS. Phytochem Anal. doi:10.1002/pca.1364
Weissteiner S, Huetteroth W, Kollmann M, Weißbecker B, Romani R, Schachtner J, Schütz S (2012) Cockchafer larvae smell host root scents in soil. PLoS One 7(10)
Wenda-Piesik A, Piesik D, Ligor T, Buszewski B (2010) Volatile organic compounds (VOCs) from cereal plants infested with crown rot: Their identity and their capacity for inducing production of VOCs in uninfested plants. Int J Pest Manag 56:377–383
Wenke K, Kai M, Piechulla B (2010) Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 231:499–506
Witcosky JJ, Schowalter TD, Hansen EM (1987) Host-derived attractants for the beetles Hylastes nigrinus (Coleoptera: Scolytidae) and Steremnius carinatus (Coleoptera: Curculionidae). Environ Entomol 16:1310–1313
Wu S, Krings U, Zorn H, Berger RG (2005) Volatile compounds from the fruiting bodies of beefsteak fungus Fistulina hepatica (Schaeffer: Fr.) Fr. Food Chem 92:221–226
Yi HS, Heil M, Adame-Alvarez RM, Ballhorn DJ, Ryu CM (2009) Airborne induction and priming of plant defenses against a bacterial pathogen. Plant Physiol 151:2152–2161
Acknowledgments
The authors thank Marc Camerman and Franck Michels for research assistance, Dr. Marie Fiers for providing standards. Gembloux Agro-Bio Tech (University of Liège) funded the present project (Rhizovol project).
Author information
Authors and Affiliations
Corresponding author
Additional information
Aurélie Gfeller and Morgan Laloux contributed equally to the work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
Dual-choice olfactometer used for the orientation experimentation of Agriotes sordidus towards all potential olfactory stimuli emitted by 7-d-old barley roots, i.e. CO2 and other VOCs. The roots were inserted through one of the lateral connections (a) and separated from the substrate with stainless gauze (b). Each wireworm was inserted into the device through the middle entry connection (c) and retrieved from the pipes with the substrate through one of the two endings (standard GL45 screwed ends and caps) (d). (DOC 48 kb)
Figure S2
Optimization of the HS-SPME-GC-MS method. Comparison of VOC profiles released by roots of barley and trapped on four types of fibre: A = polyacrylate (PA), B = polydimethylsiloxane (PDMS), C = carboxen/polydimethylsiloxane (CAR/PDMS), and D, E = divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS). (A) to D) column HP5ms, (E) column VF-WAXms. (DOC 749 kb)
Figure S3
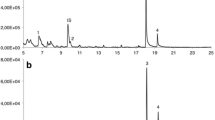
Typical GC–MS chromatogram of the SPME analysis of VOCs emitted by excised 21-d-old barley roots. Numbers correspond to the following compounds: 1, Dimethyl sulfide; 2, Hexanal; 3, Methyl hexanoate; 4, (E)-Hex-2-enal; 5, 2-Pentylfuran; 6, Pentan-1-ol; 7, 2-(Pentenyl)furan; 8, (Z)-Pent-2-en-1-ol; 9, 6-Methyl-hept-5-en-2-one; 10, Hexan-1-ol; 11, (Z)-Hex-3-en-1-ol; 12, (E)-Hex-2-en-1-ol; 13, Oct-1-en-3-ol; 14, 2-Ethylhexan-1-ol; 15, (E)-Non-2-enal; 16, Dimethyl sulfoxide; 17, Octan-1-ol; 18, (2E,6Z)-Nona-2,6-dienal; 19, Methyl (E)-non-2-enoate; 20, Methyl benzoate; 21, Nonan-1-ol; 22, (Z)-Non-3-en-1-ol; 23, (E)-Non-2-en-1-ol; 24, Nona-3,6-dien-1-ol; 25, Nona-2,6-dien-1-ol; 26, Tetradecanal; 27, Dodecan-1-ol; 28, Dihydro-5-pentyl-2(3H)-furanone; 29, Hexadecanal. (PPTX 193 kb)
Figure S4
Typical GC–MS chromatogram of the SPME analysis of VOCs emitted by excised 7 d-old barley roots grown aseptically in Hoagland fertilized vermiculite. Numbers correspond to the following compounds: 1, Dimethyl sulfide; 2, Pentan-3-one; 3, Hexanal; 4, Butan-1-ol; 5, Pent-1-en-3-ol; 6, (E)-Hex-2-enal; 7, 2-Pentylfuran; 8, Pentan-1-ol; 9, (Z)-2-(Pentenyl)furan; 10, (Z)-Pent-2-en-1-ol; 11, Oct-6-en-2-one; 12, Hexan-1-ol; 13, (E)-Hex-2-en-1-ol; 14, Oct-1-en-3-ol; 15, Heptan-1-ol; 16, 6-Methylhept-5-en-2-ol; 17, 2-Ethylhexan-1-ol; 18, (E)-Non-2-enal; 19, Dimethyl sulfoxide; 20, Octan-1-ol; 21, (2E,6Z)-Nona-2,6-dienal; 22, Methyl (E)-non-2-enoate; 23, Methyl benzoate; 24, Nonan-1-ol; 25, (Z)-Non-3-en-1-ol; 26, (E)-Non-2-en-1-ol; 27, Nona-3,6-dien-1-ol; 28, Nona-2,6-dien-1-ol; 29, 2-Phenylethanol; 30, Dodecan-1-ol; 31, Methyl tetradecanoate; 32, Dihydro-5-pentyl-2(3H)-furanone; 33, Hexadecanal. (PPTX 190 kb)
ESM 1
(DOC 33 kb)
Table S1
(DOC 33.5 kb)
Rights and permissions
About this article
Cite this article
Gfeller, A., Laloux, M., Barsics, F. et al. Characterization of Volatile Organic Compounds Emitted by Barley (Hordeum vulgare L.) Roots and Their Attractiveness to Wireworms. J Chem Ecol 39, 1129–1139 (2013). https://doi.org/10.1007/s10886-013-0302-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0302-3